Abstract
Introduction
Many factors influence the clinical course of patients with renal cell carcinoma (RCC). The most commonly used prognostic indicators are TNM stage, tumor size and RCC type. In this study we evaluated the prognostic relevance of albumin and C-reactive protein (CRP), and Glasgow Prognostic scores (GPS), in patients with primary RCC.
Methods
We retrospectively reviewed all patients surgically treated for primary RCC between 1982 and 2018 at Umeå University Hospital. There were 872 patients, 527 males and 345 females. Data on albumin, CRP and GPS points before surgery were extracted, as well as TNM stage, RCC type, tumor grade, tumor size, and primary treatment. The patients were followed for recurrence and death for up to 37.2 years. We used Kaplan-Meier estimators, Cox-proportional hazards models, to assess the relation between potentially prognostic indicators and RCC-specific death, and all-cause mortality.
Results
Of 872 patients, 708 had clear-cell RCC, 114 papillary RCC, 36 chromophobe RCC and 9 undefined RCC type while 5 patients had missing RCC type data. Except that, women had a significantly (p = 0.002) lower proportion of pRCC, no difference in RCC types and levels of albumin and CRP was observed between genders. Albumin, CRP, and GPSs were all univariately associated to RCC survival (p < 0.001). CRP demonstrated the strongest prognostic association (HR 1.67 95% Ci (1.53–1.83, overriding both albumin and GPS in multivariable models. The AUC for CRP was 0.77 (95% CI: 0.74-0.80).
Conclusion
Elevated CRP, low albumin levels, and elevated GPSs were all associated to poor survival in patients with RCC, Only CRP remained independent in multivariate analysis.
Introduction
Renal cell carcinoma (RCC) represents about 3% of all malignancies, with 100,000 new RCC diagnoses and 39,000 kidney cancer-related deaths within the European Union in 2018 [Citation1]. The overall mortality rates for RCC is approximately 40% (39–43%), but varies widely [Citation1,Citation2]. The major prognostic variables for adverse survival are local and tumor spread and histological characteristics [Citation3]. There are three major RCC types: Clear cell RCC (ccRCC), papillary RCC (pRCC) and chromophobe RCC (chRCC) with different clinical behaviour. Accurate prognostic models are crucial to guide future adjuvant or neoadjuvant treatments.
The immunological status and inflammatory response in individual patients are thought to influence tumour growth and disease progression, and related biomarkers such as albumin and c-reactive protein (CRP) may provide additional prognostic information to the standard clinical indicators [Citation4]. Previous studies have shown that high levels of albumin and CRP are associated with adverse patient survival [Citation5,Citation6]. Albumin that constitutes the majority of the total protein in human serum and body fluids is an indication of the patient's nutritional status but also an important component of the inflammatory response [Citation7]. CRP, an acute-phase protein, is part of the activation of the complement inflammatory response and plays a role in phagocytosis and T-lymphocyte function [Citation8]. CRP is also known to be an indicator of cell destruction and level of inflammation after surgery [Citation8]. CRP has been found associated with poor survival in RCC patients [Citation9]. Prognostic scores that utilize CRP and albumin levels have been developed to improve the prognostic value of these acute-phase proteins. Both the original Glasgow Prognostic Score (GPS) and the modified GPS (GPSm) has been suggested to enhance the prediction of survival in patients with RCC [Citation10,Citation11].
The aim of this study was to investigate the prognostic value of albumin and CRP, as well as the GPS scores in patients with RCC.
Materials and methods
Material
Patients surgically treated for RCC between 1982 and 2018, at the Department of Urology at Norrland University Hospital, Umeå were retrospectively retrieved from the medical records. All patients with benign histology or other malignancies than RCC were excluded. There were 872 patients with histologically confirmed RCC, 527 males and 345 females. Data on prognostic indicators and other patient characteristics were extracted from their medical records. All patients were subject to yearly follow-up, screened in the medical records and screened for being alive in the Swedish National Population Register. The last follow-up was done in December 2020. Survival time was defined as the time from diagnosis to the date of death of any cause or alive at the end of December 2020.
Histopathologic classification of RCC type and tumour grade was performed according to the Heidelberg classification and Fuhrman nuclear grading, respectively [Citation12,Citation13]. The updated TNM classification 2017 was used for tumour stage grouping [Citation14]. In the stage grouping, patients with Nx were joined with N0, and Mx joined with M0. Tumour size, defined as the largest tumour diameter, was measured primarily on the computed tomography (CT) or magnetic resonance imaging (MRI) scans.
The original GPS rated from 0 to 2. Albumin level below 37 mmol/L gave 1 point and CRP level above 10 mg/L gave 1 point [Citation11]. The GPSm scoring was similarly rated as the original, except that in GPSm no point was given for albumin level below 37 mmol/L if at the same time CRP level was below 10 mg/L. ECOG performance status was estimated at the time of the primary diagnosis [Citation15].
Ethics
The patients had informed consent, orally before year 2000, and informed and written consent from year 2000. The study was reviewed and approved by the Ethical Review Board (Dnr: 2015-146-31 M and Dnr: 2018-296-32 M) and the Ethical board of Sweden (Dnr: 2019-02579). The data used were anonymized and throughout the project all data was treated under the regulations of the General Data Protection Regulation Act.
Statistical methods
Initial statistical analysis was performed using non-parametric tests for continuous variables and χ2-test to evaluate survival differences. Cancer-specific survival (CSS) and patient overall survival (OS) and was estimated by the Kaplan-Meyer method and survival differences were analysed by the log-rank tests. We estimated Kaplan-Meyer curves by four categories of CRP (≤3 mg/L, 3–≤10, 10–≤40 and >40 mg/L), and albumin (≤37.0, 37.0–40.0, 40.0–43.0 and >43.0 mmol/L). All subsequent biomarker analyses were carried out using log-standardized concentration measures, meaning that all association were estimated per standard deviation increment by log-transformed concentrations of CRP or albumin. Cox multivariate regression analysis was used to evaluate if independent statistical information remained after testing variables with univariate significance. The ROC analysis was made according to a standardized evaluation of the four markers/scores. For albumin, the log-standardized measures were multiplied by −1 for the ROC-analysis/curves, as albumin is a negative acute-phase reactant. Potential differences were considered statistically significant when p < 0.05 using a double-sided test.
Results
Among the 872 surgically treated patients, 363 (41.5%) were diagnosed with stage I RCC, 130 (14.9%) with stage II, 182 (20.8%) with stage III and 197 (22.3%) with stage IV RCC. The majority (708, 81.2%) of the patients were diagnosed with clear cell RCC (ccRCC), followed by papillary RCC (pRCC, no 114, 13.1%), and 36 patients had chromophobe (chRCC, 4.1%) (). Primary surgery was radical nephrectomy (RN) in 653 (74.9%) patients, partial nephrectomy (PN) in 212 (24.3%), and in 7 (0.8%) patients other or combined surgeries. Mean age at diagnosis was 65.3 years for men (range: 18–87 years) and 66.8 years (ranging 25–88 years) for women. Women had lower proportion of pRCC than men (p = 0.002), but there were no other important differences by sex in RCC type.
Table 1. Distribution of patient’s characteristics in relation to gender in 872 patients with surgically treated renal cell carcinoma at Umeå University Hospital between 1982 and 2018.
There was no important difference in levels of albumin and CRP between men and women, nor were there any noticeable differences in the distribution of GPS and GPSm points between genders. The majority of patients (n = 446) had a 0 GPS score, 235 scored 1 and 190 patients scored 2.
At the last follow-up, 262 (30.0%) patients were alive with no evidence of the disease, 26 (3.0%) patients were alive with evidence of the disease, 350 (40.1%) patients had died with RCC indicated as underlying cause of death, and 234 (26.8%) patients had died of unrelated causes. The mean overall survival for alive patients were 9.5 years (range 2.1–37.2 years), and mean OS was 6.5 years (range 0.0 − 37.2 years) for the entire cohort.
RCC-specific survival
Hazard ratio estimates for the standard clinical variables and tumour characteristics are presented in . In the initial Kaplan-Meier analysis, albumin and CRP levels, GPS and GPSm scores all showed univariate prognostic information for RCC-specific death (p-value for log-rank test [prank] < 0.0001) (). In Cox-regression analyses, the hazard ratio per standard deviation increment in log-concentrations [HRstd] were 0.69 (95% CI: 0.64–0.75) for albumin, and 1.67 (95% CI: 1.53–1.83) for CRP. The HR for one GPS point was 1.67 (95% CI: 1.36–2.04) and 3.02 (95% CI: 2.45–3.71) for two GPS points. In mutually adjusted multivariate Cox-regression, the HR estimates were attenuated for each marker, but they maintained a clear association with RCC specific death (). However, when additionally adjusting for TNM-stage, RCC-type, and ECOG performance status, we observed little evidence for independent associations of albumin (p = 0.957) and GPS (p = 0.502 and 0.917) with RCC-specific death, and only CRP remained clearly associated with RCC death (HRstd: 1.34, 95% CI: 1.12–1.61). Of the two GPS scores, when tested without CRP and albumin, GPSm had the highest independent HR values: GPSm 1 point (HR 1.57 (95% CI 1.14–2.01, p = 0.002), GPSm 2 points HR 2.39 (95% CI 1.82–3.13, p < 0.001). In ROC analyses, CRP had the highest area under the curve (AUC: 0.77, 95% CI: 0.74–0.80, ).
Figure 1. Kaplan-Meyer survival curves illustrating cancer specific survival in 872 patients with RCC, (A) in relation to albumin levels, (B) in relation to CRP levels, (C) in relation to Glasgow Prognostic Score points and (D) in relation to the modified Glasgow Prognostic Score points.
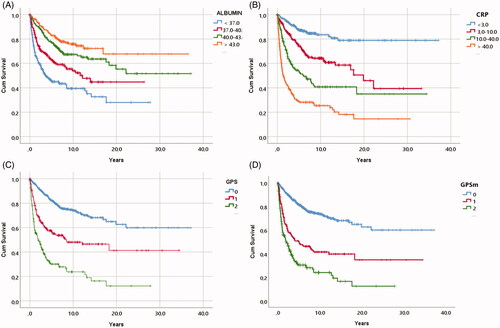
Figure 2. ROC curves showing areas under the curve for CRP, albumin, original and modified Glasgow Prognostic Scores in relation to cancer specific survival.
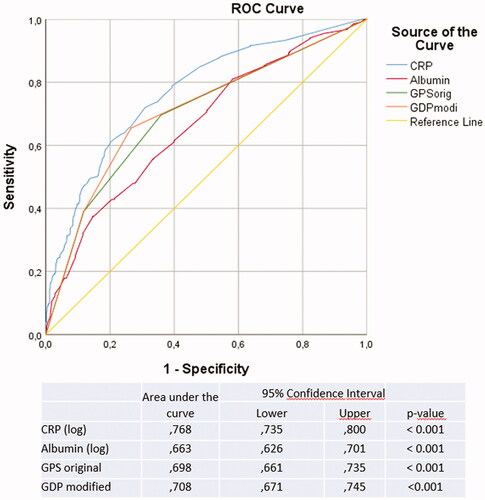
Table 2. Cox progression hazards regression analysis of cancer specific survival in 872 patients with RCC in association to age, gender, TNM stage, Tumor Size, ECOG performance status, RCC type, type of treatment, CRP, albumin and modified Glasgow prognostic scores (GPSm).
Table 3. Hazard ratios for cancer specific death for CRP, albumin and Glasgow prognostic score.
All-cause mortality
When considering all-cause mortality, all biomarkers, including albumin, CRP, GPS and GPSm, were associated with survival in Kaplan Meier analysis (). In a multivariate Cox regression analysis (), when adjusting for clinical variables and tumour characteristics, neither CRP, GPS or GPSm displayed independent associations with overall survival, whereas CRP displayed a similar association with survival as in the RCC-specific analysis (HR: 1.32, 95% CI: 1.13–1.54) ().
Figure 3. Kaplan-Meyer survival curves illustrating overall survival in relation to primary treatment in 872 patients with RCC, (A) in relation to albumin levels, (B) in relation to CRP levels, (C) in relation to Glasgow Prognostic Score points and (D) in relation to the modified Glasgow Prognostic Score points.
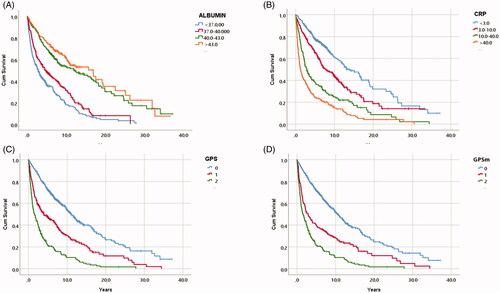
Table 4. Cox progression hazards regression analysis of Overall survival in 872 patients with RCC in association to (age, gender), TNM stage, Tumor Size, ECOG performance status, RCC type, type of treatment and Glasgow prognostic scores (GPS).
Discussion
Multiple factors are considered in RCC prognostics, including TNM stage, RCC-type, tumour size, tumour grade and performance status. However, several biomarkers are routinely measured in the clinic, including albumin and CRP. In this study we evaluated if these biomarkers may improve RCC prognostics and found that only CRP carried independent prognostic information.
In the present study, we evaluated the prognostic significance of albumin and CRP levels for both OS and CSS, confirming results as previously reported [Citation16,Citation17]. We showed that CRP and albumin levels, analysed independently, gave significant survival information. However, in multivariate Cox-regression, only CRP remained independently associated with survival when accounting for the clinical variables. Previous studies have suggested that the Glasgow Prognostic Score (GPS), which weights pre-defined cut-offs of albumin and CRP, as a better prognostic indicator [Citation10,Citation18]. Our results also confirmed the prognostic information of GPS on its own, as well as of GPSm [Citation10,Citation18] But when also taking clinical information into account, we found little evidence of an independent prognostic role of GPS or GPSm in RCC, regardless of considering RCC specific death, or all-cause mortality. Taken together, these data support the use of CRP for RCC prognostics in addition to standard clinical variables and tumour characteristics.
CRP, an acute-phase protein, is involved in the inflammatory response and has a role in T-lymphocyte function [Citation8]. CRP is also known to be an indicator of cell destruction and level of inflammation after surgery [Citation8]. Using Cox multivariate analysis, we showed that CRP levels significantly correlated to both CSS and OS in this patient cohort, confirming the results of previous studies [Citation9,Citation16]. The importance of CRP is also shown in advanced RCC patients treated with targeted therapies [Citation19]. In these patients, CRP can monitor treatment response and also predict treatment response. Similar experience with CRP as a prognostic biomarker has been observed in treatment with immune checkpoint inhibitors (ICI) [Citation20]. Patients CRP might be a useful variable to monitor T-cells activation during systemic therapy in patients with advanced RCC [Citation21]. In that study, the best result of ICI nivolumab treatment was found in the patients with CRP flare within one months of treatment and thereafter having a more than 30% decreased CRP levels. These results indicate that CRP provides powerful prognostic information that might be useful as monitor effects of ICI treatment.
With a future possibility of adjuvant treatment of patients with RCC, a number of important predictive variables will be needed for the selection of the treatment of an individual patient [Citation3], in particular when considering ICI treatment [Citation7].
Study limitations and advantages
One limitation of this study is that it is based-on patients from a single centre, which may theoretically limit the extent to which are findings translate to other settings. Another potential limitation is that the biomarker measurements were performed continuously in a clinical setting over a long period. This contrasts to studies with standardized biomarker measurements carried out in a coordinated fashion for academic purposes. Indeed, our setting is likely to encounter higher level of technical noise and drifts in biomarker measurements and may for this reason display poorer performance. However, we would argue that our data are likely to realistically reflect the performance of assessed biomarkers in this clinical setting. It is further important to highlight that during the study period, there have been continuous improvements in imaging techniques (which have greatly increased incidental detection), development of new surgical techniques, and developments of systemic therapies. All these enhancements have improved the overall survival in patients with RCC in general [Citation22].
Conclusion
The prognosis for patients with RCC is dependent on multiple factors. Inflammatory response, as indicated by circulating CRP, appears to be an important prognostic indicator for patients with RCC. It also highlight the importance of considering the immune system response in predicting the clinical course, in the era of immunotherapy.
Disclaimer
Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/World Health Organization.
| Abbreviations | ||
| CRP | = | C-reactive protein |
| RCC | = | Renal Cell Carcinoma |
| TNM | = | Tumour Node Metastasis |
| GPS | = | Glasgow Prognostic Score |
| GPSm | = | Modified Glasgow Prognostic Score |
| pRCC | = | Papillary renal cell carcinoma |
| chRCC | = | Chromophobe renal cell carcinoma |
Acknowledgements
The authors thanks nurse Kerstin Almroth for collection of blood samples. We also thanks Björn Tavelin for skilful statistical assistance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ferlay J, Colombet M, Soerjomataram I, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356–387.
- Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–1953.
- Ljungberg B, Albiges L, Abu-Ghanem Y, et al. European Association of Urology guidelines on renal cell carcinoma: the 2019 update. Eur Urol. 2019;75(5):799–810.
- Aarstad HH, Guðbrandsdottir G, Hjelle KM, et al. The biological context of C-reactive protein as a prognostic marker in renal cell carcinoma: studies on the acute phase cytokine profile. Cancers (Basel). 2020;12(7):1961.
- Steffens S, Köhler A, Rudolph R, et al. Validation of CRP as prognostic marker for renal cell carcinoma in a large series of patients. BMC Cancer. 2012;12:399.
- Chen Z, Shao Y, Wang K, et al. Prognostic role of pretreatment serum albumin in renal cell carcinoma: a systematic review and Meta-analysis. Onco Targets Ther. 2016;9:6701–6710.
- McMillan DC, Elahi MM, Sattar N, et al. Measurement of the systemic inflammatory response predicts cancer‐specific and non‐cancer survival in patients with cancer. Nutr Cancer. 2001;41(1–2):64–69.
- Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754.
- Komai Y, Saito K, Sakai K, et al. Increased preoperative serum C-reactive protein level predicts a poor prognosis in patients with localized renal cell carcinoma. BJU Int. 2007;99(1):77–80.
- Lamb GW, Aitchison M, Ramsey S, et al. Clinical utility of the Glasgow Prognostic Score in patients undergoing curative nephrectomy for renal clear cell cancer: basis of new prognostic scoring systems. Br J Cancer. 2012;106(2):279–283.
- Hu X, Wang Y, Yang WX, et al. Modified Glasgow prognostic score as a prognostic factor for renal cell carcinomas: a systematic review and Meta-analysis. CMAR. 2019;11:6163–6173.
- Kovacs G, Akhtar M, Beckwith BJ, et al. The Heidelberg classification of renal cell tumours. J Pathol. 1997;183(2):131–133.
- Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6(7):655–663.
- Brierley JD, Gospodarowicz MK, Wittekind C, editors. TNM classification of malignant tumours. 8th ed. Oxford: Wiley-Blackwell; 2017.
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern cooperative oncology group. Am J Clin Oncol. 1982;5(6):649–655.
- Hu Q, Gou Y, Sun C, et al. The prognostic value of C-reactive protein in renal cell carcinoma: a systematic review and Meta-analysis. Urol Oncol. 2014;32(1):50.e1-8–50.e8.
- Fukuda H, Takagi T, Kondo T, et al. Predictive value of inflammation-based prognostic scores in patients with metastatic renal cell carcinoma treated with cytoreductive nephrectomy. Oncotarget. 2018;9(18):14296–14305.
- Fan H, Shao ZY, Xiao YY, et al. Comparison of the glasgow prognostic score (GPS) and the modified glasgow prognostic score (mGPS) in evaluating the prognosis of patients with operable and inoperable non-small cell lung cancer. J Cancer Res Clin Oncol. 2016;142(6):1285–1297.
- Ueda K, Ogasawara N, Yonekura S, et al. The prognostic value of systemic inflammatory markers in advanced renal cell carcinoma patients treated with molecular targeted therapies. Anticancer Res. 2020;40(3):1739–1745.
- Suzuki K, Terakawa T, Furukawa J, et al. C-reactive protein and the neutrophil-to-lymphocyte ratio are prognostic biomarkers in metastatic renal cell carcinoma patients treated with nivolumab. Int J Clin Oncol. 2020;25(1):135–144.
- Fukuda S, Saito K, Yasuda Y, et al. Impact of C-reactive protein flare-response on oncological outcomes in patients with metastatic renal cell carcinoma treated with nivolumab. J Immunother Cancer. 2021;9(2):e001564.
- Lindskog M, Wahlgren T, Sandin R, et al. Overall survival in swedish patients with renal cell carcinoma treated in the period 2002 to 2012: update of the RENCOMP study with subgroup analysis of the synchronous metastatic and elderly populations. Urol Oncol. 2017;35(9):541.e15–541.e22.
