Abstract
Objective
To investigate the association between surgeon volume and urinary incontinence after radical prostatectomy.
Methods
A total of 8326 men in The National Prostate Cancer Register of Sweden (NPCR) underwent robot-assisted radical prostatectomy (RARP) between 2017 and 2019 of whom 56% (4668/8 326) had responded to a questionnaire one year after RARP. The questionnaire included the question: ‘How much urine leakage do you experience?’ with the response alternatives ‘Not at all’, ‘A little’, defined as continence and ‘Moderately’, ‘Much/Very much’ as incontinence. Association between incontinence and mean number of RARPs/year/surgeon was analysed with multivariable logistic regression including age, Charlson Comorbidity Index (CCI), PSA, prostate volume, number of biopsy cores with cancer, cT stage, Gleason score, lymph node dissection, nerve sparing intent and response rate to the questionnaire.
Results
14% (659/4 668) of the men were incontinent one year after RARP. There was no statistically significant association between surgeon volume and incontinence. Older age (>75 years vs. < 65 years, OR 2.29 [95% CI 1.48–3.53]), higher CCI (CCI 2+ vs. CCI 0, OR 1.37 [95% CI 1.04–1.80]) and no nerve sparing intent (no vs. yes OR 1.53 [95% CI 1.26–1.85]) increased risk of incontinence. There were large differences in the proportion of incontinent men between surgeons with similar annual volumes, which remained after adjustment.
Conclusions
The lack of association between surgeon volume and incontinence and the wide range in outcome between surgeons with similar volumes underline the importance of individual feedback to surgeons on functional results.
Introduction
Postoperative urinary incontinence is a common side effect after radical prostatectomy that substantially reduces quality of life [Citation1,Citation2]. In a systematic review, postoperative incontinence rates ranged from 4 to 31% depending on definition of incontinence [Citation3]. The range in the proportion of affected men has been reported to be due to differences in surgical technique, surgeon experience as well as definition of incontinence and time between surgery and assessment [Citation3–6]. Other factors that affect the proportion of men who are incontinent after radical prostatectomy include age, comorbidity, pre-operative membranous urethra length and prostate volume [Citation7].
Previously, we reported on the association between surgeon volume, i.e. number of robot-assisted radical prostatectomy (RARP’s) performed per year, and short-term outcomes including operative time, blood loss, nerve-sparing intent, negative surgical margins and readmission after RARP based on data in the National Prostate Cancer Register (NPCR) of Sweden [Citation8]. In brief, we found that the surgeons with the highest volume had, compared to those with the lowest volume, shorter operative time, less blood loss, more often applied a nerve-sparing technique and a higher proportion of negative surgical margins.
The aim of this study was to investigate the association between annual surgeon volume and risk of urinary incontinence one year after RARP by use of data from an electronic questionnaire on patient-reported outcome measures (ePROM) in NPCR.
Material and methods
The NPCR of Sweden is a nation-wide clinical cancer register with almost complete capture of all men with newly diagnosed prostate cancer in Sweden. NPCR has a capture rate of 98%, compared to the Swedish Cancer Registry to which reporting is mandated by law [Citation9]. NPCR contains data on cancer characteristics, work-up and primary treatment. Since 2015, a radical prostatectomy form, containing information on pre-, peri- and post-operative data is collected [Citation10]. Patient-reported outcomes have been collected in NPCR since 2008 for men who undergo radical prostatectomy and radical radiotherapy. Until 2018 this was done by use of a paper form that was sent by post to the patient. In 2018, an electronic online questionnaire (ePROM) was introduced. The form contains 35 questions on information/participation, general health, lower urinary tract function, bowel function, sexual function and erectile function (English version available at http://npcr.se/in-english).
Men are asked by staff at each department to fill out the ePROM at baseline before treatment. An invitation is sent from the national support centre for NPCR to all men including those who did not fill out the baseline form to fill out an ePROM one year after treatment. The invitation is triggered by the date for surgery registered in NPCR.
In NPCR, surgeons are identified by an individual code and the code key is kept at each department. A large majority of urologists in Sweden perform prostatectomy at one hospital, where he/she is employed, but a few surgeons perform surgeries at multiple hospitals and will have several codes, which we could not account for.
To investigate the association between surgeon volume and incontinence we used data from the ePROM filled 1 year after RARP performed between 1 January 2017 and 31 December 2019. In 2021, less than 7% of all radical prostatectomies were performed with open retropubic or laparoscopic technique therefore we only included ePROM collected after RARP.
Postoperative incontinence was investigated by use of three questions in the ePROM form.
Our primary endpoint was the response to the question: ‘How much urine leakage do you experience?’ The alternatives ‘Not at all’, ‘A little’ defined continence and the alternatives ‘Moderately’, ‘Much/Very much’ defined incontinence.
We also analysed two other questions within the same domain:
‘Do you have urine leakage?’ The alternatives for response were ‘Never’, ‘I leak sometimes when coughing, sneezing, and/or I use a pad when I must exert myself, e.g. sports, work in the garden or yard’, which defined continence and the alternatives ‘I use pads all the time (except possibly during the night), but they are not always wet’, ‘I use pads all the time and must change them because they are wet’, ‘I leak continuously and need large pads or diapers that must be changed continuously’, which defined incontinence.
‘How many pads do you use per 24 hours due to urinary leakage?’ ‘The alternatives for response were:’ ‘I do not use pads’, ‘Less than 1 per 24 hours’ which defined continence and the alternatives ‘Approximately 1 per 24 hours’, ‘Approximately 2 per 24 hours’, ‘Approximately 3-4 per 24 hours’, ‘Approximately 5 or more per 24 hour’ defined incontinence.
We also evaluated bother with this question: ‘If you were to live the rest of your life with your urinary tract function just as it is now, how would you experience this?’ with the responses: ‘It would not bother me at all’, ‘It would bother me a little’, ‘It would bother me moderately’ and ‘It would bother me very much’.
Information on comorbidity was collected by linkage with the Patient Registry in Prostate Cancer data Base Sweden (PCBaSe) RAPID 2019, and Charlson Comorbidity Index (CCI) was calculated as previously described [Citation11,Citation12].
The use of data in PCBaSe RAPID 2019 has been approved by the Research Ethics Authority.
Statistical analyses
We used the cut-offs for continence/incontinence currently used in the feedback to departments in ‘What’s going on’ reported by NPCR at the INCA platform [Citation13]. Surgical volume groups were determined and calculated as previously described [Citation8,Citation14]. In brief, the mean number of procedures per year was calculated by dividing the accumulated number of procedures in 2017–2019. Cut-off values for surgeon volume groups were chosen as multiples of the recommended minimum number of radical prostatectomies per surgeon and year (n = 25) in the Swedish guidelines for prostate cancer [Citation14]. The Multiple Imputation Chained Equations (MICEs) method [Citation15] was used to impute missing data on PSA, prostate volume, number of positive biopsy cores, cT stage, Gleason score, extent of lymph node dissection and nerve sparing intent. The following additional variables were included to improve predictions: region, patient age at surgery, year of surgery, total mm of cancer in biopsy cores, number of biopsies with cancer, response rate to ePROM for men operated by each surgeon, vital status and time from surgery to death or 31 October 2020, whichever event came first. Data were imputed 20 times and results from the models fitted to each dataset were pooled using Rubin’s rules [Citation16].
Univariable logistic regression was used to calculate odds ratios (ORs) for the outcomes. In the multivariable logistic regression model, we included known risk factors for incontinence; age at surgery, comorbidity, PSA, prostate volume, number of positive biopsy cores, cT stage, Gleason score, extent of lymph node dissection, nerve-sparing intent and questionnaire response rate. The result from the multivariable logistic regression models were subsequently used to construct covariate-adjusted funnel plots using the method proposed by Spiegelhalter [Citation17] with the type of symbol indicating if the value was inside or outside of the confidence intervals (CIs) instead of funnels. The reason for this was that the position of a point on the x-axis represented the surgical volume of the individual surgeon while the volume used in calculating the CIs was the number of measuring points, i.e. the number of operated men who had filled the ePROM. Surgeons with less than 10 procedures were excluded in order to calculate CIs. All statistical analyses were performed in R Statistical Software version 4.0.3 (Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 8326 men underwent RARP between 1 January 2017 and 31 December 2019 according to NPCR. Of those, 4668 men (56%) had filled the ePROM one year after RARP and were included in the analysis (). Men were included even if they had not filled the ePROM before RARP. This selection was used based on the fact that incontinence before RARP was low (2%) and that we wanted to include as many men as possible in our analysis (Supplementary Table 1). The 135 surgeons who had less than five RARP per year registered in NPCR were excluded from further analyses (). The analysed RARPs were performed at 26 hospitals and by 83 surgeons. The number of RARPs in each surgeon volume group was: very low volume surgeons 538, low volume 946, intermediate 3246, high 2 161 and very high 1 089.
Figure 1. Flow chart of men in The National Prostate Cancer Register of Sweden who underwent robot-assisted radical prostatectomy in 2017–2019. Number of hospitals included = 26 Number of surgeons included = 83.
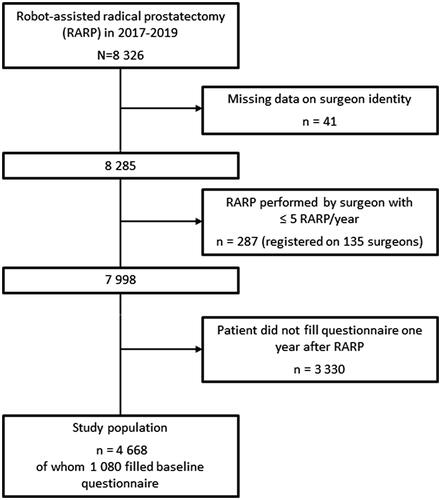
Patient and tumour characteristics for the 4668 men who filled the ePROM one year after RARP were similar to all operated men (n = 7 998) and to the 1080 men who had filled both the ePROM before and one year after RARP (). Median age at date of surgery was 66 years (IQR 61–70 years), 60% had cT1 disease and 81% had CCI = 0, i.e. no registered comorbidities (). Men who underwent RARP by surgeons who performed less than 5 RARPs/years had similar baseline characteristics as men in the analysis (data not shown).
Table 1. Baseline characteristics according to response/non-response to a PROM questionnaire for men in The National Prostate Cancer Register who underwent robot-assisted radical prostatectomy 2017–2019.
According to our primary definition, 14% (659/4668) were incontinent one year after RARP (). For the secondary definitions, the proportion of incontinent men was 27% (‘Do you have urinary leakage?’) and 32% (‘How many pads do you use per 24 h due to urinary leakage?’) (Supplementary Table 2). shows the results for the primary definition of incontinence for each individual surgeon (Supplementary Figure 1 for secondary definitions). Men who underwent RARP performed by surgeons with less than 5 RARPs/years had an incontinence rate of 17% according our primary definition, i.e. very similar to the proportion in our main analysis.
Figure 2. Bar plot of urinary incontinence one year after robot-assisted radical prostatectomy for individual surgeon according to the primary definition of urinary leakage. Incontinence based on the question ‘How much urine leakage do you experience?’ The alternatives ‘Not at all’, ‘A little’ defined continence and the alternatives ‘Moderately’, ‘Much/Very much’ defined incontinence. Each surgeon is represented by a bar.
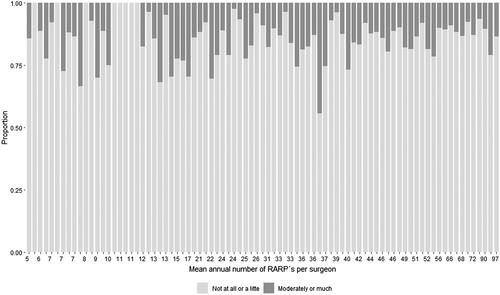
Table 2. Question on urinary leakage (primary definition) in the ePROM questionnaire in The National Prostate Cancer Register.
Patient-reported urinary tract bother increased with increasing incontinence. The proportion of men who experienced moderate or much bother were 2, 15, 77 and 94% in those who reported no, little, moderate or much leakage, respectively. In contrast, for pad use, there was no distinct threshold for the proportion of men who experienced moderate/much bother (Supplementary Table 3).
In univariable analysis, prostate volume, PSA-density, Gleason score and extended lymph node dissection were factors associated with incontinence but these associations were no longer statistically significant in multivariable analysis (). In multivariable analysis, only age, comorbidity status, PSA and no nerve sparing intent remained significantly associated with incontinence (). Men older than 75 years compared to men younger than 65 years were more likely to be incontinent postoperatively, OR incontinence 2.29 (95% CI 1.48–3.53). Men with more comorbidities were also more likely to be incontinent (CCI 2+ vs. CCI 0, OR 1.37 [95% CI 1.04–1.80]). A moderately elevated PSA was associated with a reduced risk for incontinence compared to a low PSA (PSA 3–10 ng/ml vs. PSA < 3 ng/ml, OR 0.64 [95% CI 0.45–0.92]). No nerve-sparing intent was associated with incontinence (OR 1.53 [95% CI 1.26–1.85]). In contrast, there was no statistically significant association between surgeon volume and incontinence (). Results were similar for the secondary definitions of incontinence (Supplementary Table 4).
Table 3. Risk of urinary leakage (primary definition) 1 year after robot-assisted radical prostatectomy according to PROM questionnaire.
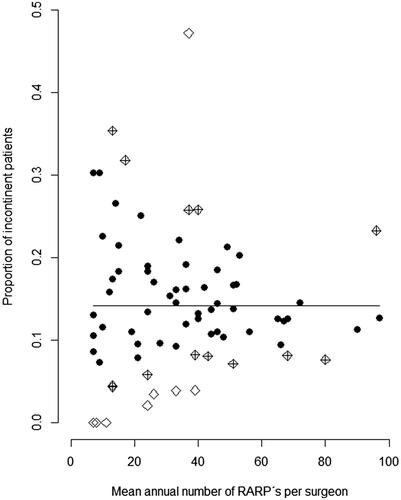
shows plots for the primary definition of incontinence adjusted for putative confounders and Supplementary Figure 2 shows results for the two secondary definitions. There was a wide range in the proportion of continent men also within same volume group across the whole range of surgeon volumes. Fifteen out of 83 surgeons had a lower proportion of urinary leakage than expected, and six surgeons had a higher proportion than expected, including one surgeon with a mean annual case load of 100 (). Ranges in outcomes were wider for the two secondary definitions of incontinence.
Figure 3. Adjusted proportions of robot-assisted radical prostatectomies with incontinence one year after surgery according to surgical volume by individual surgeon. Incontinence based on the question ‘How much urine leakage do you experience?’ Alternatives ‘Not at all’, ‘A little’ defined continence and the alternatives ‘Moderately’, ‘Much/Very much’ defined incontinence. The plot is based on the results from the multivariable model that included age at RARP, CCI, PSA, prostate volume, PSA density, number of positive biopsy cores, cT stage, Gleason score, extent of lymph node dissection, nerve sparing intent, and questionnaire response rate. Surgeons with less than 10 observations were excluded in order to construct confidence intervals. The symbol indicates if the point lies inside (dot) or outside (rhombus) of the 95% confidence interval (CI) for urinary leakage. Rhombi denote surgeons with a lower/higher than expected proportion of urinary leakage, crossed rhombi 95% CI and empty rhombi 99.9% CI.
Discussion
In this population-based study in Sweden, 14% of men reported urinary incontinence in an ePROM one year after RARP. Old age, presence of comorbid conditions, low PSA and non-nerve sparing technique were associated with a higher risk of incontinence whereas annual surgeon volume was not. The range in proportion of incontinent men was wide for all surgeon volume groups, indicating that factors other than volume are more important.
This is the first report based on ePROMs in NPCR and one of very few nation-wide, population-based register studies with PROM data on postoperative incontinence [Citation18,Citation19]. Strengths of our study include the large number of RARP’s (n = 4668) performed by 83 surgeons in 26 hospitals in a contemporary era; 2017–2019 with detailed data on cancer characteristics. We applied three definitions of incontinence and also related incontinence to the degree of bother. Importantly, the ePROM forms were sent out from an independent third party, NPCR, not by the surgeon or his/her department, and men filled the follow-up ePROM on their own at home. In large, nation-wide registers there are always some errors and missing data. We therefore excluded surgeons with less than 5 RARPs/years since these registrations had a high risk of being erroneous registrations of RARP at hospitals where there is no surgical robot or the RARP was performed by a visiting high-volume surgeon. Only half of the men who underwent RARP in Sweden during the study period filled the ePROM at one year after RARP. Since patient and cancer characteristics were similar in responders and non-responders we argue that the relatively low response rate did not bias the results. The ePROM questionnaire has not been formally validated but has been used for many years in NPCR. It is based on the principle of ‘one question, one symptom’ and was developed for the purpose of quality assurance. Another limitation is that NPCR lacks information on individual surgeon volume prior to 2015.
The lack of association between surgeon volume and incontinence in our study is in contrast to many previous studies that have reported a positive association [Citation4,Citation6,Citation20,Citation21]. A recent meta-analysis of 13 studies published between 2003 and 2020 investigated the association between surgeon volume and postoperative continence [Citation6]. Of the 13 studies, 10 reported that surgeons with higher annual volumes achieved better continence results. There were only three prospective studies and of those, two did not show any significant association. Speculatively, the lack of association between surgeon volume and incontinence in our study may be due to that surgical experience prior to the study period was as important as the annual caseload during the study period. Results from complex surgery continue to improve after the surgeon has performed hundreds of procedures [Citation22,Citation23] and the learning curve for incontinence had not reached a plateau after more than 200 RARPs [Citation24,Citation25]. Another explanation could be that there are other factors not captured in our study, such as surgical skill that are more important for incontinence than surgeon volume [Citation26].
The proportion of men who were incontinent 1 year after surgery in this study is higher than in many other series [Citation3,Citation27,Citation28]. This is a reflection of the inclusion of all men who underwent RARP in all hospitals in Sweden. We argue that our results are representative for outcomes after surgery in an entire country, in contrast to studies that have been based on data from tertial centres of excellence [Citation3]. Another population-based study using PROM data, from the English National Prostate Cancer Audit Database was recently published [Citation19]. Measured with the EPIC-26’s incontinence score and perception of urinary bother, 9% of men reported to have a ‘bad’ urinary incontinence score and 4% also reported that they had a ‘big’ problem with their urinary function. In our study, 4% of men reported to have much urinary leakage and 9% had very much bother. In the Swedish LAPPRO study, which used a definition of incontinence as having to change pad once or more per 24 h, 21% were incontinent one year after RARP [Citation27]. The corresponding number in our study was 32%. Although these two studies were carried out in similar settings there were some important differences. In LAPPRO the researchers strived to include high-volume centres, seven RARP hospitals were included compared to 26 in our study, and in LAPPRO, only surgeons who had performed >100 procedures were included and RP’s were performed between 2008 and 2011. Moreover, the pad question in the two studies was slightly different (change of pad vs. usage of pads). A striking finding in both these studies is that the proportion of incontinent men was highly dependent on definition. This number ranged from 14 to 32% in our study and from 18 to 56% in the LAPPRO study for different definitions of incontinence in accordance with the wide range in a systematic review [Citation3].
Similar to the LAPPRO trial [Citation5], we found a strong association between nerve-sparing technique and postoperative continence. It is a matter of debate if it is the nerve-sparing per se or rather the meticulous apical dissection that is part of nerve-sparing technique, that is of importance for continence recovery [Citation28]. Regardless of the exact mechanism, the efforts for preserving as much as possible of the neurovascular bundles should not be any lower in older or not sexually active men, since age and comorbidity were strong risk factors for incontinence.
Men with low PSA paradoxically had higher risk for incontinence than men with moderately elevated PSA despite that these men had similar demographics, cancer characteristics and comorbidities as the full study population (data not shown).
Patient-reported urinary bother increased with larger leakage volumes regardless if leakage was estimated as no/little/moderately/much or by the number of pads. There was a large difference in the proportion of men with moderately/much bother between those who reported little compared to moderate leakage (15 vs. 77%), which supports our choice of definition of incontinence. However, there was a fraction of men (15%) who reported little leakage but moderate/much bother. This could be due to that the bother question was not focused on bother due to leakage but rather overall urinary tract function or that even a small amount of leakage is bothersome for some men.
There was a large range in outcome also between surgeons who performed the same number of RARPs showing that centralising radical prostatectomies to high-volume surgeons needs to be supplemented with patient-reported functional outcomes in questionnaires from an independent third party. Such data are collected in NPCR and are displayed online at the secured INCA platform. ePROM data is crucial for individual feed-back to the surgeon and should also be used in patient consultations before and after RARP. These data should also be used for benchmarking between surgeons, hospitals and regions.
Conclusions
The large difference in the proportion of men with postoperative urinary incontinence between surgeons with the same annual volume of RARPs indicates that centralisation of surgery is not sufficient to improve quality of care but that use of ePROM on functional outcome is needed to improve surgical technique. Efforts should therefore be made to improve collection of ePROM data and to continuously use these data for quality improvement.
Supplemental Material
Download Zip (97.4 KB)Acknowledgements
This project was made possible by the continuous work of the National Prostate Cancer Register of Sweden (NPCR) steering group: Pär Stattin (chair), Ingela Franck Lissbrant (co-chair), Camilla Thellenberg, Eva Johansson, Magnus Törnblom, Stefan Carlsson, David Robinson, Mats Andén, Ola Bratt, Jonas Hugosson, Maria Nyberg, Per Fransson, Fredrik Sandin, Karin Hellström, Gert Malmberg and Hans Joelsson.
Disclosure statement
RAG has received lecture fees and reimbursements for travel expenses from IPSEN and Astellas. All other authors report no conflict of interest in any stage of this research work.
Data availability statement
The dataset for this study was pseudonymised, the personal identity number for men in the PCBase RAPID have been deleted and replaced by a code. The code key is kept at the National Board of Health and Welfare. Due to the large number of variables, and since there is a code key, this dataset is considered not to be fully anonymised. The following restrictions therefore apply: we are not allowed to share data on individual study subjects with other researchers, nor to upload such data on an open server. However, we can provide access to the dataset on a remote server on demand where analyses can be performed and aggregated data in Figures and Tables can be exported, but no data on individual participants can be exported. Researchers apply for collaborations based on data in PCBase contact: [email protected]. After approval, a study file will be uploaded to a remote access server. Users will be charged for software licenses, administration, and data management.
Additional information
Funding
References
- Resnick MJ, Koyama T, Fan KH, et al. Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med. 2013;368(5):436–445.
- Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358(12):1250–1261.
- Ficarra V, Novara G, Rosen RC, et al. Systematic review and Meta-analysis of studies reporting urinary continence recovery after robot-assisted radical prostatectomy. Eur Urol. 2012;62(3):405–417.
- Begg CB, Riedel ER, Bach PB, et al. Variations in morbidity after radical prostatectomy. N Engl J Med. 2002;346(15):1138–1144.
- Steineck G, Bjartell A, Hugosson J, et al. Degree of preservation of the neurovascular bundles during radical prostatectomy and urinary continence 1 year after surgery. Eur Urol. 2015;67(3):559–568.
- Trieu D, Ju IE, Chang SB, et al. Surgeon case volume and continence recovery following radical prostatectomy: a systematic review. ANZ J Surg. 2021;91(4):521–529.
- Lardas M, Grivas N, Debray TPA, et al. Patient- and tumour-related prognostic factors for urinary incontinence After radical prostatectomy for nonmetastatic prostate cancer: a systematic review and Meta-analysis. Eur Urol Focus. 2022;8(3):674–689.
- Godtman RA, Persson E, Cazzaniga W, et al. Association of surgeon and hospital volume with short-term outcomes after robot-assisted radical prostatectomy: nationwide, population-based study. PLoS One. 2021;16(6):e0253081.
- Tomic K, Sandin F, Wigertz A, et al. Evaluation of data quality in the national prostate cancer register of Sweden. Eur J Cancer. 2015;51(1):101–111.
- Cazzaniga W, Godtman RA, Carlsson S, et al. Population-based, nationwide registration of prostatectomies in Sweden. J Surg Oncol. 2019;120(4):803–812.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Van Hemelrijck M, Wigertz A, Sandin F, et al. Cohort profile: the national prostate cancer register of Sweden and prostate cancer data base Sweden 2.0. Int J Epidemiol. 2013;42(4):956–967.
- The national prostate cancer register of Sweden, NPCR. 2021. Available from: https://statistik.incanet.se/npcr/
- The confederation of Regional Cancer Centres (RCC). National prostate cancer guidelines (In Swedish). Version 6.1 [cited 22-09-2021]. 2021. Available from: https://kunskapsbanken.cancercentrum.se/globalassets/cancerdiagnoser/prostatacancer/vardprogram/nationellt-vardprogram-prostatacancer.pdf
- Van Buuren S. Flexible imputation of missing data. Boca Raton (FL): CRC Press; 2012.
- Rubin DB. Imputation of nonresponse in surveys. New York (NY): Wiley; 1987.
- Spiegelhalter DJ. Funnel plots for comparing institutional performance. Stat Med. 2005;24(8):1185–1202.
- Nossiter J, Morris M, Cowling TE, et al. Hospital volume and outcomes after radical prostatectomy: a national population-based study using patient-reported urinary continence and sexual function. Prostate Cancer Prostatic Dis. 2021. DOI: 10.1038/s41391-021-00443-z.
- Parry MG, Skolarus TA, Nossiter J, et al. Urinary incontinence and use of incontinence surgery after radical prostatectomy: a national study using patient-reported outcomes. BJU Int. 2021;130:84–91.
- Collette ERP, Klaver SO, Lissenberg-Witte BI, et al. Patient reported outcome measures concerning urinary incontinence after robot assisted radical prostatectomy: development and validation of an online prediction model using clinical parameters, lower urinary tract symptoms and surgical experience. J Robot Surg. 2021;15(4):593–602.
- Nam RK, Herschorn S, Loblaw DA, et al. Population based study of long-term rates of surgery for urinary incontinence after radical prostatectomy for prostate cancer. J Urol. 2012;188(2):502–506.
- Hopper AN, Jamison MH, Lewis WG. Learning curves in surgical practice. Postgrad Med J. 2007;83(986):777–779.
- Grivas N, Zachos I, Georgiadis G, et al. Learning curves in laparoscopic and robot-assisted prostate surgery: a systematic search and review. World J Urol. 2021;40:929–949.
- Fossati N, Di Trapani E, Gandaglia G, et al. Assessing the impact of surgeon experience on urinary continence recovery after robot-assisted radical prostatectomy: results of four high-volume surgeons. J Endourol. 2017;31(9):872–877.
- Thompson JE, Egger S, Bohm M, et al. Superior biochemical recurrence and long-term quality-of-life outcomes are achievable with robotic radical prostatectomy After a long learning Curve-Updated analysis of a prospective single-surgeon cohort of 2206 consecutive cases. Eur Urol. 2018;73(5):664–671.
- Nyberg M, Sjoberg DD, Carlsson SV, et al. Surgeon heterogeneity significantly affects functional and oncological outcomes after radical prostatectomy in the swedish LAPPRO trial. BJU Int. 2021;127(3):361–368.
- Haglind E, Carlsson S, Stranne J, et al. Urinary incontinence and erectile dysfunction After robotic versus open radical prostatectomy: a prospective, controlled, nonrandomised trial. Eur Urol. 2015;68(2):216–225.
- Michl U, Tennstedt P, Feldmeier L, et al. Nerve-sparing surgery technique, not the preservation of the neurovascular bundles, leads to improved long-term continence rates after radical prostatectomy. Eur Urol. 2016;69(4):584–589.
