?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
Papillary urothelial neoplasm of low malignant potential (PUNLMP) and stage TaG1 non-muscle invasive bladder cancer (NMIBC) represent separate categories in current WHO 1999 grade definitions. Similarly, PUNLMP and Ta low-grade are separate entities in the WHO 2004/2016 grading system. However, this classification is currently questioned by reports showing a similar risk of recurrence and progression for both categories.
Patients and methods
In this population-based study, risk estimates were evaluated in patients diagnosed with PUNLMP (n = 135) or stage TaG1 (n = 2176) NMIBC 2004–2008 with 5-year follow-up registration in the nation-wide Bladder Cancer Data Base Sweden (BladderBaSe). The risk of recurrence was assessed using multivariable Cox regression with adjustment for multiple confounders (age, gender, marital status, comorbidity, educational level, and health care region).
Results
At five years, 28/135 (21%) patients with PUNLMP and 922/2176 (42%) with TaG1 had local recurrence. The corresponding progression rates were 0.7% (1/135) and 4.0% (86/2176), respectively. A higher relative risk of recurrence was detected in patients with TaG1 tumours compared to PUNLMP (Hazard Ratio 1.6, 95% CI 1.2–2.0) at 5-year follow-up, while progression events were too few to compare.
Conclusions
The difference in risk of recurrence between primary stage TaG1 and PUNLMP stands in contrast to the recently adapted notion that treatment and follow-up strategies can be merged into one low-risk group of NMIBC.
1. Introduction
The papillary urothelial neoplasm of low malignant potential (PUNLMP) grade definition as introduced in 1998, constitutes a category of non-muscle invasive bladder cancer (NMIBC) with mild nuclear atypia and increased cell proliferation, but with preserved polarity and minimal variation among cells [Citation1]. However, data indicate similar recurrence and progression rates for PUNLMP and low-grade tumours (WHO 2004/2016) [Citation1–4]. Likewise, similar estimates for recurrence and progression for 127 PUNLMP out of 2204 Ta low-grade tumours were recently reported in an international multicenter study [Citation5]. Consequently, and in conjunction with a decreased proportion of tumours categorized as PUNLMP over time, the use of PUNLMP as a separate grade category has been questioned [Citation6].
In Sweden, the national urothelial carcinoma guidelines (https://kunskapsbanken.cancercentrum.se/diagnoser/urinblase-och-urinvagscancer/vardprogram/) recommend a combination of the 4-tiered WHO 1999 (PUNLMP, G1, G2, and G3) and the 3-tiered WHO 2004/2016 grading systems (PUNLMP, low-grade (G1), high-grade (G2), and high-grade (G3), where WHO 1999 G1 to a large extent corresponds to WHO 2004/2016 low-grade. To assess the risk of recurrence and progression in a population-based setting using the WHO 1999 grading system, we investigated all patients diagnosed with primary stage TaG1 or PUNLMP between 2004 and 2008 in the Bladder Cancer Data Base Sweden (BladderBaSe), comprising all patients in the Swedish National Registry of Urinary Bladder Cancer (SNRUBC).
2. Patients and methods
2.1. Study population
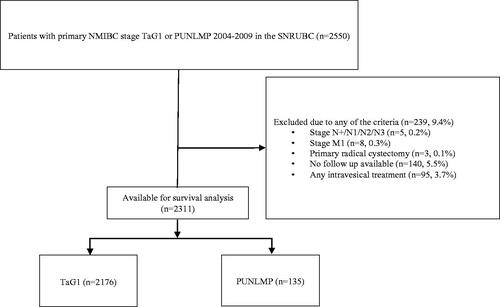
We identified 2550 patients diagnosed with primary stage TaG1 or PUNLMP between 2004 and 2008 in the Bladder Cancer Data Base Sweden (BladderBaSe), comprising all patients in the Swedish National Registry of Urinary Bladder Cancer (SNRUBC) with linked data from national registers, demographic data and 5 years follow-up data from a follow-up form distributed 5 years after diagnosis [Citation7]. We excluded patients treated with primary radical cystectomy (n = 3), primary lymph node-positive (n = 5), or metastatic disease (n = 8) as registered in the primary form at diagnosis, and patients with no available follow-up (missing 5 years follow-up form, n = 140) (CONSORT flow chart in ). Information on adjuvant intravesical treatment in the SNRUBC for the included years is incomplete with respect to the type of treatment (BCG or chemotherapy), duration of treatment, and/or the number of courses administered. Furthermore, such adjuvant treatment affects recurrence, which was our primary outcome measure. Thus, we excluded all 95 patients treated with adjuvant instillations. Consequently, the current study included 2311 patients for analyses (PUNLMP, n = 135 and TaG1, n = 2176).
2.2. Measures
Recurrence and progression data and dates were retrieved from the Swedish National Registry of Urinary Bladder Cancer (SNRUBC) 5-year follow-up form. Recurrence was defined as tumour recurrence verified by biopsy and/or TUR-B. Progression was defined as muscle-invasive local recurrence or nodal and/or distant metastasis.
Charlson Comorbidity Index (CCI) [Citation8] was calculated based on a list of diseases, with a specific weight assigned to each disease category according to data from the National Patient Register. The separate weights were collated to give an overall score, categorising morbidity as follows: 0 = none, 1 = mild, 2 = intermediate, and ≥3 = severe. Educational level was retrieved from Statistics Sweden and categorised as low (≤9 years of education), intermediate (10–12 years), and high (≥13 years), corresponding to mandatory school, high school, and college or university [Citation7].
2.3. Statistical analyses
The Kaplan–Meier technique was used for calculating the cumulative incidence of recurrence at 12, 15, and 60 months after the date of diagnosis. Hazard ratio for risk of recurrence comparing PUNLMP with TaG1 were estimated from the date of diagnosis using multivariable Cox regression with adjustment for multiple confounders (age, gender, marital status, comorbidity, educational level, and health care region). Date of death or 5-year follow-up date was considered the end of follow-up, whatever happened first. Due to only one registered case of progression in the PUNLMP group, we chose not to compare the risk of progression between groups.
As a sensitivity analysis, the regression models were also performed excluding the Northern healthcare region, motivated by the relatively higher proportion of PUNLMP diagnosed (20%). We also performed a sensitivity analysis in respect to the relatively high proportion of patients (5.5%) with no available follow-up (total n = 140; PUNLMP, n = 17, TaG1 n = 123). Hence, the multivariable Cox regression models for risk of recurrence were tested in two separate analyses with the imputation of the following hypothetical extreme outcomes: (a) all patients with no available follow-up registered with an event (recurrence) after 1 year during follow-up, and (b) all patients with no available follow-up registered with no event (recurrence) during follow-up.
For all statistical analyses, the R statistical package was used [Citation9].
2.4. Ethical review
The study was approved by the Research Ethics Board of Uppsala University, Sweden (EPN 2015/277).
3. Results
Baseline characteristics are available in . The proportion of PUNLMP in the study population (5.8%) remained consistent throughout the included years (2004, 6.3%; 2005, 7.8%; 2006, 5.5%; 2007, 4.2%; 2008, 5.4%) and beyond (data until 2015 in Supplemental Table 1). Recurrence proportions at 12, 15, and 60 months are available in Supplemental Table 2.
Table 1. Baseline characteristics for patients in the Swedish National Registry of Urinary Bladder Cancer (SNRUBC) diagnosed in 2004–2008 with papillary urothelial neoplasm of low malignant potential (PUNLMP) or TaG1 bladder cancer.
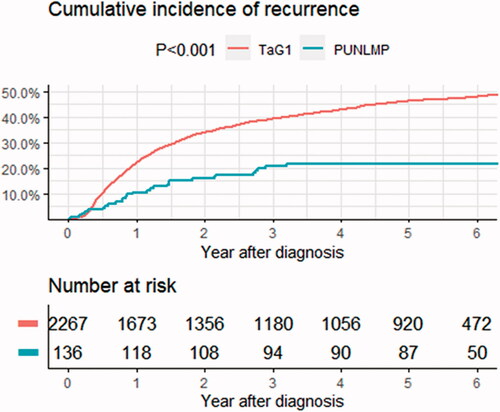
The 5-year recurrence rate was 21% (95% CI, 14–29) (28/135) for PUNLMP patients and 42% (95% CI 40–44) (922/2176) for TaG1, and the corresponding progression rates were 0.7% (95% CI 0.0–4.1) (1/135) and 4.0% (95% CI, 3.2–4.9) (86/2176) (). Using Cox multivariable regression models, TaG1-tumours were associated with increased risk of recurrence (HR 1.6, CI 1.2–2.0) compared to PUNLMP (). Proportions of recurrence at 12, 15, and 60 months for PUNLMP and TaG1 are available in Supplemental Table 2.
Table 2. Risk of recurrence evaluated in a Cox regression model.
A sensitivity analysis excluding the Northern health care region with the highest proportion PUNLMP (20%) (), revealed no changes from the main results (Supplemental Table 3). Assessing the patients with no available 5 years follow-up, revealed a higher proportion of PUNLMP compared to the included study population (17/140, 12% vs. 135/2311, 5.8%, p = 0.005). When all these patients were added to the multivariable Cox regression models with assumptions of 100% or 0% recurrence, respectively, the resulting HR for TaG1 compared to PUNLMP were 1.6 (1.3–2.1, p < 0.001) and 1.4 (1.1–1.7, p = 0.006). Thus, both risk estimates remained within the 95% CI of the main analysis suggesting no significant selection bias.
4. Discussion
This population-based study comprising the largest series of PUNLMP patients to date showed a 60% increased risk of tumour recurrence for TaG1 compared to PUNLMP after 5 years of follow-up. In contrast to the recent study by Henstchel et al. that was performed in the context of the 3-tiered WHO 2004/2016 grading system, the present study was based on the 4-tiered WHO 1999 grading system. Furthermore, we opted to exclude patients with co-existing carcinoma in situ (CIS) and any registered adjuvant instillations to rule out effects of recurrence-reducing treatments when evaluating prognostic differences between PUNLMP and TaG1-tumours [Citation5]. Another difference between that study and the present was the population-based data in the SNRUBC with high coverage and from five consecutive years, while Hentschel et al. collected data from several European countries (and Canada) diagnosed over almost three decades [Citation5]. Altogether, these three differences compared to the present study might explain why no difference in time to recurrence was found in that study. In addition, the lack of information about tumour multiplicity and tumour size limits the comparison of our findings with those in other studies. The persistent reporting of PUNLMP cases throughout study years and health care regions is also different from other studies [Citation1,Citation5] ().
Table 3. Recurrence and progression in previous studies with 50 cases of PUNLMP (adapted from Hofmann et al.) [Citation7].
The pathological distinction between PUNLMP and Ta low-grade (G1) is a challenging task with known high interobserver variability [Citation1], despite the introduction of more precise criteria in both the WHO 1999 and 2004 definitions. In the current study, pathological re-evaluation adjusting for interobserver variability was not done. On the other hand, assessing the outcome of PUNLMP applied in a clinical context without pathological re-evaluation confers the broad generalizability of our results. The lack of detailed information on the disease course in patients with progressive disease is another study limitation. Likewise, data on progression to stage T1 was not available, further limiting a more specific description of these patients.
Compared to Swedish patients diagnosed before the current study between 1987 and 1988, 5 years progression rates for PUNLMP and Ta low-grade (0 vs. 4%, respectively) were similar to our present data [Citation13] (), while corresponding recurrence rates in the older study were higher (35 vs. 71%, respectively). The similar relative difference between groups suggests improved transurethral resection quality and clinical care over time. Thus, trends in calendar time for date of diagnosis may be important to be taken into account when comparing outcomes for PUNLMP and Ta low-grade.
The EAU-NMIBC guidelines panel group recently concluded that a 4-tiered combination of the WHO 1973 and 2004/2016 outperforms each of the 3- and 2-tiered grading systems individually, when evaluating a large retrospective individual patient data study [Citation15]. Considering molecular grade as a continuous variable [Citation16], a more granular 4-tiered stratification also seems beneficial compared to a less granular 3- or even 2-tiered grade stratification. In addition, a new ‘very high risk’ group was defined comprising patients with the most elevated risk of progression to facilitate identification and consideration of primary cystectomy [Citation15].
In the other spectrum of NMIBC, the opposite strategy is applied when merging PUNLMP and low-grade in one group. The risk at stake here has not the same dignity as in high-grade disease, yet overtreatment and unnecessary cystoscopies are also important to consider [Citation17]. However, at present current guidelines recommend different follow-up schedules for Ta low-grade tumours, with NICE favouring only two cystoscopies at 3 and 12 months post-operatively [Citation17], whereas EAU proposes follow-up cystoscopies for five years. In our data, the probability of recurrence at 15 months (including the 12-month cystoscopy) was 13% (CI 7.0–19) vs. 26% (CI 24–28) for PUNLMP and TaG1, respectively. While the cumulative incidence curve for recurrence for TaG1 continues to rise, there are no recurrences after 3 years in patients with PUNLMP (). These differences suggest biological factors leading to separate clinical courses for these grade categories. Irrespective of tumour size and multiplicity, patients with PUNLMP tumours can be recommended to stop follow-up cystoscopies after three years due to the low risk of recurrence and negligible risk of progress according to our findings.
5. Conclusions
In summary, our results show a 60% increased relative risk of tumour recurrence at 5 years for TaG1 compared to PUNLMP in a population-based setting using the WHO 1999 grading system. Despite limitations in the analysed data, we argue that abandoning the PUNLMP category would neglect important biologic and prognostic information when designing trials for low-risk NMIBC and that patients with PUNLMP tumours can stop follow-up cystoscopies at three years based on the extremely low risk of further recurrences and no risk of progression.
Supplemental Material
Download MS Word (30.6 KB)Disclosure statement
The authors have nothing to disclose.
Additional information
Funding
References
- MacLennan GT, Kirkali Z, Cheng L. Histologic grading of noninvasive papillary urothelial neoplasms. Eur Urol. 2007;51(4):889–897; discussion 897–898.
- van Rhijn BW, Burger M, Lotan Y, et al. Recurrence and progression of disease in non-muscle-invasive bladder cancer: from epidemiology to treatment strategy. Eur Urol. 2009;56(3):430–442.
- Jones TD, Cheng L. Papillary urothelial neoplasm of low malignant potential: evolving terminology and concepts. J Urol. 2006;175(6):1995–2003.
- Hofmann TK-C, Hartmann R, Stöhr A, et al. Clinical implications of the 2004 WHO histological classification on non-invasive tumours of the urinary bladder. EAU-EBU Update Series. 2006;4(3):83–95.
- Hentschel AE, van Rhijn BWG, Brundl J, et al. Papillary urothelial neoplasm of low malignant potential (PUN-LMP): still a meaningful histo-pathological grade category for Ta, noninvasive bladder tumors in 2019? Urol Oncol. 2020;38(5):440–448.
- Sylvester RJ, Rodriguez O, Hernandez V, et al. European Association of Urology (EAU) prognostic factor risk groups for non-muscle-invasive bladder cancer (NMIBC) incorporating the WHO 2004/2016 and WHO 1973 classification systems for grade: an update from the EAU NMIBC guidelines panel. Eur Urol. 2021;79(4):480–488.
- Haggstrom C, Liedberg F, Hagberg O, et al. Cohort profile: the Swedish National Register of Urinary Bladder Cancer (SNRUBC) and the bladder cancer data base Sweden (BladderBaSe). BMJ Open. 2017;7(9):e016606.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- R Core Team 2020. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. Available from: https://www.R-project.org/
- Oosterhuis JW, Schapers RF, Janssen-Heijnen ML, et al. Histological grading of papillary urothelial carcinoma of the bladder: prognostic value of the 1998 WHO/ISUP classification system and comparison with conventional grading systems. J Clin Pathol. 2002;55(12):900–905.
- Cheng L, Neumann RM, Bostwick DG. Papillary urothelial neoplasms of low malignant potential. Clinical and biologic implications. Cancer. 1999;86(10):2102–2108.
- Holmang S, Andius P, Hedelin H, et al. Stage progression in ta papillary urothelial tumors: relationship to grade, immunohistochemical expression of tumor markers, mitotic frequency and DNA ploidy. J Urol. 2001;165(4):1124–1128. discussion 1128–1130.
- Holmang S, Hedelin H, Anderstrom C, et al. Recurrence and progression in low-grade papillary urothelial tumors. J Urol. 1999;162(3 Part 1):702–707.
- Fujii Y, Kawakami S, Koga F, et al. Long-term outcome of bladder papillary urothelial neoplasms of low malignant potential. BJU Int. 2003;92(6):559–562.
- van Rhijn BWG, Hentschel AE, Brundl J, et al. Prognostic value of the WHO1973 and WHO2004/2016 classification systems for grade in primary Ta/T1 non-muscle-invasive bladder cancer: a multicenter European Association of Urology non-muscle-invasive bladder cancer guidelines panel study. Eur Urol Oncol. 2021;4(2):182–191.
- Liedberg F, Lauss M, Patschan O, et al. The importance of being grade 3: WHO 1999 versus WHO 2004 pathologic grading. Eur Urol. 2012;62(4):620–623.
- Malmstrom PU. Cystoscopic surveillance of patients with non-muscle-invasive bladder cancer revisited. Scand J Urol. 2020:54(5):364–366.