Abstract
Purpose
To assess if cancer-specific survival (CSS) following curative intent treatment (CIT) for muscle-invasive bladder cancer (MIBC) differs between patients presenting with MIBC (primary) and patients presenting with non-muscle-invasive bladder cancer who progress to MIBC (secondary).
Methods
This study uses data from the Cancer Registry of Norway on patients initially diagnosed with bladder cancer in 2008–2012 and treated with radical cystectomy (RC) or radiotherapy (RT). To ensure a clinically relevant population, we selected patients with a pre-treatment histology confirming muscle-invasion. Survival models were applied to evaluate differences in observed and adjusted CSS by type of MIBC and stratified by type of CIT. Adjustment was made for age group, sex, previous cancer, diagnostic hospital’s academic status and geographical region, and type of CIT.
Results
We identified 650 eligible patients: 589 (91%) primary MIBC and 61 (9%) secondary MIBC. A total of 556 (86%) patients underwent RC and 94 (14%) RT. The 5-year CSS for primary MIBC was 56% and 59% for secondary MIBC (p = 0.68). The type of MIBC did not impact the risk of bladder cancer death (HR = 0.85, CI = 0.55–1.33, p = 0.48), nor when stratified for CIT (RC: HR = 0.93, CI = 0.57–1.53, p = 0.78); RT: HR = 0.71, CI = 0.24–2.16, p = 0.55).
Conclusion
This first nation-wide population-based study comparing CSS between primary and secondary MIBC showed no significant difference in survival regardless of type of CIT. Continued surveillance of patients with non-muscle-invasive bladder cancer is necessary to detect early progression to MIBC. Future studies should include molecular and genetic characteristics in addition to detailed clinicopathologic information.
Introduction
In Norway, 1,626 cases of bladder cancer (BC) were diagnosed in 2020, of which 1,273 (78%) cases were men, making it the 4th most frequent cancer form among Norwegian men [Citation1]. In Europe, more than 90% of BC cases are urothelial carcinomas (UC) [Citation2] and approximately 25% of all BC cases are muscle-invasive bladder cancer (MIBC) [Citation3]. MIBC can be present at first BC diagnosis (primary MIBC = priMIBC) or have a prior history of non-muscle invasive bladder cancer (NMIBC) before presenting with MIBC (secondary MIBC = secMIBC).
Regardless of type of MIBC (priMIBC or secMIBC), radical cystectomy (RC) has been the standard curative intent treatment (CIT) for the past decades. In selected patients RC is combined with neoadjuvant cisplatin-based chemotherapy (NAC) [Citation2], which in Norway became recommended as part of routine practice in 2013 [Citation4]. Pelvic radiotherapy (RT; ≥ 60 Gy) is offered as CIT to patients unfit or unwilling to undergo RC. Trimodal therapy combining transurethral resection of the bladder (TURB), chemotherapy, and RT [Citation2] was gradually introduced into clinical practice after the publication of national guidelines for treatment of bladder cancer in Norway in 2013 [Citation4].
Several studies have compared post-RC survival in patients with priMIBC and secMIBC [Citation5–18]. Results are conflicting with reports of worse [Citation6,Citation14,Citation16], better [Citation11,Citation17] or comparable survival [Citation5,Citation7–10,Citation12,Citation13,Citation15,Citation18] for secMIBC vs. priMIBC. To our knowledge, no study has compared survival in patients with priMIBC vs. secMIBC based on data from a national cancer registry on patients with histologically confirmed MIBC by TURB and included both RC and RT as CIT.
To fill this gap, the present study uses nationwide data from the Cancer Registry of Norway (CRN) and the National Patient Registry (NPR) on patients initially diagnosed with BC in 2008–2012. Our objective was to describe the patient and treatment characteristics of curatively treated, non-metastatic priMIBC and secMIBC, and to compare bladder cancer specific survival (CSS) between patients with priMIBC and secMIBC, as well as stratified by type of CIT (RC and RT).
Materials and methods
Data sources
We used data from the CRN to identify patients with a first-time morphologically verified UC of the bladder diagnosed in 2008–2012. A personal identification number has been assigned to all residents in Norway since 1960, which was used to link data from the CRN and the NPR.
Study population
Patients finally evaluable for the current study had to fulfil the following criteria:
Pre-CIT muscle-invasion demonstrated in the histological specimen from a TURB.
No distant metastases (M0) at the time of MIBC diagnosis.
Curative intent treatment (CIT) with RC or RT.
PriMIBC required proof of histological muscle-invasion present in the initial diagnostic TURB specimen. To capture patients initially under-staged who underwent a second TURB, patients presenting with histological muscle-invasion in a TURB specimen obtained ≤4 months after the first BC diagnosis were categorized as priMIBC. In patients with secMIBC, muscle-invasion had to be present in a TURB specimen acquired >4 months after the first BC diagnosis and before December 2015.
Based on previous publications using BC data from the CRN [Citation19] and other relevant studies [Citation10,Citation15,Citation16], we chose a cut-off at 4 months to separate priMIBC from secMIBC.
Assessments
From the CRN, in addition to age at BC diagnosis, sex and previous cancer diagnoses, we retrieved information and corresponding dates on BC diagnosis, TURB, RC, RT, status at last observation and cause of death. Age was divided into four groups (≤59, 60–69, 70–79 and ≥80).
For the whole BC patient cohort, all histological reports available at the CRN were quality ensured by the research team concerning muscle-invasion in the TURB specimens, though without detailed information on the depth of invasion. For RC patients, the histopathological T and N category (pT; pN) was identified without sub-classification into a and b in pT2-pT4 [Citation20]. No information on molecular or genetic markers was available.
The NPR provided information on treatment codes (medical, surgical and chemotherapy), the diagnostic hospital’s academic status (academic vs. community) and geographical region in Norway (Southeast, West, Central, North) from all patients’ contacts within public hospitals and from publicly funded private specialists.
To capture patients treated with RC but not registered in the CRN, we cross-checked the information on RC obtained from the CRN with surgical codes for RC in the NPR and identified 56 additional patients.
Statistical analysis
Descriptive statistics (mean, median, interquartile range (IQR), proportions) were applied. Patients were followed from MIBC diagnosis until death, emigration, or end of study (31 December 2019), whichever came first. The total follow-up time was 3,100 person-years (median 3.5 years). Kaplan-Meier (KM) curves were applied to illustrate crude overall survival (OS) and CSS, and a log-rank test evaluated the (unadjusted) differences between them. The association of type of MIBC (secMIBC vs. priMIBC) with CSS was evaluated by flexible parametric survival models (FPSM) [Citation21] adjusting for age group, sex, previous cancer, diagnostic hospital’s academic status and geographical region, and type of CIT (RT vs. RC). The analysis for RC treated patients was additionally adjusted for post-cystectomy pT-category (<pT2, ≥pT2, missing pT), pN-category (pN0, pN+, missing pN), and concomitant CIS (no, yes, missing). In all FPSMs, the baseline hazard was modelled using 4 degrees of freedom (4df) for the splines. Quantities reported from the model-based analyses are hazard ratios (HRs) including 95% confidence intervals (CI) and p-values.
The statistical significance level was set to ≤ 0.05. Statistical analyses were performed using Stata 17 (StataCorp, College Station, TX), stpm2 command for estimating FPSMs.
Results
Patients and treatment
From the CRN, 5,521 patients were identified with a first-time morphologically verified UC BC diagnosis from 2008 through 2012. Muscle-invasive disease was histologically verified in in 1,337 patients (24.2%). We excluded 101 patients in whom muscle-invasion was found solely in a cystectomy specimen, and 53 patients with a record of distant metastases in the CRN at the time of MIBC diagnosis. In total, 1,183 (21.4%) patients fulfilled the criteria of pre-CIT muscle-invasion demonstrated in the histological specimen from a TURB and no distant metastasis. Of those patients, 650 (55%) patients underwent CIT (Supplementary Figure S1). Out of 650 MIBC patients treated with CIT, we identified 589 (91%) patients with priMIBC and 61 (9%) patients with secMIBC. Compared to patients with secMIBC, more patients with priMIBC were treated with CIT (56% vs. 44%: Supplementary Figure S1).
Median age of the patients at BC diagnosis was 71 (IQR = 63–77) years and the patients were predominantly male (79%) (). A total of 556 (86%) patients underwent RC, of whom 56 (10%) patients received NAC. RT represented CIT in 94 (14%) patients, of whom 6 (6%) patients underwent post-RT RC. During the follow-up period 274 (42%) patients died of BC. Patient and treatment characteristics were similar in the priMIBC and secMIBC groups. In patients with secMIBC, a median time of 1.1 year (IQR = 0.5–3.1) elapsed from diagnosis of NMIBC to diagnosis of MIBC.
Table 1. Primary and secondary MIBC patients treated with curative intent: (a) All patients: Patient- and treatment characteristics, (b) Patients treated with radical cystectomy (RC): pT-category, (c) Patients treated with RC: pN-category (d) Patients treated with RC: Concomitant CIS.
Out of 556 patients undergoing RC, histopathological information from the RC was registered in the CRN for 500 (90%) patients: pT and pN were available in 496 (99%) and 411 (82%) patients, respectively. The distributions of pT- and pN-categories were similar in the priMIBC and the secMIBC group (). Concomitant CIS was present in 111 (22%) of the patients with a similar distribution in the priMIBC and secMIBC group ().
Out of 500 patients treated with RC and no NAC, 449 (90%) patients underwent RC ≤90 days of MIBC diagnosis, with no difference in elapsed median time (49 days) or the number of patients undergoing RC within 90 days (90 vs. 91%) between patients with priMIBC and secMIBC (Supplementary Table S1a).
In all cystectomized and irradiated patients, there was no difference in age and sex distributions between priMIBC and secMIBC (Supplementary Table S1b and c).
Survival
All patients: Crude 5-year OS and CSS were 44% and 57% (). The 5-year CSS was 56% for priMIBC and 59% for secMIBC (p = 0.68) (). The adjusted survival analysis revealed that the type of MIBC had no impact on the risk of BC death (HR = 0.85, CI = 0.55–1.33, p = 0.48). Sex, previous cancer, academic status and type of CIT were not associated with the risk of death, but higher age (≥80 vs. ≤59) and region (North vs. Southeast) significantly increased this risk ().
Figure 1. Survival after diagnosis of MIBC in 650 patients undergoing curative treatment: (a) All patients; Crude overall survival (OS = dashed) and cancer-specific survival (CSS = solid), (b) CSS in all patients; primary (priMIBC = solid) vs. secondary MIBC (secMIBC = dashed), (c) CSS in patients treated with radical cystectomy (RC); primary vs. secondary MIBC, (d) CSS in patients treated with radiotherapy (RT); primary vs. secondary MIBC.
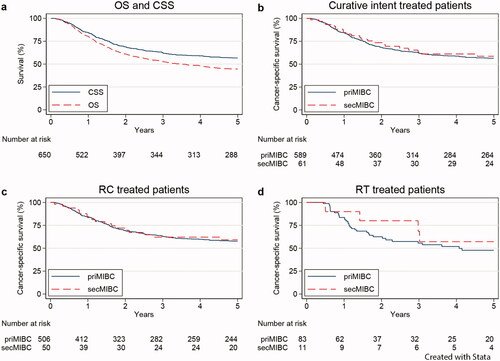
Table 2. Flexible parametric survival model evaluating associations with cancer-specific survival for all included MIBC patients (N = 650).
Radical cystectomy: Crude 5-year CSS was 58% for all 556 patients: 58% for priMIBC and 59% for secMIBC (p = 0.85) (). The type of MIBC was not associated with the adjusted CSS (HR = 0.93, CI = 0.57–1.53, p = 0.78). Sex, previous cancer, academic status and region did not impact CSS, but higher age (≥80 vs ≤59), higher pT-category (≥pT2 vs < pT2) and pN+ (vs. pN0) were significantly associated with increased risk of BC death (Supplementary Table S2).
Radiotherapy: Crude 5-year CSS for priMIBC was 48% and 57% for secMIBC (p = 0.49) (). There was no significant impact of the type of MIBC on the adjusted CSS (HR = 0.71, CI = 0.24–2.16, p = 0.55). Age, sex, previous cancer, academic status and region were not associated with CSS (Supplementary Table S3).
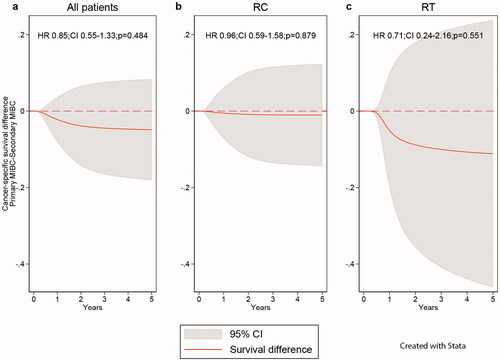
The lack of significant differences in CSS (priMIBC–secMIBC) in the adjusted analyses for all patients and those treated with RC or RT are illustrated in .
Figure 2. Adjusted difference in cancer-specific survival between patient with primary and secondary MIBC by treatment; no difference (dashed), observed survival difference (solid), confidence interval (CI = grey area); (a) All patients, both types of curative intent treatment, (b) Post-cystectomy (RC), (c) Post-radiotherapy (RT).
Discussion
To our knowledge, this paper presents the first nationwide, population-based study that compares survival between patients with priMIBC and secMIBC for all curatively treated patients and where all patients had confirmed muscle-invasion prior to treatment. We did not find a significant difference in crude or adjusted CSS between all patients with priMIBC and secMIBC, nor when stratified by type of CIT.
In agreement with our findings, two prospective studies [Citation12,Citation15] and several retrospective studies [Citation5,Citation7–10] evaluating survival in patients undergoing RC for a diagnosis of MIBC by TURB, found similar crude CSS for MIBC patients and no significant difference between priMIBC and secMIBC or association with CSS to the type of MIBC. These data are confirmed by two meta-analyses [Citation22,Citation23]. In line with our results, a recently published Canadian population-based study [Citation18] did not find a significant difference in survival between priMIBC and secMIBC. In that study, all patients undergoing RC for BC were included, and the pre-treatment pathology confirmed muscle-invasion in only 49% of patients with secMIBC (79% of priMIBC). The present population-based study therefore represents a more homogenous patient population since all patients included had pre-CIT confirmed MIBC, and thus serves as a better basis for survival comparison between curatively treated priMIBC and secMIBC.
Other studies have reported conflicting effects of secMIBC vs. priMIBC with respect to survival. Favourable post-RC survival (CSS, OS) for secMIBC vs. priMIBC was reported in two Canadian series [Citation11,Citation17]. In a multicentre study [Citation11], patients with priMIBC were more frequently diagnosed with poor prognosis factors (hydronephrosis, pT3, pT4, lymphovascular invasion, pN+) than patients with secMIBC. In our study, we found similar pT and pN distributions in priMIBC and secMIBC, which is in line with several other clinical studies reporting no difference in survival between patients with priMIBC and secMIBC [Citation10,Citation15], suggesting that the favourable outcome for patients with secMIBC in the former series [Citation11] may be related to significantly more advanced disease in patients with priMIBC. In another Canadian population-based series [Citation17] which included all BC patients undergoing RC, no histopathologic or clinical information for pre-RC staging was available. Patients were presumed to have secMIBC if they had undergone two TURBs or more over 4 months apart before RC, while all other patients were presumed to have priMIBC. No subsequent pathological review was conducted. The previously mentioned Canadian population-based study had a similar selection of the study population (RC for BC) and revealed that only 49% of the secMIBC patients had MIBC prior to RC in a subsequent pathological review [Citation18]. Thus, it is very likely that a proportion of secMIBC patients in the former study were treated with RC for NMIBC. Patients undergoing RC before muscle-invasion have a significantly better prognosis compared to priMIBC and secMIBC [Citation5,Citation7,Citation9,Citation14] and including these patients probably contributed to the superior OS for secMIBC in this study [Citation17].
On the other hand, worse survival for secMIBC compared to priMIBC has been reported in retrospective series [Citation6,Citation14,Citation16] and is supported by two meta-analyses [Citation24,Citation25]. Patients in the retrospective studies [Citation6,Citation14,Citation16] were selected based on pre-RC histopathological verification of muscle-invasion and reported similar clinicopathologic characteristics in priMIBC and secMIBC patients. However, information on the surveillance regime and time to progression was not available in two of the studies [Citation6,Citation16]. As noted by the authors in one of the studies [Citation16], the worsened prognosis of secMIBC patients compared to priMIBC patients could be caused by a proportion of secMIBC patients receiving inadequate treatment or surveillance. Delayed RC (>3 months) has been shown to have a detrimental effect on overall survival [Citation26]. In one of the studies [Citation14] surveillance cystoscopy was performed regularly but a second TURB was not routinely performed in the first half of the study period. Some of the patients in the secMIBC group may have been under-staged at initial TURB, resulting in a delayed RC which may have impacted on the worsened survival for secMIBC.
Post-RC survival (recurrence free survival, CSS, OS) and pathologic response after treatment with NAC was worse for patients with secMIBC compared to patients with priMIBC in a recent retrospective study [Citation27]. This finding was supported by a meta-analysis [Citation23]. The effect was hypothesized to be linked to the predominant occurrence of a cisplatin sensitizing DNA damage repair gene (ERCC2) [Citation28] in priMIBC tumours, predicting response to cisplatin. We did not exclude patients treated with NAC, but due to limited numbers we were not able to compare survival between NAC treated patients with priMIBC and secMIBC.
We found that the proportion of patients undergoing RC with secMIBC was 9%. In comparison, the proportion of patients with secMIBC ranges from 20% to 42% in population-based studies [Citation17,Citation18], from 22% to 38% in retrospective single- and multi-institutional studies [Citation5,Citation7–10,Citation14,Citation16] and from 16% to 23% in prospective series [Citation12,Citation15]. The lower proportion of secMIBC in our study may partly be explained by differences in patient selection and definitions of priMIBC and secMIBC. Compared to other population-based studies, we did not include patients undergoing RC before MIBC [Citation17,Citation18] as secMIBC, which potentially increased the proportion of secMIBC in these studies. Our definition of priMIBC is also slightly different compared to the most recent population-based study [Citation18], since we allowed for patients with MIBC in a TURB less than 4 months after first BC diagnosis to be included as priMIBC as opposed to less than 2 months apart. Thus, some priMIBC patients in our study would have been categorized as secMIBC in that study [Citation18]. Compared to retrospective and prospective studies, our selection of patients for study inclusion is similar but our definitions of priMIBC and secMIBC differs slightly. In some studies patients were considered priMIBC if a subsequent TURB performed within 3 months of the first BC diagnosis showed MIBC [Citation7,Citation10,Citation16], in comparison we extended this timeframe to 4 months. Some of these patients would be considered secMIBC in the previous studies [Citation10,Citation15,Citation16].
In summary, the impact of priMIBC and secMIBC on patient prognosis remains unclear as the available evidence continues to show conflicting results. Neither can we rule out the possibility of secMIBC having a worse prognosis than priMIBC. SecMIBC may be of a more aggressive nature due to the extended duration of the malignancy compared to priMIBC increasing the risk of micro-metastatic dissemination, possible tumour clone selection after prior intravesical therapy [Citation6] and possible local tumour spread after multiple TURBs [Citation29]. On the other hand, the effect may be compensated by the close follow-up of primary NMIBC by urologists and early detection and treatment of MIBC.
Our results in the RT group comprise patients treated before 2015 and do not reflect more modern radiotherapy techniques allowing dose-escalated tumour boosting with possibly improved survival [Citation30]. Today, it is important to continuously assess the real-life use of and effect of radiotherapy multimodal treatment.
A limitation of our study is the unavailability of risk factors such as smoking habits, socioeconomic status and comorbidities. On the other hand, we present a population-based cohort where we assume these factors are evenly distributed. Unfortunately, we do not have a quality register for BC in Norway with pre-treatment results of imaging or clinical findings enabling clinical TNM categorization. However, the verification of histological muscle-invasion upon study entry ensured clinically relevant and comparable patient groups. Type of operational technique (Open RC vs. robot assisted), extent of lymph node dissection, lymphovascular invasion, number of positive lymph nodes vs. numbers removed could not be assessed.
Conclusion
We found no difference in post-CIT survival in patients with priMIBC compared to those with secMIBC, regardless of type of CIT (RC, RT). With today’s knowledge, differential curative management of patients with priMIBC and secMIBC is not warranted. Continued close surveillance of patients with NMIBC is necessary to ensure early detection and management of MIBC. To improve our understanding of priMIBC vs. secMIBC, future studies should not only investigate in depth clinicopathological parameters in MIBC, but also molecular and genetic differences to aid physicians in tailoring treatment for MIBC patients.
Ethics approval
Approved by the Regional Committee for Medical and Health Research Ethics (REC), Southeast Norway. Approval number: 2016/2286/REK sør-øst A. The requirement for consent was waived by the ethics committee.
Supplemental Material
Download PDF (216.4 KB)Disclosure statement
No potential conflict of interest was reported by the authors. Dam Foundation (https://dam.no) has made this project possible.
References
- Norway CRo. Cancer in Norway 2020 - Cancer incidence, mortality, survival and prevalence in Norway [Internet]. Oslo: Cancer Registry of Norway; 2021. [cited 2021]. Available from: https://www.kreftregisteret.no/globalassets/cancer-in-norway/2020/cin-2020.pdf.
- Witjes Hmb JA, Cathomas R, Compérat E, et al. The EAU guidelines on muscle-invasive and metastatic bladder cancer 2021. [cited 2021 Jun 6]. Available from: https://uroweb.org/guideline/bladder-cancer-muscle-invasive-and-metastatic/
- Westergren DO, Gårdmark T, Lindhagen L, et al. A nationwide, population based analysis of patients with organ confined, muscle invasive bladder cancer not receiving curative intent therapy in Sweden from 1997 to 2014. J Urol. 2019;202(5):905–912.
- Helsedirektoratet. Nasjonalt handlingsprogram med retningslinjer for diagnostikk, behandling og oppfølgning av blaerekreft Oslo: Helsedirektoratet; 2013. [cited 2021 Aug 3]. Available from: https://blaerekreft.no/wp-content/uploads/Nasjonalt-handlingsprogram-bl%C3%A6rekreft.pdf.
- Yiou R, Patard JJ, Benhard H, et al. Outcome of radical cystectomy for bladder cancer according to the disease type at presentation. BJU Int. 2002;89(4):374–378.
- Schrier BP, Hollander MP, van Rhijn BW, et al. Prognosis of muscle-invasive bladder cancer: difference between primary and progressive tumours and implications for therapy. Eur Urol. 2004 Mar;45(3):292–296.
- May M, Helke C, Nitzke T, et al. Survival rates after radical cystectomy according to tumor stage of bladder carcinoma at first presentation. Urol Int. 2004;72(2):103–111.
- Ferreira U, Matheus WE, Nardi Pedro R, et al. Primary invasive versus progressive invasive transitional cell bladder cancer: multicentric study of overall survival rate. Urol Int. 2007;79(3):200–203.
- Lee CT, Dunn RL, Ingold C, et al. Early-stage bladder cancer surveillance does not improve survival if high-risk patients are permitted to progress to muscle invasion. Urology. 2007;69(6):1068–1072.
- de Vries RR, Nieuwenhuijzen JA, Vincent A, et al. Survival after cystectomy for invasive bladder cancer. Eur J Surg Oncol. 2010 Mar;36(3):292–297.
- Kotb AF, Kovac E, Kassouf W, et al. Radical cystectomy for clinically muscle invasive bladder cancer: does prior non-invasive disease affect clinical outcomes? World J Urol. 2012;30(6):761–767.
- Aziz A, Gierth M, Fritsche HM, et al. Oncological outcome of primary versus secondary Muscle-Invasive bladder cancer is comparable after radical cystectomy. Urol Int. 2013;91(1):97–102.
- Hidas G, Pode D, Shapiro A, et al. The natural history of secondary muscle-invasive bladder cancer. BMC Urol. 2013;13:23.
- Breau RH, Karnes RJ, Farmer SA, et al. Progression to detrusor muscle invasion during urothelial carcinoma surveillance is associated with poor prognosis. BJU Int. 2014;113(6):900–906.
- May M, Burger M, Brookman-May S, et al. EORTC progression score identifies patients at high risk of cancer-specific mortality after radical cystectomy for secondary muscle-invasive bladder cancer. Clin Genitourin Cancer. 2014;12(4):278–286.
- Moschini M, Sharma V, Dell’oglio P, et al. Comparing long-term outcomes of primary and progressive carcinoma invading bladder muscle after radical cystectomy. BJU Int. 2016;117(4):604–610.
- Zakaria AS, Santos F, Kassouf W, et al. Survival after radical cystectomy for bladder cancer in relation to prior non-muscle invasive disease in Quebec. Urol Int. 2016;97(1):49–53.
- Lusty A, Doiron RC, Booth CM, et al. No outcome differences after cystectomy between patients with De novo Muscle-Invasive bladder cancer compared to progressors: a retrospective population-based study. J Urol. 2021;206(2):260–269.
- Blindheim A, Fosså S, Babigumira R, et al. T1 bladder cancer in Norway: treatment and survival. Scand J Urol. 2020;54(5):370–375.
- International Union Against Cancer (UICC). TNM classification of malignant tumours. 6th ed. Sobin L, Wittekind C, editors. New York: Wiley; 2002.
- Lambert PC, Royston P. Further development of flexible parametric models for survival analysis. Stata J. 2009;9(2):265–290.
- Chen J, Zhang H, Sun G, et al. Comparison of the prognosis of primary and progressive muscle-invasive bladder cancer after radical cystectomy: a systematic review and meta-analysis. Int J Surg. 2018;52:214–220.
- Pones M, D’Andrea D, Mori K, et al. Differential prognosis and response of denovo vs. secondary muscle-invasive bladder cancer: an updated systematic review and meta-analysis. Cancers (Basel). 2021;13(10):2496.
- Ge P, Wang L, Lu M, et al. Oncological outcome of primary and secondary muscle-Invasive bladder cancer: a systematic review and meta-analysis. Sci Rep. 2018;8(1):7543.
- Zheng XN, Qiu S, Yang L, et al. Comparison of survival outcomes between primary and secondary muscle-invasive bladder cancer: an updated meta-analysis. Int J Med Sci. 2021;18(2):505–510.
- Russell B, Liedberg F, Khan MS, et al. A systematic review and meta-analysis of delay in radical cystectomy and the effect on survival in bladder cancer patients. Eur Urol Oncol. 2020;3(2):239–249.
- Pietzak EJ, Zabor EC, Bagrodia A, et al. Genomic differences between “primary” and “secondary” muscle-invasive bladder cancer as a basis for disparate outcomes to cisplatin-based neoadjuvant chemotherapy. Eur Urol. 2019;75(2):231–239.
- Van Allen EM, Mouw KW, Kim P, et al. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov. 2014;4(10):1140–1153.
- El-Abbady AA, Shoukry MS, Hanno AG, et al. Repeated transurethral resection of recurrent superficial bladder tumors-does it affect the spread and stage of the tumor? Scand J Urol Nephrol. 2002;36(1):60–64.
- Fonteyne V, Ost P, Bellmunt J, et al. Curative treatment for muscle invasive bladder cancer in elderly patients: a systematic review. Eur Urol. 2018;73(1):40–50.
