Abstract
Background
Studies describing treatment utilization for castration-resistant prostate cancer (CRPC) are limited. We aimed to describe the treatment utilization of a contemporary population-based CRPC cohort between 2006 and 2016.
Methods
We identified 1699 men with a PC diagnosis between 2005 and 2015, who developed CRPC between 2006 and 2015 in the Stockholm region of Sweden. Demographic information, stage and grade at PC diagnosis, stage at CRPC, prostate-specific antigen (PSA) nadir, PSA doubling time, treatment utilization rate within 1 year of CRPC diagnosis, reason for stopping therapy, treatment sequence trajectory, overall and PC specific survival was described.
Results
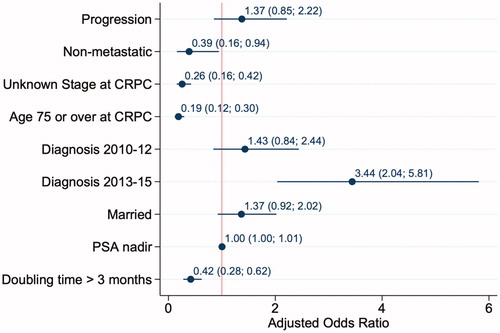
Treatment for men with de novo metastatic disease (n = 463) was 32%, treatment for men with progressive metastatic disease after PC diagnosis (n = 66) was 44%, treatment for men with nonmetastatic CRPC (n = 113) was 34% and treatment for those with an unknown stage at time of CRPC diagnosis (n = 857) was 12%. Docetaxel was used in 39%, abiraterone acetate plus prednisone in 15%, enzalutamide in 13%, cabazitaxel in 11% and radium-223 in 5% of treatments. Treatment increased from 22% in 2006–2009 for metastatic cancer to 50% in 2013–2015 (p < .001). Factors associated with treatment were an unknown stage at diagnosis (OR: 0.3, 95% CI: 0.2–0.4), age ≥75 years (OR: 0.2, 95% CI: 0.1 − 0.3), PSA doubling time >3 months (OR: 0.4, 95% CI: 0.3 − 0.6) and a diagnosis between 2013 and 2015 (OR: 3.4, 95% CI: 2.0 − 5.8).
Conclusions
Despite treatment availability, in this large real-world cohort we found treatment utilization to remain low.
Introduction
Prostate cancer (PC) is the third leading cause of cancer related death of men in Europe [Citation1]. Currently the gold standard for men diagnosed with metastatic PC is androgen deprivation, however, when treated long enough most men will develop castrate resistant PC (CRPC) [Citation2]. CRPC is the leading cause of PC related deaths, which amount to more than 30,000 annually in the United States and 375,000 annually worldwide [Citation1,Citation3]. Historically, overall survival (OS) of non-metastatic CRPC (nmCRPC) is 5 years [Citation4] and metastatic CRPC (mCRPC) is less than 2 years [Citation5,Citation6]. More recently, non-hormonal systemic treatments and novel androgen receptor treatments (ARTs) have helped control the disease, manage symptoms and improved OS [Citation7].
While it is encouraging to observe such such positive results from clinical trials, it has been important to confirm the effectiveness in real-world evidence (RWE) studies [Citation8–15]. However, few studies analyze treatment utilization for CRPC patients [Citation16,Citation17]. The only known population-based study that examined treatment utilization for CRPC patients reported only 40% utilization for metastatic CRPC patients [Citation16]. It is unclear what factors may explain a slower adaption of therapy utilization or treatment deferment in men with CRPC [Citation16–18]. In this population-based study, we aimed to understand treatment utilization in Sweden between 2006 and 2015 when non-hormonal systemic treatments and ARTs became available. Specifically, we aimed to analyze treatment patterns, OS, and PC specific survival during this 10-year span and amongst different CRPC disease stages.
Material and methods
Ethical approval was obtained from the regional ethics committee in Stockholm and informed consent was waived (Dnr: 2017/505-32). The primary patient data source was the STHLM-0 database, a population-based register containing data on every prostate-specific antigen (PSA) test and prostate biopsy for men residing in the Stockholm region (population 1.8 million in 2005 to 2.2 million in 2015) [Citation19]. The STHLM-0 register has been linked to the national cancer, cause of death and prescribed drug registers with unique national personal identification numbers. All men in STHLM-0 with a diagnosis of PC from 2005 to 2015 were considered for this study (n = 21,695).
CRPC criteria
Patients diagnosed with PC between 2005 and 2015 who met criteria for medical or surgical castration and later developed CRPC between 2006 and 2015 were included. Prior methods for determining CRPC are described previously [Citation20].
CRPC oncologic follow up
After the CRPC cohort was defined (n = 1712), a retrospective chart review was performed starting from the date of PC diagnosis. A total of 13 patients were removed due to data quality and data linkage problems resulting in a total of 1699 men. The review was performed at the oncology sites (Södersjukhuset and Karolinska Solna) in the Stockholm region that treat PC patients with chemotherapy, non-hormonal systemic treatments and ARTs with treatment exposure data through September 2016 and OS data through October 2017. No patients from clinical trials were included. Data collection included treatment start date and treatment regimen. All patients received continued androgen suppression after CRPC diagnosis. Treatment side effects and the occurrence of palliative radiation was recorded. The oncology clinics were separated by electronic medical record on 30 September 2016 and not all follow up data were observable after this date.
Stage of CRPC
Patient stage at PC and at CRPC diagnosis was determined. The American Joint Committee on Cancer TNM staging system was used. Patients were separated into two categories; distant metastasis (M1), or no/not assessed distant metastasis (M0/Mx). The interval of 3 months prior to and 6 months after the CPRC reference date was used to assess cross sectional imaging (magnetic resonance imaging, computed tomography), bone scintigraphy or positron emission tomography for evidence of metastatic disease. Importantly, there was no study protocol for imaging and was rather based on clinical decision making. Patients were then separated into three categories at CRPC: M1, M0 or Mx disease. Using metastatic information at PC diagnosis and at CRPC diagnosis, men were then separated into four groups (with two groups for mCRPC men): men with de novo metastatic disease (M1) at PC diagnosis, M0/Mx at PC diagnosis which progressed to metastatic disease (M1) at time of CRPC diagnosis, men that were nonmetastatic (M0) at the time of CRPC diagnosis (nmCRPC) and men with unknown status of metastatic disease (Mx) at time of CRPC diagnosis (Supplementary Table 1).
Table 1. Men with castration-resistant prostate cancer (n = 1699).
CRPC prognostic and outcome measures
Patient demographics were recorded at time of CRPC diagnosis. Age, stage, ISUP Gleason Grade Group, PSA at PC diagnosis and PSA doubling time at CRPC diagnosis was included. PSA nadir was calculated after castration, the first treatment and second treatment.
Statistical analysis
The distribution of number of treatments per patient and calendar year was summarized graphically using bar charts, whereas treatment sequencing patterns were illustrated using alluvial plots. In these analyses all treatments, irrespective of timing in relation to the CRPC diagnosis, were included. To formally address the temporal trends in treatment utilization and factors that were associated with treatment a 12-month follow-up window after the CRPC diagnosis was defined. Only men with at least 12 months of potential follow-up were included in the analyses of treatment utilization (n = 1665).
Chi-squared tests were used to compare treatment utilization within 12 months from CRPC, by epoch (2006–2009, 2010–2012 and 2013–2015). Multivariable logistic regression was performed to study the association with treatment within 12 months of CRPC diagnosis. Included covariates were: age, marriage status, education level, PSA nadir after castration, PSA doubling time, year of CRPC diagnosis and CRPC stage. Odds ratios were reported with 95% confidence intervals and two-sided p values.
Kaplan–Meier estimates of OS, PC-specific survival (PCSS) were estimated. The Stata software (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC) and R (R Core Team (2014). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/) were used to perform the statistical analyses.
Results
We identified 1699 men with a PC diagnosed in 2005–2015 and CRPC between years 2006 and 2015 with a median follow up of treatment utilization of 20 months after CRPC diagnosis. describes the overall CRPC cohort. We identified 27% (463) of men with de novo metastatic PC, 16% (266) of men with progressively metastatic disease after PC diagnosis, 7% (113) men with nonmetastatic CRPC, and 50% (857) men with an unknown stage of disease at the time of CRPC. A description of the patient cohort groups is described in .
Table 2. Men with castration-resistant prostate cancer (n = 1699).
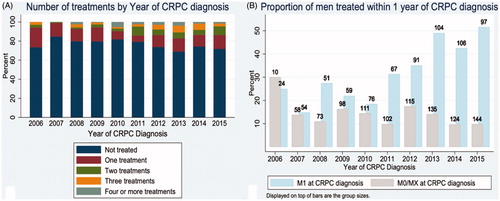
Overall, 76% (1292) of men received no treatment within 12 months of CRPC diagnosis while 24% (407) of men received at least one form of treatment for CRPC. Of men with metastatic CRPC, 36% (266/729) received treatment within 1 year of CRPC diagnosis. Of those treated, 48% (196) received a second line therapy, 26% (105) received a third line treatment and 10% (41) received 4 or more treatments during oncologic follow up (). shows the different treatment regimens prescribed over the 10-year study period. About 50% (n = 153/307) of metastatic CRPC patients received treatment within 1 year of diagnosis during the last epoch 2013–2015 compared to 22% in the first epoch from 2006 to 2009 (p < .001). shows yearly trends in treatment administration within 1 year of CRPC diagnosis if the patient had metastatic disease at the time of CRPC or was otherwise nonmetastatic or not staged at time of CRPC diagnosis.
Figure 1. Treatment utilization within 1 year of diagnosis over time – (A) The number of overall treatments lines per year of castrate resistant prostate cancer (CRPC) diagnosis and (B) the proportion of men treated within 1 year of CRPC diagnosis separated by metastatic disease (M1) at time of diagnosis and nonmetastatic (M0) or unknown stage (Mx) at time of CRPC diagnosis.
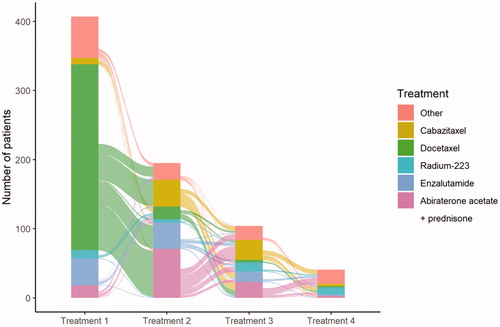
In total 747 treatments were given: 407 first-line, 195 second-line, 104 third-line and 41 fourth-line therapies were prescribed. Docetaxel was the most frequently used treatment and was used in 39% (293/747) of men who received some form of treatment, followed by abiraterone acetate plus prednisone in 15% (115/747), enzalutamide in 13% (94/747), cabazitaxel in 11% (80/747) and radium-223 in 5% (40/747) of men treated for CRPC. depicts treatment trajectories between line of therapy with an Alluvial plot.
Figure 2. Overall treatment trajectory by sequence – Alluvial plot showing treatment regimen trajectory for castrate resistant prostate cancer by line of treatment regimen.
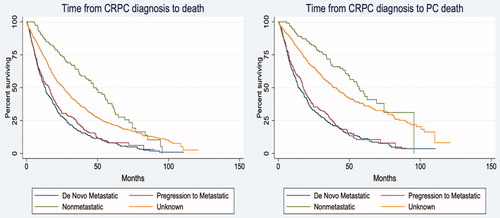
The median OS from CRPC diagnosis was 20 months and the median PCSS was 30 months. Kaplan–Meier OS and PCSS for the four CRPC stage groups are shown in . The median OS for men with de novo metastatic PC was 13 months, for progressive metastatic disease after PC diagnosis was 16 months, for nmCRPC was 47 months; for an unknown stage at time of CRPC diagnosis was 26 months ().
Figure 3. Overall and prostate cancer specific survival (A) Kaplan–Meier overall survival estimation by stage at time of castrate resistant prostate cancer (CRPC) diagnosis and (B) Kaplan-Meier prostate cancer specific survival estimation by stage at time of CRPC diagnosis.
describes the factors associated with being selected to treatment. Factors associated with not receiving treatment were having an unknown stage at diagnosis (OR: 0.3, 95% CI: 0.2 − 0.4), age ≥75 years (OR: 0.2, 95% CI: 0.1 − 0.3) and PSA doubling time >3 months (OR: 0.4, 95% CI: 0.3 − 0.6). Factors associated with receiving treatment were a diagnosis between 2013 and 2015 (OR: 3.4, 95% CI: 2.0 − 5.8).
Discussion
This study includes a large sample size of CRPC patients within the Stockholm region of Sweden and includes all patients diagnosed with PC during the years 2005 and 2015 that developed a register-based definition of CRPC. Overall the treatment utilization within 1 year after CRPC diagnosis during this 10-year period was low. Contributing factors for receiving treatment included having metastatic disease at time of CRPC, a later year of CRPC diagnosis, age less than 75 years, and a PSA doubling time ≤3 months.
Importantly, our inclusion criteria for CRPC included a PSA doubling (>100% increase) from nadir and >2 ng/mL or an absolute increase >5 ng/mL from nadir. This, more conservative, definition of CRPC likely excluded some of the lower risk CRPC men and can in part explain the short PSA doubling time observed (3 months) in this cohort and shorter OS. However, although our definition of CRPC strays from the PCWG consensus [Citation21], we found that our definition was more objective than other real world studies, where inclusion criteria may be more vague, such as PC-specific death [Citation18], progression to castrate resistance as determined by the treating physician [Citation16], the use of non-specific billing codes or insurance claims [Citation22,Citation23] or simply exposure to CRPC-specific treatments [Citation24]. Using our CRPC definition, we were able to identify 1699 men, with treatment exposure data making this cohort one of the largest RWE CRPC treatment studies.
The treatment regimens indicated for CRPC have drastically increased in the last decade. Since the landmark trials published in 2004, with an OS benefit with docetaxel for mCRPC [Citation6], five other medications have been approved for first-line therapy of mCRPC with OS benefit including: cabazitaxel (2010), sipuleucel-T (2010), abiraterone acetate plus prednisone (2013), radium-223 (2013) and enzalutamide (2014) [Citation7]. Approvals of therapies with enzalutamide (2018), apalutamide (2018) (also introduced as a mCRPC treatment) and darolutamide (2019) [Citation4], with a significant OS improvement have further expanded therapy options for nmCRPC. Importantly, our study included treatment exposure data from 2006 until 2016. In 2005, the EAU guidelines for PC first included docetaxel as a grade A recommendation for first-line treatment of metastatic CRPC [Citation25]. This recommendation was based on evidence showing a modest improvement in survival by 2 months. It was not until 2011 EAU guidelines that another agent was recommended for first-line treatment of metastatic CRPC as secondary hormonal management with abiraterone acetate with prednisone or enzalutamide, however, this was deemed only a grade C recommendation which may have also tempered utilization [Citation26]. It was not until the 2014 EAU guidelines that a grade A recommendation was given for another agent (abiraterone acetate plus prednisone) as first line therapy for metastatic CRPC [Citation27]. Importantly, treatment of non-metastatic CRPC was not included in EAU guidelines outside of clinical trials until 2019 [Citation28].
Prospective initiatives such as the Treatment Registry for Outcomes in CRPC Patients (TRUMPET)[Citation29] will help determine more contemporary clinical treatment practice patterns outside of trials, however, the true utilization of treatments have not been well described. A population-based mCRPC study in the Netherlands, described treatment utilization from 2010 to 2013 of approximately 1500 patients. In this study, the authors found trial rate of docetaxel administration was 85% versus 40% in a non-trial setting [Citation16]. George et al. similarly described treatment utilization while using a stringent inclusion criterion for mCRPC patients in the US from 2013 to 2017, and found a treatment rate of 77%, though contributing factors to treatment was unknown and median age in this study was 74 years [Citation17]. These rates are comparable to the findings from our study. When looking at mCRPC in the last epoch (2013–2015), we observed treatment rates within 1 year of CRPC diagnosis at 50%. Treatment rates were low in the earliest epoch (2006–2009), however, it is possible during this time patients with non-symptomatic or slow progressing mCRPC may not have received treatment. We observed a significant increase in the treatment utilization over the decade of study, however, considering this data lacks treatment exposure data from the most recent 5 years, we would expect higher utilization in current practice.
We know PC affects older men disproportionately, however, many believe CRPC treatment outcomes and tolerability are worse in older patients yet efficacy may actually be sustained [Citation11,Citation30,Citation31]. In this cohort, we found that younger age was an independent predictor of receiving treatment. The Dutch RWE study highlighted the younger age in trial patients (median 67 years) versus standard care patients (median 76 years) [Citation16]. In our study cohort median age was 77 years, which varied across CRPC stage types. Treatment benefit has been shown in men ≥75 years [Citation30,Citation31], as well as efficacy in octogenarians [Citation32]. Despite these findings in this study, we found low treatment rates for elderly patients, which suggests an underutilization in this group. Importantly, we did not have information on performance status, which is a known predictor of treatment response and as such has been included in guidelines for treatment guidance, whereas age has not [Citation21].
Overall, we observed treatment rates of 24% and as high as 58% for progressive mCRPC patients in the last epoch. Here we describe patterns that are consistent with other population-based approaches, however, we found much lower treatment rates than may be expected for patients presenting to an oncologist in the Stockholm region. Our findings would suggest, then, the lower treatment utilization may also be explained by a suboptimal referral from urologists and other non-oncologic providers preventing oncologic evaluation of all CRPC patients within the population. These findings further underscore the need for true population-based approach and promote the need for referral to providers that provide systemic CRPC therapy to fully estimate absolute utilization.
Strengths and limitations
This study uses national population-based registers with a retrospective analysis and has inherent limitations with missing data and nonstandard follow-up frequencies. Although we used objective measures of PSA recurrence after castration (defined by medical prescription or surgical castration billing codes), we were unable to confirm a true castrate state with serum testosterone levels. Furthermore, our study cohort definition included men diagnosed with PC from 2005 to 2015 who developed CRPC between 2006 and 2015. Therefore, by design, men diagnosed with CRPC earlier in the study may represent a more rapid disease type rather than later in the cohort, with likely a mix of both rapid CRPC development as well as more indolent CRPC disease. In addition, men with CRPC during 2006–2015 that were diagnosed with PC prior to 2005 were not included, which would likely exclude men with the slowest progression to CRPC, thus creating a more aggressive cohort in this study compared to the entire CRPC population. In terms of stage, patients were categorized into metastatic disease versus non-metastatic disease at time of CRPC, however, we do not distinguish between metastatic disease to regional lymph nodes versus skeletal disease versus visceral disease which are known to have different prognosis and is a limitation of this analysis. Additionally, in terms of treatment, men early in the study period had more time and thus more opportunity to receive treatment and multiple lines of treatment. For this reason, when analyzing treatment trends, we chose to use a distinct period within 1 year of CRPC diagnosis to eliminate this bias, however, other descriptive statistics seen in may reflect this limitation.
Conclusions
We observed an overall treatment utilization of 24% for CRPC during a decade of changing treatment availability from 2006 to 2015 and a treatment rate of 50% in the last epoch from 2013 to 2015 for mCRPC patients. We observed that treatment utilization may be associated with younger age (<75 years) and a faster PSA doubling time (≤3 months). This large real-world cohort suggests that treatment utilization for CRPC is low. Additional studies are needed to better understand why the treatment rates are low and if men with CRPC in Sweden are undertreated.
| Abbreviations | ||
| PC | = | prostate cancer |
| CRPC | = | castration-resistant prostate cancer |
| OS | = | overall survival |
| nmCRPC | = | non-metastatic castration-resistant prostate cancer |
| mCRPC | = | metastatic castration-resistant prostate cancer |
| ART | = | androgen recept0r treatments |
| RWE | = | real-world evidence |
| PSA | = | prostate-specific antigen |
| ISUP | = | International Society of Urologic Pathologists |
| PCSS | = | prostate cancer-specific survival |
| TRUMPET | = | Treatment Registry for Outcomes in CRPC Patients |
Supplemental Material
Download MS Word (5.7 MB)Disclosure statement
Level, Dearden, Liwing, Mehra and Nair are employees of Janssen. Schain is a former employee of Janssen and an owner and employed by Schain Research (a consultancy service related to studies for Janssen).
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA A Cancer J Clin. 2019;69(1):7–34.
- Denis LJ, Carneiro De Moura JL, Bono A, et al. Goserelin acetate and flutamide versus bilateral orchiectomy: a phase III EORTC trial (30853). EORTC GU Group and EORTC Data Center. Urology. 1993;42(2):119–129.
- Global Cancer Observatory, International Agency for Research on Cancer, World Health Organization. 2018. May 1, 2020]; Available from: https://gco.iarc.fr/
- Esther J, Maughan BL, Anderson N, et al. Management of nonmetastatic castration-resistant prostate cancer: recent advances and future direction. Curr Treat Options Oncol. 2019;20(2):14.
- Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513–1520.
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–1512.
- Ritch CR, Cookson MS. Advances in the management of castration resistant prostate cancer. BMJ. 2016;355:i4405.
- Boegemann M, Khaksar S, Bera G, et al. Abiraterone acetate plus prednisone for the management of metastatic castration-resistant prostate cancer (mCRPC) without prior use of chemotherapy: report from a large, international, real-world retrospective cohort study. BMC Cancer. 2019;19(1):60.
- Boni G, Mazzarri S, Cianci C, et al. (223)Ra-chloride therapy in men with hormone-refractory prostate cancer and skeletal metastases: real-world experience. Tumori. 2018;104(2):128–136.
- Joshua AM, Shore ND, Saad F, et al. Safety of enzalutamide in patients with metastatic castration-resistant prostate cancer previously treated with docetaxel: expanded access in North America. Prostate. 2015;75(8):836–844.
- Maines F, Caffo O, Giorgi UD, et al. Safety and clinical outcomes of Abiraterone acetate after docetaxel in octogenarians with metastatic castration-resistant prostate cancer: results of the Italian compassionate use named patient programme. Clin Genitourin Cancer. 2016;14(1):48–55.
- Nakano K, Ohta S, Komatsu K, et al. Docetaxel with or without estramustine for estramustine refractory castration-resistant prostate cancer: a single institution experience. BMC Urol. 2012;12(1):3.
- Pezaro CJ, Omlin AG, Altavilla A, et al. Activity of cabazitaxel in castration-resistant prostate cancer progressing after docetaxel and next-generation endocrine agents. Eur Urol. 2014;66(3):459–465.
- Sartor O, Vogelzang NJ, Sweeney C, et al. Radium-223 safety, efficacy, and concurrent use with Abiraterone or Enzalutamide: first U.S. experience from an expanded access program. Oncology. 2018;23(2):193–202.
- Shivji A, Ali R, North S, et al. Real world evidence: Abiraterone use post-docetaxel in metastatic castrate-resistant prostate cancer. J Oncol Pharm Pract. 2019;25(6):1293–1300.
- Westgeest HM, Uyl-de Groot CA, van Moorselaar RJA, et al. Differences in trial and real-world populations in the Dutch castration-resistant prostate cancer registry. Eur Urol Focus. 2018;4(5):694–701.
- George DJ, Sartor O, Miller K, et al. Treatment patterns and outcomes in patients with metastatic castration-resistant prostate cancer in a real-world clinical practice setting in the United States. Clin Genitourin Cancer. 2020;18:284–294.
- Lissbrant IF, Garmo H, Widmark A, et al. Population-based study on use of chemotherapy in men with castration resistant prostate cancer. Acta Oncol. 2013;52(8):1593–1601.
- Nordstrom T, Aly M, Clements MS, et al. Prostate-specific antigen (PSA) testing is prevalent and increasing in Stockholm County, Sweden, Despite no recommendations for PSA screening: results from a population-based study, 2003–2011. Eur Urol. 2013;63(3):419–425.
- Aly M, Leval A, Schain F, et al. Survival in patients diagnosed with castration-resistant prostate cancer: a population-based observational study in Sweden. Scand J Urol. 2020;54:115–121.
- Lowrance WT, Murad MH, Oh WK, et al. Castration-resistant prostate cancer: AUA guideline amendment 2018. J Urol. 2018;200(6):1264–1272.
- Franck Lissbrant I, Ventimiglia E, Robinson D, et al. Nationwide population-based study on the use of novel antiandrogens in men with prostate cancer in Sweden. Scand J Urol. 2018;52(2):143–150.
- Wen L, Valderrama A, Costantino ME, et al. Real-world treatment patterns in patients with castrate-resistant prostate cancer and bone metastases. Am Health Drug Benefits. 2019;12(3):142–149.
- Flaig TW, Potluri RC, Ng Y, et al. Treatment evolution for metastatic castration-resistant prostate cancer with recent introduction of novel agents: retrospective analysis of real-world data. Cancer Med. 2016;5(2):182–191.
- Aus G, Abbou CC, Bolla M, et al. EAU guidelines on prostate cancer. Eur Urol. 2005;48(4):546–551.
- Mottet N, Bellmunt J, Bolla M, et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2011;59(4):572–583.
- Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65(2):467–479.
- Cornford P, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer. Part II-2020 update: treatment of relapsing and metastatic prostate cancer. Eur Urol. 2021;79(2):263–282.
- Penson DF, Lin DW, Karsh L, et al. Treatment registry for outcomes in patients with castration-resistant prostate cancer (TRUMPET): a methodology for real-world evidence and research. Fut Oncol. 2016;12(23):2689–2699.
- Graff JN, Baciarello G, Armstrong AJ, et al. Efficacy and safety of enzalutamide in patients 75 years or older with chemotherapy-naive metastatic castration-resistant prostate cancer: results from PREVAIL. Ann Oncol. 2016;27(2):286–294.
- Horgan AM, Seruga B, Pond GR, et al. Tolerability and efficacy of docetaxel in older men with metastatic castrate-resistant prostate cancer (mCRPC) in the TAX 327 trial. J Geriatr Oncol. 2014;5(2) :119–126.
- de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005.