Abstract
Objectives
To compare time intervals to diagnosis and treatment, tumor characteristics, and management in patients with primary urinary bladder cancer, diagnosed before and after the implementation of a standardized care pathway (SCP) in Sweden.
Materials and methods
Data from the Swedish National Register of Urinary Bladder Cancer was studied before (2011–2015) and after (2016–2019) SCP. Data about time from referral to transurethral resection of bladder tumor (TURBT), patients and tumor characteristics, and management were analyzed. Subgroup analyses were performed for cT1 and cT2–4 tumors.
Results
Out of 26,795 patients, median time to TURBT decreased from 37 to 27 days after the implementation of SCP. While the proportion of cT2–T4 tumors decreased slightly (22–21%, p < 0.001), this change was not stable over time and the proportions cN + and cM1 remained unchanged. In the subgroups with cT1 and cT2–4 tumors, the median time to TURBT decreased and the proportions of patients discussed at a multidisciplinary team conference (MDTC) increased after SCP. In neither of these subgroups was a change in the proportions of cN + and cM1 observed, while treatment according to guidelines increased after SCP in the cT1 group.
Conclusion
After the implementation of SCP, time from referral to TURBT decreased and the proportion of patients discussed at MDTC increased, although not at the levels recommended by guidelines. Thus, our findings point to the need for measures to increase adherence to SCP recommendations and to guidelines.
Introduction
Urinary bladder cancer is one of the most common malignancies, of which 22% of tumors are muscle invasive (MIBC) at diagnosis and the remainders are non-muscle invasive (NMIBC) [Citation1–3]. Macroscopic hematuria is the predominant presenting symptom with a high positive predictive value for bladder cancer [Citation4]. Current clinical guidelines recommend investigation of macroscopic hematuria with cystoscopy and computed tomography–urography (CTU) [Citation1].
Delay in cancer diagnosis might have a negative impact on prognosis [Citation5], while in bladder cancer a short time from referral to transurethral resection of bladder tumor (TURBT) was associated with more advanced tumors and a negative outcome [Citation6]. Especially in women, diagnostic delay has been reported to be associated with more aggressive bladder cancer and higher cancer-specific mortality [Citation7]. Furthermore, delays to surgery have a negative impact on both quality-of-life and overall survival (OS) [Citation8]. Correspondingly, a delay in definitive surgical treatment beyond 12 weeks is associated with a higher risk of disease-specific and all-cause mortality [Citation9]. Similar results were observed in a review, albeit the definition of delay varied greatly between the studies included [Citation10]. Measures to shorten the time to diagnosis have been successfully introduced in the UK [Citation11].
In Sweden, a standardized care pathway (SCP) for patients with suspected bladder cancer, mainly due to macroscopic hematuria, was implemented in 2016. The ambition was not just to reduce the time from first symptom to diagnosis but also to provide well-organized and professional care according to guidelines without unnecessary waiting time, regardless of where in the country patients seek care [Citation12].
The aim of this study was to investigate whether the implementation of SCP was associated with changes in time to treatment and tumor stage at diagnosis and for specific subgroups management according to national guidelines and OS for the cT2–4 subgroup.
Materials and methods
Patients
Data was obtained from the Swedish National Register of Urinary Bladder Cancer (SNRUBC), a population-based, nationwide register of all patients with newly diagnosed bladder cancer [Citation2,Citation13]. All patients diagnosed from 1 January 2010 to 31 December 2019 were included and divided into two periods: before SCP 2010–2015 and after implementation of SCP 2016–2019. To examine changes over time not related to SCP, we further stratified patients into four periods: 2010–2012, 2013–2015, 2016–2017 and 2018–2019.
Patient characteristics (age and gender), tumor characteristics, clinical stage according to TNM [Citation14], tumor grade according to WHO 1999 classification [Citation15], tumor size and number of tumors, primary treatment, and OS were analyzed in relation to time from referral to TURBT, which was performed in all patients. Furthermore, time intervals from TURBT to radical cystectomy and to the start of neoadjuvant chemotherapy (NAC) were compared in relation to SCP.
A high proportion of cNX tumors was registered, although a CTU was performed in nearly all these patients, and if no regional lymph-node enlargement was observed the correct registration should have been cN0. Therefore, all cNX were grouped together with cN0 and patients with cN1–3 were analyzed together as cN+.
In stage cT1 tumors, registration of primary treatment included second-look resection (SLR, defined as re-resection within 56 days after TURBT) and intravesical instillation therapy (IVIT, defined as multiple intravesical instillations of BCG) or chemotherapy (but not including single, immediate postoperative instillations) and cystectomy if clinically indicated. In patients with cT2–4 tumors, registration of primary treatment after TURBT and in some cases SLR included cystectomy with or without NAC (defined as any systemic chemotherapy planned and given preceding cystectomy) or curative radiotherapy (RT) was carried out.
However, it should be noted that, within the group registered as having NAC treatment, a minority (89 out of 825 patients; 11%) had primary cN + tumors and should have been classified as treated by induction therapy, but this variable was not registered before 2020. Over time, TNM categories in the SNRUBC changed in accordance with the published versions of the TNM classification. Histopathological classification of tumor grade was performed according to the WHO 1999 classification, which was used for tumor grade classification for bladder cancer in Sweden [Citation15]. The study was approved by the Swedish Ethical Review Authority (File No. 2020-02397).
Standardized care pathway
The concept of SCP aimed to improve outcome for bladder cancer patients in the country by reducing the time from first symptom to definitive treatment and by performing a uniform management according to international guidelines. This management included immediate (within 24 h) referral from any care unit to cystoscopy and CTU for all eligible patients, TURBT for all patients with suspected bladder cancer, and multidisciplinary team conference (MDTC) for all patients with ≥ cT1 tumors.
The SCP was initiated in September 2015 and, in our study design, we considered it fully implemented on a nationwide basis for all patients seeking healthcare with macroscopic hematuria from the start of 2016. Patients with other reasons for suspected bladder cancer such as findings from radiological examinations were also included in SCP, while patients with asymptomatic microscopic hematuria were not. Initially, a lower age limit of 40 years or older was set to qualify for the SCP, but this was changed to 50 years or older from 2018 and onwards, due to the rarity of urothelial malignancy in patients younger than 50 years of age.
The SCP initially defined the upper time interval limit from referral to the investigation with CTU, cystoscopy and subsequent TURBT as 9–12 days, but it was adjusted to 13 days from 2018 and onwards. The other recommended upper time limits for SCP from referral were: to pathological report of TURBT specimen, 19 days, to MDTC for patients with cT1–cT4 tumors, 26 days, to cystectomy, 37 days, to NAC, 39 days, and to RT, 43 days.
Statistics
Age and different time intervals were described using the median and inter-decile range (IDR) to minimize the influence of outliers, while still representing most of the patients. Analyses were performed both for the entire study population and for each of the subgroups with cT1 tumors and cT2–T4 tumors, respectively. Furthermore, all patients, and patients in subgroups cT1 and cT2–4 were divided into two groups according to the time from the date of referral to the date of TURBT (time to TURBT). These groups were chosen as follows: 1–25th percentiles (0–20 days) and 26–100th percentiles (>20 days). This span of time was also close to the time limit indicated in the recommendations for SCP (13 days).
Differences between groups were compared using chi-squared (χ2) test or the Mann-Whitney U test as appropriate. OS was visualized by Kaplan-Meier curves for the group cT2–4. This analysis was also stratified by the time from referral to TURBT (0–20 days vs >20 days) to evaluate effects of possible changes in patient selection after SCP. For survival analyses, observation time started at TURBT and ended at death, or was censored when lost to follow-up, emigration or at the end of the observation time on 10 November 2020, whichever occurred first. p-values < 0.05 were considered statistically significant. Statistical analyses were performed using SPSS version 27 (IBM Corp., Armonk, NY).
Results
In all, 26,795 patients were diagnosed with bladder cancer, of whom 20,020 (75%) were male. The median age was 73 years (IDR = 59–86). Median time to TURBT decreased after SCP from 37 to 27 days (p < 0.001) and the proportion of patients who had a TURBT within 20 days increased from 21% to 30% (p < 0.001). There was a substantial variation in proportion of TURBT within 20 days between the different health care regions (range 16–28% before and 24–47% after SCP). Time to TURBT was decreasing already before the introduction of SCP with a median of 39 days in 2010–2012 compared to 36 days in 2013–2015 (p < 0.001) ().
Table 1. Descriptive parameters of all patients with bladder cancer stratified into four periods in relation to the implementation of the standardized care pathway.
The proportion of cTaG1–2 did not change over time (45%). There was a slight decrease in cT2–4 tumors after SCP, from 22% to 21% (p = 0.01). However, this change was not stable over time, with 20% cT2–4 tumors in 2016–2017 and 22% again in 2018–2019. The proportions of cN + and cM1 remained unchanged at 3% and 3%, respectively.
cT1 tumors
Subgroup analysis in patients diagnosed with cT1 tumors (n = 6,242) showed a shorter median time to TURBT (27 vs 37 days, p < 0.001) after SCP (). There was an increased proportion of patients managed according to guidelines, including patients discussed at MDTC increasing from 40% to 62% (p < 0.001) and patients that received both SLR and IVIT increasing from 31% to 39% (p < 0.001). However, similar trends of improved management were observed prior to the introduction of SCP, with proportions discussed at MDTC increasing from 31% to 48% and use of SLR and IVIT increasing from 29% to 33% in 2010–2012 and 2013–2015, respectively. While the proportion of cystectomies was similar before and after SCP, median time from TURBT to cystectomy decreased slightly from 96 to 90 days (p < 0.001).
Table 2. Descriptive parameters of patients with cT1 tumors stratified into two periods in relation to the implementation of the standardized care pathway.
In the subgroup with time to TURBT of 0–20 days, patients were more often younger than 73 years after compared to before SCP (51% vs 46%, p < 0.001), less frequently had cN + disease (0.7% vs 2.0%, p < 0.001), and received treatment with SLR and IVIT more often (41% vs 30%, p < 0.001). No such differences were seen in the group with time to TURBT >20 days.
cT2–4 tumors
In the subgroup of patients with cT2–4 tumors (n = 5,872), the median time to TURBT decreased from 33 to 27 days (p < 0.001) after the introduction of SCP (). There was no change in the proportion of cN + or cM1. The proportion of patients discussed at MDTC increased from 63% to 82% (p < 0.001), but there were no differences in curative treatment (48% vs 49%, p = 0.250) or the use of NAC (14% in both time periods, p = 0.250). Time from TURBT to cystectomy preceded by NAC decreased after SCP (123 vs 132 days, p = 0.039), while time to cystectomy without NAC showed no difference.
Table 3. Descriptive parameters of patients with cT2–4 tumors stratified into two periods in relation to the implementation of the standardized care pathway.
In the group with time to TURBT of 0–20 days, patients had cN + disease less frequently after compared to before SCP (16% vs 25%, p < 0.001), received treatment with radical cystectomy more often (43% vs 35%, p < 0.001) and received NAC more often (20% vs 14%, p = 0.005). In the group with time to TURBT >20, no corresponding differences were seen.
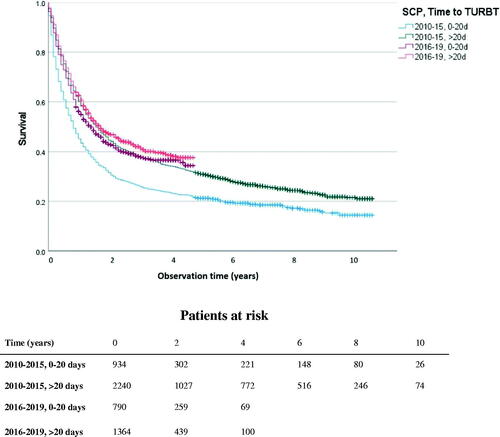
In the entire cT2–4 subgroup, Kaplan-Meier analysis showed that OS improved after the introduction of SCP (Log rank χ2 = 13.35, p < 0.001). Median OS was 16 months before SCP and 18 months after SCP. However, this improvement started before SCP, with 15 months and 17 months median OS in the periods 2010–2012 and 2013–2015, respectively. In cT2–4 patients with time to TURBT (0–20 days), Kaplan-Meier analysis showed that OS was also improved after SCP (Log rank χ2 = 35.55, p < 0.001), with 17 months median OS compared to 10 months before SCP (). An improved OS was also seen in patients with time to TURBT >20 days (Log rank χ2 = 4.02, p = 0.045), with 21 compared to 19 months median OS.
Figure 1. Overall survival curves for patients with stage cT2-4 and with information about time from referral to TURBT (n = 5,332). Patients were stratified into four groups in relation to the time to transurethral resection of bladder tumor (0–20 and >20 days), and before and after the implementation of the standardized care pathway (2010–2015 and 2016–2019). Difference between the groups 0–20 days before and after SCP (Log rank χ2 = 35.55, p < 0.001). Difference between the groups >20 days before and after SCP (Log rank χ2 = 4.02, p = 0.045).
Discussion
In this study, we report a decreased time from referral to TURBT after SCP and an increased proportion of patients discussed at MDTC during the first 4 years after the implementation of SCP for patients with macroscopic hematuria. However, these improvements were not at the levels recommended by the guidelines. No stable change in stage distribution was noted.
However, in both the cT1 and the cT2–4 subgroups of patients with a shorter time to TURBT, there was a higher proportion with clinically node positive disease before SCP. This seems to indicate a selection of patients with advanced tumors to earlier TURBT before SCP, in line with the results of Wallace et al. [Citation6]. On the other hand, patients with a shorter time to TURBT after SCP were younger and received treatment according to guidelines to a higher extent. This might indicate a selection of patients being candidates for radical treatment after SCP. Thus, in patients with cT2–4 tumors and shorter time to TURBT, the improved OS after implementation of SCP should be interpreted with caution. The results after SCP might be affected by shorter observation times, and the selection of a group of patients with a higher treatment intensity and lower age. Whether these differences after the implementation of SCP are due to selection bias, changed referral routines, more patients discussed at MDTC or a combination of factors is impossible to disentangle.
The median time from referral to TURBT in the present study decreased by 10 days with the introduction of SCP. This decrease by itself might be important for specific groups of patients having earlier treatment resulting in better outcome or more patient satisfaction by faster care. The median time from first symptom to TURBT has been reported in the literature as between 70 and 110 days [Citation6,Citation16]. Unfortunately, the SNRUBC does not contain data on time from first symptom and, therefore, we were limited to study the time from referral to TURBT. However, the SCP recommendations of immediate referral for eligible patients might have reduced the total time from first symptom to TURBT considerably.
Nilbert et al. [Citation12] compared 262 bladder cancer patients subjected to SCP with 163 patients not included in SCP for macroscopic hematuria in the Southern Healthcare Region of Sweden during the first 11 months after the implementation of SCP. The authors reported a reduction of time from referral to TURBT from 45 days to 39 days, but without any decreased time to cystectomy [Citation12]. Similarly, Blick et al. [Citation11] reported that, after the implementation of a 2-week waiting rule from referral to the first urologist consultation, a reduction of the time from 42 to 22 days was noted. However, no decrease in time to definitive treatment was observed. In line with these findings, our results after SCP of shorter time to TURBT but not to further treatment, indicates that improving management of patients with alarm symptoms is a complex multi-task procedure [Citation6,Citation10–12].
MDTC might be an important tool to optimize management of patients after diagnosis of urinary bladder cancer. A causality between increased use of MDTC after SCP and increased treatment intensity is likely in the present study, as treatment more in line with guidelines when applying MDTC has previously been reported for patients with cT2–4 bladder cancer but not in cT1 tumors [Citation11,Citation17,Citation18]. The poor adherence to guidelines regarding SLR and IVIT also suggest that time limits should not be the sole focus when implementing an SCP.
In the present study, the reduction of time to TURBT and the increased proportion of patients discussed at MDTC below the recommended levels indicate a non-adherence to guidelines. Such non-adherence to follow SCP schedules and guideline recommendations has previously been reported in 20–30% of patients in some SCP programs, leading to a discussion about economic punishment for non-adherence to SCP [Citation19,Citation20]. Our results should incite a discussion of possible measures to increase the adherence to SCP recommendations in Sweden.
In contrast to the Swedish SCP, many algorithms worldwide also include asymptomatic microscopic hematuria. The Swedish policy to disregard asymptomatic microscopic hematuria was based on a low (1.85%) diagnostic yield [Citation21–23]. Thus, the present SCP only investigates patients with macroscopic hematuria. Patients younger than 50 years of age are evaluated outside of the SCP and patients with asymptomatic microscopic hematuria are not evaluated at all. Such a strategy entails a risk of delayed diagnosis outside SCP and may contribute to decreased public awareness [Citation24,Citation25]. Furthermore, in the case of exceptional events such as the COVID-19 pandemic, an additional diagnostic delay for those individuals not fulfilling SCP-criteria can be expected [Citation25].
The main strength of our study is that it is a nationwide population-based investigation of a registration period over 10 years with a considerable number of patients, making subgroup analyses meaningful. The main limitations are incomplete data, especially during the first period, and the lack of pertinent data, e.g., time from symptoms to referral and cancer-specific survival. Another limitation is that the study design comparing two different time periods implies that changes over time in demographic and clinical variables might be due to other factors than the implementation of SCP. Furthermore, due to the observational and retrospective nature of the present study, causality cannot be proven.
In conclusion, the implementation of an SCP led to a shorter median time to TURBT and an increase in the proportion of patients discussed at MDTC. However, these improvements were not at the levels recommended by the guidelines, indicating insufficient adherence to follow the SCP recommendations. These observations and the lack of stable tumor stage improvement after SCP point to the urgent need for measures to increase adherence to SCP recommendations. Further SCP improvements should focus not just on time limits but also on adherence to management guidelines.
Ethical approval
The study was approved by the Swedish Ethical Review Authority (File No. 2020-02397) and was performed in accordance with the Declaration of Helsinki.
| Abbreviations | ||
| CI | = | Confidence interval |
| CTU | = | Computed tomography–urography |
| IDR | = | Inter-decile range (10–90%) |
| IVIT | = | Intravesical instillation therapy |
| LMP | = | Low malignant potential |
| MDTC | = | Multidisciplinary team conference |
| NAC | = | Neoadjuvant chemotherapy |
| OS | = | Overall survival |
| RT | = | Radiation therapy |
| SCP | = | Standardized care pathway |
| SLR | = | Second-look resection |
| SNRUBC | = | Swedish National Register of Urinary Bladder Cancer |
| TNM | = | Tumor-node-metastasis |
| TURBT | = | Transurethral resection of bladder tumor |
| WHO | = | World Health Organization |
Acknowledgments
The authors thank all monitors at the regional cancer centers and the staff members responsible for registration at the Departments of Urology in Sweden.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Babjuk MB, Compérat E. EAU guidelines on non muscle invasive bladder cancer. Eur Urol. 2020:8–14.
- Jahnson S, Hosseini Aliabad A, Holmang S, et al. Swedish National Registry of Urinary Bladder Cancer: no difference in relative survival over time despite more aggressive treatment. Scand J Urol. 2016;50(1):14–20.
- Svenska nationella kvalitetsregistret för Urinblåse- och urinvägscancer (SNRUBC). 2021. Available from: https://statistik.incanet.se/Urinblasecancer/.
- Shapley M, Mansell G, Jordan JL, et al. Positive predictive values of >/=5% in primary care for cancer: systematic review. Br J Gen Pract. 2010;60(578):e366–e377.
- Hansen RP, Vedsted P, Sokolowski I, et al. Time intervals from first symptom to treatment of cancer: a cohort study of 2,212 newly diagnosed cancer patients. BMC Health Serv Res. 2011;11:284.
- Wallace DMA, Bryan RT, Dunn JA, et al. Delay and survival in bladder cancer. BJU Int. 2002;89(9):868–878.
- Dobruch J, Daneshmand S, Fisch M, et al. Gender and bladder cancer: a collaborative review of etiology, biology, and outcomes. Eur Urol. 2016;69(2):300–310.
- Bourgade V, Drouin SJ, Yates DR, et al. Impact of the length of time between diagnosis and surgical removal of urologic neoplasms on survival. World J Urol. 2014;32(2):475–479.
- Gore JL, Lai J, Setodji CM, et al. Mortality increases when radical cystectomy is delayed more than 12 weeks: results from a surveillance, epidemiology, and end results-medicare analysis. Cancer. 2009;115(5):988–996.
- Russell B, Liedberg F, Khan MS, et al. A systematic review and meta-analysis of delay in radical cystectomy and the effect on survival in bladder cancer patients. Eur Urol Oncol. 2020;3(2):239–249.
- Blick C, Bailey D, Haldar N, et al. The impact of the two-week wait rule on the diagnosis and management of bladder cancer in a single UK institution. Ann R Coll Surg Engl. 2010;92(1):46–50.
- Nilbert M, Blackberg M, Ceberg J, et al. Diagnostic pathway efficacy for urinary tract cancer: population-based outcome of standardized evaluation for macroscopic haematuria. Scand J Urol. 2018;52(4):237–243.
- Malmström PU, Gårdmark T, Sherif A, et al. Incidence, survival and mortality trends of bladder cancer in Sweden 1997–2016. Scand J Urol. 2019;53(4):193–199.
- Brierley J, Gospodarowicz M, Wittekind C. TNM classification of malignant tumours. 8th ed. Hoboken (NJ): JJohn Wiley and sons; 2017.
- Busch C, Algaba F. The WHO/ISUP 1998 and WHO 1999 systems for malignancy grading of bladder cancer. Scientific foundation and translation to one another and previous systems. Virchows Arch. 2002;441(2):105–108.
- McCombie SP, Bangash HK, Kuan M, et al. Delays in the diagnosis and initial treatment of bladder cancer in Western Australia. BJU Int. 2017;120:28–34.
- Pillay B, Wootten AC, Crowe H, et al. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: a systematic review of the literature. Cancer Treat Rev. 2016;42:56–72.
- Harshman LC, Tripathi A, Kaag M, et al. Contemporary patterns of multidisciplinary care in patients with muscle-invasive bladder cancer. Clin Genitourin Cancer. 2018;16(3):213–218.
- Zhou Y, Walter FM, Singh H, et al. Prolonged diagnostic intervals as marker of missed diagnostic opportunities in bladder and kidney cancer patients with alarm features: a longitudinal linked data study. Cancers. 2021;13(1):156.
- Chawla A, Westrich K, Dai A, et al. US care pathways: continued focus on oncology and outstanding challenges. Am J Manag Care. 2019;25(6):280–287.
- Waisbrod S, Natsos A, Wettstein MS, et al. Assessment of diagnostic yield of cystoscopy and computed tomographic urography for urinary tract cancers in patients evaluated for microhematuria: a systematic review and meta-analysis. JAMA Netw Open. 2021;4(5):e218409.
- Linder BJ, Bass EJ, Mostafid H, et al. Guideline of guidelines: asymptomatic microscopic haematuria. BJU Int. 2018;121(2):176–183.
- Malmstrom PU, Skaaheim Haug E, Bostrom PJ, et al. Progress towards a Nordic standard for the investigation of hematuria: 2019. Scand J Urol. 2019;53(1):1–6.
- Hughes-Hallett A, Browne D, Mensah E, et al. Assessing the impact of mass media public health campaigns. Be clear on cancer ‘blood in pee’: a case in point. BJU Int. 2016;117(4):570–575.
- Koo MM, Swann R, McPhail S, et al. Presenting symptoms of cancer and stage at diagnosis: evidence from a cross-sectional, population-based study. Lancet Oncol. 2020;21(1):73–79.
