In the current issue of Scand J Urol, two articles on the Swedish National Guidelines on Prostate Cancer highlight new recommendations and those recommendations that differ from the EAU guidelines [Citation1,Citation2]. The creation and annual updates of the National Guidelines provided by the guidelines working group are important steps in providing high quality and equal quality of care for men with prostate cancer in all regions of Sweden. However, the compilation of data, formulation of recommendations, and publication of these guidelines are just the first steps in that direction. The recommendations in the Guidelines then have to be implemented by urologists and oncologists in the entire country. In order to survey the adherence to these guidelines rapid reporting and compilation of data on adherence and subsequent feed-back to each department need to be delivered. Such reporting is delivered by the National Prostate Cancer Register of Sweden (NPCR) with information on diagnostics, staging, and primary treatment in the primary registration in NPCR and for men with advanced prostate cancer there is a longitudinal registration in the Patient-overview Prostate Cancer [Citation3,Citation4].
One example of a rapid implementation of a change in the diagnostic work-up of men with prostate cancer is the new recommendation in the Guidelines of a magnetic resonance tomography (MRT) prior to biopsy of the prostate. The proportion of men in NPCR with PSA below 100 ng/mL who underwent MRT prior to biopsy was 10% in 2016 and increased to 80% in 2021 [Citation3].
By linking data in NPCR to other health care registers such as the Prescribed Drug Register, more comprehensive data can rapidly be captured. Such linkages show that there are still large differences in the delivery of prostate cancer care in Sweden. For example, there were large differences in the distribution of an intensified treatment of men with de novo metastatic castration sensitive prostate cancer as recommended by the Guidelines. There was two-fold difference between the region with the highest and lowest use of an additional medical treatment delivered together with androgen deprivation therapy (ADT) to these men ().
Figure 1. Proportion of men with de novo metastatic castration sensitive prostate cancer in NPCR who received additional medical treatment; docetaxel, abiraterone, enzalutamide, or apalutamide together with androgen deprivation therapy. Data from NPCR and the Prescribed Drug Register on drug treatment delivered within six months from date of diagnosis for men with de novo metastatic castration sensitive prostate cancer diagnosed between 1 July 2018 and 31 March 2022.
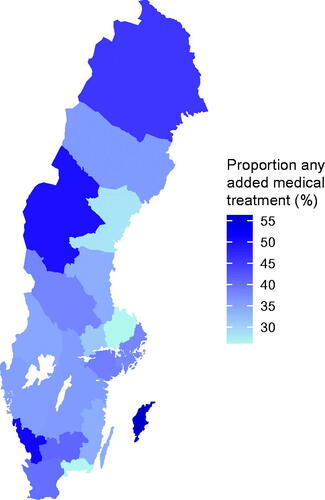
Figure 2. Proportion of men in NPCR below age 80 with locally advanced prostate cancer who received radical treatment. Data available at www.npcr.se/RATTEN.
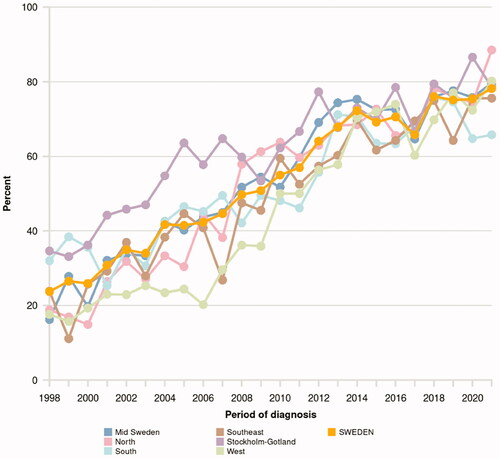
However, guidelines are merely one out of several factors that affect treatment patterns. For example, treatment of men with locally advanced Pca changed substantially during the last two decades, starting well before 2014 when the first National Guidelines for Prostate Cancer were published. In 1998, less than 30% of men below age 80 with locally advanced Pca in Sweden received radical treatment, this proportion then gradually increased and was around 50% in 2008, i.e. the year before the publication of SPCG-7, a landmark study that showed an increased survival for men who were treated with radiotherapy and ADT compared to men who were treated with ADT only [Citation3,Citation5]. Currently, as recommended by the Guidelines almost 80% of men with locally advanced Pca receive radical treatment, mostly radiotherapy (Figure 2).
Recently, Liedberg et al. published an article similar to those by Bratt et al., which was based on the Swedish Guidelines for bladder cancer [Citation6] and there is a similar set-up with a clinical cancer register to assess adherence to guidelines for bladder cancer [Citation7].
In conclusion, the combination of national guidelines for cancer care and data in clinical cancer registers are useful tools for quality assurance and quality improvement that can decrease differences in cancer care delivered by different health care providers. Such tools need to be further disseminated and their use should be encouraged.
Disclosure statement
Pär Stattin is register holder of NPCR.
References
- Bratt O, Carlsson S, Fransson P, et al. The Swedish National Guidelines on Prostate Cancer Part 1: early detection, diagnostics, staging, patient support and primary management of non-metastatic disease. Scand J Urol. 2022;2022:1–9.
- Bratt O, Carlsson S, Fransson P, et al. The Swedish National Guidelines on Prostate Cancer, part 2: recurrent, metastatic and castration resistant disease. Scand J Urol. 2022;2022:1–7.
- www.npcr.se/RATTEN. Accessed 19 July 2022.
- Franck Lissbrant I, Hjälm Eriksson M, Lambe M, et al. Set-up and preliminary results from the patient-overview prostate cancer. Longitudinal registration of treatment of advanced prostate cancer in the National Prostate Cancer Register of Sweden. Scand J Urol. 2020;54(3):227–234.
- Widmark A, Klepp O, Solberg A, et al. Scandinavian prostate cancer group study 7; Swedish association for urological oncology 3. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet. 2009;373(9660):301–308.
- Liedberg F, Kjellström S, Lind A-K, et al. Swedish national guidelines on urothelial carcinoma: 2021 update on non-muscle invasive bladder cancer and upper tract urothelial carcinoma. Scand J Urol. 2022;56(2):137–146.
- https://cancercentrum.se/samverkan/cancerdiagnoser/urinblasa-urinvagar/kvalitetsregister/rapporter/. Accessed 19 July 2022.
