 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
There is now an unprecedented amount of evidence to consider when revising prostate cancer guidelines. We believe that there is a value in publishing summaries of national clinical guidelines in English for others to read and comment on.
Methods
This is part 1 of a summary of the Swedish prostate cancer guidelines that were published in June 2022. It covers the early detection, diagnostics, staging, patient support and management of the non-metastatic disease. Part 2 covers recurrence after local treatment and management of the metastatic disease.
Results
The 2022 Swedish guidelines include several new recommendations: rectal iodine-povidone to reduce post-biopsy infections, external beam radiation with focal boost to the tumour, use of a pre-rectal spacer to reduce rectal side effects after external beam radiotherapy in some expert centres, 6 months’ concomitant and adjuvant rather than neoadjuvant and concomitant hormonal treatment together with radiotherapy for unfavourable intermediate and high-risk disease, and adjuvant abiraterone plus prednisolone together with a GnRH agonist for a subgroup of men with very high-risk disease. The Swedish guidelines differ from the European by having more restrictive recommendations regarding genetic testing and pelvic lymph node dissection, the risk group classification, recommending ultra-hypofractionated (7 fractions) external radiotherapy for intermediate and selected high-risk cancers, by not recommending any hormonal treatment together with radiotherapy for favourable intermediate-risk disease, and by recommending bicalutamide monotherapy instead of a GnRH agonist for some patient groups.
Conclusions
The 2022 Swedish prostate cancer guidelines include several new recommendations and some that differ from the European guidelines.
1. Introduction
In the past few years, a large number of important randomized clinical prostate cancer trials have been published. There is now an unprecedented amount of evidence for clinical guidelines groups to consider when revising national and international guidelines. Although the same evidence from clinical trials and other studies are available for all guideline groups, the clinical conclusions drawn from the evidence may differ – not only because the health care systems and the populations differ between countries, but also because the clinical interpretation of crude scientific data is subjective. We believe that there is a value in publishing summaries of national clinical guidelines in English for others to read and possibly comment on.
This is part 1 of 2 of a summary of the most recent version of the Swedish prostate cancer guidelines, published in June 2022. This article covers the early detection, diagnostics, staging, patient support and primary management of the non-metastatic disease. Recurrence after local treatment with curative intent and management of men with metastatic disease are covered in part 2 [Citation1]. The summary focuses on new recommendations, based on recently published pivotal clinical trials, and on recommendations that differ from the European guidelines [Citation2].
2. The national prostate cancer guidelines group
The first Swedish national prostate cancer guidelines were published in 2014. They have been annually revised since then. A draft is publicly available online and sent for comments to various organisations. When the comments have been considered by the guidelines group and the appropriate revisions have been made, the final version is approved by the Swedish Confederation of Regional Cancer Centres before publication.
Each of the 6 regional cancer centres appoints one oncologist and one urologist to the guidelines group. The group and its associated experts also include cancer nurse specialists, radiologists, a pathologist, a nuclear medicine specialist, a hospital social worker, a sexologist, a clinical chemist, a general practitioner, a clinical geneticist, patient representatives and an administrative co-ordinator.
The guidelines group closely co-operates with the National Prostate Cancer Register of Sweden (NPCR). NPCR data (publicly available in English at npcr.se) are used to evaluate the influence of the national guidelines on clinical care [Citation3,Citation4]. Hospital-level outcomes of specific quality indicators are regularly reported to all heads of urology and oncology departments in Sweden [Citation5].
3. Epidemiology
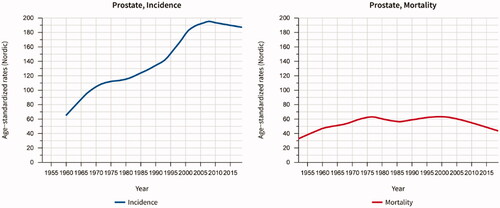
Prostate cancer is the most common cancer in Sweden. With approximately 11,000 new cases per year in a population of 10.4 million people, Sweden has the third highest age-standardized incidence in Europe [Citation6]. The pandemic caused an incidence drop to 8,900 in 2020 [Citation7]. In the past 20 years, age-standardized mortality has decreased by a third (). Among men under 80 years of age the mortality has almost halved, probably both because of earlier detection leading to more men being cured by surgery and local radiotherapy, and because of more effective treatment of metastatic disease.
Figure 1. Age-standardized incidence and mortality of prostate cancer in Sweden 1960–2020. Data source: NORDCAN (https://nordcan.iarc.fr/en).
4. Early detection and screening
The Swedish National Board of Health and Welfare, in agreement with other national healthcare authorities, recommend against population-based prostate cancer screening because of the associated issues with overdiagnosis and overtreatment. The Board does, however, acknowledge that individual men may consider the potential benefits and harms of PSA testing differently. The national guidelines recommend that men be informed about the potential consequences, and offered testing if they after proper information wish to. A national brochure about PSA testing is available online and in general practice.
In 2018, the Swedish Ministry of Health and Social Affairs commissioned the Confederation of Regional Cancer Centres to do something about the widespread, ineffective, unorganized PSA testing. The Confederation proposed regional, population-based, developmental projects with ”organized prostate cancer testing” (OPT) aiming at improving pre-testing information, reducing socioeconomic inequality, making the testing and subsequent diagnostics more effective, and gaining knowledge and experience to prepare for a future national screening programme [Citation8]. The regional projects, of which 5 are ongoing and 10 are planned to start in 2022 or 2023 (Sweden has 21 regions), involve letters with a brief description of the pros and cons of prostate cancer testing. In-depth information is available online. Men who opt for testing are managed according to a strict algorithm in a separate pathway outside routine health care, in essence just like in a formal screening programme. Birth cohorts of men are gradually invited with the aim of including the entire target population of men between 50 and 74 years of age within 7–9 years. All data are registered in regional databases for quality control, development and research.
4.1. Hereditary risk group and genetic testing
Family history is one of the strongest risk factors for prostate cancer [Citation9]. Men with two affected first-degree relatives are recommended PSA testing from the age of 40 years, with shorter intervals and a lower cut-off (2 µg/l) for urology referral than men in general.
Prostate cancer may be the one among common cancers for which inherited predisposition is most important [Citation10], but germline testing for high penetrance germline mutations has only recently become clinically important. BRCA2 and HOXB13 have emerged as the two clinically most important prostate cancer predisposition genes [Citation11,Citation12]. Men with deleterious BRCA2 or HOXB13 mutations are recommended the same follow-up as men with a strong family history of prostate cancer.
By 2020 the Swedish guidelines recommend that men younger than 60 years with metastatic or Gleason pattern 5 prostate cancer and a first-degree relative with BRCA2-associated cancer should be offered onco-genetic counselling about BRCA2 mutation testing. So should men with a family history suggesting Lynch syndrome. The reason for being more restrictive with genetic testing than the European guidelines [Citation2] is the lack of population-based studies of the practical consequences for the tested men and their families. A Swedish working group is preparing for how more extensive testing is best implemented in routine clinical care.
5. Diagnosing prostate cancer
Standardised cancer diagnostic pathways were implemented in Swedish healthcare in 2015, inspired by Danish and Norwegian examples. The prostate cancer pathway inclusion criteria are a palpable prostate nodule and/or a serum PSA value of 3 µg/l for men younger than 70 years, ≥5 µg/l for men aged 70–80 years and ≥7 µg/l for men older than 80 years. The pathway specifies procedures and optimal time frames for diagnostics and staging. Sadly, the standardised pathway has not much reduced the national average waiting times for prostate cancer patients; less than half of them are managed within the optimal time frames.
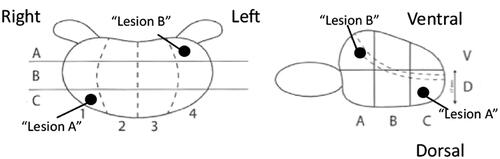
By 2020, the guidelines recommend an MRI as the first investigation for men with a raised PSA value, except for men with PSA ≥100 µg/l, verified metastasis or a short life expectancy. Whether the biopsy protocol should primarily aim at maximising the detection of Gleason score 7–10 cancer or at reducing the detection of Gleason score 6 cancer is debated. The Swedish guidelines group leans towards the latter: men with a benign DRE, a non-suspicious MRI (PI-RADS 1–3) and a PSA density <0.15 µg/l/cm3 are not routinely recommended a prostate biopsy. Targeted biopsies only are recommended for most men with a PI-RADS 4–5 lesion. The locations of the biopsies are described according to a national template ().
Figure 2. The Swedish national template for describing the localisation of prostate biopsies. In this example, the locations are described as C1D (Lesion A) and A4V (Lesion B).
Because of the low prevalence of fluoroquinolone-resistant bacteria in Sweden, the guidelines recommend a single dose of ciprofloxacin as standard prophylaxis before prostate biopsy. A recent meta-analysis suggests that rectal cleansing with povidone-iodine reduces infectious complications after transrectal prostate biopsy [Citation13]. The European guidelines since 2021 recommend rectal povidone-iodine cleansing and the Swedish guidelines now also do so. Another way to reduce post-biopsy infection is using the transperineal route. Transperineal prostate biopsy is in Sweden routinely done only at a few centres, but the 2022 guidelines stress the importance of implementing the transperineal biopsy technique to reduce the risk of post-biopsy infection.
Many bloods, urine and tissue tests predict the likelihood of Gleason score 7–10 cancer on biopsy in men with raised PSA values [Citation14]. Using these tests as “a filter” substantially reduces the number of prostate biopsies and diagnosed Gleason score 6 cancers. The Stockholm3 test, combining serum and genetic markers with clinical information [Citation15,Citation16], has recently been evaluated for an MRI-based diagnostic pathway in a screening-like setting [Citation17]. The guidelines support using the Stockholm3 test in regional projects with organized prostate cancer testing to gain evidence for its performance in repeated testing.
6. Staging
The Swedish risk group classification is based on the D’Amico classification [Citation18], Cambridge Prognostic Groups [Citation19], NPCR data [Citation20] and considerations on how targeted biopsies and MRI results affect the prognostic value of the risk group categorisation (Box 1, ). The Swedish Likert scales for describing the probability of extra-prostatic tumour extension (EPE) and seminal vesicle invasion (SVI) are described in Box 2.
Figure 3. The view of the patient-reported outcome measures after radical prostatectomy collected by the National Prostate Cancer Register of Sweden is available online for all operating departments for quality control and improvement.
Box 1 The Swedish prostate cancer risk group classification.
Box 2 The Swedish Likert scale for describing the probability of extra-prostatic tumour extension (EPE) on MRI. A similar scale is used for seminal vesicle invasion (SVI). Created by Fredrik Jäderling, Karolinska Institute.
Table
CT and MRI both have low sensitivity and specificity for N-staging [Citation21]. Only 4% of men with Cambridge Prognostic Group 4 have findings suggesting lymph node metastasis on CT/MRI [Citation22]. For this reason and because suspicion of N1 often does not affect the treatment choice, abdominal CT or MRI is not recommended for patients with Cambridge Prognostic Group 4 cancer unless the PSA is >50 ng/ml. A bone scan with SPECT-CT or an MRI of the axial skeleton is recommended for M-staging for those with high or very high-risk diseases. Although PSMA-PET-CT has higher sensitivity to detect metastasis [Citation23], there is no evidence for how limited metastatic findings on PSMA-PET-CT should affect treatment. Therefore, and because of limited PET resources, the guidelines recommend PSMA-PET-CT as optional rather than as the method of choice for staging.
7. Patient support, the treatment decision and rehabilitation
The 2009 Swedish national cancer strategy stated that every cancer patient should be offered a personal cancer nurse specialist for psychosocial support and for help with coordinating their care. The cancer nurse specialist should shortly after the notification of the diagnosis do a basic needs assessment and provide the patient with written, individualized, care and rehabilitation plans. For special needs, the patient should be offered contact with a social counsellor, urotherapist, sexologist, physiotherapist, dietitian, etc.
It is the responsibility of the diagnosing urologist and the cancer nurse specialist to ensure that the patient has been provided with sufficient verbal and written information to be able to take an active role in the treatment decision. The national guidelines’ appendix includes patient information sheets about the different treatments for non-metastatic disease, advice on self-management (physical exercise, smoking cessation, etc), sexual side-effects and their management, and hormonal treatment (methods and management of side-effects). Patients considered for either radical prostatectomy or primary radiotherapy should be offered appointments with both a prostate cancer surgeon and a radiation oncologist. Before these appointments, a structured assessment of their urinary, bowel and sexual functions should be carried out, as these may affect the risk of treatment side effects. All patients should be informed about their right to have a second opinion at another centre.
The guidelines specify clinical situations when the management should be discussed at a multidisciplinary team conference, such as high or very high-risk non-metastatic disease, metastatic disease, biochemical recurrence after surgery or primary radiotherapy, progressive castration-resistant disease, and co-morbid conditions that make the treatment choice particularly difficult.
All centres providing radical prostatectomy or radiotherapy should provide structured rehabilitation programmes for restoring the patients’ sexual, urinary and bowel functions. The guidelines include specific recommendations about managing side effects such as erectile dysfunction, urinary incontinence, strictures, radiation cystitis, radiation proctitis, lymphoedema, osteoporosis and hot flashes.
8. Active surveillance and watchful waiting
Active surveillance is defined as expectant management including repeated diagnostic investigations with the aim of offering curative treatment in case of early signs of progression, whereas watchful waiting involves PSA testing and digital rectal examination with the aim of starting hormonal treatment in case of progression to locally advanced or metastatic disease.
Active surveillance reduces overtreatment and is associated with better quality of life than radical prostatectomy and radiotherapy [Citation24], and has become the dominant management for low-risk prostate cancer in Sweden. In 2018–2020 almost all men under 80 years with a very low (93%) or low (84%) risk of the disease started active surveillance (data online at npcr.se). Those with an intermediate risk of cancer are encouraged to take part in the SPCG-17 trial, which is evaluating the safety of an MRI-based follow-up with defined triggers for intervention [Citation25].
The use of MRI and targeted plus systematic biopsies is paramount for the proper selection of men for active surveillance [Citation26], but the evidence for an entirely MRI-based follow-up is not convincing [Citation27]. The recommended active surveillance follow-up is a PSA test every sixth months, palpation annually, and, as a rule, a repeat biopsy every second to third year. Omitting repeat biopsies is acceptable in patients with low-risk disease who have had a recent unsuspicious MRI and has a stable PSA level with a density below 0.15 ng/l/cm3. The long-term safety of active surveillance depends on adherence to the follow-up protocol, but only 42% of active surveillance patients in Stockholm had in 2008 to 2017 the recommended repeat biopsy within one year, although the proportion increased in the later part of the studied time period [Citation28].
Most patients on watchful waiting are old or have severe comorbidity. Although most elderly men with a localised low or intermediate-risk prostate cancer die from other causes than their prostate cancer, local tumour progression and metastatic disease may rapidly occur more than 15 years after diagnosis [Citation29]. Active follow-up is therefore essential to detect progression early enough to start hormonal treatment, with or without local radiotherapy, in time to prevent severe symptoms and possibly also death from prostate cancer.
9. Radical prostatectomy
In Sweden, almost all radical prostatectomies are done laparoscopically with robot assistance (93% in 2021). Because high surgeon and hospital volumes are associated with better functional and oncological outcomes [Citation30–32], the Swedish guidelines recommend that radical prostatectomy be done only by surgeons who do at least 25 procedures per year at departments with at least two such surgeons. As outcomes vary considerably also between high-volume surgeons [Citation32], patient-reported outcome measures (PROMs) should systematically be collected with the NPCR’s online questionnaire to facilitate continuous quality control. The heads of the departments have access to their own individual surgeons’ results (), as well as aggregated results on departmental, regional and national levels.
Figure 4. National register data from the National Prostate Cancer Register of Sweden for the use of different radiotherapy modalities in 2021 (npcr.se).
Extended pelvic lymph node dissection (ePLND) gives prognostic information and may guide postoperative radiotherapy, but is unlikely to be of therapeutic value [Citation33] and increases the risk of postoperative complications [Citation34,Citation35]. The Swedish guidelines group draws a different conclusion from the evidence than the European guidelines [Citation2] and does not recommend routine PLND, not even for patients with high-risk disease, partially because postoperative radiotherapy can now be guided by PSMA-PET/CT.
10. Radiotherapy: primary and adjuvant
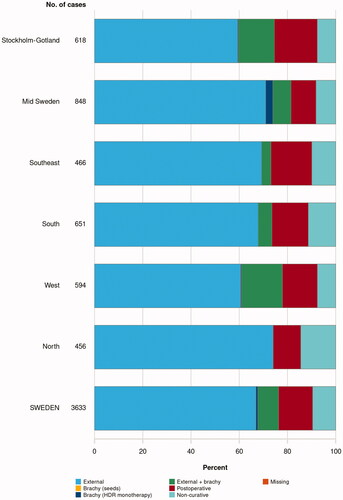
Recent research and technical developments have led to a wide spectrum of different ways to deliver radiation treatment with curative intent. These are briefly described below. Specific treatment recommendations based on risk grouping are summarized in Box 3. Swedish national register data for the use of different radiotherapy modalities in 2021 are shown in .
Box 3. Summary of recommended radiotherapy modalities and combinations with doses and fractionation for different risk groups. Please see Box 1 for definitions of the risk groups. Abbreviations: EBRT: external beam radiation therapy, Gy: Gray, fx: fraction, HDR: high dose-rate, BT: brachytherapy, MRI: magnetic resonance imaging.
Intermediate risk:
First choice: Ultra-hypofractionated EBRT (6.1 Gy/7fx)
Moderately hypofractionated EBRT (3 Gy/20fx) may be considered
HDR-BT monotherapy (13–14Gy/2fx) may be considered, particularly when EBRT or surgery is not suitable (e.g. for patients with an ileoanal reservoir).
High risk
EBRT with HDR-BT boost: EBRT (2 Gy/25fx or 3 Gy/13–14fx) + HDR-BT (10 Gy/2fx or 14–15Gy/1fx)
Moderately (3 Gy/20–22fx) or ultra-hypofractionated EBRT (6.1 Gy/7fx) when HDR-BT is not suitable
EBRT with focal boost (2.2Gy/35fx to whole prostate + up to total 95 Gy to tumour) may be considered.
Very high risk
EBRT with HDR-BT boost: EBRT (2 Gy/25fx or 3 Gy/13–14fx) + HDR-BT (10 Gy/2fx or 14–15Gy/1fx).
EBRT with focal boost (2.2Gy/35fx to whole prostate + up to total 95 Gy to tumour) may be considered
EBRT (2 Gy/39fx) in patients not eligible for combination therapy or focal boost
10.1. External beam radiotherapy (EBRT)
MRI-based dose-planning with the use of fiducial markers for image-guided RT (IGRT) with volumetric arc radiation treatment (VMAT) or intensity-modulated radiation treatment (IMRT) techniques are recommended as standard of care. Using a pre-rectal hydrogel spacer should be considered in patients with an increased risk of significant rectal toxicity [Citation36]. Recommended doses for EBRT with curative intent are either 78 Gray in 39 fractions (conventional fractionation), 66 Gray in 20 fractions (moderate hypofractionation) as in the CHHIP trial [Citation37] or, since 2020, ultra-hypofractionated radiotherapy with 42.7 Gray in 7 fractions. The ultra-hypofractionated was shown to be equally effective as the standard radiotherapy in the Nordic HYPO-RT-PC trial [Citation38]. Most Swedish radiotherapy centres participated in this trial and are therefore highly experienced in planning and delivering ultra-hypofractionated radiotherapy. Not least patients living far away from a radiation centre appreciate avoiding the otherwise long radiation treatment course. Ultra-hypofractionation is the radiation treatment of choice for men with intermediate-risk and is considered a valid option for some, selected men with favourable localised high-risk cancer. This contrasts with the European guidelines, which still recommend ultra-hypofractionated radiotherapy in prospective clinical trials only [Citation2].
10.2. High dose-rate brachytherapy
Combination therapy using EBRT with a high dose-rate brachytherapy boost (EBRT-BT) is available in all but one university hospital in Sweden. Randomized prospective trials [Citation39], as well as retrospective observational studies, have reported reduced rates of progression to metastatic disease and prostate cancer mortality compared with EBRT and radical prostatectomy, particularly for high-risk and poorly differentiated cancer [Citation40–42]. EBRT-BT is therefore the preferred radiotherapy method for men with high or very high-risk disease, provided that individual patient and cancer characteristics make the patient well suited for EBRT-BT. Examples of when a BT boost may not be the best option are gross tumour extension into the distal part of a seminal vesicle, a very large prostate (> 50 mm breadth), a large middle lobe, severe lower urinary tract symptoms, and a recent TUR-P.
HDR BT monotherapy can be considered for localised intermediate-risk prostate cancer, based on a recent systematic review with a meta-analysis [Citation43] and national experience [Citation44] showing high rates of disease control and low rates of severe side-effects.
10.3. Neoadjuvant, concomitant and adjuvant hormonal treatment
Patients with unfavourable intermediate-risk cancer were for many years recommended 6 months of neoadjuvant and concomitant GnRH agonist plus bicalutamide, but a recent meta-analysis showed better effect if the hormonal treatment starts at the same time as the radiotherapy [Citation45]. The guidelines are now changed accordingly: a 6-month course of concomitant and adjuvant GnRH plus bicalutamide is recommended for patients with cancer in more than half of systematic biopsy cores, Gleason score 4 + 3 = 7 and/or 2–3 of the factors defining intermediate risk (T2b-c, Gleason score 7, PSA 10–20 ng/ml) [Citation46]. The European guidelines recommend adding 4–6 months of hormonal treatment for all intermediate-risk patients, but studies suggest that the benefit, if any, is small for those with favourable intermediate-risk disease [Citation46,Citation47].
All patients with high-risk disease are recommended the same 6-month course of hormonal treatment as patients with intermediate-risk diseases. Patients with a bulky high-risk tumour or very high-risk disease are additionally recommended adjuvant hormonal treatment for 2–3 years [Citation48,Citation49]. The European guidelines recommend adjuvant GnRH agonist [Citation2], but the Swedish guidelines favour bicalutamide because of its side-effect profile [Citation50,Citation51].
A recent report from the STAMPEDE multi-trial showed a substantially improved overall survival of adding 2 years of abiraterone to concomitant and adjuvant GnRH agonist treatment for a subgroup of men with very high-risk disease: those with at least 2 of the risk factors T3–4, Gleason score 8–10 or PSA > 40 ng/ml, or lymph-node metastasis [Citation52]. This treatment concept is now recommended in the Swedish guidelines.
10.4. Adjuvant radiotherapy
Although randomized trials have shown no benefit from giving adjuvant radiotherapy after radical prostatectomy compared with radiotherapy at the time of biochemical relapse [Citation53], adjuvant radiotherapy (64–70 Gy in 32–35 fractions) should be considered for the small group of patients with >10 mm cancer in the surgical margins in the absence of lymph node metastasis (pNx or pN0), particularly if there is Gleason grade 5 cancer in the margin. Radiotherapy should commence at least 3–6 months after surgery to reduce the risk of permanent urinary incontinence.
11. Primary hormonal treatment for non-metastatic disease
In men with localised prostate cancer (cT1-2) with a moderately high PSA value (<30–50 ng/ml) and a PSA doubling time over 12 months, primary hormonal treatment does not prolong survival [Citation48,Citation49]. Watchful waiting is therefore the preferred management option for these patients if they are not candidates for curative treatment. In men with higher or more rapidly increasing PSA values and in men with locally advanced, non-metastatic disease, early hormonal treatment does prolong life, provided that their life expectancy is more than 5 years [Citation48,Citation49]. The Swedish guidelines recommend bicalutamide 150 mg once daily over GnRH agonists, based on bicalutamide’s more favourable side-effect profile [Citation50,Citation51], which the European guidelines do not. Single-dose breast irradiation should be given before the start of bicalutamide treatment [Citation54].
12. Conclusions
The evidence-base for diagnosing and managing men with prostate cancer is rapidly progressing. The 2022 Swedish prostate cancer guidelines include several new recommendations and some that differ from the European guidelines. Some recommendations may be controversial and we would appreciate it if those who have different opinions would share them in a letter to the Editor of the Scandinavian Journal of Urology.
Disclosure statement
In accordance with Taylor & Francis policy and my ethical obligation as a researcher, I am reporting that none of the authors have any ongoing financial and/or business interests in any company that may be affected by the research reported in the enclosed paper.
Additional information
Funding
References
- Bratt O, Carlsson S, Fransson P, et al. The Swedish national guidelines on prostate cancer, part 2: recurrent, metastatic and castration resistant disease. Scand J Urol. 2022.
- EAU – EANM – ESTRO – ESUR – ISUP – SIOG. Guidelines on prostate cancer. European Association of Urology. 2022. https://uroweb.org/guidelines/prostate-cancer.
- Cazzaniga W, Ventimiglia E, Alfano M, et al. Mini review on the use of clinical cancer registers for prostate cancer: the national prostate cancer register (NPCR) of Sweden. Front Med. 2019;6:51.
- Nugin H, Folkvaljon Y, Damber JE, et al. Work-up and treatment of prostate cancer before and after publication of the first national guidelines on prostate cancer care in Sweden. Scand J Urol. 2018;52(4):277–284.
- Stattin P, Sandin F, Sandback T, et al. Dashboard report on performance on select quality indicators to cancer care providers. Scand J Urol. 2016;50(1):21–28.
- Gandaglia G, Leni R, Bray F, et al. Epidemiology and prevention of prostate cancer. Eur Urol Oncol. 2021;4(6):877–892.
- Fallara G, Sandin F, Styrke J, et al. Prostate cancer diagnosis, staging, and treatment in Sweden during the first phase of the COVID-19 pandemic. Scand J Urol. 2021;55(3):184–191.
- Alterbeck M, JärburE, Bjartell A, et al. Designing and implementing a population-based organized prostate cancer testing program. Eur Urol Focus. 2022.
- Bratt O, Drevin L, Akre O, et al. Family history and probability of prostate cancer, differentiated by risk category: a nationwide population-based study. J Natl Cancer Inst. 2016;108(10):djw110.
- Lichtenstein P, Holm NV, Verkasalo PK, et al. Environmental and heritable factors in the causation of cancer–analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343(2):78–85.
- Nyberg T, Tischkowitz M, Antoniou AC. BRCA1 and BRCA2 pathogenic variants and prostate cancer risk: systematic review and meta-analysis. Br J Cancer. 2022;126(7):1067–1081.
- Nyberg T, Govindasami K, Leslie G, et al. Homeobox B13 G84E mutation and prostate cancer risk. Eur Urol. 2019;75(5):834–845.
- Pradere B, Veeratterapillay R, Dimitropoulos K, et al. Nonantibiotic strategies for the prevention of infectious complications following prostate biopsy: a systematic review and meta-analysis. J Urol. 2021;205(3):653–663.
- Eyrich NW, Morgan TM, Tosoian JJ. Biomarkers for detection of clinically significant prostate cancer: contemporary clinical data and future directions. Transl Androl Urol. 2021;10(7):3091–3103.
- Grönberg H, Adolfsson J, Aly M, et al. Prostate cancer screening in men aged 50-69 years (STHLM3): a prospective population-based diagnostic study. Lancet Oncol. 2015;16(16):1667–1676.
- Ström P, Nordström T, Aly M, et al. The stockholm-3 model for prostate cancer detection: algorithm update, biomarker contribution, and reflex test potential. Eur Urol. 2018;74(2):204–210.
- Nordström T, Discacciati A, Bergman M, et al. Prostate cancer screening using a combination of risk-prediction, MRI, and targeted prostate biopsies (STHLM3-MRI): a prospective, population-based, randomised, open-label, non-inferiority trial. Lancet Oncol. 2021;22(9):1240–1249.
- D'Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969–974.
- Gnanapragasam VJ, Bratt O, Muir K, et al. The cambridge prognostic groups for improved prediction of disease mortality at diagnosis in primary non-metastatic prostate cancer: a validation study. BMC Med. 2018;16(1):31.
- Bratt O, Folkvaljon Y, Loeb S, et al. Upper limit of cancer extent on biopsy defining very low-risk prostate cancer. BJU Int. 2015;116(2):213–219.
- Hovels AM, Heesakkers RA, Adang EM, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol. 2008;63(4):387–395.
- Stenman C, Abrahamsson E, Redsäter M, et al. The proportions of positive abdominal computerized tomography and bone scan in men with Cambridge Prognostic Group 4 and 5 prostate cancer – a nationwide register study. Eur Urol Open Sci. 2022;41:123–125.
- Hofman MS, Lawrentschuk N, Francis RJ, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395(10231):1208–1216.
- Venderbos LDF, Aluwini S, Roobol MJ, et al. Long-term follow-up after active surveillance or curative treatment: quality-of-life outcomes of men with low-risk prostate cancer. Qual Life Res. 2017;26(6):1635–1645.
- Ahlberg MS, Adami HO, Beckmann K, et al. PCASTt/SPCG-17-a randomised trial of active surveillance in prostate cancer: rationale and design. BMJ Open. 2019;9(8):e027860.
- Klotz L, Pond G, Loblaw A, et al. Randomized study of systematic biopsy versus magnetic resonance imaging and targeted and systematic biopsy in men on active surveillance (ASIST): 2-year postbiopsy follow-up. Eur Urol. 2020;77(3):311–317.
- Hettiarachchi D, Geraghty R, Rice P, et al. Can the use of serial multiparametric magnetic resonance imaging during active surveillance of prostate cancer avoid the need for prostate biopsies?-A systematic diagnostic test accuracy review. Eur Urol Oncol. 2021;4(3):426–436.
- Olsson H, Nordstrom T, Clements M, et al. Intensity of active surveillance and transition to treatment in men with low-risk prostate cancer. Eur Urol Oncol. 2020;3(5):640–647.
- Popiolek M, Rider JR, Andren O, et al. Natural history of early, localized prostate cancer: a final report from three decades of follow-up. Eur Urol. 2013;63(3):428–435.
- Leow JJ, Leong EK, Serrell EC, et al. Systematic review of the volume-outcome relationship for radical prostatectomy. Eur Urol Focus. 2018;4(6):775–789.
- Lantz A, Bock D, Akre O, et al. Functional and oncological outcomes after open versus robot-assisted laparoscopic radical prostatectomy for localised prostate cancer: 8-year follow-up. Eur Urol. 2021;80(5):650–660.
- Nyberg M, Sjoberg DD, Carlsson SV, et al. Surgeon heterogeneity significantly affects functional and oncological outcomes after radical prostatectomy in the Swedish LAPPRO trial. BJU Int. 2021;127(3):361–368.
- Preisser F, van den Bergh RCN, Gandaglia G, et al. Effect of extended pelvic lymph node dissection on oncologic outcomes in patients with D'Amico intermediate and high risk prostate cancer treated with radical prostatectomy: a multi-institutional study. J Urol. 2020;203(2):338–343.
- Fossati N, Willemse PM, Van den Broeck T, et al. The benefits and harms of different extents of lymph node dissection during radical prostatectomy for prostate cancer: a systematic review. Eur Urol. 2017;72(1):84–109.
- Wallerstedt Lantz A, Stranne J, Tyritzis SI, et al. 90-Day readmission after radical prostatectomy-a prospective comparison between robot-assisted and open surgery. Scand J Urol. 2019;53(1):26–33.
- Miller LE, Efstathiou JA, Bhattacharyya SK, et al. Association of the placement of a perirectal hydrogel spacer with the clinical outcomes of men receiving radiotherapy for prostate cancer: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(6):e208221.
- Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17(8):1047–1060.
- Widmark A, Gunnlaugsson A, Beckman L, et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet. 2019;394(10196):385–395.
- Morris WJ, Tyldesley S, Rodda S, et al. Androgen suppression combined with elective nodal and dose escalated radiation therapy (the ASCENDE-RT Trial): an analysis of survival endpoints for a randomized trial comparing a low-dose-rate brachytherapy boost to a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2017;98(2):275–285.
- Kishan AU, Cook RR, Ciezki JP, et al. Radical prostatectomy, external beam radiotherapy, or external beam radiotherapy with brachytherapy boost and disease progression and mortality in patients with gleason score 9-10 prostate cancer. JAMA. 2018;319(9):896–905.
- Kishan AU, Karnes RJ, Romero T, et al. Comparison of multimodal therapies and outcomes among patients with high-risk prostate cancer with adverse clinicopathologic features. JAMA Netw Open. 2021;4(7):e2115312.
- Pettersson A, Alm D, Garmo H, et al. Comparative effectiveness of different radical radiotherapy treatment regimens for prostate cancer: a population-based cohort study. JNCI Cancer Spectr. 2020;4(2):pkaa006.
- Anderson EM, Kim S, Sandler HM, et al. High-dose-rate fractionated brachytherapy monotherapy for localized prostate cancer: a systematic review and meta-analysis. J Contemp Brachytherapy. 2021;13(4):365–372.
- Johansson B, Olsen JS, Karlsson L, et al. High-dose-rate brachytherapy as monotherapy for low- and intermediate-risk prostate cancer: long-term experience of Swedish single-center. J Contemp Brachytherapy. 2021;13(3):245–253.
- Spratt DE, Malone S, Roy S, et al. Prostate radiotherapy with adjuvant androgen deprivation therapy (ADT) improves metastasis-free survival compared to neoadjuvant ADT: an individual patient meta-analysis. J Clin Oncol. 2021;39(2):136–144.
- Nabid A, Carrier N, Vigneault E, et al. Optimizing treatment in intermediate-risk prostate cancer: secondary analysis of a randomized phase 3 trial. Int J Radiat Oncol Biol Phys. 2021;111(3):732–740.
- Zumsteg ZS, Spratt DE, Daskivich TJ, et al. Effect of androgen deprivation on long-term outcomes of intermediate-risk prostate cancer stratified as favorable or unfavorable: a secondary analysis of the RTOG 9408 randomized clinical trial. JAMA Netw Open. 2020;3(9):e2015083.
- Iversen P, McLeod DG, See WA, et al. Antiandrogen monotherapy in patients with localized or locally advanced prostate cancer: final results from the bicalutamide early prostate cancer programme at a median follow-up of 9.7 years. BJU Int. 2010;105(8):1074–1081.
- McLeod DG, Iversen P, See WA, et al. Bicalutamide 150 mg plus standard care vs standard care alone for early prostate cancer. BJU Int. 2006;97(2):247–254.
- Tyrrell CJ, Kaisary AV, Iversen P, et al. A randomised comparison of 'Casodex' (bicalutamide) 150 mg monotherapy versus castration in the treatment of metastatic and locally advanced prostate cancer. Eur Urol. 1998;33(5):447–456.
- Beckmann K, Garmo H, Adolfsson J, et al. Androgen deprivation therapies and changes in comorbidity: a comparison of gonadotropin-releasing hormone agonists and antiandrogen monotherapy as primary therapy in men with high-risk prostate cancer. Eur Urol. 2019;75(4):676–683.
- Attard G, Murphy L, Clarke NW, et al. Abiraterone acetate and prednisolone with or without enzalutamide for high-risk non-metastatic prostate cancer: a meta-analysis of primary results from two randomised controlled phase 3 trials of the STAMPEDE platform protocol. Lancet. 2021;399(10323):447–460.
- Vale CL, Fisher D, Kneebone A, et al. Adjuvant or early salvage radiotherapy for the treatment of localised and locally advanced prostate cancer: a prospectively planned systematic review and meta-analysis of aggregate data. Lancet. 2020;396(10260):1422–1431.
- Widmark A, Fossa SD, Lundmo P, et al. Does prophylactic breast irradiation prevent antiandrogen-induced gynecomastia? Evaluation of 253 patients in the randomized scandinavian trial SPCG-7/SFUO-3. Urology. 2003;61(1):145–151.
