?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
To evaluate the success rate of Extracorporeal Shock Wave Lithotripsy (ESWL) therapy and identify relevant treatment-specific factors affecting stone-free rate (SFR) after ESWL.
Materials and methods
All ESWL treatments in the years 2016–2019, in Ängelholm Hospital, Skåne, Sweden were analysed retrospectively. Primary outcome was stone-free rate (SFR) at 3 months. Univariate logistic regression was used followed by multivariable regression. Lasso analysis was made to adjust for treatment-specific factors such as age, stone size, skin-to-stone distance (SSD), stone attenuation, number of treatments, stone location and presence of a urinary stent.
Results
Factors affecting successful ESWL treatment were lower age (p < 0.001), smaller stone size and volume (both p = 0.001). SSD, stone attenuation, sex, laterality and drainage did not have an effect on SFR in this study. After the first ESWL treatment session, 46.7% of the patients were stone-free.
Conclusion
Results indicate that stone size and age are the most predictive factors for ESWL outcome. Based on this, we present a simple model for prediction of SFR after ESWL, to be used when counseling patients before ESWL treatment.
Introduction
Renal colic, caused by urolithiasis, accounts for 1% of emergency department (ED) visits in Europe [Citation1,Citation2]. Surgical treatment of urolithiasis includes ureteroscopy (URS), extracorporeal shock wave lithotripsy (ESWL) and percutaneous nephrolithotomy (PCNL). The European Association of Urology (EAU) recommends the use of URS and ESWL for removal of ureteric stones [Citation3]. International guidelines usually divide stones into three groups, depending on size (<10 mm, 10–20 mm and >20 mm). ESWL is currently an important part of urolithiasis treatment [Citation4]. ESWL can be recommended for all stones smaller than 20 mm.
ESWL in an outpatient setting is cost-efficient and clinically more effective compared to URS in treating renal stones <10 mm [Citation3,Citation5]. SFR is measured as the proportion of patients with residual stone fragments smaller than 4 mm after up to three ESWL treatments for the same stone. Factors previously shown to improve the SFR are smaller stone size, shorter skin-to-stone distance (SSD), lower stone attenuation measured as Hounsfield units (HU) and possibly the absence of a ureteric stent before ESWL [Citation3,Citation5–8]. Many studies are also relatively small and the treatment strategy after unsuccessful ESWL is often unclear.
The aim of this study is to identify easily accessible and objective factors known before ESWL that influence the SFR of ESWL treatment.
Materials and methods
This retrospective study included all patients treated with ESWL in the years 2016–2019, at the Urolithiasis Centre of Ängelholm hospital in Skåne County, Sweden. The stone centre serves approximately 450,000 inhabitants in the north-western part of Skåne. The ESWL machine used was Stortz lithotripter MODULITH® SLX-F2, 3rd generation with electromagnetic shockwave generator, and dual focus option. Treatment was performed under X-ray monitoring. EAU Guidelines and contraindications for ESWL treatment were followed [Citation3,Citation9]. We followed Stortz Medical’s recommendations regarding the number of shockwaves and energy level. Power ramping and 1.5 Hz was routinely performed during the study period. Patients with diabetes, a positive urine culture or dipstick test, an indwelling stent or a catheter were given a single dose of antibiotic prophylaxis (Ciprofloxacin 500 mg) orally approximately 30 min before ESWL [Citation3,Citation9,Citation10].
Pre-ESWL stenting was not done routinely, but sometimes used to relieve obstruction or pain, and on rare occasions due to stone size alone ().
Figure 1. STROBE flowchart of the study (STrengthening the Reporting of OBservational studies in Epidemiology).
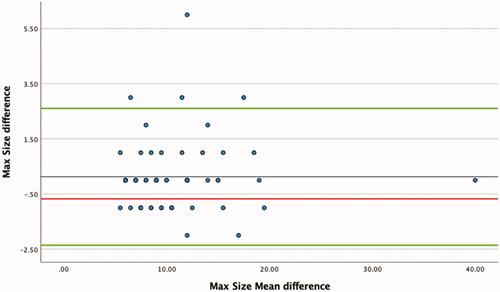
All patients were evaluated using non-contrast computer tomography (NCCT) before and 3–4 weeks after ESWL. Fragmentation was assessed as either (1) complete fragmentation/successful treatment (stone size < 4 mm), (2) partial fragmentation (stone size 4–5 mm) or (3) incomplete fragmentation (stone size ≥ 6 mm). This study defines SFR patients with residual stone fragments ≤ 4 mm. Complete fragmentation was not followed up further. Partial fragmentation was followed up with an annual NCCT. Incomplete fragmentation was generally re-treated with ESWL up to three times in total, whereafter another modality was chosen. The CT protocol made reconstructions of 3/2 mm in three planes possible, making reconstruction possible with 1 mm thickness and 0.8 mm intervals, thus detecting all but very small and insignificant stones. When measuring Hounsfield density (HU) with the ‘region of interest’ (ROI) measurement, we included 2/3 of the stone to avoid partial volume effects. Two consultants in urology measured all stones. The measurements were performed in the same way and inter-operator differences are presented in a Bland-Altman diagram ().
Figure 2. Bland-Altman diagram presented interindividual measuring difference of max size (randomly selected cases, n = 64).
During the study period, 727 ESWL treatments were performed. Three treatments (0.4%) were excluded from the study (patient’s death or mandatory follow-up was not performed). We further excluded 17 treatments on stones > 2 cm (following the EAU guidelines), leaving 707 treatments (patients treated one to three times) for evaluation ().
Patient characteristics are presented in . The majority (697; 98.6%) received small focus shock waves. The median machine energy level/power was 6 and used in 548 (77.5%) of the treatments. The median shock wave frequency was 1.5 Hz, used in 695 (98.3%) of treatments. During the ESWL, 18% had drainage (13.2% pigtail and 4.8% nephrostomy). The mean age in the study population was 61 years and the mean stone density was 939 HU and 82% had their stone located in the renal pelvis, leaving only 18% of the stones located in the ureter. The cohort of this study had a low percentage of ureteric stones, but we found no significant correlation between location and SFR after ESWL treatment (p = 0.78).
Table 1. Descriptive statistics for ESWL treatment.
This study was approved by the local ethics committee at Lund University (Dnr. 2016/254).
Previous number of ESWL treatments, presence of an indwelling stent, stone location (kidney, upper ureter, lower ureter), number of shockwaves, shockwave frequency, energy level and use of prophylactic antibiotics were prospectively registered in a medical chart system (Melior, Siemens).
Complementary treatment modalities (URS, PCNL, or percutaneous nephrostomy), stone size (measured in three dimensions with the longest measurement documented), stone attenuation in HU and SSD (measured as the length from stone to skin at the outer border of psoas/paraspinal muscles or, when in prone position, the shortest measure from the abdominal side of the skin) were retrospectively collected.
Statistics
Patient and stone specific factors were analysed with both univariate analyses (Chi-squared or t-test comparing patients with successful treatment to those with unsuccessful treatment) and multivariate analyses (logistic and lasso regression) to evaluate if they were significant independent factors. Subgroup analyses was also made looking at first and second treatment analysing differences in factors affecting SFR. The data was randomly split into two datasets of equal size, which we refer to as ‘training data’ and ‘test data’ for the analysis to minimise the risk of assumption bias. Lasso analysis stands for ‘least absolute shrinkage and selection operator’. It is a regression analysis method that performs both variable selection and regularization in order to enhance the prediction accuracy and interpretability of the resulting statistical model. Using the training data, we applied lasso regression to select the best predictors. The lambda-value for the lasso regression was chosen using cross-validation. The results are presented as odds ratios with 95% confidence intervals and p-values. The selected model was then evaluated using the test data and using receiver operating characteristic (ROC) curves to calculate the area under the curve (AUC). Statistical analysis was performed using computations in R: A Language and Environment for Statistical Computing version 4.0.2.
Results
Stone free rate (SFR)
Successful ESWL treatment (residual stone < 4 mm in longest measurement and no re-intervention needed) was achieved in 47% (n = 331) of all treatments. The success rate with treatment no. 1 was 50.1% (n = 253), treatment no. 2 was 38.3% (n = 62), and at treatment no. 3 40% (n = 16). On the follow-up NCCT, 143 patients (20.2%) had 4–5 mm stones and 247 (35%) had residual stones ≥ 6 mm. Patients with residual stones ≥ 6 mm were either followed by NCCT or they received an additional treatment with ESWL or other modality. The ability of the various baseline variables to predict a successful treatment outcome (stones < 4 mm) was examined using both univariate (Chi-squared or t-test) and multivariable logistic regression. In the univariate analysis, age (p < 0.001), maximum size (p < 0.001), volume (p < 0.001), and SSD (p = 0.047) were significantly correlated to treatment success ().
Table 2. Univariable analysis of factors that could affect SFR after ESWL.
The multivariate analysis showed significance on only age and max size/volume (both p < 0.001, ). The lasso regression model ( and ) verified that age and the similar factors maximum stone size and stone volume are strongly associated to SFR (p < 0.001).
Table 3. Multivariable analysis of factors that could affect SFR after ESWL, using volume not max stone size.
Table 4. Lasso regression model for the most predictive factors for SFR after ESWL, using volume as the measurement of stone size.
Table 5. Lasso regression model for the most predictive factors for SFR after ESWL, using max size.
To investigate the interindividual measure difference between the two consultants, a one sample T-test was performed. Approximately 10% of patients (n = 64) were randomly chosen to measure differences in measurement. There was no significant differences in maximum size (p = 0.43, Pearson’s r = 0.97) or SSD (p = 0.186, Pearson’s r = 0.97) measurements. There was, however, a significant difference in HU measurements (p < 0.001, Pearson’s r = 0.76). A Bland-Altman diagram of measuring differences in max size is presented in .
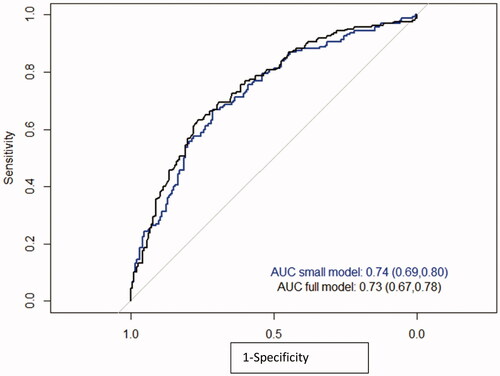
A ROC curve is presented in . The full model including SSD, HU and maximum size had an AUC of 0.72, while a model that included age and either maximum stone size or stone volume had an AUC of 0.74. Maximum stone size and stone volume correlated strongly to each other and therefore only maximum stone size was included in the model for further analysis. The reason for this was that maximum stone size is a simpler and faster measurement to perform.
Figure 3. ROC analysis of the full model (blue) and a model including only age and maximum size (black) (n = 707).
Finally, we then used the most predictive factors identified in the above models to create an equation for calculation of odds and probability of being stone-free after up to three ESWL sessions. We suggest the following equation to calculate odds for success:
Calculation of probability (P) can be made through:
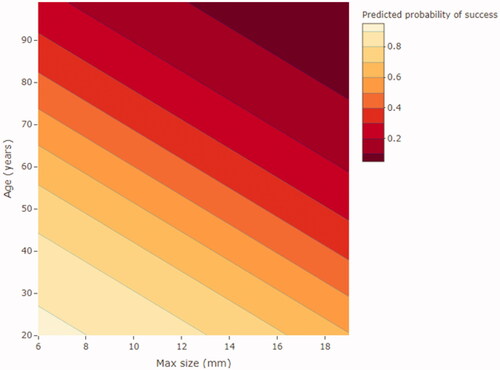
In we present a visual diagram predicting SFR/treatment success with age and maximum stone size as objective factors.
Discussion
This study found that younger age and smaller stones were predictors of success when performing ESWL on stones less than 20 mm. Size, measured as the maximal diameter in millimetres, and volume measurements are equally associated with SFR.
It is well known and accepted that smaller stone size increases spontaneous stone passage [Citation11,Citation12]. Our data suggests that the largest stone size together with age are the best and strongest predictors of SFR and thus might be sufficient for prediction of treatment success. The parameters are easily accessible and can be used when calculating odds or probability of a successful treatment.
We found that younger age was a significant predictor of successful ESWL, a finding shown previously in other studies [Citation13–18]. A weakness regarding age in this study is that the follow-up for measuring SFR does not exceed 3 months. It has been shown that the age effect is reduced if the follow-up is longer (>24 months) [Citation19]. Age being such a strong predictor of SFR after ESWL treatment could influence clinical practice. The mean age in our study population was 61 years. We know from other studies that the peak age for stone disease in our part of the world is between 40 and 50 years [Citation20], indicating that in this cohort there was a tendency to choose ESWL for the more fragile elderly patients, which could affect our results. Although the underlying mechanisms remain unknown, it is suggested that sclerotic changes in the renal parenchyma occur with aging, leading to increased acoustic impedance and poor fragmentation, and consequently low SFR after ESWL therapy for kidney calculi [Citation14,Citation15]. Sexual activity varies but is generally reduced with age, and a previous study shows that sexual activity has a positive effect on SFR after ESWL [Citation21]. Finally a selection bias is possible in our cohort, with simple stone cases in younger patients, and complex cases in older groups preferentially chosen for ESWL treatment. We unfortunately cannot check this possibility in this cohort since data on PCNL and URS during this period is lacking.
Stone size was the other factor strongly associated with higher SFR after ESWL. Multiple studies have shown that bigger stones have a lower SFR, as SFR is defined as fragments less than 4 mm [Citation11,Citation22–24]. Stone size as a predictor of SFR or successful treatment seems to unite most studies on outcome after ESWL. Other factors such as skin-to-stone distance (SSD), body-mass index (BMI), Hounsfield units (HU), and even location varies between studies investigating SFR after ESWL.
We expected that stone attenuation measured in Hounsfield Units (HU) could be a factor affecting SFR results. We did not find any significant association between HU and SFR. Previous studies have found that a density value below 600−1,000 HU relates positively to SFR [Citation6,Citation25–36]. These studies vary in their mean HU value, but most had a lower mean HU value than our cohort. One other study did not find a correlation between HU and SFR [Citation37]. The mean HU in this study (957 HU) was also comparable to ours. We found that HU was difficult to measure in a consistent way. Stone attenuation was measured by only two doctors, which should reduce variability in measurements. Even so, the interindividual difference in HU measurement was significant, indicating difficulties to standardize these measurements. Interindividual measures of difference are rarely accounted for in previous studies. Interindividual variability could be a major source of error in HU values, limiting its credibility as a prognostic factor for SFR.
SSD is a predictor of SFR in several studies [Citation7,Citation32,Citation37,Citation38], but not all [Citation25]. The cut-off value for a successful treatment in these studies ranges from 9–11 cm. We used a more pragmatic way of measuring the SSD compared to other studies. SSD was measured from the skin, directed towards the stone passing on the edge of the psoas/paraspinal muscles. This is based on the individualized anatomy but it represents an approximate angle of 22.5°. This correlates to the true SSD used in the clinical setting. Studies normally use measurements from 0°, 45°, and 90°, calculating a mean of these three values or the value at 45°, presented as SSD. In the univariate analysis we found a significant association of SSD with SFR, however in the more complex multivariate logistic regression and lasso regression analyses this effect disappeared. This indicates that the SSD, using our method of measurement, is linked to the other strongly significant factors affecting SFR, namely age and size.
Indwelling stents cause bothersome symptoms for many patients. The positive effect of reducing stone-related symptoms after ESWL are of limited and debated value, both in terms of complications and SFR. Acute treatment for ‘Steinstrasse’, a complication where multiple fragments are blocking the ureter after ESWL, is reduced by stenting but the need for complementary treatments is not reduced [Citation39,Citation40]. One confounding factor for the effects of stenting is that patients who receive indwelling stents have larger stones and/or symptoms from them [Citation39–41]. Hirsch et al. [Citation7] found that indwelling ureteral stents decrease SFR, contemplating that stents can absorb shock waves, like Goel et al. [Citation42] suggested previously. In this study we found no correlation between preoperative stenting and maximum stone size and showed no effect of ureteral stenting on SFR after ESWL.
There is a lack of consensus on how to define a successful ESWL treatment. We chose to define it as having residual stones < 4 mm and we used this definition when calculating SFR. Most studies use a stone size < 4 mm, but one could choose a smaller measurement like < 3 mm, as discussed in some studies [Citation6,Citation25,Citation33,Citation34,Citation42]. Proposals for a new definition of SFR with a level of SFR described in millimetres up to the limit of 4 mm have been suggested [Citation43]. The SFR in this study after one ESWL treatment was 46%, corresponding well to another study where retreatment was needed in up to 50% of patients [Citation16] in order to reach a typical SFR of 70–80% [Citation3,Citation22]. A number of earlier studies focus on identifying all prognostic factors affecting ESWL results [Citation6,Citation7,Citation16,Citation44–46]. Hirsch et al. [Citation7] presented a predictive model that includes variables that many prior studies found to be significant when predicting treatment success and suggested cut-off values for each (≤987 HU, stone size ≤11 mm and SSD (measured at 45 degrees) ≤ 88 mm). The AUC of their model using these three predictors was 0.74, identical to AUC using only age and maximum stone size. We have therefore presented a predictive model that we believe to be a valuable tool in clinical everyday practice. It is a simple model using age and stone size to predict SFR after ESWL. An example calculation is shown in .
A possible weakness of this study is the retrospective design. Additionally, the inclusion criteria in this cohort allowed us to look at ESWL treatment alone but excluded comparisons to other stone treatments. SSD was measured in a clinically relevant way but measurements may not be comparable to previous studies and HU measured by two consultants, which showed significant interindividual measuring difference. A strength of the study was the inclusion of a sufficient sample size for multivariate analysis. Additionally, all ESWL treatments included in our study were evaluated with NCCT before and after treatment.
Conclusion
The results of this study indicate that stone size and age are the most important factors for predicting SFR after ESWL. Stone attenuation (HU) and skin-to-stone distance (SSD) did not significantly affect the SFR following ESWL treatment in this study. With this work we present a simple predictive model for the calculation of SFR after ESWL that may contribute to the counselling of stone patients in the future.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Brown J. Diagnostic and treatment patterns for renal colic in US emergency departments. Int Urol Nephrol. 2006;38(1):87–92.
- Cupisti A, Pasquali E, Lusso S, et al. Renal colic in Pisa emergency department: epidemiology, diagnostics and treatment patterns. Intern Emerg Med. 2008;3(3):241–244.
- Türk (Chair) An C, Petřík A, Seitz C, et al. Gambaro (Consultant nephrologist), Guidelines Associates: N.F. Davis JFD, R. Lombardo, L. Tzelves. EAU Guidelines on Urolithiasis 2021. Available from: https://uroweb.org/guideline/urolithiasis/.
- Zumstein V, Betschart P, Abt D, et al. Surgical management of urolithiasis – a systematic analysis of available guidelines. BMC Urol. 2018;18(1):25.
- NICE Guideline – Renal and ureteric stones: assessment and management: NICE (2019) Renal and ureteric stones: assessment and management. BJU Int. 2019;123(2):220–232. Epub 2019/01/19.
- El-Nahas AR, El-Assmy AM, Mansour O, et al. A prospective multivariate analysis of factors predicting stone disintegration by extracorporeal shock wave lithotripsy: the value of high-resolution noncontrast computed tomography. European Urology. 2007;51(6):1688–1693; discussion 93-4.
- Hirsch B, Abt D, Gusewell S, et al. Outcome groups and a practical tool to predict success of shock wave lithotripsy in daily clinical routine. World J Urol. 2021;39(3):943–951.
- Assimos D, Krambeck A, Miller NL, et al. Surgical management of stones: American urological association/endourological society guideline, PART I . J Urol. 2016;196(4):1153–1160.
- Türk CN, Petrik A, Seitz C, et al. EAU Guidelines on Urolithiasis 2020. European Association of Urology Guidelines 2019 Edition. 2020.
- Lu Y, Tianyong F, Ping H, et al. Antibiotic prophylaxis for shock wave lithotripsy in patients with sterile urine before treatment may be unnecessary: a systematic review and Meta-analysis. J Urol. 2012;188(2):441–448.
- Jendeberg J, Geijer H, Alshamari M, et al. Size matters: the width and location of a ureteral stone accurately predict the chance of spontaneous passage. Eur Radiol. 2017;27(11):4775–4785.
- Yallappa S, Amer T, Jones P, et al. Natural history of conservatively managed ureteral stones: analysis of 6600 patients. J Endourol. 2018;32(5):371–379. doi: https://doi.org/10.1089/end.2017.0848.
- Ichiyanagi O, Nagaoka A, Izumi T, et al. Age-related delay in urinary stone clearance in elderly patients with solitary proximal ureteral calculi treated by extracorporeal shock wave lithotripsy. Urolithiasis. 2015;43(5):419–426.
- Ng CF, Wong A, Tolley D. Is extracorporeal shock wave lithotripsy the preferred treatment option for elderly patients with urinary stone? A multivariate analysis of the effect of patient age on treatment outcome. BJU Int. 2007;100(2):392–395.
- Ng CF. The effect of age on outcomes in patients undergoing treatment for renal nal stones. Curr Opin Urol. 2009;19(2):211–214.
- Abdel-Khalek M, Sheir KZ, Mokhtar AA, et al. Prediction of success rate after extracorporeal shock-wave lithotripsy of renal stones-a multivariate analysis model. Scand J Urol Nephrol. 2004;38(2):161–167.
- Abdel-Khalek M, Sheir K, Elsobky E, et al. Prognostic factors for extracorporeal shock-wave lithotripsy of ureteric stones-a multivariate analysis study. Scand J Urol Nephrol. 2003;37(5):413–418. Epub 2003/11/05.
- Wiesenthal JD, Ghiculete D, Ray AA, et al. A clinical nomogram to predict the successful shock wave lithotripsy of renal and ureteral calculi. J Urol. 2011;186(2):556–562.
- Christian C, Thorsten B. The preferred treatment for upper tract stones is extracorporeal shock wave lithotripsy (ESWL) or ureteroscopic: pro ESWL. Urology. 2009;74(2):259–262.
- Romero V, Akpinar H, Assimos DG. Kidney stones: a global picture of prevalence, incidence, and associated risk factors. Rev Urol. 2010;12(2–3):e86-96–e96.
- Li W, Mao Y, Lu C, et al. Role of sexual intercourse after shockwave lithotripsy for distal ureteral stones: a randomized controlled trial. Urol J. 2020;17(2):134–138.
- Wagenius M, Jakobsson J, Stranne J, et al. Complications in extracorporeal shockwave lithotripsy: a cohort study. Scand J Urol. 2017;51(5):407–413.
- Polat F, Yeşil S, Ak E, et al. Safety of ESWL in elderly: evaluation of independent predictors and comorbidity on stone-free rate and complications. Geriatr Gerontol Int. 2012;12(3):413–417.
- Abdelghany M, Zaher T, El Halaby R, et al. Extracorporeal shock wave lithotripsy of lower ureteric stones: Outcome and criteria for success. Arab J Urol. 2011;9(1):35–39.
- Nakasato T, Morita J, Ogawa Y. Evaluation of hounsfield units as a predictive factor for the outcome of extracorporeal shock wave lithotripsy and stone composition. Urolithiasis. 2015;43(1):69–75.
- Wiesenthal JD, Ghiculete D, John D’A Honey R, et al. Evaluating the importance of mean stone density and skin-to-stone distance in predicting successful shock wave lithotripsy of renal and ureteric calculi. Urol Res. 2010;38(4):307–313.
- Yoshida S, Hayashi T, Ikeda J, et al. Role of volume and attenuation value histogram of urinary stone on noncontrast helical computed tomography as predictor of fragility by extracorporeal shock wave lithotripsy. Urology. 2006;68(1):33–37.
- Pareek G, Armenakas NA, Fracchia JA. Hounsfield units on computerized tomography predict stone-free rates after extracorporeal shock wave lithotripsy. J Urol. 2003;169(5):1679–1681.
- Wang L-J, Wong Y-C, Chuang C-K, et al. Predictions of outcomes of renal stones after extracorporeal shock wave lithotripsy from stone characteristics determined by unenhanced helical computed tomography: a multivariate analysis. Eur Radiol. 2005;15(11):2238–2243.
- Gupta NP, Ansari MS, Kesarvani P, et al. Role of computed tomography with no contrast medium enhancement in predicting the outcome of extracorporeal shock wave lithotripsy for urinary calculi. BJU Int. 2005;95(9):1285–1288. doi:.
- Joseph P, Mandal AK, Singh SK, et al. Computerized tomography attenuation value of renal calculus: Can it predict successful fragmentation of the calculus by extracorporeal shock wave lithotripsy? A preliminary study. J Urol. 2002;167(5):1968–1971.
- Ng C-F, Siu DY-W, Wong A, et al. Development of a scoring system from noncontrast computerized tomography measurements to improve the selection of upper ureteral stone for extracorporeal shock wave lithotripsy. J Urol. 2009;181(3):1151–1157.
- Perks AE, Schuler TD, Lee J, et al. Stone attenuation and skin-to-Stone distance on computed tomography predicts for stone fragmentation by shock wave lithotripsy. Urology. 2008;72(4):765–769.
- Tanaka M, Yokota E, Toyonaga Y, et al. Stone attenuation value and Cross-Sectional area on computed tomography predict the success of shock wave lithotripsy. Korean J Urol. 2013;54(7):454–459.
- Pareek G, Armenakas NA, Panagopoulos G, et al. Extracorporeal shock wave lithotripsy success based on body mass index and hounsfield units. Urology. 2005;65(1):33–36.
- Ouzaid I, Al-Qahtani S, Dominique S, et al. A 970 hounsfield units (HU) threshold of kidney stone density on non-contrast computed tomography (NCCT) improves patients’ selection for extracorporeal shockwave lithotripsy (ESWL): evidence from a prospective study. BJU Int. 2012;110(11b):E438–42.
- Mullhaupt G, Engeler DS, Schmid HP, et al. How do stone attenuation and skin-to-stone distance in computed tomography influence the performance of shock wave lithotripsy in ureteral stone disease? BMC Urol. 2015;15:72.
- Patel T, Kozakowski K, Hruby G, et al. Skin to stone distance is an independent predictor of stone-free status following shockwave lithotripsy. J Endourol. 2009;23(9):1383–1385.
- Ather MH, Shrestha B, Mehmood A. Does ureteral stenting prior to shock wave lithotripsy influence the need for intervention in steinstrasse and related complications? Urol Int. 2009;83(2):222–225.
- Musa AA. Use of double-J stents prior to extracorporeal shock wave lithotripsy is not beneficial: results of a prospective randomized study. Int Urol Nephrol. 2008;40(1):19–22.
- Madbouly K, Sheir KZ, Elsobky E, et al. Risk factors for the formation of a steinstrasse after extracorporeal shock wave lithotripsy: a statistical model. J Urol. 2002;167(3):1239–1242.
- Goel H, Gahlawat S, Bera M, et al. Role of clinical and radiological parameters in predicting the outcome of shockwave lithotripsy for ureteric stones. Urol Ann. 2018;10(2):159–164.
- Somani BK, Desai M, Traxer O, et al. Stone-free rate (SFR): a new proposal for defining levels of SFR. Urolithiasis. 2014;42(2):95Epub 2013/12/10.
- Petrides N, Ismail S, Anjum F, et al. How to maximize the efficacy of shockwave lithotripsy. Turk J Urol. 2020;46(Supp1):S19–S26.
- Tokas T, Habicher M, Junker D, et al. Uncovering the real outcomes of active renal stone treatment by utilizing non-contrast computer tomography: a systematic review of the current literature. World J Urol. 2017;35(6):897–905.
- Knoll T, Buchholz N, Wendt-Nordahl G. Extracorporeal shockwave lithotripsy vs. percutaneous nephrolithotomy vs. flexible ureterorenoscopy for lower-pole stones. Arab J Urol. 2012;10(3):336–341.