Abstract
Introduction and objectives
Bladder cancer is primarily a disease of older age and little is known about the differences between patients diagnosed with bladder cancer at a younger versus older age. Our objectives were to compare bladder cancer specific survival in patients aged <50 versus those aged 50–70 at time of diagnosis.
Materials and methods
The Swedish bladder cancer database provided data on patient demographics, clinical characteristics and treatments for this observational study. Cox proportional hazard regression models were adjusted for appropriate variables. All analyses were stratified by disease stage (non-muscle-invasive bladder cancer and muscle-invasive bladder cancer. Furthermore, we compared the frequency of lower urinary tract infections within 24 months prior to bladder cancer diagnosis by sex and age groups.
Results
The study included 15,452 newly-diagnosed BC patients (1997–2014); 1,207 (8%) patients were <50 whilst 14,245 (92%) were aged 50–70. Patients aged <50 at diagnosis were at a decreased risk of bladder cancer death (HR = 0.82, 95%CI: 0.68–0.99) compared to those aged 50–70. When stratified by non-muscle-invasive and muscle-invasive bladder cancer, this association remained in non-muscle-invasive patients only (<50, HR = 0.43, 95% CI: 0.28–0.64). The frequency of lower urinary tract infection diagnoses did not differ between younger and older patients in either men or women.
Conclusions
Patients diagnosed with non-muscle-invasive bladder cancer when aged <50 are at decreased risk of bladder cancer-specific death when compared to their older (50-70) counterparts. These observations raise relevant research questions about age-related differences in diagnostic procedures, clinical decision-making and, not least, potential differences in tumour biology.
Keywords:
Introduction
Bladder cancer (BC) is primarily a disease of older age with a median age at diagnosis of 74 in Sweden [Citation1]. Since the majority of BC patients are aged over 50, published literature naturally focuses on older patients. Consequently, limited information is available on the demographics, clinical characteristics, and survival outcomes for younger BC patients and how these compare to older patients.
Recently, studies have attempted to answer such research questions; however, the majority have been undertaken on single-centre or regional data and are therefore limited in their cohort sizes and external validity [Citation2–6]. To our knowledge, our study is the first European nationwide study investigating clinical outcomes of young BC patients.
In this study, we aimed to compare the prognosis of BC patients aged <50, to those aged 50–70 in terms of BC-specific death. Age 50 was the cut-off age as this is the age for commencing standardized care pathways for individuals with macroscopic haematuria in Sweden, although also younger individuals with macroscopic haematuria are referred to a urologist but outside standardized care pathways due to a considerably lower risk of cancer [Citation7]. We used 50–70 as the main comparator since, within this age-span, general health in the majority of patients still permits all treatments with curative intent, as in younger patients and in contrast to those patients in older age-groups. This assumption is valid also for non-muscle invasive bladder cancer where age above 70 years is associated with independently higher risk of disease progression [Citation8].
Materials and methods
Study population and variables
The Bladder Cancer Data Base Sweden (BladderBaSe) was created in 2015. It links information from the Swedish National Register of Urinary Bladder Cancer (SNRUBC) from 1997 to 2014, with a number of national health care and demographic registers through personal identification numbers [Citation9,Citation10]. The research was approved by the Research Ethics Board of Uppsala University, Sweden (File no. 2015/277).
In Sweden a National Board of Health and Welfare consensus from 1999 recommended that microhaematuria testing in adults should be abandoned [Citation11], and primary care physicians refer only individuals with macroscopic haematuria or those with urinary tract symptoms and microscopic haematuria. Thus, the proportion of patients diagnosed with bladder cancer based on microscopic haematuria has been below 4% in Swedish population-based series [Citation12], although information about reasons behind referrals are lacking in the current study.
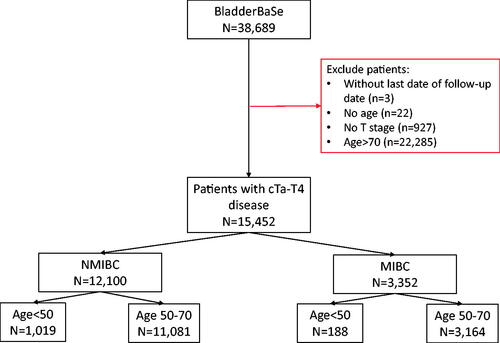
All patients diagnosed with BC (any T, any N, any M) between 1 January 1997 and 31 December 2014 were included. Data on the patients’ demographics and clinical characteristics were extracted: age, sex, education (low (≤9 years of school), intermediate (10–12 years), high (≥13 years)), civil status (unmarried, married, widowed, divorced), Charlson Comorbidity Index (CCI) (0, 1, 2, 3+), clinical TNM stage, tumour grade (WHO 1973 (1997–2002) and WHO 1999 (2003 onwards)). Patients with missing age or clinical T stage were excluded. Information was extracted regarding patients’ treatments at diagnosis. To adjust for possible confounding by LUTIs, we obtained information for ICD-10 codes N30 (cystitis), N30.9 (cystitis unspecified), N34 (urethritis) and N39 (disorder of urinary system caused by infection with unspecified location). NMIBC patients were stratified as low/high risk. Low risk NMIBC was defined as TaG1-G2, whilst high risk was defined as any of TaG3/Tis/T1. MIBC patients were also stratified by non-metastatic vs. metastatic. Treatments for MIBC patients with N + disease were analysed separately.
Statistical analyses
Descriptive analyses were undertaken for demographic and clinical information and stratified by age groups (<50 and 50–70). Chi-squared tests were used to identify differences between demographic and clinical characteristics for the age groups. To refine the Chi-squared significance, tests of proportions were subsequently performed on those variables identified as varying between age groups.
Using age <50 as the exposure of interest, Cox proportional hazards regression models were performed to calculate hazard ratios (HRs) as a measure of relative risk of BC death. All analyses were adjusted for sex, CCI, civil status, education, tumour grade and clinical (c)TNM stage. Adjustments were determined through the use of a directed acyclic graph using the DAGitty tool [Citation13]. We additionally adjusted for the number of LUTIs diagnosed in the two years prior to BC diagnosis. Furthermore, two sensitivity analyses were performed: (1) age ≤40 was used as the exposure, and (2) exclusion of patients with M + and N + disease.
All data analysis was performed using STATA 16.1 (Texas, USA).
Results
Cohort characteristics
We identified 15,452 patients in BladderBaSe: 1,207 (8%) were aged <50; 14,245 (92%) aged 50-70. summarizes the cohort demographics. The age groups differed in sex distribution with a higher proportion of females in the 50–70 age group than the younger age group. The <50 group had a higher proportion of cTa patients compared to the 50–70 s; there was also a higher frequency of G1 tumours in the <50 group. A higher proportion of patients in the <50 had a CCI of 0 (90%) compared to the 50–70 s. The cohort demographics showed a similar pattern of distribution when stratified by sex (Supplementary Table 1). Furthermore, there was an even distribution of patients within each age group across the study time-frame from 1997 to 2014.
Table 1. Cohort demographics when stratified by age groups.
Six percent (n = 902) of patients experienced a LUTI during the two years prior to their BC diagnosis. When stratified by age, 5% of patients aged <50 had experienced 1-2 LUTIs, compared to 6% in the 50–70 s. Across all age groups, the proportion of women experiencing at least one LUTI in the two years prior to BC diagnosis was statistically significantly larger than for men (8% vs. 5%, p < 0.0294).
Treatments – NMIBC
Of all NMIBC patients, 8% (aged < 50) and 12% (50–70) of patients had received intravesical treatment. Four percent of low risk NMIBC patients aged <50 received intravesical therapy with serial instillations, compared to 5% of patients age 50–70 (). In the high risk group, 20% of patients aged <50 received intravesical therapy with serial instillations compared to 26% of patients aged 50–70. Four percent of NMIBC patients had received a single-dose of post-operative chemotherapy with similar proportions observed between the age groups. In those with high-risk NMIBC, 13% aged <50 underwent radical cystectomy compared to 10% aged 50–70.
Table 2. Treatment types when stratified by age groups and NMIBC (low and high risk) and MIBC (non-metastatic and metastatic).
With respect to external-beam radiotherapy in the non-metastatic MIBC patients, three (2%) patients aged <50 received such treatment compared to 105 (4%) patients aged 50–70 (). Sixty-eight percent of non-metastatic MIBC patients underwent radical cystectomy (77% in those aged <50 and 67% in those aged 50–70). The proportion of non-metastatic MIBC patients who received neoadjuvant chemotherapy (NAC) in conjunction with radical cystectomy was 15% in those aged <50 and 12% in those aged 50–70. When the use of NAC was assessed over time in the non-metastatic MIBC patients, there was a steady increase in usage of NAC across age groups (1997–2014) (Supplementary Figure 1).
The proportion of patients with metastatic disease (N+/M1) on best supportive care was lowest in those aged <50 (20%) compared to those aged 50–70 (38%) (). When the MIBC N + patients were analysed separately, similar proportions of patients underwent radical cystectomy and perioperative chemotherapy among the <50 and 50–70 s (47 vs. 49% and 21 vs. 21% respectively).
Risk of bladder cancer death
Overall median follow-up was 5.30 years (IQR:1.92–10.14). Patients aged <50 at diagnosis were at a decreased risk of BC death (HR = 0.82, 95%CI: 0.68–0.99) compared to those aged 50–70 (). When survival analyses were adjusted for number of LUTI diagnoses in the two years prior to diagnosis, the results remained unchanged.
Table 3. Hazard ratios (HR) and 95% confidence intervals (CIs) for risk of bladder cancer death.
Risk of bladder cancer death – NMIBC
In the NMIBC patients, those aged <50 at diagnosis were at a decreased risk of BC death (HR = 0.43, 95%CI:0.28-0.64) compared to those aged 50–70 (). Kaplan Meier analyses also showed similar associations, including when stratified by sex ( and and supplementary Figures 2 and 3). The 5- and 10-year survival proportions were 98 and 96% for those aged <50, and 95% and 92% in those aged 50–70 (). The same pattern of associations remained when the NMIBC patients were stratified by low and high risk patients, and when adjusted for number of LUTI diagnoses in the two years prior to diagnosis.
Figure 2. Kaplan–Meier curves for bladder cancer specific survival when stratified by age group (<50 vs. 50–70). (A) All patients, (B) non-muscle invasive bladder cancer patients (NMIBC), and (C) muscle invasive bladder cancer patients (MIBC).
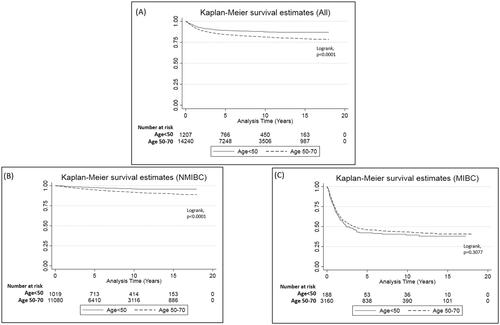
Table 4. 5 and 10-Year survival proportions and 95% confidence intervals when stratified by age groups.
When considering patients aged <40 in the younger group, the results were no longer statistically significant for all NMIBC and when stratified by low and high risk NMIBC (). Furthermore, when excluding patients with N+/M + disease from analyses, the results were no longer statistically significant for high-risk NMIBC. Other sensitivity analyses did not statistically significantly alter the results for NMIBC patients.
Risk of bladder cancer death – MIBC
For MIBC patients, the risk of death in the <50 group did not differ to those aged 50–70 (HR = 0.99, 95%CI:0.79–1.23) (). We found the same pattern of association when MIBC patients were stratified by non-metastatic and metastatic patients, and when adjusted for number of LUTI diagnoses in the two years prior to diagnosis. The sensitivity analyses did not statistically significantly alter any of the results for the MIBC patients.
Discussion
In this nationwide observational study, BC patients diagnosed aged <50 had a statistically significantly decreased risk of BC-specific death when compared to patients aged 50–70. When stratified by stage, this association remained in patients with NMIBC, both low and high risk. In those with MIBC, the risk of BC-specific death did not differ between age categories.
Previous studies have investigated clinical outcomes for younger BC patients although, to our knowledge, this is the first study using population-based national data. With respect to risk of death, a study by Lara et al. [Citation3] concluded that younger patients had a 58% reduced risk of BC death (p < 0.001) compared to older patients. These results are in-line with the results from our study, although we only observed reduced risk in NMIBC patients. The study by Lara et al., however, did not stratify their analysis by T stage. Furthermore, the lack of information on cisplatin-eligibility in that and the present study diminishes the possibility to further disentangle survival differences in younger patients with MIBC [Citation14]. Cisplatin-based chemotherapy constitutes a guideline-driven and integral part of MIBC treatment in all eligible patients [Citation15]; however, above 70 years of age the proportion of patients eligible for cisplatin decreases largely due to impaired renal function [Citation16].
We identified different associations for age-related risk of death between NMIBC and MIBC patients. Janisch et al. conducted a study in MIBC patients treated with radical cystectomy and reported a null association between age <50 vs. >50 and risk of cancer specific death [Citation4]. The study by Feng et al. also investigated the effect of age on risk of death stratified by tumour stage and reported a longer survival time (BC-specific) for those aged <50 for all stages, thereby diverging from the current results [Citation17]. In our study, the <50 s had the highest proportion of patients with Ta tumours, whilst the 50–70 s had the highest proportion of patients with invasive tumours, especially stages T1–2. However, the proportion of T3–T4, as well as metastatic patients (M+/N+), did not differ greatly between the age groups. Therefore, one possible explanation behind the observed association is that there is a disease stage cut-off (representing local tumour biology and metastatic potential) beyond which the benefits of youth are negated. A study by Tian et al. [Citation18] concluded that younger MIBC patients were at increased risk of locoregional lymph node metastasis compared to their older counterparts in a selected population treated with radical cystectomy and lymphadenectomy (where at least one lymph node was examined). In the present study we have utilised clinical lymph node staging, rather than the number of positive excised lymph nodes at cystectomy.
Since patients with higher stage grade have higher mortality [Citation19], the statistically significant difference between stages and grades between the age groups may in part explain the differences in BC-specific mortality risk observed - in the current study the <50 s had more cTa and low grade tumours compared to their older counterparts, as also reported (for grade) by both de la Calle et al. [Citation5] and Telli et al. [Citation20]. These differences in stages and grades may suggest different disease biology between the age groups; hence, investigating the distribution of taxonomic subgroups is of merit for further future research, similar to that of Shelekhova et al. [Citation21]. Here the authors concluded that more aggressive molecular subtypes were more frequent in older patients. Similarly, the basal-squamous like subtype has been associated with higher age [Citation22]. The molecular pathobiology of BC is complex [Citation23], however, it is feasible to consider that, in the absence of age-related immunosenescence [Citation24], younger patients may be better able to corral transformed cells within the urothelium and limit migration beyond the basal membrane. Equally, symptom-related health-seeking behaviour may differ between age groups and this may be reflected subsequently by the observed differences in stages at diagnosis. Furthermore, the allocation of perioperative chemotherapy for non-metastatic patients differed between age groups (higher proportion aged <50 receiving such treatments than those aged 50–70). It is also worth mentioning that there were some regional differences in treatment allocation. For example, there appeared to be a higher proportion of younger patients who received intravesical therapy in the South when compared to the background population of NMIBC in that region (37 vs. 21%). Meanwhile, in Stockholm and Uppsala, the proportion of young patients receiving intravesical therapy appears to be smaller than the background population (10 vs. 22% and 9 vs. 17%, respectively).
There is heterogeneity in how to define ‘younger patients’ in the existing literature, with <40 used elsewhere [Citation3,Citation5]. Our sensitivity analyses did however change the statistical significance of the results for NMIBC patients albeit with lower numbers (n = 296) than in previous studies by Lara et al. (n = 1,688) [Citation3] and de la Calle et al. (n = 3,314) [Citation5]. Since the presence of gross haematuria in ≥50 s triggers standardized care pathways in Sweden, we considered this as an appropriate cut-off for this dataset.
The occurrence of LUTIs before diagnosis did not confound the comparison between those <50 with those 50–70. As a higher proportion of women than men are diagnosed with LUTI prior to BC diagnosis, and women also are diagnosed with BC at a later stage [Citation25], further study is warranted of the association between LUTI and misinterpretation of symptoms and delayed diagnosis [Citation26–28]. However, to investigate how the age and sex distribution noted in our study compare to the one expected in a background non-BC population, controls would be necessary. Controls will be added to the next version of BladderBaSe hence making such a study possible in the future.
The strengths of this study include the use of data from the BladderBaSe for over 15,000 newly-diagnosed BC cases in Sweden with the possibility to stratify by age groups and by NMIBC and MIBC. Furthermore, linkage of the BladderBaSe to both inpatient and outpatient registers permitted analysis of LUTI diagnoses during the two years prior to BC diagnosis. Limitations include missingness of some variables such as N stages (65% were either NX or missing) related to the lack of cross-sectional imaging in patients with a low risk of lymph node metastases or substantial comorbidity (where presence of lymph node metastases would not alter the treatment plan). Therefore, we were not able to confirm or refute the results from the Chi-squared test for this variable [Citation18]. We also did not have access to any data regarding tobacco smoking or occupation therefore were not able to study or adjust for these variables within our analyses. There is also difficulty in separating the clinical stages T2 and T3 in routine practice, though here we only report descriptive data and do not make any firm conclusions based on clinical stage. For LUTIs, the patients captured may have encompassed the most severe infections and may not be representative of all LUTIs or upper tract UTIs related to ureteric obstruction by yet-to-be-diagnosed BC. We also note that, for all stages and age groups, there appears to be low utilisation of adjuvant peri-operative therapies (both intravesical and systemic) which may limit the generalisability of our findings.
Conclusion
This study has demonstrated a decreased risk of BC death in patients who are diagnosed with NMIBC aged <50 when compared to those diagnosed at age 50–70. The distribution of stage and grade among these younger patients may in part explain these differences as well as possible differences in tumour biology associated with onset of disease in different age groups either as result of, or in parallel with, diagnostic biases, treatment allocations, and differences. It is also possible that the change in behaviours over the years with habits such as tobacco smoking may have influenced these results. These observations raise relevant research questions regarding age-related differences in diagnostic delay, clinical decision-making, and tumour biology.
Ethical approval
Research was approved by the Research Ethics Board of Uppsala University, Sweden (File no. 2015/277).
Author contributions
Study design – All authors. Data analysis – BR, OH, FL, LH, MVH. Writing and review of the manuscript – All authors.
Supplemental Material
Download MS Word (490.9 KB)Acknowledgments
This project was made possible with help of the data collected in the SNRUBC, and we would like to thank the members of the SNRUBC: Viveka Ströck, Firas Abdul-Sattar Aljabery, Johan Johansson, Per-Uno Malmström, Malcolm Carringer, Abolfazl Hosseini-Aliabad, Truls Gårdmark, Amir Sherif, Roland Rux, Markus Johansson, Petter Kollberg, Anna-Karin Lind, Jenny Wanegård, Magdalena Cwikiel, Elisabeth Överholm, Anders Ullen, Erika Jonsson, Helena Thulin, Gun Danielsson, Helene Hummer, Fredrik Liedberg, and Staffan Jahnson.
Disclosure statemet
No potential conflict of interest was reported by the author(s).
Data availability statement
The BladderBaSe data is held on a secure server and is therefore not publicly available. However, applications to access the data can be made by contacting [email protected].
References
- Swedish National Quality Register for Bladder and Urinary Tract Cancer (SNRUBC) [Internet]. 2019. Available from: https://statistik.incanet.se/Urinblasecancer/.
- Katafigiotis I, Sfoungaristos S, Martini A, et al. Bladder cancer to patients younger than 30 years: a retrospective study and review of the literature.Urologia]. 2017;84(4):231–235.
- Lara J, Brunson A, Keegan TH, et al. Determinants of survival in adolescents and young adults with urothelial bladder cancer: results from the California cancer registry. J Urol. 2016;196(5):1378–1382.
- Janisch F, Yu H, Vetterlein MW, et al. Do younger patients with muscle-invasive bladder cancer have better outcomes?J Clin Med. 2019;8(9):1–10.
- de la Calle CM, WashingtonSL, LonerganPE, et al. Bladder cancer in patients younger than 40 years: outcomes from the national cancer database. World J Urol. 2021;39(6):1911–1916.
- Wang ZH, Li YY, Hu ZQ, et al. Does urothelial cancer of bladder behave differently in young patients? Chin Med J. 2012;125(15):2643–2648.
- Nilbert M, Bläckberg M, Ceberg J, et al. Diagnostic pathway efficacy for urinary tract cancer: population-based outcome of standardized evaluation for macroscopic haematuria. Scand J Urol. 2018;52(4):237–243.
- Sylvester RJ, Rodríguez O, Hernández V, et al. European association of urology (EAU) prognostic factor risk groups for non–muscle-invasive bladder cancer (NMIBC) incorporating the WHO 2004/2016 and WHO 1973 classification systems for grade: an update from the EAU NMIBC guidelines panel[formula presented]. Eur Urol. 2021;79(4):480–488.
- Haggstrom C, Liedberg F, Hagberg O, et al. Cohort profile: the swedish national register of urinary bladder cancer (SNRUBC) and the bladder cancerData Base Sweden (BladderBaSe)). BMJ Open. 2017;7(9):e016606.
- Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, et al. The swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24(11):659–667.
- Malmström PU. Time to abandon testing for microscopic haematuria in adults? Br Med J. 2003;326(7393):813–815.
- Månsson Å, Anderson H, Colleen S. Time lag to diagnosis of bladder cancer-influence of psychosocial parameters and level of health-care provision. Scand J Urol Nephrol. 1993;27(3):363–369.
- Textor J, van der Zander B, Gilthorpe MK, et al. Robust causal inference using directed acyclic graphs: the R package “dagitty. Int J Epidemiol. 2016;45(6):1887–1894.
- Jiang DM, Gupta S, Kitchlu AK, et al. Defining cisplatin eligibility in patients with muscle-invasive bladder cancer. Nat Rev Urol. 2021;18(2):104–114.
- Witjes JA, Bruins HM, Cathomas R, et al. European association of urology guidelines on muscle-invasive and metastatic bladder cancer: Summary of the 2020 guidelines [internet. Eur Urol. 2021;79(1):82–104.
- Canter D, Viterbo R, Kutikov A, et al. Baseline renal function status limits patient eligibility to receive perioperative chemotherapy for invasive bladder cancer and is minimally affected by radical cystectomy. Urology. 2011;77(1):160–165.
- Feng H, Zhang W, Li J, et al. Different patterns in the prognostic value of age for bladder cancer-specific survival depending on tumor stages. Am J Cancer Res. 2015;5(6):2090–2097.
- Tian Z, Meng L, Wang X, et al. Young age increases the risk of lymph-node metastasis in patients with muscle-invasive bladder urothelial carcinoma. BMC Cancer. 2020;20(1):1–6.
- Kirkali Z, Chan T, Manoharan M, et al. Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology. 2005;66(6):4–34.
- Telli O, Sarici H, Ozgur BC, et al. Urothelial cancer of bladder in young versus older adults: clinical and pathological characteristics and outcomes. Kaohsiung J Med Sci. 2014;30(9):466–470.
- Shelekhova KV, Krykow KA, Mescherjakov IA, et al. Molecular pathologic subtyping of urothelial bladder carcinoma in young patients. Int J Surg Pathol. 2019;27(5):483–491.
- Sun X, Hoadley KA, Kim WY, et al. Age at diagnosis, obesity, smoking, and molecular subtypes in muscle-invasive bladder cancer. Cancer Causes Control. 2017;28(6):539–544.
- Ward DG, Arnold R, Bryan RT. Molecular subtypes of T1 bladder cancer: biomolecular characteristics versus clinical utility [internet]. Eur Urol. 2020;78(4):538–539.
- Lian J, Yue Y, Yu W, et al. Immunosenescence: a key player in cancer development [internet]. J Hematol Oncol. 2020;13: 1–18.
- Andreassen BK, Grimsrud TK, Haug ES. Bladder cancer survival: women better off in the long run. Eur J Cancer. 2018;95:52–58.
- Cumberbatch MGK, Jubber I, Black PC, et al. Epidemiology of bladder cancer: a systematic review and contemporary update of risk factors in 2018. Eur Urol. 2018;74(6):784–795.
- Bryan RT, Evans T, Dunn JA, et al. A comparative analysis of the influence of gender, pathway delays, and risk factor exposures on the long-term outcomes of bladder cancer. Eur Urol Focus. 2015;1(1):82–89.
- Foxman B. Urinary tract infection in postmenopausal women. Curr Infect Dis Rep. 1999;1(4):367–370.