Abstract
Aims
Examine the outcome of urodynamic studies in a regional prevalence group of patients with traumatic spinal cord injury (SCI), explore relationships between urodynamic parameters and renal complications/other SCI-related dysfunctions, assess the role of urodynamics in SCI follow-up.
Methods
In a cross-sectional study, 211 patients were included, who attended a yearly check-up and had urodynamics performed as part of the follow-up in addition to S-creatinine, S-cystatin-C, renal ultrasound, and a questionnaire regarding complications. Relationships between urodynamic parameters and renal complications/other SCI-related dysfunctions were explored by descriptive and comparative statistics.
Results
Neurogenic detrusor overactivity (NDO) was found in 150, underactive/acontractile detrusor in 44, normal detrusor function in 17 patients. Maximum detrusor pressures during filling/voiding in NDO attained mean levels of >40 cm H2O in 68% of patients and >25 cm H2O in 83%. Reduced compliance of the bladder wall, cystometric bladder capacity <250 mL, and detrusor overactivity leakage during filling were associated with higher detrusor pressures. Detrusor overactivity during more than one-third of the filling phase was significantly related to signs of renal complications among patients with SCI duration 11–20 years. No significant relationships were found between other urodynamic parameters and renal/other SCI-related complications. Renal complications in underactive/acontractile and normal detrusor function co-varied with evidence of previous NDO and other co-morbidities which may negatively influence kidney function.
Conclusions
Maximum detrusor pressures during the filling/voiding phases attained high levels in a majority of patients. Detrusor overactivity during more than one-third of the filling phase was significantly related to signs of renal complications.
Introduction
The Stockholm Spinal Cord Uro Study [Citation1] is a comprehensive survey of urinary tract function in a regional prevalence population of patients with chronic traumatic spinal cord injury (SCI). It was carried out as a cross-sectional study based on a yearly check-up visit at the Spinalis out-patient clinic, including a national program for follow-up of SCI complications, a regional program for follow-up of neurogenic bladder dysfunction post-SCI, and a study-specific questionnaire on patient-reported complications of the urinary tract during the preceding year. The basic characteristics of the patient group have been presented [Citation1], and a second report looked more closely at urinary tract infection (UTI), the most common complication reported by patients in our prevalence group [Citation2] as well as in many studies worldwide [Citation3,Citation4]. In this article, we report on the urodynamic characteristics as found in a subset of the total study population.
Urodynamic studies are recommended in international guidelines [Citation5,Citation6] as the most important tool to monitor risk factors for renal deterioration secondary to SCI. Traditionally, intravesical filling pressures of <40 cm H2O have been considered safe, based on findings in a group of myelodysplastic children [Citation7]. This reference level has later been questioned [Citation8] and a detrusor pressure of <25 cm H2O has been proposed as a cut-off level in adults [Citation5]. Additional urodynamic parameters, which have previously been reported as potential risk indicators, are reduced compliance of the bladder wall, a small bladder capacity, a high amplitude of detrusor overactivity leak-point pressure, or the duration of uninhibited bladder contractions during voiding by reflex stimulation [Citation9–13]. The length of detrusor contractions during bladder filling has been identified as associated with long-term renal deterioration [Citation14]. The relative importance of amplitude and duration of detrusor contractions as potential risk factors has not yet been clarified. There is consensus that regular urodynamic follow-up after SCI is essential, but the optimal intervals and urodynamic method need further elucidation.
The results of urodynamics are also used as a guide for choice of bladder management, and pharmaceutical, minimal invasive, or surgical intervention. In neurogenic detrusor overactivity (NDO) efforts are made to reduce high-amplitude detrusor pressures during filling and/or voiding, increase functional bladder capacity, and counteract detrusor sphincter-dyssynergia. The objective is to reduce the risk of recurring UTIs [Citation15], incontinence, and other SCI-related dysfunctions, such as spasticity, neuropathic pain, and autonomic dysreflexia which may be driven by visceral stimuli from the urinary tract [Citation16]. In neurogenic underactive or acontractile bladder, the focus is on maintaining a normal bladder capacity through regular intervals of complete bladder emptying and supporting the urethral sphincter closure mechanism to minimize the number of UTIs, preventing the formation of bladder stones, and promoting continence.
In this article, we report on the urodynamic characteristics in a subset of the regional prevalence population of the Stockholm Spinal Cord Uro study. We explore relationships with signs of renal complications and other patient-reported SCI-related dysfunctions and possible implications for further follow-up of persons with SCI.
Materials and methods
Participants
Participants were 211 persons with a post-traumatic SCI for at least one year, who attended a yearly check-up visit at the regional outpatient center the Spinalis clinic, and had urodynamics performed as part of the follow-up. These participants (Urodynamics group) constitute a subset of the Stockholm Spinal Cord Uro Study (total study population), which includes 412 patients and has been presented previously [Citation1].
Methods
In the Stockholm Spinal Cord Uro study, we applied two programs for follow-up of chronic SCI: a national Swedish program for follow-up of medical SCI complications [Citation17], and a regional program for neurogenic bladder dysfunction [Citation1], complemented with a study-specific questionnaire regarding urinary tract complications during the preceding year [Citation1]. Objective measurements and patient-reported data were collected for each individual, as previously reported [Citation1]. Neurological levels and AIS grades of injury [American Spinal Injury Association (ASIA) Impairment Scale] were registered [Citation18].
Urodynamic studies which were completed within two years of the check-up visit were included, provided bladder management had not changed. All studies were performed by a specialist nurse according to the International Continence Society (ICS) Good Urodynamic Practice [Citation19] and interpreted by experienced neuro-urologists. Patients were asked to pause bladder relaxant medication before the examination. Urodynamic parameters were evaluated by the current terminology of the ICS [Citation20]. The duration of detrusor contractions during the filling phase was determined as a ratio of the entire filling phase. Relationships were explored between urodynamic parameters and signs of renal complications, numbers of patient-reported UTIs, patient-reported presence of spasticity, neuropathic pain, and autonomic dysreflexia in the Urodynamics group as a whole and subgroups of urodynamic observations and successive duration of SCI. Patient files were reviewed for notes on primary urodynamic observations post-SCI.
In the regional follow-up program for neurogenic bladder dysfunction secondary to SCI urodynamics and ultrasound of kidneys was recommended in intervals based on stratification by neurological level and severity of the spinal cord lesion and previous urodynamic findings, as described in a previous article [Citation1]. S-creatinine, S-cystatin-C, and cystatin-C-related glomerular filtration were checked annually. Patients with a suprasacral SCI, AIS grades A–C, and/or previously established NDO were recommended urodynamics and a renal ultrasound every two years, whereas those with a sacral SCI or an AIS grade D without previous NDO were recommended these examinations when indicated by new clinical symptoms or by laboratory signs suggesting an impairment of kidney function.
In the current study, signs of renal complications were pathological levels of S-creatinine, S-cystatin-C, or cystatin-C related glomerular filtration, ultrasound verified dilatation of upper urinary tracts and/or pathology of renal parenchyma, as described previously [Citation1].
Data analyses
Data were analysed using SPSS for Windows 25 software (IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.) Descriptive and comparative statistics were calculated. The independent samples t-test was applied for the comparison of groups with normally distributed variables and the Mann–Whitney U test for non-normally distributed variables. p-Values <0.05 were considered statistically significant. Logistic regression analysis was used to estimate urodynamic parameters as risk factors for renal and other SCI-related complications in groups of injury duration 1–10, 11–20, 21–30, and 31–49 years.
Results
Patient characteristics of the Urodynamics group, the total Stockholm Spinal Cord Uro Study population, and patients who did not have a urodynamic study are presented in . All groups were similar regarding gender distribution. The Urodynamics group included patients with a lower mean/median age, a higher proportion of patients with injury classification C5–C8, A–C, and T1–S3, A–C than the non-urodynamics group, and a smaller representation of patients with AIS D at any level. It also included a higher proportion of persons who voided by clean intermittent catheterisation (CIC) and bladder reflex stimulation and a smaller percentage of patients who used normal voiding or had indwelling catheters. Reasons for not having urodynamics were patients’ choice (67%), the existence of a urinary diversion (15%), a high neurological level and severity of the injury and/or frailty (8%) which prevented the implementation of the examination or the referral was missed at the annual check-up (10%). Patients who declined the examination in most cases did not explain, but some stated that they ‘had no urinary problems for a long time’ or ‘had experienced discomfort as a result of a previous urodynamic study’.
Table 1. Characteristics of patients in the Urodynamics group, the total study population and the non-urodynamics group.
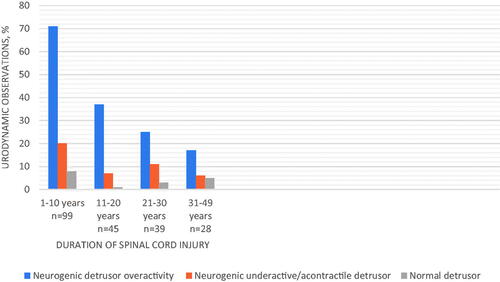
Urodynamic observations and the neurological level and severity of SCI are displayed in . Urodynamic parameters are exhibited in . The distribution of urodynamic observations was similar between sub-groups of successive SCI duration ().
Table 2. Urodynamic observations and neurological level and severity of spinal cord injury.
Table 3a. Urodynamic parameters in neurogenic overactive detrusor, underactive/acontractile detrusor and normal detrusor.
Table 3b. Patients with neurogenic overactive detrusor and SCI duration 11–20 years.
Overall, urodynamic parameters which have been described as potential risk indicators for renal deterioration were frequent. Among patients with NDO two-thirds had a maximum detrusor pressure during filling which was >40 cm H2O and four-fifths had >25 cm H2O. Detrusor overactivity leak point pressure was registered in 48 patients and among them, 92% attained an amplitude of >40 cm H2O. Among the sixty-one patients where voluntary voiding was registered 41% had a maximum detrusor pressure during voiding of >55 cm H2. The highest detrusor pressure was obtained among persons who used normal voiding, bladder reflex stimulation, or sacral anterior root stimulation.
Twenty-four patients (12%) displayed reduced compliance of the bladder wall. A cystometric bladder capacity of <250 mL was noted in 30%.
According to clinical files, 89% of patients had a urodynamic observation established within the first year post-SCI. All patients with a current NDO had a primary NDO. Approximately half of them had previous anticholinergic treatment, however detailed information on doses and treatment periods was not available. Among patients with a current underactive/acontractile or normal detrusor 36% had a primary NDO consistent with a cervical/thoracic neurological level of injury and AIS grades A–C. These patients had a median SCI duration of 28 years. Initial bladder management was reflex stimulation or combinations of reflex stimulation, Valsalva manoeuvres, and normal voiding for a median of 15 years. Several patients reported a long-standing practice of voluntary overdistension of the bladder. Current bladder management was CIC, normal voiding, or reflex stimulation.
Signs of renal complications were present in 24% of the Urodynamics group, 28% in the non-Urodynamics group, and 25% in the total population of the Stockholm Spinal Cord Uro Study ().
Figure 2. The Stockholm Spinal Cord Uro Study and co-variation with signs of renal complications, patients grouped by urodynamic observation and successive duration of spinal cord injury.
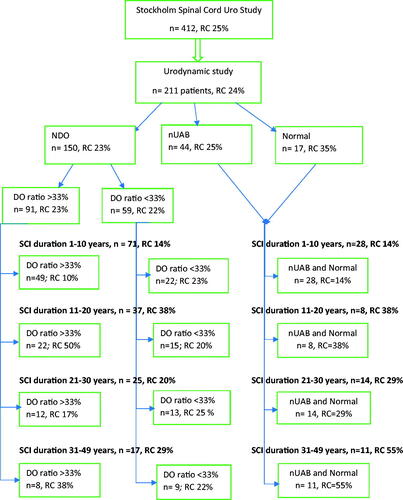
In the cross-sectional perspective of the Urodynamics group, there were no statistically significant relationships between any urodynamic parameters and signs of renal impairment. Among patients with NDO and an SCI duration of 11–20 years we found a statistically significant relationship between the duration of detrusor overactivity during the filling phase (DO ratio) and signs of renal complications. In this group, the mean DO ratio in persons with/without signs of kidney complications was 46 vs. 32% (p = 0.031). Sixty-one percent of all patients with NDO had a DO ratio of more than 33%.
Logistic regression verified the DO ratio as a risk factor for renal complications (odds ratio, OR 1.3), whereas there was no risk difference with other urodynamic parameters. Co-variation between DO ratios >33% and signs of renal complications in sub-groups of SCI duration is illustrated in .
Among patients with a current underactive/acontractile or normal detrusor there were no statistically significant relationships between urodynamic parameters and signs of renal complications with any duration of SCI. The rate of kidney complications was 29% overall, and 39% among those with an injury duration >10 years. These patients had a mean age/duration of SCI of 49/16.6 years, as compared to 43/14.4 years among patients with a current NDO. Hypertension and cardiovascular disease were present in a majority, several persons had a history of open renal stone surgery, and half of them were tobacco users. In comparison, these co-morbidities were found only in single cases in the NDO group and tobacco use was present in 28%.
Among patients with NDO, 46% reported current use of anticholinergics, mirabegron, or botulinumtoxin. Fourteen patients (9%) had not paused the medication before urodynamics. Patients with/without ongoing medication at the time of the examination had a mean maximum detrusor pressure during filling of 26/60 cm H2O (p < 0.001), but a mean DO ratio of 40/43% (p = 0.455).
Relationships between urodynamic parameters and other SCI-related complications were explored. Twenty-one percent reported more than 3 UTI’s during the preceding year, and among them, one-third had a febrile UTI requiring hospitalization. NDO was present in 85% of these patients and in 93% of those who had been hospitalized. Forty-six percent of the Urodynamics study population reported spasticity, 34% neuropathic pain, and 16% autonomic dysreflexia. There were no statistically significant relationships or co-variation in proportions between individual urodynamic parameters and the patient-reported numbers of these complications.
Discussion
The Urodynamics group included in this study is a subset from the original Stockholm Spinal Cord Uro Study, which in turn encompasses 75% of the regional population of persons with traumatic SCI in the Stockholm area. The inherent limitations and selection restrictions of the study are described in the Results section. Bearing this in mind, we believe that the urodynamic observations are reasonably representative of the prevalence population and our findings may apply to an average urban SCI population in Scandinavia today.
In the total population of the Stockholm Spinal Cord Uro Study, the main risk profiles for renal complications were identified as cervical lesions with AIS grades A–B, age, and SCI duration [Citation1]. In this study, the aim was to further examine the role of urodynamic parameters.
Among patients with NDO, urodynamic risk indicators for renal deterioration were frequent, however, in the cross-sectional perspective, no statistically significant relationships with renal complications were discovered. When the duration of SCI and the neurogenic bladder dysfunction were considered, a DO ratio of more than one-third of the filling phase emerged as statistically significant among patients with an SCI duration of 11–20 years. Also, there was co-variation between the proportions of patients with a DO ratio of more than one-third and signs of renal complications in the successive subgroups of SCI duration. Other urodynamic risk indicators did not exhibit such relationships in any subgroup of injury duration. The rate of renal complications increased with the duration of SCI and was highest in the 11–20 year group for patients with all urodynamic observations. The lower rates in the populations with longer SCI duration may partly be attributed to the so-called ‘healthy subjects’ effect’, namely that patients in these subgroups had remained relatively healthy and did not develop renal deterioration, and partly to lack of power as groups of increasing SCI duration were successively smaller.
Previous periods of anticholinergic treatment may have influenced the current rate of renal complications, but patient file information was insufficient to extract these data. It is also conceivable that detrusor overactivity of long duration may have been present during anticholinergic therapy, albeit with a lowered amplitude of the maximum detrusor pressure, thus contributing to renal complications. This reasoning needs corroboration in further studies.
Our results support the previous findings by Elmelund et al. that the length of detrusor contractions during bladder filling is associated with negative effects on the upper urinary tracts [Citation14] and perhaps the only urodynamic parameter which can be statistically related to the development of renal complications over time. These authors suggested that registration of the area under the curve of the detrusor pressure may become a useful urodynamic measurement. This type of calculation can add to a more dynamic view of the relative importance of amplitude and duration of detrusor overactivity, and further research is warranted.
Stationary urodynamics naturally provides only a snapshot of bladder function. A more dynamic representation may be achieved by increased use of patient-reported parameters, such as voiding charts providing real-life measurements, symptom reports, validated questionnaires [Citation21], and increased use of ambulatory urodynamics. With technical development user-friendly ambulatory systems may soon become a reality [Citation22], making home recordings feasible, as well as enhanced computations of urodynamic parameters.
Timely establishment of urodynamic observations during the initial period post-injury and continued life-long monitoring of detrusor overactivity is paramount after SCI [Citation23], given the negative effects of NDO on the upper urinary tracts and the association with increased frequency and severity of UTIs. We propose yearly investigations until a stable urodynamic situation and stable bladder management have been reached. Attention should be focused on the duration as well as amplitudes of detrusor overactivity. Patients with urodynamically verified NDO may then continue with biannual investigations, while those with an established underactive or normal bladder may be monitored according to symptomatology and laboratory signs. Urodynamic examinations should be performed in specialist centers. The urodynamic setting should aim to provide a patient-friendly and comfortable situation, and precautions should be taken to avoid UTIs in connection with the procedure [Citation5,Citation19]. Active patient involvement by self-reporting of functional parameters is important to get the full picture of lower urinary tract function. We propose an increased use of ambulatory urodynamics in routine follow-up, and standards for relevant ambulatory measurements need to be determined.
Urodynamic monitoring is essential for the assessment of interventions to treat NDO. The clinical result may not be sufficient to gauge the effect on the amplitude and duration of detrusor overactivity [Citation24]. In this study, bladder relaxant medication was associated with a lower maximum detrusor pressure during filling, but the DO ratio was unchanged, implying a possible insufficient effect for long-term renal protection.
Rates of renal complications increased with the duration of SCI, also among patients with a current underactive/acontractile bladder or normal detrusor function where the present urodynamic parameters were not risk indicators. There was a history of longstanding NDO in more than one-third of this population along with higher mean age, longer duration of SCI, and a higher proportion of other risk factors for chronic kidney disease [Citation25]. These findings illustrate the importance of a holistic view toward secondary complications post-SCI. While long-term NDO emerges as the most important urodynamic risk factor other health issues must also be taken into account in the context of aging with an SCI.
Conclusion
Among patients with NDO, a majority displayed maximum detrusor pressures which have traditionally been regarded as critical for long-term renal function. Detrusor contractions occurred with a mean duration of ∼40% of the filling phase, also in patients on bladder relaxant medication.
Detrusor overactivity during more than one-third of the filling phase was significantly related to signs of renal complications in patients with an SCI duration of 11–20 years. There were no significant relationships between other urodynamic parameters and renal complications.
Among patients with underactive/acontractile or normal detrusor function and an SCI duration of more than fifteen years signs of renal complications co-varied with rates of previous NDO as primary urodynamic condition post-SCI and also with cardiovascular co-morbidities, previous renal surgery, and tobacco use.
Ethics approval
The study was approved by the Regional Human Ethics Committee at the Karolinska Institute, Stockholm (2007/35-31/3).
Geolocation statement
This study was carried out at the Spinalis clinic, Aleris Rehab Station, Stockholm, Sweden.
| Abbreviations | ||
| AIS | = | American Spinal Injury Association (ASIA) Impairment Scale |
| CIC | = | clean intermittent catheterisation |
| ICS | = | International Continence Society |
| NDO | = | neurogenic detrusor overactivity |
| SCI | = | spinal cord injury |
| UTI | = | urinary tract infection |
Acknowledgements
The authors wish to thank Professor Richard Levi for instrumental help in setting up the study, and the staff at the Spinalis clinic for performing interviews, database registration, and physical examinations. We wish to especially acknowledge the work of Associate Professor Hans Wijkström who was part of the research team and actively engaged in the analysis of study data but sadly passed away during the preparation of the manuscript.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Farrelly E, Lindbo L, Wijkström H, et al. The Stockholm Spinal Cord Uro Study: 1. Basic characteristics and problem inventory. Scand J Urol. 2019;53(6):403–410.
- Farrelly E, Lindbo L, Wijkström H, et al. The Stockholm Spinal Cord Uro Study: 2. Urinary tract infections in a regional prevalence group: frequency, symptoms and treatment strategies. Scand J Urol. 2020;54(2):155–161.
- Stillman MD, Barber J, Burns S, et al. Complications of spinal cord injury over the first year after discharge from inpatient rehabilitation. Arch Phys Med Rehabil. 2017;98(9):1800–1805.
- New PW. Secondary conditions in a community sample of people with spinal cord damage. J Spinal Cord Med. 2016;39(6):665–670.
- Przydacz M, Chlosta P, Corcos J. Recommendations for urological follow-up of patients with neurogenic bladder secondary to spinal cord injury. Int Urol Nephrol. 2018;50(6):1005–1016.
- Kreydin E, Welk B, Chung D, et al. Surveillance and management of urologic complications after spinal cord injury. World J Urol. 2018;36(10):1545–1553.
- McGuire EJ, Woodside JR, Borden TA, et al. Prognostic value of urodynamic testing in myelodysplastic patients. J Urol. 1981;126(2):205–209.
- Musco S, Padilla-Fernández B, Del Popolo G, et al. Value of urodynamic findings in predicting upper urinary tract damage in neuro-urological patients: a systematic review. Neurourol Urodyn. 2018;37(5):1522–1540.
- Gerridzen RG, Thijssen AM, Dehoux E. Risk factors for upper tract deterioration in chronic spinal cord injury patients. J Urol. 1992;147(2):416–418.
- Shin JC, Lee Y, Yang H, et al. Clinical significance of urodynamic study parameters in maintenance of renal function in spinal cord injury patients. Ann Rehabil Med. 2014;38(3):353–359.
- Weld KJ, Graney MJ, Dmochowski RR. Differences in bladder compliance with time and associations of bladder management with compliance in spinal cord injured patients. J Urol. 2000;163(4):1228–1233.
- Nseyo U, Santiago-Lastra Y. Long-term complications of the neurogenic bladder. Urol Clin North Am. 2017;44(3):355–366.
- Linsenmeyer TA, Bagaria SP, Gendron B. The impact of urodynamic parameters on the upper tracts of spinal cord injured men who void reflexly. J Spinal Cord Med. 1998;21(1):15–20.
- Elmelund M, Klarskov N, Bagi P, et al. Renal deterioration after spinal cord injury is associated with length of detrusor contractions during cystometry – a study with a median of 41 years follow-up. Neurourol Urodyn. 2017;36(6):1607–1615.
- García Leoni ME, Esclarín De Ruz A. Management of urinary tract infection in patients with spinal cord injuries. Clin Microbiol Infect. 2003;9(8):780–785.
- Eldahan KC, Rabchevsky AG. Autonomic dysreflexia after spinal cord injury: Systemic pathophysiology and methods of management. Auton Neurosci. 2018;209:59–70.
- Levi R, Ertzgaard P. Quality indicators in spinal cord injury care: a Swedish collaborative project. The Swedish Spinal Cord Injury Council 1998. Scand J Rehabil Med Suppl. 1998;38:1–80.
- Biering-Sørensen F, DeVivo MJ, Charlifue S, et al. International Spinal Cord Injury Core Data Set (version 2.0)-including standardization of reporting. Spinal Cord. 2017;55(8):759–764.
- Rosier P, Schaefer W, Lose G, et al. International Continence Society Good Urodynamic Practices and Terms 2016: urodynamics, uroflowmetry, cystometry, and pressure-flow study. Neurourol Urodyn. 2017;36(5):1243–1260.
- Gajewski J, Schurch B, Hamid R, et al. An International Continence Society (ICS) report on the terminology for adult neurogenic lower urinary tract dysfunction (ANLUTD). Neurourol Urodyn. 2018;37(3):1152–1161.
- Bonniaud V, Bryant D, Parratte B, et al. Development and validation of the short form of a urinary quality of life questionnaire: SF-Qualiveen. J Urol. 2008;180(6):2592–2598.
- Kocher NJ, Damaser MS, Gill BC. Advances in ambulatory urodynamics. Curr Urol Rep. 2020;21(10):41.
- Schurch B, Iacovelli V, Averbeck MA, et al. Urodynamics in patients with spinal cord injury: a clinical review and best practice paper by a working group of the International Continence Society Urodynamics Committee. Neurourol Urodyn. 2018;37(2):581–591.
- Koschorke M, Leitner L, Sadri H, et al. Intradetrusor onabotulinumtoxinA injections for refractory neurogenic detrusor overactivity incontinence: do we need urodynamic investigation for outcome assessment? BJU Int. 2017;120(6):848–854.
- Gasparini A, Evans M, Coresh J, et al. Prevalence and recognition of chronic kidney disease in Stockholm healthcare. Nephrol Dial Transplant. 2016;31(12):2086–2094.