Abstract
Objective
To overview the updated Swedish National Guidelines on Urothelial Carcinoma 2021, with emphasis on non-muscle-invasive bladder cancer (NMIBC) and upper tract urothelial carcinoma (UTUC).
Methods
A narrative review of the updated version of the Swedish National Guidelines on Urothelial Carcinoma 2021 and highlighting new treatment recommendations, with comparison to the European Association of Urology (EAU) guidelines and current literature.
Results
For NMIBC the new EAU 2021 risk group stratification has been introduced for non-muscle invasive bladder cancer to predict risk of progression and the web-based application has been translated to Swedish (https://nmibc.net.). For patients with non-BCG -responsive disease treatment recommendations have been pinpointed, to guide patient counselling in this clinical situation. A new recommendation in the current version of the guidelines is the introduction of four courses of adjuvant platinum-based chemotherapy to patients with advanced disease in the nephroureterectomy specimen (pT2 or higher and/or N+). Patients with papillary urothelial neoplasms with low malignant potential (PUNLMP) can be discharged from follow-up already after 3 years based on a very low subsequent risk of further recurrences.
Conclusions
The current version of the Swedish national guidelines introduces a new risk-stratification model and follow-up recommendation for NMIBC and adjuvant chemotherapy after radical surgery for UTUC.
Introduction
The first Swedish national guidelines on urothelial carcinoma were published in 2013. Until then, regional treatment recommendations had been compiled separately in the majority of the six health care regions in Sweden. The European Association of Urology has also provided guidelines for the diagnosis and treatment of bladder cancer with the first guidelines published in 2000, and subsequently guidelines were also developed for both upper tract urothelial carcinoma and urethral cancer. Despite being a disease with limited funding for research [Citation1], research priorities have been stated [Citation2] and in the past few years our understanding of bladder cancer has increased with the description of molecular subtypes [Citation3]. In addition, the approval of checkpoint-inhibitors in the setting of advanced bladder cancer in Sweden in 2017 has led to a new standard in the management of urothelial carcinoma. Thus, the evidence-base for diagnosing and treating urothelial carcinoma is growing and necessitates frequently updated clinical guidelines for clinicians. The process in the national guidelines group also considering adjustments from EAU-guidelines and bringing national experts together is also facilitating uniform implementation of new procedures and therapies.
The current summary of the most recent version of the Swedish urothelial carcinoma guidelines published in November 2021 are motivated to highlight the most relevant changes but also as a reference to other national guidelines groups. This first part of two describes the most relevant changes of recommendations and those with highest level of evidence for non-muscle invasive bladder cancer (NMIBC) and upper tract urothelial carcinoma, whereas the second part will describe the corresponding disease management in muscle-invasive and metastatic disease.
The Swedish National Register for Urinary Bladder Cancer
The Swedish National Register of Urinary Bladder Cancer (SNRUBC) was initiated in 1997 with registration of detailed information on tumour characteristics and primary treatment and is administered by the Regional Cancer Centre (RCC) in each of the six health care regions in Sweden. The registration in SNRUBC expanded in 2009 to also register data from a 5-year follow-up of non-muscle invasive bladder cancer, and in 2011 to include registration on data in conjunction with radical cystectomy. Subsequently in 2015, also UTUC and urethral urothelial carcinomas were added and finally in 2017 registration of systemic oncologic treatments and radiation were included. The main aim of the SNRUBC is to perform quality assurance of bladder cancer health care in collaboration with the national guidelines group and to use the data for clinical research to further improve the care of patients with urothelial carcinoma.
Introduction and epidemiology
In Sweden, 3,200 patients were diagnosed with urothelial carcinoma in 2018 [Citation4] and 700 individuals died from bladder cancer the same year. The bladder cancer mortality in Sweden has remained stable for decades. The age-standardized incidence has increased for several years (). Today the median age at diagnosis in Sweden has raised to 75 years of age [Citation5] and currently 25,850 individuals diagnosed with urothelial carcinoma are living in Sweden [Citation6].
Heredity and Lynch syndrome
Studies in twins suggest familiar aggregation of bladder cancer, although the genetic mechanisms are not known [Citation7]. Lynch syndrome, on the other hand, is the most common hereditary cancer syndrome and caused by inherited mutations in mismatch-repair (MMR) genes, causes 7–15% of all UTUCs but only a minority of urothelial carcinomas in the bladder [Citation8]. Given existing control programmes for colorectal cancer and endometrial cancer and improved outcomes in these diseases, two out of three deaths associated with cancer are currently due to the more unusual Lynch-associated malignancies such as UTUC [Citation9]. The recommendation in the current guidelines to perform MMR-screening in all UTUC-specimens differs from the lack of such recommendation in the EAU-guidelines [Citation10]. However, screening for MMR-deficiency is motivated by the simplicity and availability of the immunohistochemical screening method (MSH2, MSH6, MLH1 and PSM2) that is already set up for colorectal carcinoma and that MMR-deficiency predicts a possible exceptional response on checkpoint-inhibition [Citation11]. The guidelines on which patients and how such monitoring of individuals with MMR-deficiency varies, where US-guidelines (NCCN and USMSTF) are recommending annual microhaematuria testing early on. However, the Swedish practice to refrain from screening patients with microscopic haematuria for urinary tract cancer in the absence of symptoms [Citation12] and the low sensitivity of the microhaematuria test are reasons for not implementing this NCCN/USMSTF-strategy for patients with Lynch syndrome. Danish data from a large Lynch cohort also suggests that urinary cytology has too low sensitivity [Citation13], which is the reason behind the recommendation to add UroVysion (together with urinary cytology) with higher sensitivity than urinary cytology [Citation14] in the current Swedish guidelines. Furthermore, the risk-population is further defined in the current Swedish guidelines by starting annual monitoring at 50 years of age and to include MHS2/EPCAM-genotype, but only include patients with MLH1, MSH6 or PMS2 if urothelial carcinoma is diagnosed in a first degree relative [Citation8].
Diagnosing non-muscle invasive bladder cancer (NMIBC)
The standardized care pathways for macroscopic haematuria for individuals above 50 years of age are applicable for all patients with urothelial carcinoma. An initial CT-urography together with cystoscopy, where manual ‘irrigation bag squeeze’ decreases the discomfort for the patient [Citation15,Citation16], and combined with a voided urinary cytology to improve the detection of high-grade urothelial carcinoma, constitutes a standard (as opposed to cytologic examination of bladder washings that is unlikely to capture malignant cells from the upper urinary tract). The current guidelines recommendation to apply the Paris system when reporting urinary cytology [Citation17] is an effort to increase the detection of high-grade disease and simultaneously decrease the proportion of urinary cytologies reported with atypia UNS. A patient with NMIBC and urinary cytology suggesting high-grade urothelial carcinoma has an increased risk of harbouring carcinoma in situ (CIS), which increases the rationale to use photodynamic diagnostics or narrow band imaging during transurethral resection (TURB) and to obtain resection-biopsies from the prostatic urethra in males to rule out CIS or intraductal CIS in the prostate.
Diagnosing upper tract urothelial carcinoma (UTUC)
Both high sensitivity (92%) and specificity (95%) for CT-urography to detect UTUC has recently been reported in a systematic review and meta-analysis [Citation18]. Other diagnostic modalities are MR-urography and selective upper tract urinary cytology obtained from a ureteric catheter placed under local anaesthesia together with a retrograde pyelography when appropriate. A ureteroscopy with selective cytology and biopsy is only indicated if nephron-sparing treatment is considered (i.e., in a low-risk tumor less than 2 cm, unifocal disease and cytology negative for high-grade urothelial carcinoma), or diagnostic uncertainties. To balance the increased risk of intravesical recurrence related to a diagnostic ureteroscopy with possible gain in diagnostic information, the guidelines recommend that the patient is discussed in a multidisciplinary tumour board (MDT) before a diagnostic ureteroscopy is considered. Additional information when reviewing a voided urinary cytology or CT-urography might also suggest locally advanced disease relevant when deciding if radical nephroureterectomy should be combined with a template-based regional lymphadenectomy. Locally advanced disease is also a setting where screening for distant metastases with CT-thorax can be considered to be exchanged to an FDG-PET-CT instead, that can detect lymph node metastases with noteworthy accuracy [Citation19]. FDG-PET-CT is also useful in patients where iodine contrast media are contraindicated (due to impaired renal function or allergy).
TNM and EAU 2021 risk group stratification of non-muscle invasive bladder cancer (NMIBC)
In Sweden the current TNM-classification is applied [Citation20], and additionally in the setting of stage T1-disease further substaging to quantify the extent of lamina propria invasion is recommended [Citation21]. The WHO 1999 and WHO 2016 grading systems have been used together since 2003, which is different from the EAU-guideline recommendation proposing the combination of WHO 1973 and WHO 2016 grading systems, despite that international pathologist organisations promote the three-tiered WHO 2016 as the only grading system. The reasons for keeping with the WHO 1999 are the additional prognostic information for progression harboured in the grade 3 (G3)-entity compared to the broader high-grade (HG) definition and the corresponding biological differences between grade 2 (G2) and G3 [Citation22].
For risk group stratification, the new EAU 2021 stratification has been introduced and translated to Swedish (https://nmibc.net) [Citation23]. The individual patient data set used to develop this system, which aims to assess probabilities of progression to muscle-invasive disease or distant metastases is by far larger than previous systems. However, the effect of the non-validated approximation between the WHO 1973 and 1999 systems when used in the Swedish context is currently not known. Patients with very high risk of progression should be recommended primary radical cystectomy, whereas patients with high risk disease need to be individually counselled based on additional risk factor assessment performed in the setting of a MDT ( and ). However, using the current EAU 2021 risk stratification one has to be aware that it gives the calculated risk for individuals without considering the risk reduction for progression obtained with BCG-treatment (or cystectomy) [Citation24].
Table 1. The EAU 2021 prognostic risk groups based on WHO 1973 and/or WHO 2016 grading system.
Table 2. Probabilities of disease progression to muscle invasion or distant metastases at 1, 5 and 10 years according to the EAU 2021 prognostic risk groups.
Multidisciplinary tumour board (MDT)
The guidelines continue to recommend that all patients with bladder cancer stage T1–T4 and UTUC should be discussed in a MDT, based on current evidence suggesting that altered treatment recommendations are frequently a consequence of a MDT-referral for these patients [Citation25–32]. The proportion of these patients discussed at a MDT has increased from 68% in 2016 to 75% in 2020 () [Citation5], although relevant differences are still present between regions ().
Figure 2. Annual proportion (%) of patients with bladder cancer stage T1–T4 or upper tract urothelial carcinoma discussed at a multidisciplinary tumor board (MDT) between 2016 and 2020 in Sweden stratified by health care region (Uppsala-Örebro (
 ), Southern (
), Southern ( ), Stockholm-Gotland (
), Stockholm-Gotland ( ), Southeastern (
), Southeastern ( ) and Western (
) and Western ( ) health care regions) as well as visualizing the compiled national proportion (
) health care regions) as well as visualizing the compiled national proportion ( ).
).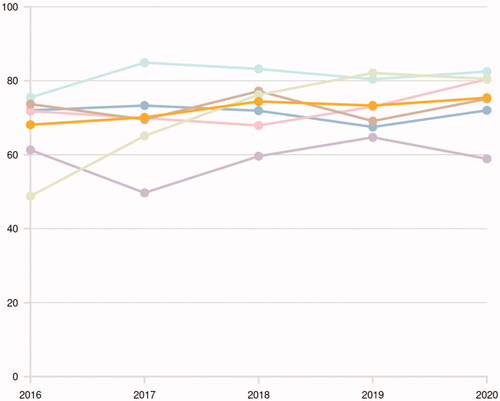
Figure 3. Proportion (%) of patients with bladder cancer stage T1–T4 or upper tract urothelial carcinoma discussed at a multidisciplinary tumor board (MDT) between 2016 and 2020 in Sweden stratified by region. Each blue bar represents a region and the yellow bar the whole Swedish population, where the colours delineate p-values compared to the national proportions with the following colour-codes 
 p < 0.05.
p < 0.05.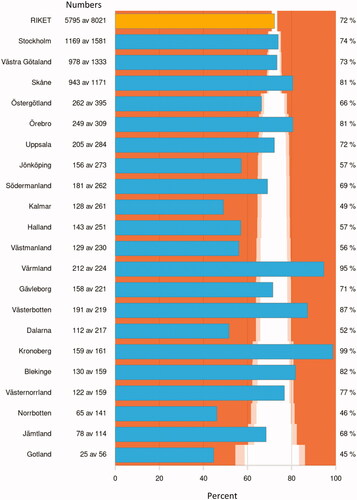
Primary treatment of non-muscle invasive bladder cancer (NMIBC)
The risk stratification of patients with NMIBC has implications for treatment recommendations but also for follow-up (see follow-up section below). To practically apply the EAU 2021 risk group stratification the use of automatic calculators are recommended, such as a recently developed app (for iOS, https://apps.apple.com/us/app/eau-nmibc-risk-calculator/id1578482687 or for android, https://play.google.com/store/apps/details?id=net.ydeal.nmibc).
Patients with TaG1 tumors have a very low risk of progression but a considerable risk of recurrence. Thus, in conjunction with transurethral resection (TURB), immediate postoperative instillation of a single chemotherapy dose can be considered. Postoperative irrigation with sterile water or NaCl is associated with less side-effects than postoperative chemotherapy instillations and can be considered postoperatively if chemotherapy instillations are not feasible to perform or contraindicated, although the level of evidence is weaker [Citation33]. The quality of the TURB-procedure is essential for outcomes in all patients with bladder cancer and, in the setting of NMIBC, the presence of detrusor muscle in the resected specimen is a proxy for recurrence-free survival [Citation34]. The presence of detrusor muscle in the resected specimen is a prioritized quality measure that is also monitored by patient representatives (Urinblåscancer Sverige). The patient representative organization Urinblåsecancer Sverige are currently displaying these results from the Swedish National Register of Urinary Bladder Cancer (SNRUBC) on their website (https://blascancerforbundet.se), to pinpoint prevailing differences between hospitals in Sweden ().
Figure 4. Proportion of patients with non-muscle invasive bladder cancer with the presence of detrusor muscle in the resected specimen between 2018 and 2020 stratified by treating hospital. Each blue bar represents a hospital and the yellow bar the whole Swedish population, where the colours delineate p-values compared to the national proportions with the following colour-codes 
 p < 0.05 .
p < 0.05 .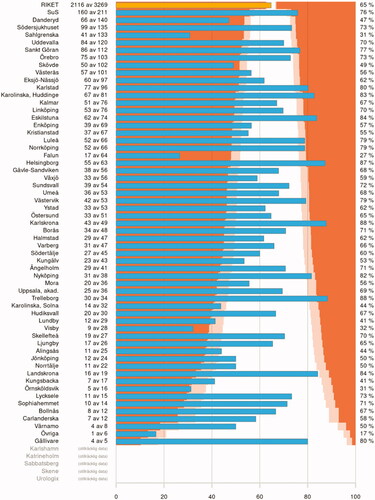
For patients with intermediate risk of progression according to the EAU 2021 prognostic model six adjuvant weekly instillations with mitomycin are recommended to decrease the risk of recurrence. The optimal number of instillations is not known, however maintenance instillations therapy beyond 12 months is not recommended. The rationale for not recommending BCG-instillations in these intermediate risk patients is the preferable side-effect profile associated with chemotherapy instillations [Citation35] and the global shortage of BCG. However, if the individual patient is considered to have a considerable risk of progression or recur after initial mitomycin-instillations, adjuvant BCG-instillations (induction and maintenance for 12 months) are recommended.
Patients with high risk of progression are recommended a re-resection of the tumor base in stage T1 disease (but not in TaG3 if the primary TURB was macroscopically radical and with detrusor muscle in the resected specimen) and subsequently adjuvant BCG-instillations with six induction courses and maintenance with an additional three courses at 3, 6 and 12 months. This strategy is applied provided no other risk factors for progression that not are included in the EAU 2021 risk group stratification are present, such as residual T1-disease at re-resection, lymphovascular invasion, deep lamina propria invasion (T1e), T1-disease in a diverticulum, variant histology or concomitant CIS in the prostatic urethra. Under these circumstances primary cystectomy is another and more valid treatment option. Patients with very high risk of progression according to the EAU 2021 risk group stratification are recommended primary cystectomy upfront, without performing a re-resection.
Recurrence after BCG treatment including non-BCG-responsive non-muscle invasive bladder cancer (NMIBC)
To stratify the different categories of local recurrence after BCG-treatment, the non-BCG-responsive setting has recently been proposed, to define patients where further BCG-instillations are not meaningful [Citation36]. In this situation radical cystectomy is the treatment recommendation. The non-BCG-responsive category is defined by recurrent T1 or TaG3-disease within 6 months of adequate BCG (i.e., at least five induction instillations and two maintenance instillations). BCG-refractory disease is also recommended radical cystectomy and is defined by T1 disease at first control after BCG induction, or TaG3 at first control at 3 months and/or 6 months after BCG induction or CIS persisting after 3 and 6 months or any TaG3 during BCG maintenance. The recently published HYMN-trial investigated radiofrequency device assisted thermo-chemotherapy with mitomycin instillations compared to reinduction with BCG in patients with recurrence after BCG instillations. It was shown that the experimental device assisted mitomycin instillation treatment strategy had inferior disease-free survival in the subgroup of patients having concomitant carcinoma in situ (HR = 2.1; 1.2–3.6) [Citation37]. Given that no suitable bladder-sparing trial protocol is available and the current standard recommendation radical cystectomy is not an option for a patient in this clinical situation, sequential instillations with gemcitabine and docetaxel is a treatment alternative without the need for a device to perform instillations [Citation38].
Primary treatment of upper tract urothelial carcinoma (UTUC)
The standard treatment for UTUC is radical nephroureterectomy including the ureteric orifice. In locally advanced disease (cT3/cT4 and/or N+) open surgery is recommended based on superior survival reported in a small randomized trial [Citation39,Citation40]. There are furthermore low levels of evidence supporting superior survival in units performing more than six annual nephroureterectomies compared to fewer procedures [Citation41]. With the advent of strong evidence supporting four courses of adjuvant platinum-based combination chemotherapy for patients with pT2 or higher and/or node positive disease [Citation42], supported by level I evidence to improve disease-free survival, a template-based lymphadenectomy is recommended in patients with invasive UTUC.
After radical nephroureterectomy a single postoperative instillation with chemotherapy decreases the risk of later intravesical tumor recurrence [Citation43,Citation44].
Selected patients with low risk tumours (for a definition see the section on diagnosing UTUC) should be considered for endourologic nephron-sparing treatment. Similarly, ureteric resection with ureteroneocystostomy with/without regional lymphadenectomy is also a nephron-sparing option for patients with distal ureteric tumors [Citation45].
Aspects on nursing, rehabilitation and supportive care including patient support for patients with non-muscle invasive bladder cancer (NMIBC)
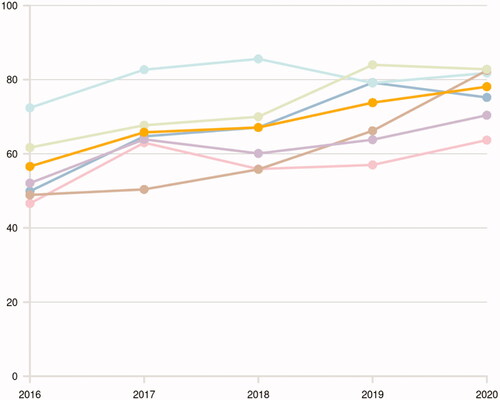
All patients with urothelial carcinoma should be offered a bladder cancer patient navigator. The proportion of patients that were actually offered this is registered in the SNRUBC and has increased during the last 5 years (). When adjuvant instillations are given in NMIBC, it is recommended that a nurse with adequate experience and education is available (optimally a bladder cancer patient navigator), to interpret symptoms and consider side-effects that can alter the treatment plan. The patient navigator is also crucial to continuously monitor needs for rehabilitation and supportive care. A close cooperation with the treating urologist is also needed in the setting of adjuvant instillations to consider specific rehabilitation measures such as prescription of anticholinergics (solifenacin) after TURB if needed [Citation46] or mirabegron during BCG-instillations [Citation47].
Figure 5. Annual proportion (%) of patients with bladder cancer stage T1–T4 or upper tract urothelial carcinoma receiving a patient navigator at diagnosis in Sweden stratified by health care region (Uppsala-Örebro (
 ), Southern (
), Southern ( ), Stockholm-Gotland (
), Stockholm-Gotland ( ), Southeastern (
), Southeastern ( ) and Western (
) and Western ( ) health care regions) as well as visualizing the compiled national proportion (
) health care regions) as well as visualizing the compiled national proportion ( ).
).
Follow-up in patients with non-muscle invasive bladder cancer (NMIBC)
A previously defined NMIBC low risk group (one tumor below 30 mm and no recurrence at first cystoscopy) can be discharged from further follow-up with cystoscopies after 5 years. Recent national data suggest that patients with papillary urothelial neoplasms with low malignant potential (PUNLMP) can be discharged from follow-up already after 3 years based on a very low risk of further recurrences [Citation48], although a recent multicenter study reported a decreased use of PUNLMP by pathologists during recent years in an international setting in the context of WHO 1973 and/or 2004 grading systems [Citation49]. The actual risk of recurrence for the individual patient is difficult to foresee, but for example in patients with intermediate risk NMIBC treated with intravesical chemotherapy further risk-stratification can probably be accomplished by including five clinical factors in a model that can stratify the risk of recurrence [Citation50], however up to now no such models are used to stratify follow-up recommendations in the Swedish national guidelines or the EAU guidelines. Furthermore, for individuals with high risk NMIBC regular monitoring of the upper urinary tracts with CT-urography is recommended at 1 or 2-year intervals, which is based on the low level of evidence being less intense than the current EAU guidelines recommending annual CT-urographies in these patients. Individual risk-assessment for risk of developing metachronous upper tract urothelial carcinomas based on risk factors is thus necessary and consequently not to a large extent different from the current EAU guidelines. A summary of relevant differences between these two guidelines are given in .
Table 3. Main differences between the new Swedish national guidelines and the current EAU guidelines from 2021.
Conclusions
The current review of the updated Swedish national guidelines on urothelial carcinoma highlights the new EAU 2021 risk group stratification for non-muscle invasive bladder cancer. The definition of non-BCG-responsive disease is also pinpointed, to guide patient counselling in this clinical situation. Another new recommendation in the current version of the guidelines is the introduction of four courses of adjuvant platinum-based chemotherapy to patients with advanced disease in the nephroureterectomy specimen (pT2 or higher and/or N+).
The following individuals were affiliated with the national guideline group and participated during the work of updating the current version of the guidelines:
PhD Truls Gårdmark, Department of Clinical Sciences, Danderyd Hospital, Karolinska Institute, Stockholm
PhD Christina Kåbjörn Gustafsson, Department of Pathology, Ryhov County Hospital, Jönköping
PhD Pär Dahlman, Department of Radiology, Uppsala Akademiska Hospital, Uppsala
Associate professor, Elin Trägårdh, Department of Nuclear Medicine, Skåne University Hospital, Malmö
PhD Gunilla Malm, Örestadskliniken Primary Care Unit, Malmö.
PhD Anders Edsjö, Department of Clinical Genetics and Pathology, Skåne University Hospital, Lund
MD Carl Henrik Sundin, Sundsvall, Chairman of the patient representative organization Urinblåsecancer Sverige
Regional Cancer Center (RCC) South is the regional cancer center for urothelial carcinoma in Sweden, with a supportive role for the Swedish National Register of Urinary Bladder Cancer (SNRUBC) and the National guidelines for urothelial carcinoma in Sweden. Under the auspices of RCC, statistician Pia Löthgren-Mårtensson updates the publicly available data from the SNRUBC (5) and Annika Wendt-Wesén is coordinating the updates of the current guidelines.
Disclosure statement
Anna-Karin Lind has received lecture honorarium from Astellas. Amir Sherif received honorarium as PI for trial from Bristol Myers Squibb. Karin Söderkvist received honorarium as PI for trial from Glaxo Smith Kline. Helena Thulin received economic compensation for developing educational material for Bactiguard AB. Dimitrious Papantonio has received lecture honorarium from Ipsen. Ingrida Verbiene has received lecture honoraria from different pharma companies. Anders Ullén has received honoraria or research funding from Pierre-Fabre, Merck KGaA, Astellas and Bayer. Sofia Kjellström, Fredrik Liedberg, Karin Falkman, Johan Sandzen, Tomas Jerlström, Viveka Ströck and Firas Aljabery have no conflicts of interest.
References
- Boormans JL, Zwarthoff EC. Limited funds for bladder cancer research and what can we do about it. Bladder Cancer. 2016;2(1):49–51.
- Bessa A, Maclennan S, Enting D, et al. Consensus in bladder cancer research priorities between patients and healthcare professionals using a four-stage modified Delphi method. Eur Urol. 2019;76(2):258–259.
- Black PC. Seeking the molecular truth in bladder cancer: biology = genome×(transcriptome)2. Eur Urol. 2017;72(3):366–367.
- https://sdb.socialstyrelsen.se/if_can/resultat.aspx.
- https://statistik.incanet.se/Urinblasecancer/.
- https://www-dep.iarc.fr/NORDCAN/SW/table11.asp?cancer=300&sex=0&stat=2&age_from=1&age_to=18®istry=752&sYear=2015&eYear=2016&submit=%A0%A0Utf%F6r%A0%A0.
- Koutros S, Decker KL, Baris D, et al. Bladder cancer risk associated with family history of cancer. Int J Cancer. 2021;148(12):2915–2923.
- Joost P, Therkildsen C, Dominguez-Valentin M, et al. Urinary tract cancer in lynch syndrome; increased risk in carriers of MSH2 mutations. Urology. 2015;86(6):1212–1217.
- Pylvänäinen K, Lehtinen T, Kellokumpu I, et al. Causes of death of mutation carriers in Finnish lynch syndrome families. Fam Cancer. 2012;11(3):467–471.
- Rouprêt M, Babjuk M, Burger M, et al. European association of urology guidelines on upper urinary tract urothelial carcinoma: 2020 update. Eur Urol. 2021;79(1):62–79.
- Le DT, Uram H, Wang BR, et al. PD-1 blockade in tumors with Mismatch-Repair deficiency. N Engl J Med. 2015;372(26):2509–2520.
- Malmström PU, Skaaheim Haug E, Boström PJ, et al. Progress towards a Nordic standard for the investigation of hematuria: 2019. Scand J Urol. 2019;53(1):1–6.
- Myrhøj T, Andersen M-B, Bernstein I. Screening for urinary tract cancer with urine cytology in Lynch syndrome and familial colorectal cancer. Fam Cancer. 2008;7(4):303–307.
- Jin H, Lin T, Hao J, et al. A comprehensive comparison of fluorescence in situ hybridization and cytology for the detection of upper urinary tract urothelial carcinoma: a systematic review and Meta-analysis. Medicine (Baltimore)). 2018;97(52):e13859.,.
- Berajoui MB, Aditya I, Herrera-Caceres J, et al. A prospective randomized controlled trial of irrigation "bag squeeze" to manage pain for patients undergoing flexible cystoscopy. J Urol. 2020;204(5):1012–1018.
- Gunendran T, Briggs RH, Wemyss-Holden GD, et al. Does increasing hydrostatic pressure ("bag squeeze") during flexible cystoscopy improve patient comfort: a randomized, controlled study. Urology. 2008;72(2):255–258.
- Rosenthal DL. The Paris system for reporting urinary cytology. 2016. http://www.springer.com/us/book/9783319228631.
- Janisch F, Shariat SF, Baltzer P, et al. Diagnostic performance of multidetector computed tomographic (MDCTU) in upper tract urothelial carcinoma (UTUC): a systematic review and Meta-analysis. World J Urol. 2020;38(5):1165–1175.
- Voskuilen CS, Schweitzer D, Jensen JB, et al. Diagnostic value of 18F-fluorodeoxyglucose positron emission tomography with computed tomography for lymph node staging in patients with upper tract urothelial carcinoma. Eur Urol Oncol. 2020;3(1):73–79.
- Brierly J, Gospodarowicz M, Wittekind C. TNM classification of malignant tumours. 8th ed. Oxford: UICC International Union Against Cancer; 2017.
- Fransen van de Putte EE, Otto W, Hartmann A, et al. Metric substage according to micro and extensive lamina propria invasion improves prognostics in T1 bladder cancer. Urol Oncol. 2018;36(8):361.e7-361–361.e13.
- Liedberg F, Lauss M, Patschan O, et al. The importance of being grade 3: WHO 1999 versus WHO 2004 pathologic grading. Eur Urol. 2012;62(4):620–623.
- Sylvester RJ, Rodríguez O, Hernández V, et al. European association of urology (EAU) prognostic factor risk groups for non-muscle-invasive bladder cancer (NMIBC) incorporating the WHO 2004/2016 and WHO 1973 classification systems for grade: an update from the EAU NMIBC guidelines panel. Eur Urol. 2021;79(4):480–488.
- Lobo N, Hensley PJ, Bree KK, et al. Updated European Association of Urology (EAU) prognostic factor risk groups overestimate the risk of progression in patients with non-muscle-invasive bladder cancer treated with bacillus Calmette-Guérin. Eur Urol Oncol. 2021.doi: https://doi.org/10.1016/j.euo.2021.11.006.
- Gil M, Guerra J, Andrade V, et al. The impact of multidisciplinary team conferences in urologic cancer in a tertiary hospital. Int Urol Nephrol. 2021;53(1):41–47.
- Gordetsky J, Collingwood R, Lai WS, et al. Second opinion expert pathology review in bladder cancer: implications for patient care. Int J Surg Pathol. 2018;26(1):12–17.
- Wayment RO, Bourne A, Kay P, et al. Second opinion pathology in tertiary care of patients with urologic malignancies. Urol Oncol. 2011;29(2):194–198.
- Traboulsi SL, Brimo F, Yang Y, et al. Pathology review impacts clinical management of patients with T1-T2 bladder cancer. Can Urol Assoc J. 2017;11(6):188–193.
- Luchey AM, Manimala NJ, Dickinson S, et al. Change in management based on pathologic second opinion among bladder cancer patients presenting to a comprehensive cancer center: implications for clinical practice. Urology. 2016;93:130–134.
- Kurpad R, Kim W, Rathmell WK, et al. A multidisciplinaryapproach to the management of urologic malignancies: does it influence diagnostic and treatment decisions? Urol Oncol. 2011;29:78–82.
- Van Rhijn BW, van der Kwast TH, Kakiashvili DM, et al. Pathological stage review is indicated in primary pT1 bladder cancer. BJU Int. 2009;106(2):206–211.
- Tay LJ, Chatterton K, Colemeadow J, et al. Improving management of upper tract urothelial carcinoma. BJU Int. 2020;126(1):5–6.
- Onishi T, Sugino Y, Shibahara T, et al. Randomized controlled study of the efficacy and safety of continuous saline bladder irrigation after transurethral resection for the treatment of non-muscle-invasive bladder cancer. BJU Int. 2017;119(2):276–282.
- ) Mariappan P, Finney SM, Head E, et al. Good quality white-light transurethral resection of bladder tumours (GQ-WLTURBT) with experienced surgeons performing complete resections and obtaining detrusor muscle reduces early recurrence in new non-muscle-invasive bladder cancer: validation across time and place and recommendation for benchmarking. BJU Int. 2012;109:1666–1673.
- Van der Meijden AP, Sylvester RJ, Oosterlinck W, et al. EORTC Genito-Urinary tract cancer group. Maintenance bacillus Calmette-Guérin for ta, T1 bladdertumours is not associated with increased toxicity: results from a EORTC phase III trial. Eur Urol. 2003;44(4):429–434.
- Kamat AM, Sylvester RJ, Böhle A, et al. Definitions, end points, and clinical trial designs for Non-muscle-Invasive bladder cancer: recommendations from the international bladder cancer group. J Clin Oncol. 2016;34(16):1935–1944.
- Tan WS, Panchal A, Buckley L, et al. Radiofrequency-induced thermo-chemotherapy effect versus a second course of bacillus Calmette-Guérin or institutional standard in patients with recurrence of non-muscle-invasive bladder cancer following induction or maintenance bacillus Calmette-Guérin therapy (HYMN): a phase III, open-label, randomised controlled trial. Eur Urol. 2019;75(1):63–71.
- Steinberg RL, Thomas LJ, Brooks N, et al. Multi-Institution evaluation of sequential gemcitabine and docetaxel as rescue therapy for nonmuscle invasive bladder cancer. J Urol. 2020;203(5):902–909.
- Peyronnet B, Seisen T, Dominguez-Escrig JL, et al. Oncological outcomes of laparoscopic nephroureterectomy versus open radical nephroureterectomy for upper tract urothelial carcinoma: an European Association of Urology guidelines systematic review. Eur Urol Focus. 2019;5(2):205–223.
- Simone G, Papalia R, Guaglianone S, et al. Laparoscopic versus open nephroureterectomy: perioperative and oncologic outcomes from a randomised prospective study. Eur Urol. 2009;56(3):520–526.
- Sui W, Wallis CJD, Luckenbaugh AN, et al. The impact of hospital volume on short-term and long-term outcomes for patients undergoing radical nephroureterectomy for upper tract urothelial carcinoma. Urology. 2021;147:135–142.
- Birtle A, Johnson M, Chester J, et al. Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): a phase 3, open-label, randomised controlled trial. Lancet. 2020;395(10232):1268–1277.
- O'Brien T, Ray E, Singh R, et al. Prevention of bladder tumours after nephroureterectomy for primary upper urinary tract urothelial carcinoma: a prospective, multicenter, randomized clinical trial of single postoperative intravesical dose of mitomycin C. Eur Urol. 2011;60(4):703–710.
- Ito A, Shintaku I, Satoh M, et al. Prospective randomized phase II trial of a single early intravesical instillation of pirarubicin (THP) in the prevention of bladder recurrence after nephroureterectomy for upper urinary tract urothelial carcinoma: the THP monotherapy study group trial. J Clin Oncol. 2013;31(11):1422–1427.
- Seisen T, Peyronnet B, Dominguez-Escrig JL, et al. Oncologic outcomes of kidney-sparing surgery versus radical nephroureterectomy for upper tract urothelial carcinoma: a systematic review by the EAU non-muscle invasive bladder cancer guidelines panel. Eur Urol. 2016;70(6):1052–1068.
- Zhang Z, Cao Z, Xu C, et al. Solifenacin is able to improve the irritative symptoms after transurethral resection of bladder tumors. Urology. 2014;84(1):117–121.
- Sun K, Wang D, Wu G, et al. Mirabegron improves the irritative symptoms caused by BCG immunotherapy after transurethral resection of bladder tumors. Cancer Med. 2021;10(21):7534–7541. Sep 21. Epub ahead of print.
- Bobjer J, Hagberg O, Aljabery F, et al. Bladder cancer recurrence in primary papillary urothelial neoplasm of low malignant potential (PUNLMP) compared to G1 WHO 1999: a population-based study scand. J Urol. 2022;56:14–18.
- Hentschel AE, van Rhijn BWG, Bründl J, et al. Papillary urothelial neoplasm of low malignant potential (PUN-LMP): still a meaningful histo-pathological grade category for ta, noninvasive bladder tumors in 2019? Urol Oncol. 2020;38(5):440–448.
- Lammers RJ, Hendriks JC, Rodriguez Faba OR, et al. Prediction model for recurrence probabilities after intravesical chemotherapy in patients with intermediate-risk non-muscle-invasive bladder cancer, including external validation. World J Urol. 2016;34(2):173–180.

