Abstract
Objective
Inflammation of the urinary bladder may cause burdensome pain also called bladder pain syndrome (BPS). A limitation in understanding BPS pathophysiology is the lack of appropriate preclinical model. Previously published clinical and preclinical studies revealed positive impact of Cernitin™ on pain relief in chronic prostatitis. The objective of this study was to evaluate the effects of Cernitin™ on induced inflammation of the urinary bladder in rats. We also sought to identify biomarkers which might play a role in the management of BPS.
Materials and methods
Cystitis was induced by injection of cyclophosphamide (CYP) in female rats. Thereafter, animals were randomly divided into four treatment groups and two control groups. Evaluation of pain scores was assessed by von Frey assay. Expression of pain- and pro-inflammatory biomarkers was determined by enzyme-linked immunosorbent assay (ELISA) and immunohistochemistry.
Results
Treatments with Cernitin™ displayed significant anti-nociceptive effects on CYP-induced visceral pain (p < .01). In contrast, vehicle-treated animals showed high pain score even at the lowest force. Furthermore, results of ELISA showed that Cernitin™-treated animals had significantly reduced levels of COX-2 (T60, p < .01; GBX, p < .05) in bladder tissue homogenate. Immunohistochemical (IHC) staining of bladder tissues showed that Cernitin™-treated animals exhibited less CD45-positive cells, while massive CD45-positive cells infiltration was detected in vehicle-treated animals. IHC also revealed lower SP and PGD2 expression levels in Cernitin™-treated tissues.
Conclusions
Cernitin™ components reduced pain score and inflammatory marker COX-2. Our findings suggest a potential therapeutic role for Cernitin™ in the management of BPS.
Keywords:
Introduction
Bladder pain syndrome (BPS) is defined as persistent or recurrent pain or discomfort perceived in the urinary bladder region and represent a heterogenous spectrum of disorders impacting on the bladder (https://uroweb.org/guideline/chronic-pelvic-pain/ 2022). Earlier, the term Interstitial cystitis (IC) denoted an inflammatory disease of the bladder wall requiring histopathological examination for proper diagnosis. Current consensus suggest that the classical ulcerative type (previously Hunner cystitis) is denoted IC, while the non-ulcerative form is part of BPS, often denoted in the literature as BPS/IC. BPS dominates in females (10:1) [Citation1] and affects millions of women worldwide.
The treatment approach to BPS is complex and target chronic inflammation of the bladder, overactivity of the pelvic floor muscles and the peripheral as well as the central nervous systems. As pain or discomfort of the pelvic region are the cardinal symptoms, medication that alleviate the perception of this disorder would be beneficial.
The lack of experimental models that manifest clinical features of human BPS hampered research in this area until recently. In this study, we have taken the advantage of the new and newly developed CYP-induced cystitis model in female rats [Citation2] and examined for the first time the impact of CernitinTM components on treatment of the induced inflammation. CernitinTM pollen extract constitutes water‐soluble fraction CernitinTM T60 (hereafter referred to as T60) and fat‐soluble fraction of CernitinTM GBX (hereafter referred to as GBX). The extracts have been used in fixed combination for treatment of benign prostatic diseases for decades with beneficial outcome and minimum adverse effects [Citation3,Citation4]. However, the effect of CernitinTM in the female lower urinary tract symptom, especially non-infectious BPS/IC, has not been investigated. The rationale for using CernitinTM treatment for BPS is based on the pharmacological properties of CernitinTM and the results reported with regard to inhibition of benign prostatic hyperplasia (BPH) progression, anti-inflammatory effect and pain relief [Citation5–9]. Importantly, there is a noticeable clinical similarity between non-bacterial chronic prostatitis NIH-type IIIB and BPS in terms of persistent or recurrent pelvic pain, frequency, urgency and nocturia experienced [Citation10]. Furthermore, both conditions are known to be poorly defined in terms of pathophysiology and have limited therapeutic options. With this background, we found it rational to examine whether CernitinTM would reduce the inflammation and alleviate nociceptive properties in CYP-induced BPS female rats.
Cyclophosphamide-induced BPS in rodents is a well-characterized model [Citation11–13]. Systemic CYP is metabolized in the liver but eliminated primarily through the kidneys. CYP’s major uro-toxic metabolite is acrolein, which causes mucosal inflammation as indicated by microscopic changes in the bladder and the presence of inflammatory cell infiltrations as well as visceral pain [Citation2,Citation14]. This experimental model makes CYP-induced BPS the optimal choice to elucidate mechanisms, identify specific biomarkers related to this chronic condition and subsequently finding effective therapeutic options. A chronic condition, inducing inflammation and tissue damage, can change the properties of sensory pathways leading to a reduction in pain threshold (allodynia) and an amplification of painful sensations (hyperalgesia) [Citation2]. Furthermore, chronic CYP-induced BPS may involve alterations in neurotrophic factors, chemokines [Citation12,Citation15] and cytokines [Citation16]. The presence of pro-inflammatory cytokines can induce cyclooxygenase-2 (COX-2) enzyme, an inflammatory early response gene [Citation17] and generate prostaglandins, a substance that plays a role in the inflammation process. In this study, we hypothesize that CYP-induced BPS upregulates COX-2 [Citation18] and prostanoids [Citation19] in the urinary bladder, which contributes to altered urodynamic function. Cyclooxygenase-2 has also been reported to have a nociceptive (analgesic) effect in both the central and peripheral nervous systems. They show the improvement in bladder function with administration of a specific COX-2 inhibitor [Citation20] suggesting the pivotal role of COX-2 and prostanoids in BPS.
In the current study, we validated nociceptive score by von Frey testing. In addition, we determined the inflammation markers COX-2 and MCP-1 level in bladder tissue homogenates and prostaglandin (PGD2) and pain marker substance P (SP) in the bladder tissue by immunohistochemistry. Infiltration of inflammatory cells was examined by CD45-positive cells in bladder tissue sections. The study was intended to provide insight into human bladder disorders such as inflammatory diseases and to identify biomarkers that could be used as prognostic disease markers and predictors of a therapeutic response.
Materials and methods
Experiments were performed on 60 adult female Sprague Dawley (SD) rats, 8 weeks old (with initial body weights 235 ± 35 g. Rats were purchased from Janvier Labs (Saint Berthevin, France). Procedure for cyclophosphamide- (CYP) (Sigma-Aldrich, Saint-Quentin Fallavier, France) induced bladder inflammation and manual Von Frey test were carried out at Urosphere (Toulouse, France). All experiments were performed according to the European Community Council Directive 2010/63/UE and the French Ministry for Agriculture, Agrifood and Forestry (Decree 2013-118). Experimental protocols were reviewed by CEEA-122 Ethical Committee for Protection of Animals used for Scientific Purposes and approved by French Ministry for National Education, Higher Education and Research under the number APAFIS #16506-2018082411278474.
Induction of cystitis and drug administration
Bladder inflammation was induced in rats as previously described [Citation2,Citation21,Citation22]. Briefly, animals were injected with CYP intraperitoneally (i.p.) at a dose of 40 mg/kg in three doses; every third day (on days 0, 3 and 6) of experiment. Animals were randomly divided into six groups as follows: group 1 and 2 were given the vehicles of the components (H2O and DMSO/corn oil, respectively), group 3 were given CernitinTM component T60, group 4 the CernitinTM component GBX, group 5 the combination of the components T60 and GBX and group 6 gabapentin as control (Sigma-Aldrich) prepared in saline (centravet, Lapalisse, France). CernitinTM T60 was prepared in H2O. CernitinTM GBX was prepared in 2% DMSO (Sigma-Aldrich) and 98% corn oil. Test substances, gabapentin or vehicles were administered orally (p.o.) in a final volume of 4 ml/kg, once at days; 7, 8, 9 and 10 (1 h prior von Frey test) for a total of four administrations. Dose concentration and distribution of substances are depicted in .
Table 1. Experimental design.
von Frey test
In order to decrease stress due to the new environment, rats were allowed to acclimatize to the environment for 30 min prior to von Frey testing. At day 0, von Frey testing was performed before CYP administration in order to obtain basal values. von Frey filaments (Bioseb, Vitrolles, France) were then applied to the lower abdomen, close to the urinary bladder as previously described [Citation2,Citation21]. Each filament was tested through the mesh floor with enough strength to cause the filament to slightly bend for 1–2 s 3 times with an interval of 5 s between each application. The scoring of nociceptive response was as follows: 0 = no response, 1 = reaction of the abdomen, 2 = trampling or change of position and 3 = reaction of the animal, change of position, licking of the area stimulated and/or vocalization. Nociceptive scores were expressed as the percentage of the maximal score for the three pooled applications. The experimental design is summarized in .
Figure 1. Experimental designs used in the study. Vehicles 1 and 2, gabapentin and test substances as indicated in . was administered on day (D) 7, D8, D9 and D10. Von Frey test was first performed at day 7 (before pharmacological treatment) to confirm presence of visceral pain and then at day 10 to analyze compounds effect on CYP-induced chronic bladder pain.
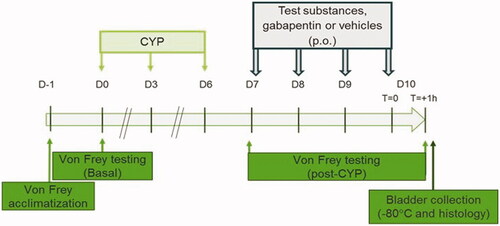
Just after the final von Frey test on day 10, animals were sacrificed and the bladders were collected and weighed. Each bladder was divided in half, one half was fixed in 4% formalin and embedded in paraffin for immunohistochemistry assay and the other half was stored at −80 °C until further use. Thereafter, five randomly selected bladder tissues from each group to further analyses with ELISA and Immunohistochemistry.
Measurement of tissue inflammatory cytokine levels
The expression levels of COX-2 and MCP-1 in bladder tissue were measured with enzyme-linked immunosorbent assay (ELISA) kits (MyBioSource, Inc, San Diego, CA). The procedures were performed according to the manufacturer’s instructions. Briefly, rat bladder tissues were homogenized in tissue protein extraction reagent with Complete protease inhibitor tablets (Roche, Indianapolis, IN) using a Polytron homogenizer. After low-speed centrifugation to remove debris, aliquots of the homogenates were removed and protein concentrations were measured using BCA Protein Assay Kit (Pierce, UK) and stored at 80 °C until use.
To measure COX-2 and MCP-1, bladder homogenates were diluted in a 1:5 ratio. Standards and samples containing plates were read using a wavelength of 450 nm on a microplate reader SpectraMax i3 microplate reader (Molecular Devices LLC, Sunnyvale, CA, USA). The results of MCP-1 and COX-2 were expressed as pg/ml. The standard curve was constructed by plotting the mean absorbance obtained from each standard against its concentration. Thus, the corresponding concentration for each sample was determined from the standard curve.
Histopathology and immunohistochemistry
Formalin-fixed, paraffin embedded bladder tissue sections (4 µm) were stained with hematoxylin and eosin (HE) in order to examine and document bladder inflammation. The sections were evaluated by one of us (ND) while blind to the experimental treatment. The histopathologic changes include edema, inflammation (interstitial leukocytes, lymphocyte infiltration) or hemorrhages. The bladder tissues were microscopically examined and scored as previously described [Citation21]: (0) no evidence of inflammatory cell infiltrates or interstitial edema; (1) mild, few inflammatory cell infiltrates and little interstitial edema; (2) moderate amount of inflammatory cell infiltrates and moderate interstitial edema and (3) severe, extensive presence of large amount of inflammatory cell infiltrates and severe interstitial edema.
Immunohistochemistry was performed according to a previously published study [Citation5]. Briefly, sections were deparaffinized, subjected to antigen retrieval (citrate buffer, pH 6) and then exposed to using primary antibodies: CD45 (PA5-96061) and substance P (SP) (PA5-106934) were from Thermo Scientific (Waltham, MA) and PGD2 (MAB10099) from (R&D Systems, Inc., MN, USA). Thereafter, immunoreaction was detected by di-aminobenzidine (DAB). The staining intensity was determined semi-qualitatively by color intensity as follows; strongly stained as score 3, moderately stained as score 2, low stained as score 1 and no staining as score 0.
Statistical analysis
For nociceptive scores, a two-way analysis of variance (ANOVA) with repeated measures was used to determine whether there was a significant difference among groups. SEM was applied in the statistics to compare the means among three or more groups. Before carrying out any statistical test, the data were tested for normal distribution (Shapiro-Wilk normality test) and their variance evaluated (F test or Bartlett’s test for two or more groups, respectively). The appropriate statistical test was consequently applied. For ELISA results we have used a Student’s t-test to compare means between two groups and data were expressed as mean ± SD. The accepted level of significance was p < .05.
Results
Effects of Cernitin™ on pain behavior
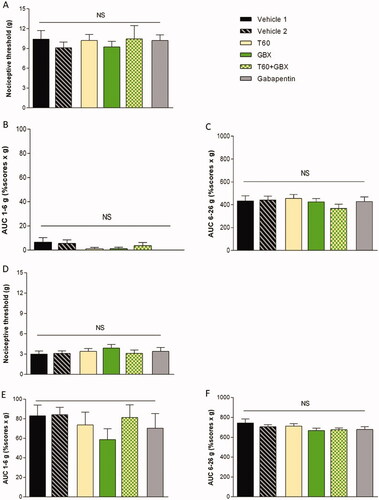
The three repeated CYP injections induced bladder inflammation and severe painful behavior in rats measured by von Frey testing. CYP-induced bladder pain was characterized by a painful response to normally innocuous 1–6 g von Frey forces (allodynia) and by an increased response to a noxious 6–26 g von Frey forces (hyperalgesia) (). Von Frey test was performed before CYP induction (basal) and then after induction but prior to pharmacological treatment (D7) (). No significant difference was observed among groups before treatment (D7) indicating that pain response occurred similarly in all groups. Time course pharmacological treatments revealed that nociceptive response was significantly reduced in Cernitin™ components-treated groups for all von Frey forces compared to vehicle-treated animals (). No difference was observed between the two components T60 and GBX treatment groups on the nociceptive and threshold score when exposed to von Frey filaments (low force, p < .05) and (high force, p < .01). A statistical significance level (p < .05) was obtained in the combination group () although no additive enhancement in nociceptive threshold observed. When compared to Cernitin™, animals exhibit a more significant response to gabapentin treatment (p < .001) ().
Figure 2. Nociceptive scores measured with von Frey filaments. (A–C) Repeated injection of CYP produced bladder pain at 1–6 g and 6–26 g von Frey forces performed before chronic cystitis induction (basal). (D–F) von Frey test was evaluated after induction but prior pharmacological treatment (day 7). No significant difference was observed among groups before treatment indicating that inflammation occurred similarly in all groups. (J–G) Nociceptive response was significantly reduced in Cernitin™-treated groups for all von Frey forces compared to vehicle-treated animals. Most significant reduction in gabapentin-treated animals. *p < .05 considered significant. Data are presented as mean ± S.E.M. (n = 9–10), determined by statistical method ANOVA.
COX-2 expression in bladder with CYP-induced inflammation
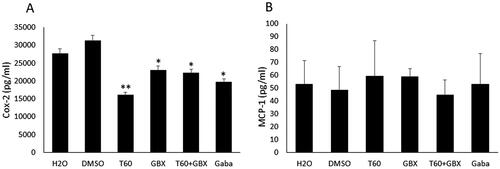
To determine the role of COX-2 after induction of chronic (10 day) CYP-induced BPS, we examined the level of COX-2 protein expression in bladder tissue homogenates. Results revealed that treatment with Cernitin™ components significantly decreased COX-2 levels compared to vehicle-treated animals (). Most significant reduction was observed in the animals treated with 22.5 mg/kg T60 (p < .01), whereas no difference was found between GBX (1.2375 mg/kg) alone and GBX combined with T60, both treatments showing similar effect on COX-2 expression (p < .05). Gabapentin treatment (100 mg/kg) showed significant decrease in COX-2 level (p < .05) (). In contrast to COX-2, the levels of MCP-1 protein seemed to be unaffected by the treatment with T60 or GBX alone, but a slight decrease was observed in the animals treated with combined T60 and GBX ().
Figure 3. Mean expression level of COX-2 and MCP-1 in the bladder tissue homogenates. (A) a significant decrease in COX-2 levels was observed in the Cernitin™T60 (**p < .01) and Cernitin™ GBX (*p < .05) groups compared to vehicle-treated groups, combination treatment did not enhance the effect of Cernitin™ (*p < .05). (B) MCP-1 levels remained unchanged. Gabapentin (Gaba)-treated group produced a significant reduction of COX-2 level (*p < .05). Data are presented as mean ± SD (n = 5).
Histology and immunostaining in bladder tissues and effect of treatment with CernitinTM
Examination of the bladder tissue from five rats of each group showed signs of inflammation in vehicle-treated tissue samples (). Microscopic analysis of the bladder tissues revealed that vehicle 1-treated rats showed cystitis characterized by inflammation with edema in five of five rats (5/5) and in vehicle 2 (4/5), both presented score 2. Hemorrhage was only found in rat treated with vehicle 1 (1/5). Following treatment with Cernitin™, the inflammatory changes were reversed in most of bladder tissues. Very mild edema and inflammation (score 1) observed in T60 (1/5), GBX (2/5), combination of both components (2/5) and in gabapentin edema (3/5) and inflammation (1/5).
Table 2. Hematoxylin–eosin staining analyses of tissue sections for presence of bladder inflammation and edema performed in five animals from each treatment group.
We thereafter examined the tissues for the expression of CD45, PGD2 and SP. Immunohistochemistry analyses demonstrated that CD45-positive cells were present abundantly within the lamina propria and submucosal regions and in the perivascular areas of the vehicle-treated bladder tissues (, arrows). Infiltrates of few CD45-positive cells were also found in the lamina propria of Cernitin™ treatment groups, whereas number of CD45-positive cells in gabapentin-treated group was higher than Cernitin™-treated groups but less than vehicle-treated groups (, arrows).
Figure 4. Immunohistochemical analysis of the bladder tissues. Effects of Cernitin™ on the expression of (A) CD45, (B) PGD2 and (C) SP. T + G = T60 + GBX. Immunostaining was performed in the five animals mentioned in . intensity was determined by color intensity as follows; strongly stained as score 3, moderately stained as score 2 and weakly stained as score 1, no staining as score 0.
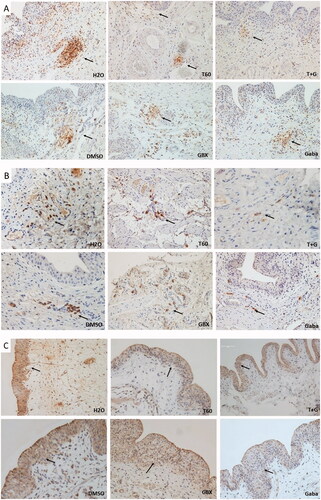
Moreover, a strong PGD2 immunostaining was observed in the number of cells grouped and located in the lamina propria layer in vehicle-treated rats (, arrows). Following Cernitin™ treatment, there were PGD2 moderately stained-positive cells distributed in the muscular and adventitia layers of the bladder (, arrows). Conversely, gabapentin-treated bladder tissue demonstrated weakly stained and few PDG2 positive in the lamina propria. Moreover, immunostaining analyses of SP demonstrated weak to moderate expression in the urothelial cells of the treatment groups and in nerve cells within the submucosal and lamina propria regions (, arrows). Especially weak staining was observed in gabapentin-treated tissues. Expression of SP in the vehicle-treated bladder tissues showed moderate to strong staining ().
Discussion
Challenges to set proper diagnosis and select effective treatment of inflammation of the bladder, stem from a poorly defined pathophysiology and a paucity of well-designed experimental animal model. In this study we used previously developed animal models of cystitis that mimics features of human non-ulcerative BPS/IC [Citation2]. The current study revealed that intra peritoneal injection of cyclophosphamide (CYP) in female rats causes pain plausibly comparable to BPS. Treatment with Cernitin™ components reduced pain score. Pain relief was associated with a significant decrease in COX-2 level in bladder tissue homogenate. Results also showed a decrease in CD45 expressing cell infiltration and edema and reduced number of cells expressing PGD2 following treatment with Cernitin™. Our findings suggest that Cernitin™ components may exploit the COX-2/prostaglandin pathway to alleviate bladder pain.
We recently reported that Cernitin™ had been shown to significantly attenuate pain score caused by the carrageenan-induced chronic prostatitis in rats [Citation5]. Moreover, Cernitin™ treatment markedly reduced the levels of pain and inflammatory associated with COX-2 and MCP-1 in prostatic tissues. Therefore, we further sought to identify an objective biomarker(s) associated with the inflammation-induced pain. It is noteworthy to mention that in the previous study we treated animals with induced chronic prostatitis with CernitinTM extracts in a fixed combination only. Since this is the first time we examine the effects of CernitinTM on the female rat model for inflammation in the bladder wall, we concluded that it would be logical to use each component alone as well as a combination of both to better characterize the impact of the drug.
Various animal models have been employed in respect of an inflammatory-mediated cystitis [Citation23]. A range of chemicals, including cyclophosphamide and lipopolysaccharide [Citation24] that induce both acute and longer lasting bladder hyperactivity was deployed. Whilst there are many limitations to the use of inflammatory animal models, in the study of symptom defined disorders such as over active bladder and BPS/IC these animals show enhanced visceral response plus the allodynia and hyperalgesia observed in humans [Citation2,Citation25] suggesting suitability of the models. The histological findings from the current study was in accordance with previous studies [Citation11,Citation14] of animals demonstrating the damage caused by CYP to the urinary bladder. The damages were manifested in edema in the lamina propria, focal urothelial disruption and infiltration of CD45-positive cells. Therefore, repeated CYP treatment represents a noxious, chemical irritation of the bladder mucosa.
Pain is an unpleasant sensory and emotional experience and in humans pain studies are difficult to perform, are subjective and are limited by ethical considerations, leading to the wide spread use of animals as models to study pain [Citation24]. The most commonly used species are mice and rats [Citation12,Citation25]. In line with the mentioned study by Augé et al. [Citation2], von Frey testing showed that chronic injection of CYP produced visceral pain which was characterized by a painful response to normally innocuous and increased response to the noxious ones. Cernitin™ components T60 and GBX each alone had significantly reduced nociceptive score but to lesser extent than gabapentin. Similarly to the results obtained from chronic prostatitis, the combined treatment reduced nociceptive score significantly but did not further enhance the effect of Cernitin™. Gabapentin effects was more pronounced in reducing pain score than on the inflammatory marker, COX-2. Clinically, an anticonvulsant gabapentin confirmed to be effective for treating chronic neuropathic pain [Citation26] was used as a control to validate pain score. Interestingly, CernitinTM treatment showed more efficiently reduced COX-2 levels compared to gabapentin.
The visceral pain coincided with the bladder inflammation as examined for the inducible enzyme, cyclooxygenase-2 (COX-2). Cyclooxygenase-2 and its metabolites, prostaglandins, contribute to changes in lower urinary tract function following chronic CYP-induced BPS [Citation21]. In accordance with our reported study on the chronic prostatitis, results revealed that water-soluble T60 inhibited COX-2 relatively strongly compared to GBX (). It has previously been shown that treatment with a selective COX-2 inhibitor,5,5-dimethyl-3-(3-fluorophenyl)-4-(4-methylsulfonyl)phenyl-2(5H)-furanone(DFU), relieved the symptoms of CYP-induced BPS in rats [Citation18]. These data indicate that the mechanism of anti-inflammatory action of the CernitinTM involves the inhibitory activity of COX-2. In contrast low expression levels of MCP-1 detected in the bladder tissue homogenate and these levels remained unchanged by CernitinTM treatment. MCP-1 is a member of the chemokine superfamily that plays a critical role in the recruitment and activation of monocytes during inflammation [Citation27]. Our result was in contrast to the studies by others who reported the increased MCP-1 level in cystitis models [Citation28,Citation29]. The discrepancy in the results is mainly due to the different animal model or chemicals, such as lipopolysaccharide, used to induce BPS. It could also be explained by a cytotoxic effect of CYP on monocytes resulting in low levels of MCP-1, as the treatment did not exhibit any change in the level. In chronic prostatitis and current BPS preclinical models, Cernitin™ has shown to induce its anti-inflammatory effects through prostaglandin/COX-2 pathway, although other pathways cannot be excluded. Another aspect of reducing symptoms of BPS is the likely effect of Cernitin™ on other tissues. Notably, Kiyokazu et al. reported that Cernitin™ components inhibited contraction of mice urethral smooth muscles induced by noradrenaline [Citation30]. This shows another pharmacological effect that can be considered as a therapeutic mechanism of Cernitin™.
Neurogenic inflammation contributes to bladder afferent hypersensitivity and stromal fibrotic and edematous changes in BPS without ulcerous lesions, suggesting the potential involvement of neurogenic inflammation in its pathophysiology. Bladder afferent fibers contain a variety of neuropeptides, including substance P (SP) [Citation31,Citation32]. We have demonstrated that expression is increased after cyclophosphamide (CYP)-induced cystitis in rats. In line with these studies, we observed SP expression in the urothelial cells and some nerve cells in the subepithelial connective tissues. Cernitin™ treatments showed a moderate downregulation of SP levels, especially T60 compared to gabapentin treatment which showed pronounced reduction.
The limitation of this study is the challenges relating to the applicable quantification of behavioral responses that could be considered equivalent to pain in humans. Despite these limitations, we were able to show that animal model of pain has provided a potential tool in clinical scenario. Nevertheless, the advantages and disadvantages of each model and behavioral test should be taken into account to obtain objective and meaningful results that will improve our understanding and management of pain.
Conclusion
the results represent a step toward better understanding lower urinary tract symptoms and the potential development of more targeted therapies. Furthermore, the current study provides evidence suggesting that Cernitin™ may represent a beneficial therapeutic approach for conditions associated with bladder pain. However, more studies are required to be able to translate the effect of Cernitin™ presented in this pre-clinical model to clinical scenario.
Author contributions
ND, LR and PL designed the experiments and supervised the study. CA performed the in vivo experiments. ND performed microscopy, immunohistochemistry and histopathological analysis. ND and LR wrote the manuscript incorporating all of the authors' editorial input. MG and PL provided clinical view to the manuscript.
Acknowledgements
We thank the expert technical assistance at Urosphere, Toulouse, France for performing the pain assessment experiment. We thank ImaGene-iT AB, Medicon Village, Lund, Sweden for performing immunohistochemistry.
Disclosure statement
The authors have the following conflicts of interest to disclose. Dr. Dizeyi declares financial support from AB Cernelle for carrying out, in a specialized laboratory, the series of tests presented in the present document. Author Mrs. Ramnemark is employed by AB Cernelle; Associate Professor Grabe are advisors to the project. Dr. Augé and Dr. Lluell received financial support from Cernelle to perform animal model.
Additional information
Funding
References
- Lamvu G, Carrillo J, Ouyang C, et al. Chronic pelvic pain in women: a review. J Am Med Assoc. 2021;325(23):2381–2391.
- Augé C, Gamé X, Vergnolle N, et al. Characterization and validation of a chronic model of cyclophosphamide-Induced interstitial cystitis/bladder pain syndrome in rats. Front Pharmacol. 2020;11(August):1305–1311.
- Togo Y, Ichioka D, Miyazaki J, et al. Oral administration of cernitin pollen extract (Cernilton®) for 30 days might be useful to avoid unnecessary biopsy in prostate biopsy candidates: a preliminary study. Int J Urol. 2018;25(5):479–485.
- Wagenlehner FME, Bschleipfer T, Pilatz A, et al. Pollen extract for chronic prostatitis-chronic pelvic pain syndrome. Urol Clin North Am. 2011;38(3):285–292.
- Chabot S, Dizeyi N, Ramnemark L, et al. Impact of CernitinTM on induced chronic prostatitis in animal model for understanding management of lower urinary tract symptoms. Phytomedicine Plus. 2021;1(4):100057.
- Iwamura H, Koie T, Soma O, et al. Eviprostat has an identical effect compared to pollen extract (Cernilton) in patients with chronic prostatitis/chronic pelvic pain syndrome: a randomized, prospective study. BMC Urol. 2015;15(1):5–10.
- Lu CF, Meng XH, Li HB, et al. Effect of phellodendron chinense extract on carrageenan-induced chronic prostatitis in rats. Trop J Pharm Res. 2015;14(2):257–262.
- Talpur N, Echard B, Bagchi D, et al. Comparison of Saw Palmetto (extract and whole berry) and Cernitin on prostate growth in rats. Mol Cell Biochem. 2003;250(1/2):21–26.
- Wagenlehner FME, Schneider H, Ludwig M, et al. A pollen extract (Cernilton) in patients with inflammatory chronic prostatitis-chronic pelvic pain syndrome: a multicentre, randomised, prospective, double-blind, placebo-controlled phase 3 study. Eur Urol. 2009;56(3):544–551.
- Shoskes DA, Nickel JC, Rackley RR, et al. Clinical phenotyping in chronic prostatitis/chronic pelvic pain syndrome and interstitial cystitis: a management strategy for urologic chronic pelvic pain syndromes. Prostate Cancer Prostatic Dis. 2009;12(2):177–183.
- Ayhanci A, Yaman S, Sahinturk V, et al. Protective effect of seleno-L-methionine on cyclophosphamide-induced urinary bladder toxicity in rats. Biol Trace Elem Res. 2010;134(1):98–108.
- Guo M, Chang P, Hauke E, et al. Expression and function of chemokines CXCL9-11 in micturition pathways in cyclophosphamide (CYP)-induced cystitis and somatic sensitivity in mice. Front Syst Neurosci. 2018;12(April):9–16.
- Tooke K, Girard B, Vizzard MA. Functional effects of blocking VEGF/VEGFR2 signaling in the rat urinary bladder in acute and chronic CYP-induced cystitis. Am J Physiol Renal Physiol. 2019;317(7):F43–F51.
- Ozyuvali E, Yaman T, Kosem B, et al. Protective effect of intravesical platelet-rich plasma on hemorrhagic cystitis. CIM. 2016;39(6):116–121.
- Vera PL, Iczkowski KA, Wang X, et al. Cyclophosphamide-induced cystitis increases bladder CXCR4 expression and CXCR4-macrophage migration inhibitory factor association. PLoS One. 2008;3(12):e3898.
- Malley SE, Vizzard MA. Changes in urinary bladder cytokine mRNA and protein after cyclophosphamide-induced cystitis. Physiol Genomics. 2002;9(1):5–13.
- Mitchell JA, Warner TD. Cyclo-oxygenase-2: pharmacology, physiology, biochemistry and relevance to NSAID therapy. Br J Pharmacol. 1999;128(6):1121–1132.
- Hu VY, Malley S, Dattilio A, et al. COX-2 and prostanoid expression in micturition pathways after cyclophosphamide-induced cystitis in the rat. Am J Physiol Regul Integr Comp Physiol. 2003;284(2):53–52.
- Ricciotti E, Fitzgerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31(5):986–1000.
- Hannan TJ, Roberts PL, Riehl TE, et al. Inhibition of cyclooxygenase-2 prevents chronic and recurrent cystitis. EBioMedicine. 2014;1(1):46–57.
- Augé C, Basso L, Blanpied C, et al. Pain management in a model of interstitial cystitis/bladder pain syndrome by a vaccinal strategy. Front Pain Res. 2021;2(March):1–8.
- Augé C, Chene G, Dubourdeau M, et al. Relevance of the cyclophosphamide-induced cystitis model for pharmacological studies targeting inflammation and pain of the bladder. Eur J Pharmacol. 2013;707(1-3):32–40.
- Birder L, Andersson KE. Animal modelling of interstitial cystitis/bladder pain syndrome. Int Neurourol J. 2018;22(Suppl 1):S3–S9.
- Bjorling DE, Wang ZY, Bushman W. Models of inflammation of the lower urinary tract. Neurourol Urodyn. 2011;30(5):673–682.
- Meotti FC, Forner S, Lima-Garcia JF, et al. Antagonism of the transient receptor potential ankyrin 1 (TRPA1) attenuates hyperalgesia and urinary bladder overactivity in cyclophosphamide-induced haemorrhagic cystitis. Chem Biol Interact. 2013;203(2):440–447.
- Lee JW, Han DY, Jeong HJ. Bladder pain syndrome treated with triple therapy with gabapentin, amitriptyline, and a nonsteroidal anti-inflammatory drug. Int Neurourol J. 2010;14(4):256–260.
- White FA, Feldman P, Miller RJ. Chemokine signaling and the management of neuropathic pain. Mol Interv. 2009;9(4):188–195.
- Lv J, Huang Y, Zhu S, et al. MCP-1-induced histamine release from mast cells is associated with development of interstitial cystitis/bladder pain syndrome in rat models. Mediators Inflamm. 2012;2012:358184.
- Xu S, Wang X, Wang Y, et al. Transgenic mice expressing MCP-1 by the urothelium demonstrate bladder hypersensitivity, pelvic pain and voiding dysfunction: a multidisciplinary approach to the study of chronic pelvic pain research network animal model study. PLoS One. 2016;11(9):e0163829.
- Kiyokazu N, Ikuko K, Masayasu K. Effects of pollen-extract components, diamines and derivatives of feruloylputrescine on isolated bladder and urethral smooth muscles of mice. Jpn J Pharmacol. 1990;53(2):157–164.
- Arms L, Vizzard MA. Neuropeptides in lower urinary tract function. Handb Exp Pharmacol. 2011;202(202):395–423.
- Girard BM, Wolf-Johnston A, Braas KM, et al. PACAP-mediated ATP release from rat urothelium and regulation of PACAP/VIP and receptor mRNA in micturition pathways after cyclophosphamide (CYP)-induced cystitis. J Mol Neurosci. 2008;36(1-3):310–320.