Abstract
Objective
There is now an unprecedented amount of evidence to consider when revising prostate cancer guidelines. We believe that there is a value in publishing summaries of national clinical guidelines in English for others to read and comment on.
Methods
This is part 2 of a summary of the Swedish prostate cancer guidelines that were published in June 2022. This part covers recurrence after local treatment and management of metastatic and castration resistant disease. Part 1 covers early detection, diagnostics, staging, patient support and management of non-metastatic disease.
Results
The 2022 Swedish guidelines include several new recommendations. Among these is a recommendation of a period of observation with repeated PSA tests for patients with approximately 10 years’ life expectancy who experience a BCR more than 2–5 years after radical prostatectomy, to allow for estimating the PSA doubling time before deciding whether to give salvage radiotherapy or not. Recent results from the PEACE-1 trial led to the recommendation of triple-treatment with a GnRH agonist, abiraterone plus prednisolone and 6 cycles of docetaxel for patients with high-volume metastatic disease who are fit for chemotherapy. The Swedish guidelines differ from the European ones by having more restrictive recommendations about genetic testing of and high-dose zoledronic acid or denosumab treatment for men with metastatic prostate cancer, and by recommending considering bicalutamide monotherapy for selected patients with low-volume metastatic disease.
Conclusions
The 2022 Swedish prostate cancer guidelines include several new recommendations and some that differ from the European guidelines.
1. Introduction
A large number of important randomized clinical prostate cancer trials have been published the past few years. There is now an unprecedented amount of evidence for clinical guidelines groups to consider when revising national and international guidelines. Although the same evidence from clinical trials and other studies are available for all guidelines groups, the clinical conclusions drawn from the evidence may differ – not only because the health care systems and the populations differ between countries, but also because the clinical interpretation of crude scientific data is subjective. We believe that there is a value in publishing summaries of national clinical guidelines in English for others to read and comment on.
This is part 2 of a summary of the most recent version of the Swedish prostate cancer guidelines, published in June 2022. Both parts focus on new recommendations, based on recently published pivotal clinical trials, and on recommendations that differ from the European guidelines [Citation1]. This part covers the management of men with recurrent, metastatic and/or castration-resistant disease. Organisational aspects, diagnostics, staging and primary management of non-metastatic disease are covered by part 1 [Citation2]. So is rehabilitation and supportive care, although these are utterly important aspects of care for men with recurrent or metastatic cancer. For instance, an interview study suggests that men who experience biochemical recurrence of prostate cancer are more anxious and concerned about the treatability of their disease than they were at the time of the initial diagnosis [Citation3].
2. Recurrent disease
2.1. Biochemical recurrence after radical prostatectomy
Following radical prostatectomy, 25–50% of the patients will eventually experience biochemical (PSA-only) recurrence. As the biochemical recurrence usually precedes progression to metastatic disease and prostate cancer death by many years, treatment decisions need to be individualized. The treatment decision has traditionally been based on predictive nomograms based on clinical factors such as the pT stage, tumour grade and serum PSA level, but the use of PSMA-PET/CT imaging is becoming more and more common for guiding the decision making. PSMA-PET/CT guided salvage radiotherapy is currently evaluated in a Swedish randomised multi-centre trial (NCT04794777). For patients not included in the trial, the Swedish guidelines recommend considering a PSMA-PET/CT scan for patients with BCR, particularly for those with a high risk of metastatic recurrence, provided that they are candidates for salvage radiotherapy in the absence of metastatic disease.
The national guidelines’ recommendation of offering early salvage radiotherapy to patients with more than 10 years’ life expectancy agree well with the European guidelines. A treatment dose of 66-70 Gy has been the standard in Sweden since the 1990s, but since a recent randomised trial showed as good effect with 64 Gy in 32 fractions for patients with Gleason score ≤ 7 and a serum PSA < 0.5 ng/ml, 64 Gy is now recommended for this group of patients [Citation4]. For patients with approximately 10 years’ life expectancy who experience a biochemical recurrence more than 2–5 years postoperatively, a period of observation with repeated PSA tests is recommended to allow for estimating the PSA doubling time before deciding whether to give salvage radiotherapy or not. For patients with PSA ≥ 0.7 ng/ml, 2 years of adjuvant hormonal bicalutamide treatment is recommended [Citation5], but 6 months of adjuvant GnRH-agonist may also be considered [Citation6].
2.2. Recurrence after primary radiotherapy
The Swedish recommendations on the management of patients with biochemical recurrence after primary radiotherapy also agree well with the European guidelines. Because of the infrequent use of local salvage treatment and its associated risks of complications [Citation7], the management should be discussed at a multidisciplinary team (MDT) meeting. Patients with more than 10 years life-expectancy and a histologically confirmed local recurrence without metastasis on PSMA-PET/CT should be considered for local salvage treatment (cryotherapy, radical prostatectomy or high-dose rate brachytherapy). As few Swedish centres have experience of local salvage treatment after primary radiotherapy, the guidelines recommend they are offered within a prospective study protocol at one national centre per treatment modality only.
Most patients with a biochemical recurrence after primary radiotherapy are recommended either watchful waiting or hormonal treatment. Those with a Gleason score 6-7 cancer and slowly rising PSA values are recommended watchful waiting until the PSA is over 10 ng/ml, whereas those with Gleason score 8–10, higher PSA values or a PSA doubling time < 6 months are usually recommended bicalutamide monotherapy as first-line treatment.
3. Metastatic prostate cancer: primary management
3.1. Primary oligometastatic disease
There is no consensus for the upper limit of the number of metastases defining oligometastatic disease, but most definitions allow up to 3–5 metastases. Using PET/CT for primary staging affects the categorisation of disseminated prostate cancer as oligometastatic or high-volume disease: many patients whose disease would be categorised as oligometastatic on a standard CT and bone scan have wide-spread metastasis on PSMA-PET/CT [Citation8]. All randomised trials that support the treatment guidelines have been based on staging with CT and bone scan, not PET/CT, so there is minimal evidence for how we best manage patients with just over 3-5 metastases on PSMA-PET/CT. This is one reason for the absence of a general recommendation to use PSMA-PET/CT for primary staging in the Swedish guidelines [Citation2].
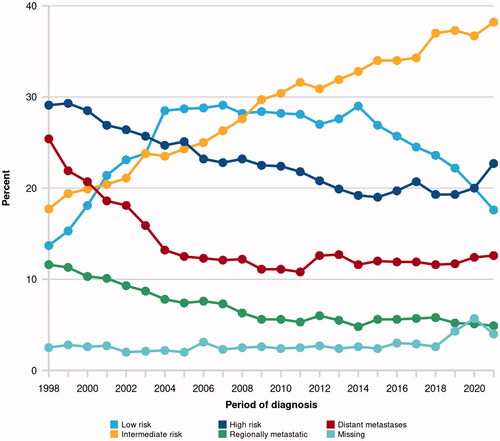
The proportion of Swedish prostate cancer patients with distant metastasis at the time of diagnosis has been stable over the past 15 years (11–13%, ). More than one-fourth of the patients with M1 disease have 1–3 skeletal metastases according to standard imaging, according to the National Prostate Cancer Register of Sweden (unpublished data). On top of the 11–13% with distant metastasis another 5-6% are diagnosed with ‘regionally metastatic disease’, defined as clinical stage T4 and/or N1and/or PSA 50–99 ng/ml in the absence of confirmed distant metastases (M0 or MX).
Figure 1. The proportional distribution of clinical risk groups at the time of prostate cancer diagnosis, as registered in the National Prostate Cancer Register of Sweden (NPCR, website npcr.se). Regionally metastatic disease is defined as clinical stage T4 and/or N1 and/or PSA 50–99 ng/ml in the absence of confirmed distant metastasis (i.e. disease categorised as M0 or MX).
Men with primary oligometastatic prostate cancer in a good general condition with good urinary and bowel functions should be offered local radiotherapy to the primary tumour. Including the regional lymph-nodes in the radiotherapy field is optional in cN1 M0 disease, whereas radiotherapy targeting distant metastases should be offered in prospective trials only. The radiotherapy should be combined with hormonal treatment [Citation2]. In December 2021, the STAMPEDE trial reported substantially longer survival for men with cN1 M0 disease who in addition to 3 years of adjuvant GnRH agonist also received 2 years of adjuvant abiraterone plus prednisolone [Citation9]. This new, intensified adjuvant hormonal treatment is now recommended in the Swedish guidelines for fit men with cN1 M0 disease. A radical prostatectomy with an extended lymph-node dissection is an alternative to radiotherapy for selected men with cN1 M0 disease, for example for those with outflow obstruction, a concomitant gross benign prostatic enlargement or inflammatory bowel disease.
The recommended hormonal treatment of men with oligometastatic prostate cancer have up until 2021 been either bicalutamide monotherapy or a GnRH-agonist. For those with a good performance status, GnRH agonist treatment may be combined with 6 cycles of docetaxel [Citation10]. In 2021 combined hormonal treatment with GnRH agonist plus either apalutamide or enzalutamide became reimbursed and hence also recommended in Sweden, as this combination treatment results in substantially longer progression-free and overall survival [Citation11–13].
Bicalutamide monotherapy for treating locally advanced prostate cancer, with or without regional lymph-node metastasis, has been used more in Sweden and the other Nordic countries than the rest of the world, possibly because of the favourable results of the Scandinavian Prostate Cancer Group 6 trial [Citation14]. The Swedish guidelines may be alone in recommending that bicalutamide monotherapy is considered as an alternative to castration treatment also for asymptomatic men with a few bone metastases who are not fit for modern combination treatment. Other guidelines, including the European ones [Citation1], make a strong recommendation against bicalutamide monotherapy for men with distant metastasis because of inferior results compared with GnRH agonist treatment. The survival difference in the only comparative study was, however, only 6 weeks and the difference was significant only in patients with an initial PSA value over 400 ng/ml [Citation15]. This, in combination with bicalutamide’s more favourable side-effect profile [Citation16], makes us believe that bicalutamide monotherapy may be considered in selected patients with a few distant metastases who are not fit for modern combination treatment.
3.2. Oligometastatic recurrence
The recommendations for hormonal and additional systematic treatment for men with oligometastatic recurrence are similar to those described just above and below. The guidelines stress the importance of including patients with oligometastatic recurrence in prospective trials, but open up for radiotherapy to the lymph-nodes or a pelvic lymph-node dissection for selected patients with regional lymph-nodes only recurrence on PSMA-PET/CT who wish to postpone hormonal treatment.
3.3. High-volume metastatic disease
The guidelines group acknowledge that any cut-off between low- and high-volume metastatic disease is arbitrary and non-biological. Moreover, if metastatic disease is categorised as low-volume based on conventional imaging rather than PSMA-PET/CT, it is probably also inaccurate. Nonetheless, the Swedish guidelines’ recommendations follow the definition of high-volume metastatic disease used in the CHAARTED trial: either ≥4 bone metastases of which at least 1 outside vertebral column or pelvis, or visceral metastasis [Citation17].
The addition of either 6 cycles of docetaxel 75 mg/m2 every third week [Citation10,Citation17], abiraterone plus prednisolone [Citation18], apalutamide [Citation12] or enzalutamide [Citation11,Citation13] to castration therapy for men with previously untreated metastatic prostate cancer prolongs their median overall survival with approximately one year. In Sweden, reimbursement for using abiraterone on this indication is restricted to patients with “high-risk” metastatic disease who are not fit for chemotherapy. “High-risk” metastatic prostate cancer is defined as in the LATITUDE trial: at least 2 of the 3 criteria Gleason score 8–10, ≥ 3 metastases on bone scan and visceral metastases [Citation18]. In 2021, both apalutamide and enzalutamide became approved and reimbursed in Sweden for use in addition to castration therapy in men with metastatic prostate cancer, without any specification of the number or type of metastases. The decision was based on three large randomised trials (TITAN, ENZAMET and ARCHES) that all showed a clear overall survival benefit, as well as clinically relevant effects on secondary outcome measures such as radiographic progression-free survival [Citation11–13]. All these trials included patients with oligometastatic disease and metachronous metastasis. No direct comparison of the individual agents’ effects has been done, but all the trials showed similar survival benefits of the addition of them to castration therapy, compared with castration therapy alone.
The guidelines group conclude that all patients with metastatic prostate cancer and a good performance status should be offered the combination of castration therapy plus either six cycles of docetaxel or continuous treatment with abiraterone/prednisolone, apalutamide or enzalutamide until radiological or clinical progression. Combination treatment should be considered regardless of whether the patient has high- or low-volume, synchronous or metachronous disease. The evidence for combination treatment of men with low-volume or metachronous metastatic disease is, however, stronger for apalutamide and enzalutamide than for the other treatments. The choice between chemotherapy with docetaxel and any of the new androgen receptor targeting agents (ARTAs) need to be thoroughly discussed with the patient because of their different toxicity profiles and requirements for monitoring. Life-expectancy, comorbidities and other medications need also be considered.
Results from the PEACE-1 trial were reported at the European Society of Medical Oncology (ESMO) congress in September and published in spring 2022 [Citation19]. The study shows that patients with high-volume disease who are fit for chemotherapy benefit from triple-treatment with a GnRH agonist, abiraterone plus prednisolone and six cycles of docetaxel, compared with combining a GnRH agonist with docetaxel only. None of these treatments are approved specifically for use in this triple-combination, but they all have a registered indication for hormone-sensitive metastatic prostate cancer and it’s uncertain whether the pharmaceutical companies will apply for a specific indication for triple-treatment. The Swedish guidelines group therefore now recommend this triple-treatment for patients with high-volume metastatic disease, who in PEACE-1 had a survival benefit 19 months. Similar results for a triple- treatment with darolutamide, docetaxel and a GnRH agonist were reported from the ARASENS trial in February 2022 [Citation20]. The draft version of the 2022 Swedish guidelines was released just before ARASENS was reported and does therefore not consider its results in the recommendations.
3.4. Follow-up investigations
Modern treatment of metastatic prostate cancer includes combining the castration therapy with some other systemic treatment. Evaluating the effect of the additional primary systemic treatment is essential for the subsequent management of the patient, both for the timing of the change to the next line of systemic treatment and for and the choice of the type of the next treatment. If there is no imaging between the initial, pre-treatment assessment and when PSA later starts to rise, it is often not possible to know whether there has been any radiological progression over the past few months. The Swedish guidelines therefore recommend a CT scan and a bone scan after 6 months of continuous additional systemic treatment, alternatively a few weeks after completing additional docetaxel treatment. In addition to routine clinical follow-up, a bone mineral density assessment is recommended as described in detail below.
4. Castration-resistant prostate cancer
4.1. General considerations
Castration-resistant disease is defined as three consecutive rises in PSA, measured at least one week apart, and a PSA value over 2 ng/ml or objective local or distant progression or radiologically new metastases in patients with a plasma testosterone value under 1,7 nmol/l. The guidelines strongly recommend that patients entering the castration resistant phase are discussed in a multidisciplinary team conference. If possible, the patient should be offered to participate in a clinical trial.
The treatment decision should always be individualized and made together with the patient himself. Factors that need to be taken into account include the patient’s personal preferences, comorbidities, current medication, previous prostate cancer treatments and the response thereof. Of growing importance are histologic variants, such as neuroendocrine differentiation, and genetic alterations (germline as well as somatic). New effective treatments are now being registered almost on an annual basis. This is of course positive for both the treating doctors and the patients, but the registration and reimbursement processes often take a considerable length of time during which financial issues and ethical conflicts arise.
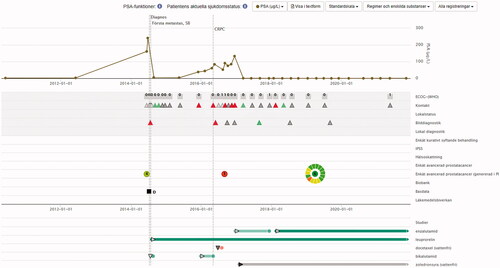
Over the past several years Patient Overview Prostate Cancer has been increasingly used all over Sweden to help patients and their physicians to get an overview of the disease, diagnostic investigations, treatments, treatment responses, and the patient’s symptoms and quality-of-life (). The Patient Overview Prostate Cancer is currently being introduced also for earlier stages of the disease, including diagnostic investigations in men without any confirmed prostate cancer diagnosis [Citation2].
Figure 2. An example of a view from Patient Overview Prostate Cancer. The graphic display gives an immediate apprehension of the progression of the disease, previous diagnostic investigations, previous and ongoing treatments, treatment responses, and the patient’s symptoms and quality-of-life, as registered in an online questionnaire before the appointment. The circle represents the answers to 15 symptom domains. The colour of the circle’s separate sectors is either green (no problems), yellow (moderate problems) or red (severe problems), which allows the physician to direct their attention to what bothers the patient most. The number in the middle of the circle represents overall quality-of-life, rated from 0 to 10.
4.2. Non-metastatic castration-resistant prostate cancer
The increased number of prostate cancer patients undergoing primary treatment with curative intent has led to a growing number of patients entering a castration-resistant disease stage with no detectable metastases on bone and CT scans, i.e. with non-metastatic castration resistant prostate cancer (nmCRPC). Based on the results from three large randomised trials (SPARTAN [Citation21], PROSPER [Citation22], ARAMIS [Citation23]), the Swedish health authorities in 2021 approved of reimbursement for the addition of either apalutamide, darolutamide or enzalutamide to castration therapy in nmCRPC patients with good performance status, a PSA over 2.0 ng/ml and a PSA doubling time under 10 months. The combined treatment should continue until objective metastatic progression. Patients with nmCRPC who do not fulfil these criteria should be closely monitored and offered combination treatment with bicalutamide, despite the low level of evidence for a clinically important effect.
4.3. Metastatic castration resistant prostate cancer
The number of available treatments for of metastatic castration-resistant prostate cancer (mCRPC) is increasing, but the evidence is poor for their optimal sequence in individual patients. As first-line treatment of mCRPC either docetaxel, enzalutamide or abiraterone with prednisolone is are recommended. Patients entering the mCRPC phase have now often been exposed to one or more of these or similar treatments in the disease’s previous hormone sensitive or nmCRPC phase. The disease-specific effects and side-effects of these treatments must be considered when choosing first line mCRPC treatment.
As second-line treatment, the guidelines recommend against the use of sequential treatment with ARTAs (abiraterone, apalutamide, darolutamide and enzalutamide) as there is no evidence of any clinically meaningful effect [Citation24]. Provided that the patient is fit for chemotherapy, the recommended approach is to use docetaxel in case of previous ARTA treatment in the hormone sensitive setting and vice versa. For patients with bone metastatic disease only, who are not suitable for chemotherapy, radium-223 is an alternative. Radium-223 may also be used as third-line treatment. The combination of radium-223 treatment with abiraterone and prednisolone is associated with an increased risk of fractures [Citation25], which led the European Medicines Agency to recommend that radium-223 treatment should always be combined with a bisphosphonate or denosumab plus vitamin D and calcium [Citation26]. Cabazitaxel may also be used as second- or third-line treatment of mCRPC, but only in patients who have previously been treated with docetaxel [Citation27]. The recommended dose for cabazitaxel is 20 mg/m2 because this dose has been shown to be as effective as and less toxic than 25 mg/m2 [Citation28].
A PARP inhibitor, olaparib, has been shown to be an effective second- or third-line treatment for mCRPC patients with BRCA1 or BRCA2 mutated tumours [Citation29]. Olaparib is approved by the European Medicines Agency on this indication and is since May 2022 reimbursed in Sweden for patients with such mutations who have tried and failed all other available standard systematic treatments. This imposes a rising demand for testing for BRCA mutations, both for germline mutations in blood samples and for somatic mutations in tumour tissue. The guidelines group will in autumn 2022 consider how this is best done, including how the patients and their families should be informed when they are offered genetic testing and how they should be notified of the test results [Citation2].
Radionuclide treatment with PSMA-177lutetium has proved effective in heavily pre-treated men with mCRPC [Citation30], but this treatment was not yet approved by the European Medicines Agency when the Swedish 2022 guidelines were finalized.
A central oncological principle is to stop cancer treatment when it is no longer benefitting the patient. It is, however, often difficult to know when this breaking point is reached for an individual patient. With the exception of castration treatment, stopping the ongoing treatment for mCRPC is recommended if two or more of the following criteria are present: deterioration of performance status, increased or new cancer-related symptoms, biochemical progress and radiological progress. The Swedish guidelines stress that treatment should not be stopped because of biochemical progression only (PSA, ALP or other biomarkers). This is particularly important the first few months of treatment, as rising PSA and ALP values may be a flare phenomenon associated with cancer cell death or normal tissue regeneration [Citation31,Citation32].
5. Bone health
5.1. Prevention of osteoporosis related fractures
Long-term castration treatment increases the risk of osteoporosis related fractures over time [Citation33,Citation34], particularly in elderly and frail men [Citation35]. The addition of new androgen receptor pathway treatments (ARTA) further increases this risk [Citation12]. The Swedish prostate cancer guidelines recommend individualised physical exercise for all men on castration treatment. Immediate treatment with a bisphosphonate or denosumab plus vitamin D and calcium is recommended for men with
additional treatment with apalutamid, darolutamide, enzalutamide, abiraterone or radium-223
glucocorticoid treatment equivalent to 5 mg prednisolone daily for more than 3 months
a previous low-energy fracture
osteoporosis, defined as a T-value below minus 2.5 on dual X-ray absorptiometry (DXA)
Men older than 80 years and those with other risk factors for fractures should have a bone mineral density assessment (DXA) shortly after starting on castration treatment, whereas a DXA is recommended after 2–3 years of castration treatment for younger men without risk factors. The DXA should be repeated every 2–3 years unless bisphosphonate or denosumab treatment is started.
5.2. Prevention of cancer-related skeletal complications
High-dose treatment with either zoledronic acid or denosumab is in the European guidelines recommended for all men with mCRPC to reduce their risk of skeletal morbidity. The Swedish guidelines are more restrictive: although high-dose zoledronic acid or denosumab treatment should be considered for all men with mCRPC and a life-expectancy over 6 months, the benefit of such treatment has not been investigated in men receiving modern disease-specific mCRPC treatment. After the trials evaluating high-dose zoledronic acid and denosumab treatment were reported, it has been shown that effective cancer-specific treatment is more important than the use of these bone-targeting agents for men with mCRPC [Citation10]. Although not specifically studied in men with mCRPC, the experience from trials in patients with other types of cancer suggests that patients with lytic metastases represent a group that is particularly likely to benefit from treatments that reduce cancer-related skeletal morbidity [Citation36,Citation37]. Patients with wide-spread skeletal metastasis are also more likely to experience skeletal complications and should be prioritized for zoledronic acid or denosumab treatment. A randomized trial has shown that treatment with zoledronic acid every 12th week is as effective as treatment every 4th week [Citation38], so the Swedish guidelines recommend treatment every 12th week.
Acknowledgement
Fredrik Sandin, statistician at Regional Cancer Centre Mid-Sweden in Uppsala, provided data from the National Prostate Cancer Register of Sweden (NPCR).
Disclosure statement
In accordance with Taylor & Francis policy and my ethical obligation as a researcher, I am reporting that none of the authors have any ongoing financial and/or business interests in any company that may be affected by the research reported in the enclosed paper.
References
- EAU - EANM - ESTRO - ESUR - ISUP - SIOG Guidelines on Prostate Cancer [Internet]. European Association of Urology. 2022.
- Bratt O, Carlsson S, Fransson P, et al. The Swedish national guidelines on Prostate Cancer, Part 1: Early Detection, Diagnostics, Staging, Patient Support and Primary Management of Non-metastatic Disease. Scand J Urol. 2022.
- Shen MJ, Nelson CJ, Peters E, et al. Decision-making processes among Prostate Cancer Survivors with Rising PSA Levels: Results from a Qualitative Analysis. Med Decis Making. 2015;35(4):477–486.
- Ghadjar P, Hayoz S, Bernhard J, Swiss Group for Clinical Cancer Research (SAKK), et al. Dose-intensified versus conventional-dose salvage radiotherapy for biochemically recurrent prostate cancer after prostatectomy: the SAKK 09/10 randomized phase 3 trial. Eur Urol. 2021;80(3):306–315.
- Dess RT, Sun Y, Jackson WC, et al. Association of presalvage radiotherapy PSA levels after prostatectomy with outcomes of long-term antiandrogen therapy in men with prostate cancer. JAMA Oncol. 2020;6(5):735–743.
- Carrie C, Magne N, Burban-Provost P, et al. Short-term androgen deprivation therapy combined with radiotherapy as salvage treatment after radical prostatectomy for prostate cancer (GETUG-AFU 16): a 112-month follow-up of a phase 3, randomised trial. Lancet Oncol. 2019;20(12):1740–1749.
- Valle LF, Lehrer EJ, Markovic D, et al. A systematic review and Meta-analysis of Local Salvage Therapies After Radiotherapy for Prostate Cancer (MASTER). Eur Urol. 2021;80(3):280–292.
- Hofman MS, Lawrentschuk N, Francis RJ, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395(10231):1208–1216.
- Attard G, Murphy L, Clarke NW, et al. Abiraterone acetate and prednisolone with or without enzalutamide for high-risk non-metastatic prostate cancer: a meta-analysis of primary results from two randomised controlled phase 3 trials of the STAMPEDE platform protocol. Lancet. 2022;399(10323):447–460.
- James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;1389(10024):1163–1177.
- Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N Engl J Med. 2019;381(2):121–131.
- Chi KN, Chowdhury S, Bjartell A, et al. Apalutamide in patients with metastatic castration-sensitive prostate cancer: final survival analysis of the randomized, double-blind, phase III TITAN Study. J Clin Oncol. 2021;39(20):2294–2303. 10
- Armstrong AJ, Shore ND, Szmulewitz RZ, et al. Efficacy of enzalutamide plus androgen deprivation therapy in metastatic hormone-sensitive prostate cancer by pattern of metastatic spread: ARCHES post hoc analyses. J Urol. 2021;205(5):1361–1371.
- Thomsen FB, Brasso K, Christensen IJ, Scandinavian Prostate Cancer Group, et al. Survival benefit of early androgen receptor inhibitor therapy in locally advanced prostate cancer: long-term follow-up of the SPCG-6 study. Eur J Cancer. 2015;51(10):1283–1292.
- Tyrrell CJ, Kaisary AV, Iversen P, et al. A randomised comparison of 'Casodex' (bicalutamide) 150 mg monotherapy versus castration in the treatment of metastatic and locally advanced prostate cancer. Eur Urol. 1998;33(5):447–456.
- Beckmann K, Garmo H, Adolfsson J, et al. Androgen deprivation therapies and changes in comorbidity: a comparison of gonadotropin-releasing hormone agonists and antiandrogen monotherapy as primary therapy in men with high-risk prostate cancer. Eur Urol. 2019;75(4):676–683.
- Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737–746.
- Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377(4):352–360.
- Fizazi K, Foulon S, Carles J, et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): a multicentre, open-label, randomised, phase 3 study with a 2 x 2 factorial design. Lancet. 2022;399(10336):1695–1707.
- Smith MR, Hussain M, Saad F, et al. Darolutamide and survival in metastatic, hormone-sensitive prostate cancer. N Engl J Med. 2022;386(12):1132–1142. Epub 20220217.
- Smith MR, Saad F, Chowdhury S, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378(15):1408–1418.
- Sternberg CN, Fizazi K, Saad F, PROSPER Investigators, et al. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2020;382(23):2197–2206.
- Fizazi K, Shore N, Tammela TL, ARAMIS Investigators, et al. Nonmetastatic, castration-resistant prostate cancer and survival with darolutamide. N Engl J Med. 2020;383(11):1040–1049.
- Maughan BL, Antonarakis ES. Androgen pathway resistance in prostate cancer and therapeutic implications. Expert Opin Pharmacother. 2015;16(10):1521–1537.
- Smith M, Parker C, Saad F, et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(3):408–419.
- Agency EM. EMA restricts use of prostate cancer medicine Xofigo https://www.ema.europa.eu/en/news/ema-restricts-use-prostate-cancer-medicine-xofigo2018. [2022-02-14].
- de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147–1154.
- Eisenberger M, Hardy-Bessard AC, Kim CS, et al. Phase III study comparing a reduced dose of cabazitaxel (20 mg/m(2)) and the currently approved dose (25 mg/m(2)) in postdocetaxel patients with metastatic castration-resistant prostate cancer-PROSELICA. J Clin Oncol. 2017;35(28):3198–3206.
- de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091–2102.
- Sartor O, de Bono J, Chi KN, et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med. 2021;385(12):1091–1103.
- Sella A, Sternberg CN, Skoneczna I, et al. Prostate-specific antigen flare phenomenon with docetaxel-based chemotherapy in patients with androgen-independent prostate cancer. BJU Int. 2008;102(11):1607–1609.
- Mikah P, Krabbe LM, Eminaga O, et al. Dynamic changes of alkaline phosphatase are strongly associated with PSA-decline and predict best clinical benefit earlier than PSA-changes under therapy with abiraterone acetate in bone metastatic castration resistant prostate cancer. BMC Cancer. 2016;16(1):214.
- Thorstenson A, Bratt O, Akre O, et al. Incidence of fractures causing hospitalisation in prostate cancer patients: results from the population-based PCBaSe Sweden. Eur J Cancer. 2012;48(11):1672–1681.
- Wu CC, Chen PY, Wang SW, et al. Risk of fracture during androgen deprivation therapy among patients with prostate cancer: a systematic review and meta-analysis of cohort studies. Front Pharmacol. 2021;12:652979.
- Li G, Thabane L, Papaioannou A, et al. An overview of osteoporosis and frailty in the elderly. BMC Musculoskelet Disord. 2017;18(1):46.
- Lipton A, Zheng M, Seaman J. Zoledronic acid delays the onset of skeletal-related events and progression of skeletal disease in patients with advanced renal cell carcinoma. Cancer. 2003;98(5):962–969.
- Borggrefe J, Giravent S, Campbell G, et al. Association of osteolytic lesions, bone mineral loss and trabecular sclerosis with prevalent vertebral fractures in patients with multiple myeloma. Eur J Radiol. 2015;84(11):2269–2274.
- Himelstein AL, Foster JC, Khatcheressian JL, et al. Effect of longer-interval vs standard dosing of zoledronic acid on skeletal events in patients with bone metastases: a randomized clinical trial. JAMA. 2017;317(1):48–58.
