Abstract
Objectives
To assess treatment response (PSA < 0.2 ng/ml), need for additional therapy and complication rate after robot assisted salvage pelvic lymph node dissection (sPLND)
Material and Methods
Analysis of outcomes data from radical prostatectomy (RP) patients consecutively operated with robot assisted sPLND due to biochemical recurrence and positron-emission tomography (PET)/computed tomography (CT)-detected nodal recurrence of pelvic lymph nodes.
Results
Sixty-nine patients underwent robotic sPLND after a median time of 47 months post- RP. Sixty-four patients (93%) had malignant lymph nodes upon histological assessment of sPLND specimen. Twenty patients (29%) achieved PSA < 0.2 ng/ml 6 weeks postoperatively. After median (IQR) follow-up of 15 months (10–27), fourteen patients (20%) still had PSA < 0.2 ng/ml without additional therapy and forty-one patients (59%) had started additional therapy. No significant predictor for treatment response was found. Postoperative complications occurred in 14 patients (20%). Eleven of these complications were classified as Clavien-Dindo grade 1
Conclusion
Oncological benefit of sPLND as the only salvage procedure seems to be limited, though almost one third of patients achieved treatment response. Clinical trials are needed to determine if sPLND as part of a multimodal treatment may improve outcome.
Introduction
After radical prostatectomy (RP), 33–40% of patients experience biochemical recurrence (BCR) [Citation1,Citation2]. Improved imaging, particular positron-emission tomography (PET)/computed tomography (CT) techniques, has enabled early detection of lymph node metastasis [Citation3–5]. In this context, metastasis directed therapy (MDT) either as stereotactic ablative radiotherapy (SABR) or salvage lymph node dissection (sLND), has emerged as an alternative option to standard androgen deprivation therapy (ADT). Several retrospective systematic reviews and studies on the safety and effectiveness of open pelvic- and retroperitoneal sLND, are available [Citation6–9]. Common for many these reports are the high rate of biochemical recurrence (BCR) after sLND. Recently, Bravi et al. [Citation10] reported results from a long-term follow-up (FU) study in 189 patients operated with sLND between 2002 and 2011. At a median FU of 87 months, 45 patients (24%) had died from PCa. Clinical recurrence-free survival and BCR-free survival at 10 years were 31% and 11%, respectively, and a total of 145 patients (77%) had received ADT. Such data indicate that MDT alone offer limited oncological benefit.
One argument often used in favour of sLND is that it may delay start of ADT. In a randomizedphase II trial by Ost et al. [Citation11], ADT-free survival was 13 months for the surveillance group and 21 months for the MDT group. In this study MDT was given as sLND or SABR.
In most of the reported sLND studies an open surgical approach was performed, which might be associated with increased perioperative morbidity compared to minimal-invasive approaches [Citation12]. To date, a few retrospective series on robotic sLND have been published [Citation13–16]. These studies included between 16 and 36 patients. One of these studies covered robotic salvage pelvic lymph node dissection (sPLND) only [Citation14], similar to our study, while the others [Citation13,Citation15,Citation16] also included retroperitoneal sLND.
At our institution fluorine-18-labeled -fluciclovine PET/CT was introduced in October 2012 as imaging of RP patients with BCR. Recently this tracer was replaced with fluorine-18 labelled prostate-specific membrane antigen (PSMA).
In this study we assess our current experience with robotic sPLND. Our aims are to investigate treatment response after robotic sPLND, the time to start of additional treatment and factors associated with these two events. Furthermore, we assessed complications related to this mini-invasive procedure.
Material and methods
Patients
Our study population consists of 69 patients consecutively operated with sPLND at the Oslo University Hospital from June 2014 to December 2018. All patients had post-RP BCR (PSA ≥ 0.2 ng/ml) and lymph node recurrence detected by PET/CT. None of the patients used ADT upon referral for sPLND, and none had distant metastases detected on MRI or bonescan.
PET imaging
All patients underwent PET/CT before sPLND, either with 18 F-fluciclovine (49 patients), 18 F-PSMA-1007 (19 patients) or 18 F-fluorodeoxyglucose(FDG) (1 patient) [Citation17,Citation18]. Imaging was performed on a Siemens Biograph mCT 40 or on a GE Discovery 690 PET/CT-scanner for all but two patients (PET preformed at St.Olavs Hospital,Trondheim and in Aarhus, Denmark). Routine PET/CT protocols and reports were applied pre-sPLND.
Surgical procedures
The da Vinci Surgical system was used with a six‐port configuration to perform the transperitoneal sPLND procedures (Intuitive Surgical, Sunnyvale, CA, USA). Depending upon previous treatments and findings on imaging, the extent and mode of sPLND was left to the discretion of the surgeon and ranged from targeted removal of the PET positive node(s) to a bilateral or unilateral extended sPLND. Targeted removal of nodes was preferred in patients previously operated with PLND concomitant to RP. Extended sPLND encompassed the internal iliac, obturator, external iliac and common iliac landing areas. Two patients had removal of vesicula seminalis and sPLND due to recurrence in both sites. The length of stay in the hospital after sPLND was routinely one night, the patient arrived at the hospital the same day they were operated and went home the day after without urinary catheter.
Histopathology
Histopathological evaluation of the sPLND specimen assessed the total number of excised lymph nodes, the number of metastatic lymph nodes and the laterality of the excised metastatic nodes. Information of previous RP specimens was retrieved from the medical records (pathological T stage, surgical margins and Gleason ISUP Gleason Grade Groups (GGG)).
Follow up
Postoperative follow-up (FU) consisted of outpatient visits at 6 and 12 weeks at our institution with clinical examination and PSA evaluation. Thereafter the patients were followed by their general practitioner with 3 months intervals, in collaboration with the urological department at the local hospital. PSA, clinical recurrence, postoperative complications and start of the first additional treatment post-sPLND were recorded. Eventual start of ADT was generally offered to patients with rising PSA post-sPLND with or without clinically detected recurrence, but was left to the physician’s discretion and patient preference.
Outcome variables
The primary endpoint was treatment response defined as the percentage of patients obtaining PSA < 0.2 ng/ml 6 weeks after sPLND. The type and time of any additional treatment was recorded. Clinical recurrence was based on metastatic findings on MRI or PET/CT imaging post sPLND. Finally, complications were reported post-sPLND according to the Clavien–Dindo classification system [Citation19]. The study was approved by the Regional Ethical Committee (REK 2016/1977), The Data Protection Officer, and the Institutional Review Board.
Statistics
Frequencies and percentages of categorical variables and medians and interquartile ranges (IQR) for continuous variables were calculated. Intergroup differences between targeted sPLND, unilateral extended or bilateral extended sPLND template, were assessed by chi-squared tests for categorical variables and Wilcoxon rank sum test for skewed continuous variables. Multivariable Cox regression was used to predict additional treatment after SLND. Predictors consisted of GGG < 4 vs GGG ≥ 4, time from RP to sPLND, number of positive lesions on PET/CT scan (two and three or more vs one as reference), and PSA level at sPLND. The patients were followed up until December 31, 2019 (end of the study). The Kaplan-Meier estimates displayed survival free of additional treatment. The level of significance was set at 0.05. Data were analysed using the IBM Statistical Package for the Social Sciences (SPSS) Statistics version 25.
Results
Background characteristics
Histology of the RP specimen showed ≥ pT3a in 76% of the patients and 73% with GGG ≥ 3 (). Thirty patients (43%) had concomitant PLND at RP and of these patients almost one third had metastatic lymph nodes. Thirty-seven patients (54%) had undergone post-RP pelvic radiotherapy before referral for sPLND ().
Table 1. Patient characteristics, n = 69.
sPLND results
Median age pre-sPLND was 67 years (). Sixty patients (85%) had only 1 or 2 positive lesions on PET/CT. Median number of positive lesions was 1 (range 1–20 lesions). The median number of excised lymph nodes was 19 for bilateral extended sPLND (). The median number of metastatic lymph nodes removed was three nodes both for unilateral- and bilateral extended sPLND (). No metastatic lymph nodes were described in the sPLND histopathological specimen of five patients (7%). Re-evaluation of the PET images pre-sPLND for these five patients showed that in three patients the PET reported positive pelvic nodes were outside the surgical field (mesorectal nodes in two patients and an inguinal node in one patient). For the other two patients the re-evaluation changed the description of the PET image from positive to equivocal findings. Three patients had a new sPLND (re-sPLND) due to BCR and new findings on PET/CT after the first sPLND ().
Table 2. Pre-, peri- and postoperative sPLND data stratified on mode of sPLND.
Table 3. Oncological results during follow-up (n = 69).
Oncological outcomes
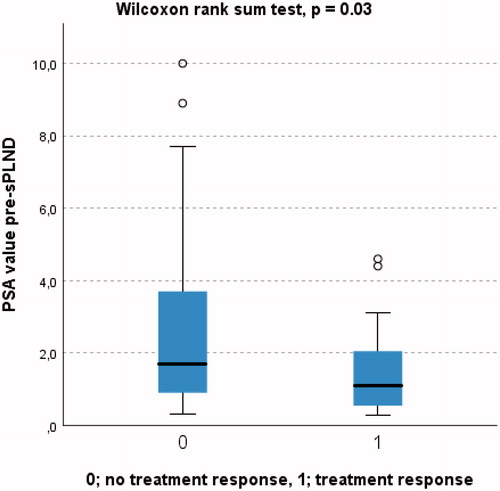
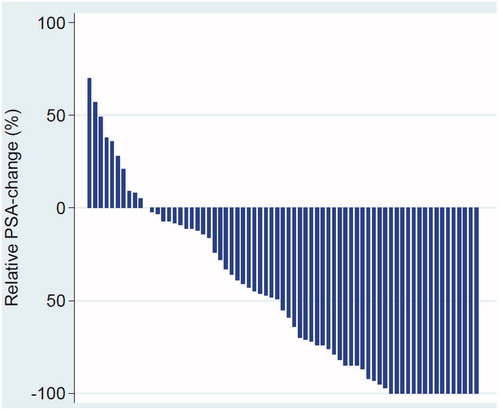
Twenty patients (29%) achieved treatment response () independently of mode of sPLND (). At 6 weeks post sPLND more than 50% reduction of PSA value was detected in 38 patients (54%) while 10 patients (14%) had an increase of their pre-sPLND PSA value ().
Figure 1. Relative PSA-changes (post- versus preoperative values) after robotic sPLND are plotted in descending order from left to right with each bar depicting one patient.
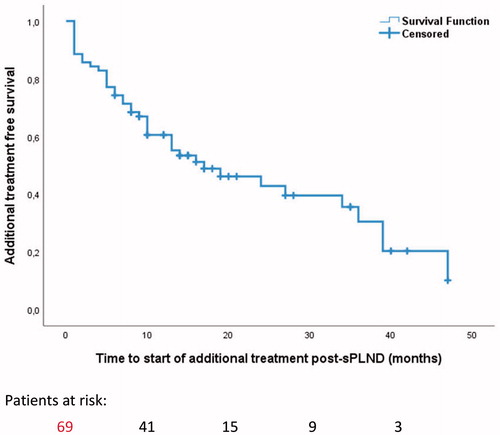
At a median follow-up of 15 months, 14 patients (20%) still had PSA < 0.2 ng/ml (). Forty-one patients (59%) had started additional treatment at median follow-up 15 months.
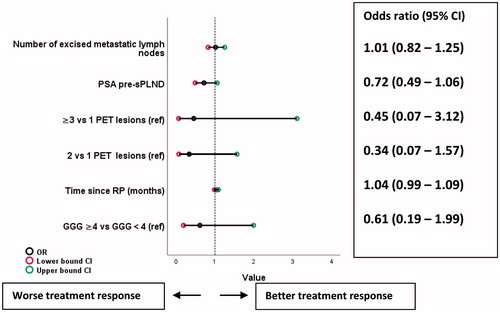
Median (IQR) PSA for patients with and without treatment response was 1.1 ng/ml (0.5–2.2) and 1.8 ng/ml (0.9–3.8), respectively (p = 0.03) (). In univariable logistic regression none of clinically relevant variables predicted treatment response expect for pre-sPLND PSA (odds ratio 0.71, 95% CI 0.50–1.01, p = 0.05). No covariates emerged as statistically significant predictors for treatment response in multivariable logistic regression analysis (), but there was a tendency towards association between less likelihood of treatment response and increasing pre-sPLND PSA and increased numbers of PET positive nodes.
Figure 3. Covariates associated with treatment response (PSA < 0.2 ng/ml) in multivariate logistic regression. GGG: ISUP Gleason Grade Group.
Median survival time without additional treatment was 17 months (95% CI 6–28) (). The pre-sPLND PSA value, number of excised positive lymphnodes and time between RP and sPLND significantly predicted the time to additional treatment post-sPLND ().
At the end of study 2 patients had died, one of them due to prostate cancer.
Postoperative complications
There were no perioperative complications. Post-sPLND complications occurred in 14 patients (20%) (). In eleven of these patients, the complications were classified as Clavien-Dindo grade 1 (). Complications occurred in 9 of 38 patients who had undergone postoperative radiation therapy after primary RP, compared to 5 complications in 32 patients without postoperative radiation (Chi-squared p = 0.5).
Discussion
To our knowledge, this is the largest single institution study of robotic sPLND. Twenty patients (29%) achieved treatment response. At a median follow-up of 15 months 14 of these patients (20%) still had PSA < 0.2 ng/ml. Decreasing levels of pre-sPLND PSA predicted treatment response. Finally, 20% of patients experienced postoperative complications, mostly Clavien-Dindo grade 1.
Other studies have found similar discouraging rates of treatment response rates. Siriwardana et al. [Citation14] reported 31% treatment response in a series of 35 patients. In another study Linxweiler et al. [Citation15] found treatment response in 33% of patients in a series of 36 patients. The distribution of percentage PSA decline in our series was similar to a recent study with 68 patients [Citation20]. Siriwardana et al. [Citation14] and Linxweiler et al. [Citation15] found that 50% of patients had started additional treatment after a median FU of 12 months, which is in accordance with our results which showed that 41 patients (59%) had received additional treatment at median follow-up 15 months.
The rate of postoperative complications is comparable to Siriwardana et al. [Citation14] who reported complications in 23% of 35 patients, all Clavien–Dindo grade ≤2. In a study by Devos et al. [Citation12] comparing open sLND with robotic sLND, postoperative complications within 30 days after surgery were more prevalent in the open sLND group compared to the robotic group (41.6% vs. 20%, p = 0.04). The robotic approach might be favorable due to more meticulous dissection and better control of lymphatic vessels because of higher magnification and in depth tissue visualization.
As in the paper by Siriwardana et al. [Citation14], we did not find that GGG in RP specimen was a predictor of treatment response. Similarly, Bravi et al. [Citation10] found that GGG was not a predictor for cancer-specific mortality. We found that pre-sPLND PSA value, number of excised positive lymphnodes and time between RP and sPLND significantly predicted the time to additional treatment post-sPLND. However, the initiation of such treatment after sPLND represents a rather uncertain endpoint because it is often based on vague criteria and left to the discretion of the patient and physician.
One argument often used in favour of sPLND is that this intervention may delay start of ADT. However, without comparison to a control group it is impossible to adequately assess the oncologic benefit of sPLND. This is illustrated by the remarkable observation in the only randomized study of MDT [Citation11], where 35% of patients undergoing surveillance experienced spontaneous PSA decline without receiving any therapy.
One may also question if delaying ADT is of any benefit. A report last year suggests that 30% of improvement in prostate cancer specific survival during the recent years is due to adjuvant ADT [Citation21]. Messing et al. [Citation22] showed already in 1999 in a randomized study that in RP patients with metastatic lymph nodes immediate ADT has beneficial effect on survival and progression compared to deferred ADT.
The median yield of lymph nodes in bilateral extended sPLND was 19 nodes (IQR 11–24) which is similar to a systematic review by Ploussard et al. [Citation7]. In our study most of the clinical recurrence after sPLND was due to new lymph node metastasis detected on PET/CT which implicate that sPLND did not remove all metastatic lesions.
In their long-term outcome study Bravi et al. [Citation10] found a significant survival benefit associated with the administration of ADT after sLND, suggesting that sLND as a local intervention should be considered part of a multimodal approach. In the ongoing STORM study [Citation23] the aim is to investigate if additional whole pelvic radiotherapy after MDT plus adjuvant ADT may more effectively treat micro metastasis and thereby improve oncological outcomes.
Although 20% of patients still had PSA < 0.2 ng/ml after median 15 months, we failed to find any significant predictor for treatment response; hence an important clinical question is how to better select patients for MDT. A promising field of research in this respect is liquid biopsy, such as miRNA derived from oligometastatic cancers which may identify patients who most likely not will benefit from MDT due to high numbers of circulating tumor cells [Citation24–25] and thereby be spared aggressive local treatment.
There are some important limitations to our study. The most important ones are the retrospective nature of the study, a relatively small sample size yet large for a robotic sPLND series, short median FU and the lack of a control group. Another weakness is the heterogenic diagnostic work-up pre-sPLND with different PET/CT tracers and that five different surgeons alternated in performing the sPLND procedures.
Conclusion
Oncological benefit of robotic sPLND as the only salvage procedure seems to be limited, though almost one third of patients achieved treatment response. Clinical trials are needed to determine if sPLND as part of a multimodal treatment may improve outcome.
Supplemental Material
Download MS Word (16.5 KB)Acknowledgements
Tor Åge Myklebust MSc Phd for excellent help creating waterfall figure. Dr Jon Roar Hoff for collection of follow up data.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- EAU guidelines: Management of PSA-only recurrence after treatment with curative intent. Available from: https://uroweb.org/guideline/prostate-cancer/#6_3. 2021.
- Bjartell A, Bottai M, Persson J, et al. Prediction of clinical progression after radical prostatectomy in a nationwide population-based cohort. Scand J Urol. 2016;50(4):255–259. Aug
- Lars J Petersen LJ, Zacho HD. Gallium-68 prostate-specific membrane antigen positron emission tomography/computed tomography for staging of high-risk prostate cancer. Scand J Urol. 2017;51(6):498–501. Dec
- Rauscher T, Maurer AJ, Beer FP, et al. Value of 68Ga-PSMA HBED-CC PET for the assessment of lymph node metastases in prostate cancer patients with biochemical recurrence: comparison with histopathology after salvage lymphadenectomy. J Nucl Med. 2016;57(11):1713–1719.
- Perera M, Papa N, Christidis D, et al. Sensitivity, Specificity, and predictors of positive 68Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70(6):926–937.
- Ost P, Bossi A, Decaestecker K, et al. Metastasis-directed therapy of regional and distant recurrences after curative treatment of prostate cancer: a systematic review of the literature. Eur Urol. 2015;67(5):852–863.
- Ploussard G, Gandaglia G, Borgmann H, et al. Salvage lymph node dissection for nodal recurrent prostate cancer: a systematic review. Eur Urol. 2019;76(4):493–504.
- Suardi N, Gandaglia G, Gallina A, et al. Long-term outcomes of salvage lymph node dissection for clinically recurrent prostate cancer: results of a single-institution series with a minimum follow-up of 5 years. Eur Urol. 2015;67(2):299–309.
- Fossati N, Suardi N, Gandaglia G, et al. Identifying the optimal candidate for salvage lymph node dissection for nodal recurrence of prostate cancer: results from a large, multi-institutional analysis. Eur Urol. 2019;75(1):176–183.
- Bravi CA, Fossati N, Gandaglia G, et al. Long-term outcomes of salvage lymph node dissection for nodal recurrence of prostate cancer after radical prostatectomy: not as good as previously thought. Eur Urol. 2020;78(5):661–669.
- Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36(5):446–453.
- Devos G, Muilwijk T, Raskin Y, et al. Comparison of peri-operative and early oncological outcomes of robot-assisted vs. open salvage lymph node dissection in recurrent prostate cancer. Front Oncol. 2019;9:781.
- Montorsi F, Gandaglia G, Fossati N, et al. Robot-assisted salvage lymph node dissection for clinically recurrent prostate cancer. Eur. Urol. 2017;72(3):432–438.
- Siriwardana A, Thompson J, van Leeuwen PJ, et al. Initial multicenter experience of (68) gallium-PSMA PET/CT guided robotassisted salvage lymphadenectomy: acceptable safety profile but oncological benefit appears limited. BJU Int. 2017;120(5):673–681. Nov
- Linxweiler J, Saar M, Al-Kailani Z, et al. Robotic salvage lymph node dissection for nodal-only recurrences after radical prostatectomy: perioperative and early oncological outcomes. Surg Oncol. 2018;27(2):138–145.
- Abreu F, Fay C, Park D, et al. Robotic salvage retroperitoneal and pelvic lymph node dissection for 'node-only' recurrent prostate cancer: technique and initial series . BJU Int. 2017;120(3):401–408.
- Schuster DM, Nanni C, Fanti S, et al. Anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid: physiologic uptake patterns, incidental findings, and variants that may simulate disease. J Nucl Med. 2014;55(12):1986–1992.
- Giesel FL, Hadaschik B, Cardinale J, J, et al. F-18 labelled PSMA-1007: biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur J Nucl Med Mol Imaging. 2017;44(4):678–688.
- Clavien PA, Sanabria JR, Strasberg SM. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery. 1992;111(5):518–526.
- Linxweiler J, Sprenk J, Cascetta K, et al. Robotic salvage lymph node dissection in recurrent prostate cancer: lessons learned from 68 cases and implications for future clinical management. J Urol. 2021;206(1):88–96.
- Welch HG, Albertsen PC. Reconsidering prostate cancer mortality - the future of PSA screening. N Engl J Med. 2020;382(16):1557–1563.
- Messing EM, Manola J, Sarosdy M, et al. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N Engl J Med. 1999;341(24):1781–1788.
- De Bruycker A, Spiessens A, Dirix P, et al. PEACE V - Salvage Treatment of OligoRecurrent nodal prostate cancer Metastases (STORM): a study protocol for a randomized controlled phase II trial. BMC Cancer. 2020;20(1):406.
- Lussier YA, Xing HR, Salama JK, et al. MicroRNA expression characterizes oligometastasis(es). PLoS One. 2011;6(12):e28650.
- Uppal A, Wightman SC, Mallon S, et al. 14q32-encoded microRNAs mediate an oligometastatic phenotype. Oncotarget. 2015;6(6):3540–3552.