Abstract
Objective
Differences in outcome after radical prostatectomy for prostate cancer can partly be explained by intersurgeon differences, where degree of experience is one important aspect. This study aims to define the learning curve of robot-assisted laparoscopic prostatectomy (RALP) regarding oncological and functional outcomes.
Materials and methods
Out of 4003 enrolled patients in the LAPPRO trial, 3583 met the inclusion criteria, of whom 885 were operated on by an open technique. In total, 2672 patients with clinically localized prostate cancer from seven Swedish centres were operated on by RALP and followed for 8 years (LAPPRO trial). Oncological outcomes were pathology-reported surgical margins and biochemical recurrence at 8 years. Functional outcomes included patient-reported urinary incontinence and erectile dysfunction at 3, 12 and 24 months. Experience was surgeon-reported experience before and during the study. The relationship between surgeon experience and functional outcomes and surgical margin status was analysed by mixed-effects logistic regression. Biochemical recurrence was analysed by Cox regression, with robust standard errors.
Results
The learning curve for positive surgical margins was relatively flat, with rates of 21% for surgeons who had performed 0–74 cases and 24% for surgeons with > 300 cases. Biochemical recurrence at 4 years was 11% (0–74 cases) and 13% (> 300 cases). Incontinence was stable over the learning curve, but erectile function improved at 2 years, from 38% (0–74 cases) to 53% (> 300 cases).
Conclusions
Analysis of the learning curve for surgeons performing RALP showed that erectile function improved with increasing number of procedures, which was not the case for oncological outcomes.
Introduction
The learning curve in surgery refers to how the increasing experience of a surgeon in performing a specific operation affects outcomes. Prostate cancer surgery is a balance between the oncological and functional outcomes, where the primary aim is cancer cure. As disease recurrence may occur more than 10 years after surgery, short-term outcome measures associated with the definitive oncological outcome, such as surgical margins, biochemical recurrence and time to metastases, are used as surrogate markers. A poor concordance between positive surgical margin and biochemical recurrence rate has been reported [Citation1], and biochemical recurrence may be a more reliable surrogate variable for prostate cancer-specific mortality.
The surgeon has to take into account tumour severity and local extension in planning and performing the operation. The decision whether to preserve the neurovascular bundles or not is a balance between oncological control and avoiding side effects, such as urinary incontinence and impotence [Citation2]. Studies have reported that the overall risk for incontinence and erectile dysfunction after surgery is relatively high, even when performing surgery on small tumours [Citation3], and that such functional impairments seriously affect the quality of life [Citation4]. The oncological and functional outcomes after surgery are affected by a number of different factors, such as tumour characteristics and surgical technique, but also by surgeon heterogeneity [Citation5].
A cohort of 9076 patients treated by open radical prostatectomy between 1987 and 2003 at Memorial Sloan–Kettering Cancer Center, Cleveland Clinic and Wayne State University, USA, has been analysed for various aspects of surgical experience and reported in several publications [Citation1,Citation6–9]. The overall conclusions from these studies were summarized [Citation10] as follows: the risk of recurrence and positive surgical margin was reduced by increased experience of the surgeon [Citation1,Citation6]. However, the mechanisms of learning for the two different outcomes appeared to differ [Citation9]. The effect of learning was independent of preoperative risk or pathological T stage [Citation7,Citation8], and outcomes remained heterogeneous across surgeons after accounting for experience [Citation9].
The LAPPRO trial involves several surgeons, all of whom have performed several surgeries over time. This hierarchical study design enables a decomposition of the observed variability of outcome into three different sources: between patient, between surgeon and within surgeon, where the last one can partly be explained by increasing experience, i.e. a learning curve. Previous analyses in the LAPPRO trial have shown that heterogeneity among surgeons was substantial regarding both functional and oncological outcomes, and was, to some extent, explained by experience [Citation5]. The aim of this article is to investigate the effect of increasing surgeon experience on oncological and functional outcomes after robot-assisted laparoscopic prostatectomy (RALP).
Materials and methods
The basis for this study was the LAPPRO study, a prospective, non-randomized multicentre trial comparing RALP with retropubic open prostatectomy [Citation11]. Patients were included at 14 urological centres in Sweden from September 2008 to December 2011; RALP was performed in seven centres [Citation11,Citation12]. In this analysis, patients had to meet the following inclusion criteria: age < 75 years, clinical tumour stage ≤ T3, prostate-specific antigen (PSA) < 20 ng/ml, no signs of distant metastasis and operated on by a robot-assisted laparoscopic technique [Citation12–14]. The LAPPRO study was approved by the regional ethical review board, Gothenburg (number 277-07) and registered in the Current Controlled Trials database (ISRCTN06393679).
Surgeon experience
Surgeon experience was defined as the surgeon-reported experience prior to the study and the total number of surgeries within the study; that is, the surgeon’s total number of operations also included those performed before LAPPRO. The perioperative clinical report form documented the number of surgeon-reported procedures performed before the day of each surgical procedure according to the following intervals: ‘0–50’, ‘51–100’, ‘101–150’ and ‘above 150’. This information was recorded each time the surgeon performed an operation within the trial, as was the date of the procedure. Surgeons indicating ‘above 150’ at the first procedure within the trial were later contacted and asked for the exact number of performed procedures at the time of the first procedure registered in the trial. An integer-valued counting variable of experience was derived using a set of three rules:
Experience can only increase (e.g. a surgeon first reporting 100–150 and then 50–100 will have the lower value recorded).
A maximum of 50 procedures can be reported in each interval. If > 50 procedures are reported in the same interval, the recoding will involve simple counting (one by one) to result in consistent numbering of the procedures. Surgeons reporting all the surgeries within a single interval were defined as starting at the lowest values of that interval; for example, for the interval ‘51–100’, counting starts at 51.
The variable counting the number of past surgeries, hence starting at the estimated number of operations performed before LAPPRO, thereafter includes those performed within LAPPRO.
Clinical data
Clinical data were collected before surgery, perioperatively, and at 6–12 weeks and 12 and 24 months after surgery using clinical report forms, collecting information on PSA, clinical and pathological T stage, Gleason score on biopsy and surgical specimen, prostate weight, length of cancer in biopsy, presence of a positive surgical margin and body mass index (BMI). Information on residual and recurrent disease was documented by information on PSA, any adjuvant or salvage treatment (androgen deprivation therapy, antiandrogens, radiation, chemotherapy) and diagnosis of metastases.
Patient-reported outcomes
At baseline and at 3, 12 and 24 months and 8 years postoperatively, patient-reported outcomes were collected through questionnaires mailed to the patients and returned to a third party [Citation11]. The questionnaires included questions on education, marital status, comorbidity, urinary and erectile function, and quality of life. Six years postoperatively, information on recurrent disease was collected through structured telephone interviews with the patients [Citation15]. The construction of the questionnaires has been described in detail in previous publications [Citation5,Citation12–14].
Outcomes
Oncological outcomes were surgical margin status, as described in the pathology report of the prostatectomy specimen, and biochemical recurrence within 8 years, defined as either residual or recurrent disease. See for definitions. Biochemical recurrence was derived as time to any event, with follow-up times of 3, 12 and 24 months and 6 and 8 years. The exact date of the events (PSA increase or initiation of adjuvant/salvage treatment) or censoring (dropout or no recurrence at 8 years) are interval-censored between the current and previous follow-up dates. For this reason, the event time was set to the midpoint between the two consecutive follow-up dates. For example, a PSA increase reported at 12 months but not at 3 month follow-up will be assumed to have occurred at 7.5 months. Differences between this approach and an interval-censored approach were found to be negligible [Citation16]. Functional outcomes were urinary incontinence and erectile dysfunction at 3, 12 and 24 months after surgery. The different follow-up occasions (6–12 weeks, 3, 12 and 24 months, and 6 and 8 years) where the different outcomes were assessed are presented in . The prespecified statistical analysis plan stated all four outcomes as equally important.
Table 1. Definitions of study outcomes.
Statistical analysis
For surgical margin status, urinary incontinence and erectile dysfunction, the effect of surgeon experience was quantified using a hierarchical logistic regression model, with experience measured by number of past surgeries (before and within the study) included as a natural cubic spline with two knots, and surgeon as a random intercept. Each follow-up was analysed separately. For recurrence, a Cox proportional hazards model was used. Surgeon was included as a cluster and standard errors were estimated using robust variance estimation. Including a random effect/cluster for surgeon ensures that the dependency structure in the data is accounted for, such that the uncertainty in the data is not underestimated.
Variables considered to be confounding were included in the regression models for adjustment. For all outcomes, adjustment was made for clinical T stage, preoperative PSA, biopsy Gleason score and length of cancer. For surgical margin status and biochemical recurrence, prostate weight was also adjusted for. For urinary incontinence, age at surgery, prostate weight, BMI and diabetes were adjusted for. For erectile dysfunction, the models also adjusted for preoperative erectile dysfunction, age at surgery, diabetes and cardiovascular disease. Nerve-sparing technique is considered a mediator on the causal path between experience and outcome and is therefore not adjusted for. However, to assess the direct effect of the learning curve on the outcome adjusted for nerve-sparing approach, a sensitivity analysis was performed in which nerve sparing (yes, no) was added to the set of adjustment variables (Supplementary analysis 1). All continuous variables, including surgical experience, were standardized prior to analyses. Patients with preoperative incontinence were excluded from the analysis of incontinence. The extent to which the relationship between experience and outcome is modified by the intensity at which the surgeries are performed, i.e. annual volume or case load, was addressed by an additional analysis (Supplementary analysis 2).
The bias that missing values may induce was investigated to be reduced by 10-time multiple imputations using predictive mean matching [Citation17] and subsequently pooled using Rubin’s rule [Citation18]. The results were presented graphically as predicted (fixed effect) curves and associated confidence intervals of the chance of favourable outcome conditional on fixed levels of the adjustment variables. The levels used were median and most frequent values in the cohort values for continuous and categorical variables, respectively. For biochemical recurrence, the cumulative incidence (1 − survival) at 4 years was presented. Both adjusted and unadjusted curves were presented, as well as raw proportions, for experience, categorized as 1–74, 75–149, 150–299 and ≥ 300, respectively. The extent to which experience could explain the total variability (both between- and within-surgeon) in outcome was assessed by pseudo R-square. The models were compared with reduced models without experience included by a likelihood ratio test. For the Cox model with robust variance, a Wald test was used. This is different from the procedure used by Nyberg et al. [Citation5], where the part of between-surgeon heterogeneity accounted for by experience was quantified. R software was used for the analyses, with the packages lme4 [Citation19] and survival [Citation20] for parameter estimation, mice [Citation21] for multiple imputations and ggeffects [Citation22] for the calculation of predicted mean curves, using the functions ggpredict and pool_predictions.
Results
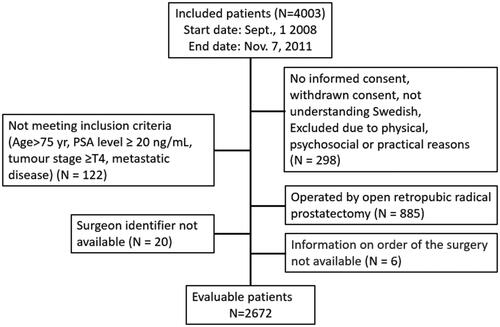
Twenty-five surgeons at seven urological centres in Sweden performed RALP on 2672 patients between 2008 and 2011 (). Before and within LAPPRO, the median number of operations performed by a surgeon was 21 (minimum–maximum range 0–228) and 185 (range 8–1026), respectively. Of all 25 surgeons, 21, nine, three and three had performed at least a total of 200, 500, 750 and 1000 surgeries, respectively, as depicted in . The median annual volume was 40 procedures (range 5–84).
Figure 2. Left panel: percentage of patients with a negative surgical margin (no cancer cells in the surgical margin of specimens) as found in the pathology report. Right panel: predicted cumulative chance of freedom from biochemical recurrence 4 years after surgery. The unadjusted curve (blue dashed line) represents estimates from regression with no covariates included. The adjusted curve (red solid line) represents estimates from regression with covariates included. The grey area is the 95% confidence interval (CI) for adjusted estimates. Black dots depict raw proportions with 95% CI. The number of surgeons performing at least 1, 50, 200, 500 and 750 surgeries is given at the bottom of the left panel.
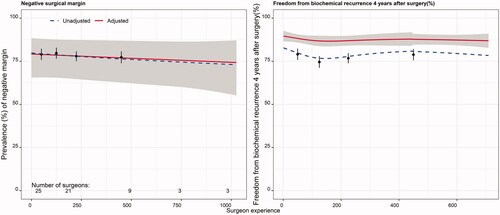
Figure 3. Percentage of patients reporting urinary continence (defined as change of sanitary pad less than once per 24 h) at 3, 12 and 24 months postoperatively. The unadjusted curve (blue dashed line) represents estimates from regression with no covariates included. The adjusted curve (red solid line) represents estimates from regression with covariates included. The grey area is the 95% confidence interval (CI) for adjusted estimates. Black dots depict raw proportions with 95% CI. The number of surgeons performing at least 1, 50, 200, 500 and 750 surgeries is given at the bottom of the left panel.
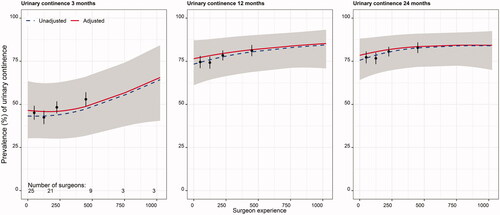
Figure 4. Percentage of patients reporting erectile function (defined as having a stiff enough penis for penetration after sexual stimulation at least 50% of times) at 3, 12 and 24 months postoperatively. The unadjusted curve (blue dashed line) represents estimates from regression with no covariates included. The adjusted curve (red solid line) represents estimates from regression with covariates included. The grey area is the 95% confidence interval (CI) for adjusted estimates. Black dots depict raw proportions with 95% CI. The number of surgeons performing at least 1, 50, 200, 500 and 750 surgeries is given at the bottom of the left panel.
Patient characteristics were, in most aspects, not different between surgeons in the early part of training and experienced surgeons, but a larger proportion of patients operated on by an experienced surgeon had a higher level of education. Preoperative incontinence was reported by 26 patients ().
Table 2. Patient, tumour and surgeon characteristics.
For the oncological outcome measure positive surgical margin, the learning curve was relatively flat, with an adjusted risk among the inexperienced surgeons (< 75 operations) of 21% compared to 24% for the most experienced (> 300 operations). Biochemical recurrence was stable across degrees of experience, with an adjusted risk of 11% for the inexperienced surgeons and 13% for the most experienced surgeons (). The models had limited ability to explain variability, as demonstrated by the low values of R2 (6% and 11% for surgical margin and biochemical recurrence, respectively), and adding surgeon’s experience made no improvement (p = 0.504 and 0.570) (). Adjusted and unadjusted predictions and raw proportions are presented in the Supplement (Table S2).
The incidence of urinary incontinence at 3 months was lower if the operation was performed by the most experienced surgeons, at 44% (> 300 cases), compared with 54% among inexperienced surgeons (< 75 cases) (). At 24 months, the adjusted risk for incontinence was 16% and 21%, respectively, for surgeons with experience above 300 cases compared with those with experience of less than 75 cases. The models had limited ability to explain variability (5–6%) ().
Erectile dysfunction occurred less in those operated on by experienced surgeons at all three time-points, with an adjusted risk of 64% (> 300 operations) versus 80% (< 75 operations) at 3 months, to 47% (> 300 operations) versus 62% (< 75 operations) at 24 months’ follow-up (). The models had good ability to explain variability (30–38%) and experience made a significant improvement at 24 months (p = 0.022) ().
In the supplementary analyses, adjusting for nerve-sparing surgery did not have any major impact on the predicted learning curve. Furthermore, variation in annual volume did not give rise to any pronounced modification of the learning curve.
Discussion
The analyses of the effect of surgeon experience in this prospectively followed, large, multicentre cohort of patients undergoing RALP showed that there was a learning curve regarding the functional outcomes, meaning that outcomes improved with the increasing number of operations performed. For the oncological outcomes, we did not observe such a positive development. The learning curve for urinary continence was relatively short until a plateau was reached, but for erectile function the upward slope was constant over the entire follow-up period. For urinary continence, the effect of inexperience was attenuated after a longer period of follow-up, meaning that the negative effect of being operated on by an inexperienced surgeon was rather short lived. The intersurgeon variability is manifested in the width of the confidence intervals of the predicted learning curves. The relative contribution of the various sources to this variability was quantified by Nyberg et al. [Citation5] for the outcomes at 24 month follow-up. It was found that differences in experience explained 42%, 11% and 19% of the between-surgeon variability in incontinence, erectile dysfunction and recurrence, respectively. This is different from our approach, where we explore the impact of experience on the total (both within- and between-surgeon) variability.
In a 2019 report from a single centre, a risk reduction with increasing experience was found for surgical margins but not for biochemical recurrence [Citation23]. A single-centre, single-surgeon study reported that the risk of positive surgical margins decreased with increasing experience in RALP, but the surgeon had extensive experience of open radical prostatectomy before turning to a robotic technique [Citation24]. Thus, the external validity of those results is low, although a large cohort was studied. In a study on learning curves for open radical prostatectomy, a poor concordance between surgical margin status and recurrence rates was found [Citation1]. The authors suggested that the improvement in the two different oncological outcomes was driven by different mechanisms. Since the outcome regarding surgical margin status can be evaluated right after the operation, there is an opportunity for immediate feedback, which is beneficial for learning. Biochemical recurrence may occur many years later, which gives less opportunity for feedback. It seems reasonable that there could be differences in the learning curve between open and robotic radical prostatectomy. A comparison of the learning curves between open and robot-assisted techniques should preferably have been made in our study, as presented by others [Citation24], but this was not possible as all surgeons practising an open technique within LAPPRO were already experienced at the start of inclusion. At that time (2008–2011), almost no surgeons were in training on the open prostatectomy technique.
Our results of functional outcomes are in line with an earlier report of the learning curve for one surgeon turning from an open to a robotic technique [Citation24]. The learning curve for preservation of erectile function was continuous, without a clear plateau. One contributory factor may be that increasing experience adds not only dexterity but also insight into the planning of a procedure, where many factors should be taken into account. Even though the primary aim of radical prostatectomy is cancer cure, analyses of functional outcomes are of importance in view of their negative impact on the patient’s quality of life [Citation4].
We observed that patients with a university education were to a larger extent operated on by experienced surgeons. One explanation could be related to demography; patients with higher educational level live in urban areas near tertiary academic hospitals, where experienced surgeons work. Others have reported worse outcomes of treatment for colorectal cancer for socioeconomically deprived patients in both the Netherlands and England [Citation25,Citation26].
The strengths of this study include the size of the cohort, the prospectively collected data and the multicentre design. Patients from three tertiary referral (university) hospitals were included along with those from four ‘county’ hospitals, giving high external validity. Since functional outcomes were reported by the patients to a third party, an interviewer effect was avoided [Citation27,Citation28]. Limitations include that these analyses were secondary endpoints in the trial, and thus the study sample size was not optimized for the current objective. Since the distribution of the surgeons’ total experience was skewed, with the majority having limited experience, all surgeons will contribute to the characterization of the beginning of the learning curve (the left-hand part) but few will contribute to the right-hand part, meaning that this part is determined with greater uncertainty.
Our results suggest that the primary goal of prostate cancer surgery, cancer cure, is relatively unaffected by the surgeon’s experience, but that the quality of life-affecting side effects after surgery decreased with experience.
Author contributions
David Bock had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Bock, Nyberg, Lantz, S. V. Carlsson, Sjoberg, S. Carlsson, Stranne, Steineck, Wiklund, Haglind and Bjartell. Acquisition of data: Nyberg, Lantz, S. V. Carlsson, Sjoberg, S. Carlsson, Stranne, Steineck, Wiklund, Haglind and Bjartell. Analysis and interpretation of data: Bock, Nyberg, Lantz, S. V. Carlsson, Sjoberg, S. Carlsson, Stranne, Steineck, Wiklund, Haglind and Bjartell. Drafting of the manuscript: Bock and Haglind. Critical revision of the manuscript for important intellectual content: Bock, Nyberg, Lantz, S. V. Carlsson, Sjoberg, S. Carlsson, Stranne, Steineck, Wiklund, Haglind and Bjartell. Statistical analysis: Bock. Obtaining funding: Haglind, Lantz, S. V. Carlsson and Sjoberg. Administrative, technical or material support: none. Supervision: S. Carlsson, Stranne, Steineck, Wiklund, Haglind and Bjartell. Other: none.
Supplemental Material
Download Zip (1,005.6 KB)Acknowledgements
The authors want to thank all study participants in LAPPRO. The personnel at the Scandinavian Surgical Outcomes Groups, Department of Surgery, Institute of Clinical Sciences, Sahlgrenska Academy at Gothenburg University are acknowledged for their excellent work interacting with participants, and personnel at the participating urology sites for their work contribution.
Disclosure statement
No potential conflict of interest was reported by the authors. David Bock certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (e.g. employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties or patents filed, received, or pending), are the following: none.
Additional information
Funding
References
- Vickers A, Bianco F, Cronin A, et al. The learning curve for surgical margins after open radical prostatectomy: implications for margin status as an oncological end point. J Urol. 2010;183(4):1360–1365.
- Steineck G, Bjartell A, Hugosson J, et al. Degree of preservation of the neurovascular bundles during radical prostatectomy and urinary continence 1 year after surgery. Eur Urol. 2015;67(3):559–568.
- Carlsson S, Berglund A, Sjoberg D, et al. Effects of surgeon variability on oncologic and functional outcomes in a population-based setting. BMC Urol. 2014;14(1):25.
- Wallerstedt A, Nyberg T, Carlsson S, et al. Quality of life after open radical prostatectomy compared with robot-assisted radical prostatectomy. Eur Urol Focus. 2019;5(3):389–398.
- Nyberg M, Sjoberg DD, Carlsson SV, et al. Surgeon heterogeneity significantly affects functional and oncological outcomes after radical prostatectomy in the Swedish LAPPRO trial. BJU Int. 2021;127(3):361–368.
- Vickers AJ, Bianco FJ, Serio AM, et al. The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Cancer Inst. 2007;199(15):1171–1177.
- Klein EA, Bianco FJ, Serio AM, et al. Surgeon experience is strongly associated with biochemical recurrence after radical prostatectomy for all preoperative risk categories. J Urol. 2008;179(6):2212–2216.
- Vickers AJ, Bianco FJ, Gonen M, et al. Effects of pathologic stage on the learning curve for radical prostatectomy: evidence that recurrence in organ-confined cancer is largely related to inadequate surgical technique. Eur Urol. 2008;53(5):960–966.
- Bianco FJ, Jr., Vickers AJ, Cronin AM, et al. Variations among experienced surgeons in cancer control after open radical prostatectomy. J Urol. 2010;183(3):977–982.
- Vickers AJ, Cronin AM. Data and programming code from the studies on the learning curve for radical prostatectomy. BMC Res Notes. 2010;3:234.
- Thorsteinsdottir T, Stranne J, Carlsson S, et al. LAPPRO: a prospective multicentre comparative study of robot-assisted laparoscopic and retropubic radical prostatectomy for prostate cancer. Scand J Urol Nephrol. 2011;45(2):102–112.
- Haglind E, Carlsson S, Stranne J, et al. Urinary incontinence and erectile dysfunction after robotic versus open radical prostatectomy: a prospective, controlled, nonrandomised trial. Eur Urol. 2015;68(2):216–225.
- Lantz A, Bock D, Akre O, et al. Functional and oncological outcomes after open versus robot-assisted laparoscopic radical prostatectomy for localised prostate cancer: 8-year follow-up. Eur Urol. 2021;80(5):650–660.
- Nyberg M, Hugosson J, Wiklund P, LAPPRO group, et al. Functional and oncologic outcomes between open and robotic radical prostatectomy at 24-month follow-up in the Swedish LAPPRO trial. Eur Urol Oncol. 2018;1(5):353–360.
- Nyberg M, Akre O, Bock D, et al. Risk of recurrent disease 6 years after open or robotic-assisted radical prostatectomy in the prospective controlled trial LAPPRO. Eur Urol Open Sci. 2020;20:54–61.
- Axen E, Godtman RA, Bjartell A, et al. Degree of preservation of neurovascular bundles in radical prostatectomy and recurrence of prostate cancer. Eur Urol Open Sci. 2021;30:25–33.
- Little RJA. Missing-data adjustments in large surveys. J Business Econ Statist. 1988;6(3):287–296.
- Marshall A, Altman DG, Holder RL, et al. Combining estimates of interest in prognostic modelling studies after multiple imputation: current practice and guidelines. BMC Med Res Methodol. 2009;9:57.
- Bates D, Mächler M, Bolker B, et al. Fitting linear mixed-effects models using lme4. J Stat Soft. 2015;67(1):1–48.
- Therneau TM, Grambsch PM. Modeling survival data: extending the Cox model. New York: Springer; 2000.
- van Buuren S, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Soft. 2011;45(3):1–67.
- Lüdecke D. ggeffects: tidy data frames of marginal effects from regression models. JOSS. 2018;3(26):772.
- Bravi CA, Tin A, Vertosick E, et al. The impact of experience on the risk of surgical margins and biochemical recurrence after robot-assisted radical prostatectomy: a learning curve study. J Urol. 2019;202(1):108–113.
- Thompson JE, Egger S, Bohm M, et al. Superior quality of life and improved surgical margins are achievable with robotic radical prostatectomy after a long learning curve: a prospective single-surgeon study of 1552 consecutive cases. Eur Urol. 2014;65(3):521–531.
- van den Berg I, Buettner S, van den Braak R, et al. Low socioeconomic status is associated with worse outcomes after curative surgery for colorectal cancer: results from a large, multicenter study. J Gastrointest Surg. 2020;24(11):2628–2636.
- Moller H, Sandin F, Robinson D, et al. Colorectal cancer survival in socioeconomic groups in England: variation is mainly in the short term after diagnosis. Eur J Cancer. 2012;48(1):46–53.
- Mansson A, Henningsohn L, Steineck G, et al. Neutral third party versus treating institution for evaluating quality of life after radical cystectomy. Eur Urol. 2004;46(2):195–199.
- Litwin MS, Lubeck DP, Henning JM, et al. Differences in urologist and patient assessments of health related quality of life in men with prostate cancer: results of the CaPSURE database. J Urol. 1998;159(6):1988–1992.