Abstract
Objective
We evaluated long-term risk for biochemical recurrence and subsequent prognosis in a population-based cohort.
Material and Methods
We used register-based data to evaluate 6 675 consecutive patients having radical prostatectomy in Västra Götaland county in Sweden during 1995–2014. Patients were followed until death or end of study, 31 December 2014. Data were collected from registers on national, regional and local level and linked by means of the Swedish personal identity number. Biochemical recurrence was defined as PSA ≥0.2 ng/ml; failure as hormonal treatment, metastasis or prostate cancer death. Survival analysis was used to estimate time to biochemical recurrence and time to failure after biochemical recurrence for patients with 0 − 2 years, 2–5 years, 5–10 years and >10 years interval to biochemical recurrence, respectively.
Results
A total of 1214 men had biochemical recurrence during follow-up. Biochemical recurrence-free survival was 83% (95% confidence interval [CI] 82–84%), 75% (95% CI 74–77%) and 69% (95% CI 67–71%) at 5, 10 and 15 years, respectively. Cumulative incidence of failure for all patients 15 years after biochemical recurrence was 50% (95% CI 43–55%) in competing risk analysis. The risk of failure after biochemical recurrence was highest among patients having biochemical recurrence within 2 years from surgery. Incomplete data on PSA-history is a limitation.
Conclusions
The risk for biochemical recurrence persists 15 years after surgery. Follow-up should continue as long as treatment would be considered in case of recurrent disease.
Introduction
For how long follow-up should be continued for patients with undetectable PSA after radical prostatectomy remains an unanswered question [Citation1]. There are two main objectives for follow-up after treatment with curative intent; to assess and care for needs of physical and psychosocial rehabilitation, and to detect treatable disease progression. Presuming that needs of rehabilitation are met at an early stage, the rationale behind extended follow-up is to detect recurrence in a subclinical stage and thereby change the course of the disease. This implies two questions: Does the risk for biochemical recurrence (BCR) cease after a certain time, and if not, does the risk for subsequent progression decline with increased time to BCR?
In the literature there are several reports on long-term risk for BCR, but few authors report recurrence rates beyond 10 years after surgery. With longer follow-up, results are inconsistent regarding further risk for BCR [Citation2,Citation3]. Shorter interval to BCR has been associated with higher risk for distant metastasis and prostate cancer specific mortality, but effect size varies and the definite impact of interval to BCR on prognosis has not been established [Citation4]. Though different thresholds for early vs late BCR have been used in the literature [Citation4], most studies contrast BCR occurring over the first few years with later occurring BCR and there is very limited data on the potential impact of interval to BCR for recurrences after the first few years after surgery [Citation5–8].
Furthermore, the majority of studies on recurrence and prognosis consists of single-surgeon or single-institution series from tertiary referral centres, while there is considerable lack of population-based data. Existing population-based studies tend to show higher recurrence rates than studies from highly specialized centres [Citation3,Citation8–10].
In this large, whole-of-population cohort, we have studied patterns of long-term biochemical recurrence and the influence of time to recurrence on prognosis. Our study is based on aggregation of data from multiple registers with up to twenty years of follow-up. The primary objective was to estimate long-term risk for biochemical recurrence in an unselected population, and secondary to estimate risk for progression after BCR occurring >10 years after surgery.
Materials and methods
Overview
This study was based on the WSOP – Western Sweden study of Opportunistic Prostate Cancer Screening database. WSOP includes all men from the age of 18 registered as living in the Western Medical Care Region in Sweden on 31 December any year from 1995 to 2014 and consists of PSA values analysed at the hospital laboratories and the largest private laboratory in addition to data from several nation-wide and regional registers, linked by means of the Swedish personal identity number. A detailed description of the database can be found in supplementary 1. The study was approved by the Regional Ethical Review Board in Gothenburg (number 467-15).
Patients
For the present study, we included all patients in WSOP undergoing radical prostatectomy in Västra Götaland county between 1995 and 2014, irrespective of surgical modality (open, laparoscopic or robot assisted radical prostatectomy). For details of inclusion, see supplementary table 1. Exclusion criteria were neoadjuvant hormonal treatment, evidence of distant metastasis at surgery, persistent postoperative PSA ≥0.2 ng/ml, lack of postoperative PSA values, and hormonal treatment, distant metastasis or prostate cancer death without a recorded PSA value ≥0.2 ng/ml.
Data collection
Data on prostate cancer diagnosis, distant metastasis and radical prostatectomy was collected from the National Prostate Cancer Registry (NPCR), the National Patient Registry (PAR) and the Regional administrative healthcare database VEGA. Time and cause of death were collected from the Cause of Death Registry. Data on neoadjuvant, adjuvant and salvage treatment were collected from NPCR, the Prescribed Drugs Registry and local registries at the regional radiotherapy departments. Coverage of Swedish national registers is generally high [Citation11–13]. Availability of PSA values differs between laboratories depending on year, for details see supplement 1. The Prescribed Drugs Registry and the VEGA database started in 2005, data on surgical pathology was included in NPCR in 2007.
Statistical analysis
BCR was defined as a postoperative PSA value ≥0.2 ng/ml after an initial value <0.2 ng/ml. Failure after BCR was defined as first occurrence of hormonal treatment (antiandrogen or androgen deprivation therapy (ADT)), skeletal metastasis or prostate cancer death. Cause of death was defined based on data from death certificates, where prostate cancer mentioned as immediate or underlying cause of death was considered as prostate cancer death as previously described [Citation14].
BCR-free survival after radical prostatectomy and failure-free survival after BCR were estimated with 95% confidence intervals, using Kaplan–Meier survival analysis. For BCR-free survival, patients were censored at death from other causes or 31 December 2014, whatever occurred first. An alternative analysis with censoring at last known PSA measurement was made as a sensitivity analysis, as was a supporting competing risk analysis [Citation15,Citation16].
Kaplan-Meier estimates of failure-free survival after BCR were stratified on time to BCR in four categories; 0-2 years, 2-5 years, 5-10 years and >10 years. Cut-offs were chosen prior to analysis, based on intervals considered as clinically relevant. A supporting competing risk analysis was performed in order to examine if the effect of other deaths on the estimates were of clinical importance [Citation15,Citation16]. Differences between groups were compared with log-rank test, where the level of statistical significance was set at 5%. No adjustment for multiple comparisons was performed. Subgroup analysis on patients having BCR from year 2005 when data on hormonal treatment was first available was made as a sensitivity analysis. An additional analysis of BCR-free survival and failure-free survival was made, stratified on clinical risk groups: ‘Low-risk’ (PSA ≤ 10, Gleason score ≤6, and clinical stage T1), ‘Intermediate risk’ (PSA < 20, Gleason score ≤7, and clinical stage ≤ T2, not qualifying for low risk), or ‘High-risk’ (any of PSA >20, Gleason score ≥8, or clinical stage ≥ T3). All statistical analyses were made in R [Citation17].
Results
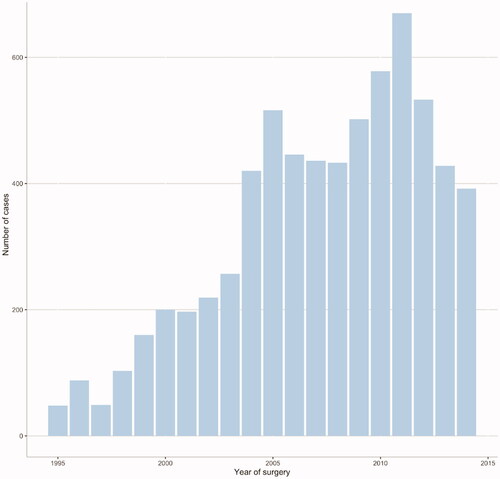
A total of 11 483 patients in WSOP were assessed for eligibility and 8 414 met the inclusion criteria; after exclusions 6 675 patients remained for the main analysis of whom 1 214 had BCR during follow-up (). Median follow-up was 5.6 years for patients without BCR (mean 6.4, max 19.9 years). Data on age, tumour characteristics and post-operative radiotherapy for all patients according to time to BCR are shown in . 491 patients died during follow-up, 64 from prostate cancer and 427 from unrelated causes. Median time from surgery to death was 8.3 and 7.2 years for death from prostate cancer and unrelated causes respectively. 334 patients had hormonal treatment, either ADT, antiandrogen or a combination of both. 80 patients developed skeletal metastasis. Annual number of radical prostatectomies increased ten-fold during the first ten years of the study and peaked in 2011 with 670 cases (). Postoperative radiotherapy was uncommon during the first half of the study period; in total 694 adjuvant or salvage treatments were given, of which 50 before 2005.
Figure 1. Flow chart of patient inclusion in the study. BCR: Biochemical recurrence; HT: Hormonal therapy; PC: Prostate Cancer.
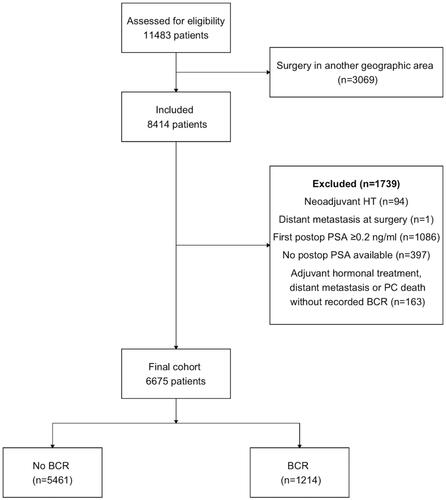
Table 1. Age, tumour characteristics and postoperative radiotherapy according to years from surgery to biochemical recurrence.
The risk for BCR was highest within the first two years after surgery, after which it plateaued and was constant up to 15 years (). BCR-free survival at 5, 10 and 15 years was 83% (95% confidence interval [CI] 82–84%), 75% (95% CI 74–77%) and 69% (95% CI 67–71%), respectively. Competing risk analysis did not show any substantial differences from the Kaplan-Meier approach (Supplementary figure 1). In the sensitivity analysis, with censoring at time of last PSA, incidence rates were largely the same for the first five years, after which they increased compared to censoring only for death (Supplementary figure 2).
Figure 3. (a) Kaplan–Meier plot of biochemical recurrence-free survival after radical prostatectomy with 95% confidence intervals. At least one postoperative PSA <0.2 mg/ml was required for inclusion, hence there are no recurrences within the first months. Curve is truncated when number at risk falls below ten. BCR – biochemical recurrence. (b) Kaplan–Meier plot of failure-free survival after BCR according to time from surgery to BCR with 95% confidence intervals. Each curve is truncated when number at risk in the group falls below ten. BCR: Biochemical recurrence.
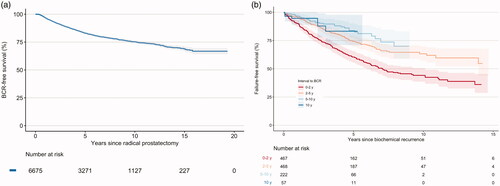
Failure after BCR occurred in all groups, regardless of time to BCR. The risk of failure was significantly higher among patients having BCR within two years from surgery compared to the other groups. Statistically significant difference was also seen between patients with 2–5 and 5–10 years to BCR. Confidence intervals for patients with >10 years to BCR were wide and the risk did not significantly differ from the other groups, except for BCR within two years (, ). Sensitivity analysis of patients having BCR from 2005 and forward showed the same relationships as when all patients were included; follow-up after BCR was shorter due to the applied restriction (Supplementary figure 3). Cumulative incidence of failure for all patients 15 years after BCR was 53% (95% CI 46–60%), vs 50% (95% CI 43–55%) in competing risk analysis; this effect was seen in all groups, except for the group with > 10 years to BCR where the incidence remained largely the same in competing risk analysis. In analysis based on clinical risk groups, there was a continued risk for BCR throughout the follow-up in all risk groups; failure after BCR also occurred in all risk groups (Supplementary figure 4a,b).
Table 2. Pairwise comparison of the distribution of failure-free survival according to category of time to biochemical recurrence as in .
Discussion
The aim of radical prostatectomy is to achieve cure from prostate cancer. In this large, population-based study, we demonstrate a continued risk for BCR throughout the study period of up to twenty years. The risk was highest within the first two years after surgery, after which it declined slightly. After five years, the risk did no longer decrease but remained the same up to 15 years. For patients alive 10 years after surgery, the risk for BCR is still substantial. Median age at radical prostatectomy in Sweden in 2016 was 65 years (interquartile range [IQR] 60–69 years) and 67 years (IQR 62–70 years), for robot assisted and open radical prostatectomy, respectively [Citation18]; life expectancy at age 65 was 19 years the same year [Citation19]. Hence, the risk for long-term BCR is relevant for a large group of patients. Patients with BCR within two years of surgery had the most unfavourable prognosis; for patients with BCR after more than two years, the risk for failure was still substantial. Even though the majority of patients with salvage hormonal treatment neither developed distant metastasis nor died from prostate cancer during follow-up, we consider such treatment as failure. Adverse effects of ADT are potentially severe, showing a wide range of manifestations including secondary morbidity and reduced quality of life [Citation20]. While side-effects of antiandrogen monotherapy are less pronounced, with exception for gynecomastia [Citation21], hormonal treatment of any kind is not consistent with cure.
In a study in 2003, Han et al showed 5, 10 and 15-year BCR-free survival rates of 84%, 72% and 61% respectively [Citation3]. Their cohort of 2091 consecutive patients underwent radical prostatectomy in one centre between 1982 and 1999. Our population-based cohort is mainly of later date, starting in the early days of PSA testing in Sweden; nevertheless, their results are comparable to our findings. In contrast, Bianco et al in 2005 showed rates of cancer control, defined as freedom from BCR or any other therapeutic interventions, similar between 10 and 15 years, 77% and 75% respectively, while the 5-year rate was 82% [Citation2]. Their cohort of 1746 patients were all undergoing surgery by one single surgeon over the course of twenty years. Due to the great heterogeneity of oncological results between surgeons [Citation22], these results are thus not necessarily applicable to an unselected population.
Van den Broeck et al. in a systematic review in 2019, concluded that there is an inverse relationship between interval to BCR and oncological outcomes after radical prostatectomy; albeit this association was not uniformly reported in included studies [Citation4]. In 2011, Boorijan et al. in a study of 2426 patients with BCR, showed that though cancer-specific mortality was higher for patients with shorter interval to BCR, this was attributable to adverse clinicopathologic features, and that time to BCR did not independently predict neither systemic progression nor prostate cancer mortality[Citation6]. As in our study, BCR was more common within the first years after surgery. Our results are not adjusted for clinicopathologic risk factors; nevertheless, this supports our findings that there were no statistically significant differences in prognosis according to interval to BCR for patients with BCR occurring more than two years after surgery.
Incomplete data on PSA-history is a limitation in our material. Exclusion due to lack of postoperative PSA values constituted 5% of the initial sample, and was in part caused by short interval from surgery to end of study. In general, availability differs between participating laboratories and is thus mainly depending on location; dropout due to lack of PSA is not considered as systematic in relation to the study endpoints. The lack of data on medication prescribed before 2005 is another limitation. Since failure is in part defined as hormonal treatment, this might falsely lengthen the interval to failure for patients with short interval to BCR who had surgery during the first half of the study period, resulting in underestimation of their risk. However, sensitivity analysis of patients having BCR from 2005 showed the same results regarding risk for subsequent progression as in the main analysis. Since there has been no standardized criteria for when hormonal treatment should be started after BCR, timing of treatment in relation to clinical progression might differ over time and between locations. We consider systematic differences related to time to BCR unlikely. Data on surgical pathology is only available for patients having surgery from 2007, for whom we lack long-term follow-up. Therefore, no adjustment for pathological features has been possible. Since this is a geographical cohort having surgery during two decades, follow-up has not been standardized and practice have varied regarding time of discontinuation. After discontinuation of PSA follow-up, BCR is likely to remain undetected until symptoms arise, resulting in underestimation of risk for recurrence when patients are censored at death or end of study. Censoring at last PSA on the other hand is likely to overestimate the risk, since patients with symptoms that may indicate recurrence will be PSA-tested whether regular follow-up have been discontinued or not. Regardless of method of censoring, we see the same pattern; the risk for recurrence persists fifteen years after surgery. We suggest that length of follow-up after radical prostatectomy should be related to remaining life expectancy, with discontinuation at a time point when treatment for BCR, if it occurs, would no longer be considered. Such an approach would agree with current guidelines regarding choice of primary therapy. Strengths of our study includes the large sample size, the population-based design and the length of follow-up.
Conclusions
Time from surgery alone can not determine a safe time point after which follow-up can be discontinued. After the first few years, BCR has similar impact on the risk of significant disease progression, regardless if five, ten or 15 years have passed since surgery. This is especially important to younger patients with a long life-expectancy.
Supplemental Material
Download TIFF Image (8 MB)Supplemental Material
Download TIFF Image (9.6 MB)Supplemental Material
Download TIFF Image (9.6 MB)Supplemental Material
Download TIFF Image (2.4 MB)Supplemental Material
Download MS Word (31.3 KB)Acknowledgement
The authors thank Associate Professor Andreas Josefsson for his efforts to initialize the WSOP database.
Disclosure statement
Johan Stranne has received lecture fees from IPSEN, Astellas, Bayer, Jansen and Ferring and worked as surgical proctor for Intuitive Surgery. Rebecka Arnsrud Godtman has received lecture fees from IPSEN and Astellas.
Additional information
Funding
References
- Mottet N, Bellmunt J, Briers E, et al. EAU – ESTRO – ESUR – SIOG guidelines on prostate cancer. Online resource. EAU Guidelines Office, Arnhem, The Netherlands. https://uroweb.org/guidelines/prostate-cancer.2022.
- Bianco FJ, Jr., Scardino PT, Eastham JA. Radical prostatectomy: long-term cancer control and recovery of sexual and urinary function (“trifecta”). Urology. 2005; 66(5 Suppl):83–94. 94.
- Han M, Partin AW, Zahurak M, et al. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol. 2003;169(2):517–523.
- Van den Broeck T, van den Bergh RCN, Arfi N, et al. Prognostic value of biochemical recurrence Following treatment with curative intent for prostate cancer: a systematic review. Eur Urol. 2019;75(6):967–987.
- Antonarakis ES, Feng Z, Trock BJ, et al. The natural history of metastatic progression in men with prostate-specific antigen recurrence after radical prostatectomy: long-term follow-up. BJU Int. 2012;109(1):32–39.
- Boorjian SA, Thompson RH, Tollefson MK, et al. Long-term risk of clinical progression after biochemical recurrence following radical prostatectomy: the impact of time from surgery to recurrence. Eur Urol. 2011;59(6):893–899.
- Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294(4):433–439.
- Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281(17):1591–1597.
- Andersen S, Richardsen E, Nordby Y, et al. Disease-specific outcomes of radical prostatectomies in Northern Norway; a case for the impact of perineural infiltration and postoperative PSA-doubling time. BMC Urol. 2014;14(1):49.
- Bolton DM, Ta A, Bagnato M, et al. Interval to biochemical recurrence following radical prostatectomy does not affect survival in men with low-risk prostate cancer. World J Urol. 2014;32(2):431–435.
- Brooke HL, Talbäck M, Hörnblad J, et al. The swedish cause of death register. Eur J Epidemiol. 2017;32(9):765–773.
- Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the swedish national inpatient register. BMC Public Health. 2011;11:450.
- Tomic K, Berglund A, Robinson D, et al. Capture rate and representativity of The national prostate cancer register of Sweden. Acta Oncol. 2015;54(2):158–163.
- Godtman R, Holmberg E, Stranne J, et al. High accuracy of swedish death certificates in men participating in screening for prostate cancer: a comparative study of official death certificates with a cause of death committee using a standardized algorithm. Scand J Urol Nephrol. 2011;45(4):226–232.
- Choudhury JB. Non-parametric confidence interval estimation for competing risks analysis: application to contraceptive data. Stat Med. 2002;21(8):1129–1144.
- Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. New York: Wiley; 1980.
- R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; 2021. https://www.R-project.org/.
- Cazzaniga W, Godtman RA, Carlsson S, et al. Population-based, nationwide registration of prostatectomies in Sweden. J Surg Oncol. 2019;120(4):803–812.
- Statistics Sweden [Internet]. Life expectancy at birth and age 65 by sex 1970–2020 and projection 2021–2070. Available from: https://www.scb.se/en/finding-statistics/statistics-by-subject-area/population/population-projections/population-projections/pong/tables-and-graphs/life-expectancy-at-birth-and-age-65-by-sex-and-projection/.
- Nguyen PL, Alibhai SMH, Basaria S, et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol. 2015;67(5):825–836.
- Wirth MP, Hakenberg OW, Froehner M. Antiandrogens in the treatment of prostate cancer. Eur Urol. 2007;51(2):306–314.
- Nyberg M, Sjoberg DD, Carlsson SV, et al. Surgeon heterogeneity significantly affects functional and oncological outcomes after radical prostatectomy in the swedish LAPPRO trial. BJU Int. 2021;127: 361–368.