Abstract
Background
99mTc-Sestamibi Single Photon Emission Computed Tomography/Computed Tomography (SPECT/CT) contributes to the non-invasive differentiation of renal oncocytoma (RO) from renal cell carcinoma (RCC) by characterising renal tumours as Sestamibi positive or Sestamibi negative regarding their 99mTc-Sestamibi uptake compared to the non-tumoral renal parenchyma.
Purpose
To determine whether 99mTc- Sestamibi uptake in renal tumour and the non-tumoral renal parenchyma measured using Standard Uptake Value (SUV) SPECT, has a beneficial role in differentiating RO from RCC.
Material and Methods
Fifty-seven renal tumours from 52 patients were evaluated. In addition to visual evaluation of 99mTc-Sestamibi uptake, SUVmax measurements were performed in the renal tumour and the ipsilateral non-tumoral renal parenchyma. Analysis of the area under the receiver operating characteristic curve identified an optimal cut-off value for detecting RO, based on the relative ratio of 99mTc- Sestamibi uptake.
Results
Semiquantitative evaluation of 99mTc-Sestamibi uptake did not improve the performance of 99mTc- Sestamibi SPECT/CT in detecting RO. 99mTc- Sestamibi SPECT/CT identifies a group of mostly indolent Sestamibi-positive tumours with low malignant potential containing RO, Low-Grade Oncocytic Tumours, Hybrid Oncocytic Tumours, and a subset of chromophobe RCCs.
Conclusion
The imaging limitations for accurate differentiation of Sestamibi-positive renal tumours mirror the recognised diagnostic complexities of the histopathologic evaluation of oncocytic neoplasia. Patients with Sestamibi-positive renal tumours could be better suited for biopsy and follow-up, according to the current active surveillance protocols.
Introduction
Benign and malignant solid renal tumours overlap in their standard imaging features [Citation1]. The inability to accurately diagnose benign renal neoplasia results in up to 30% of unnecessary partial nephrectomies [Citation2]. Currently, it is not possible to preoperatively differentiate between different types of solid renal tumours using the existing molecular imaging techniques [Citation3,Citation4]. The introduction of 99mTc-Sestamibi single photon emission computed tomography/computed tomography (SPECT/CT) improves the characterisation and the distinction of solid renal tumours, as demonstrated in the recent meta-analysis by Wilson et al. [Citation5]. 99mTc-Sestamibi SPECT/CT, followed by renal biopsy, improved the management of benign kidney tumours, such as renal oncocytoma (RO), by reducing unnecessary surgeries [Citation6] and by introducing cost-saving efficiencies compared with the established clinical routines [Citation7].
Histopathological and molecular characterisation of renal neoplasms is continuously evolving, as reflected in the 2016 and 2022 World Health Organization (WHO) classification by introducing new, provisional and emerging renal entities [Citation8–10]. For example, clear cell papillary RCC (ccpRCC), a tumour of low malignant potential, is now classified as a separate entity [Citation11]. The so-called “hybrid chromophobe oncocytic tumour” (HOCT), showing features that overlap between RO and chromophobe renal cell carcinoma (chRCC), considered a subtype of chRCC in the 2016 WHO classification, has been found to demonstrate a molecularly variable profile, with genomic features intermediate between RO and chRCC [Citation12]. Other provisional and emerging entities, such as low-grade oncocytic tumour (LOT) and eosinophilic vacuolated tumour (EVT), are also expanding the spectrum of renal oncocytic neoplasia [Citation13]; both entities have been recently listed in the 2022 5th edition of WHO classification as “emerging renal entities” [Citation10]. For example, LOT is an indolent, typically sporadic and solitary tumour [Citation14]; rare cases have been documented in tuberous sclerosis complex (TSC) patients [Citation15,Citation16]. Although it has some similar morphologic features to RO and ChRCC, it also has some differences and an unusual CK7 positive/CD117 negative immunoprofile [Citation17]. Recent studies have found frequent TSC/MTOR mutations in LOT, but a uniform lack of multiple chromosomal losses, as seen in ChRCC, has not been found [Citation15,Citation16].
Prior studies have published findings regarding the visual differentiation of RO from RCC utilising 99mTc-Sestamibi SPECT/CT [Citation18] in line with the previous findings by Gorin et al. [Citation19]. According to the initial hypothesis of Rowe et al. [Citation20], renal tumours, such as RO that demonstrate greater mitochondrial content, exhibit an increased uptake of 99mTc-Sestamibi, given that Sestamibi acts as a mitochondrial agent. Therefore, renal tumours with increased 99mTc-Sestamibi uptake can be characterised as ‘Sestamibi-positive’. In contrast, renal tumours with decreased 99mTc-Sestamibi uptake can be considered ‘Sestamibi-negative’.
In the present study, we sought to evaluate if implementing additional quantitative tools will aid in the visual assessment of 99mTc-Sestamibi SPECT/CT in patients with solid renal tumours and whether this could improve the diagnostic performance in differentiating RO from other RCCs. In particular, we investigated if standardised uptake value (SUV) SPECT could strengthen the preoperative characterisation of solid renal tumours.
Material and methods
Study design and image acquisition
This explorative, non-randomised prospective study started in September 2015 [Citation18] and ended in September 2019. All eligible candidates were discussed at the kidney tumour conference in the Radiology Department of Karolinska University Hospital, Huddinge. Patients with T2, T3 or T4 renal tumours, renal tumours > 7 cm in maximum diameter, and/or patients with metastatic disease were not included in the study. Patients who agreed to participate underwent a 99mTc-Sestamibi SPECT/CT examination before nephrectomy or renal biopsy, following the same imaging protocol previously described and published by our group [Citation21]. In brief, a CT scan was performed after a SPECT acquisition with projections reconstructed using Hermes SUV SPECT Hybrid Recon™ Oncology software (v.1.2) (HERMES Medical Solutions AB, Stockholm, Sweden). To enable quantitative evaluation, we collected the available data on syringe activity before administration, syringe residual activity post-administration, patient weight, time points of injection, and scan start, all used in the reconstruction software.
Evaluation of 99mTc-Sestamibi SPECT/CT examination
Two readers independently and simultaneously performed the visual evaluation: a Consultant in Radiology and Nuclear Medicine and a Consultant in Radiology. To mitigate the effects of interobserver variation, we redefined the uptake of 99mTc-Sestamibi in the examined renal tumour as follows: a tumour was classified as Sestamibi-positive if the 99mTc-Sestamibi uptake was visually higher compared to the ipsilateral normal renal parenchyma. If the 99mTc-Sestamibi uptake in the tumour was visually equal to or lower than the ipsilateral renal parenchyma, the tumour was classified as a Sestamibi-negative. In cases with a disagreement between the readers, a third reader was added, and all three independently performed a blind re-evaluation of these cases.
The two main readers also performed SUVmean and SUVmax measurements in the renal tumour, as well as assessed the ipsilateral non-tumoral renal parenchyma. Freehand regions of interest (ROIs) of complete tumours were drawn in the axial CT images of the SPECT/CT examination. Volumes of interest (VOIs) were automatically generated based on the manually drawn ROIs. The VOIs were copied onto the SPECT images, and the SUV parameters were annotated. To obtain SUVmean and SUVmax values, fixed 1 cm3 VOIs spheres were similarly placed in the regions of high renal 99mTc-Sestamibi uptake in the ipsilateral non-tumoral renal parenchyma. We excluded the SUVpeak measurements because many renal tumours were too small (volume approximately 1 cm3 or less) to be evaluated using this parameter because, by definition, SUVpeak represents the maximum activity concentration in 1 cm3 volume.
Histopathological analysis of 99mTc-Sestamibi SPECT/CT examined renal tumours & immunohistochemical investigation of renal oncocytic tumours
In cooperation with the Clinical Pathology-Cytology Department of our hospital, the diagnostic tissue material of the study (i.e. surgical resections and/or biopsies) was re-evaluated. Two Consultant Pathologists independently and simultaneously reviewed the haematoxylin & eosin (H&E), as well as the immunohistochemical (IHC) slides of all tumours in a blinded manner and without any knowledge of the prior visual or quantitative evaluation of the 99mTc-Sestamibi SPECT/CT dataset. The confirmed histopathological diagnoses and/or updated diagnoses, based on consensus, were used as the gold standard to correlate with the results from 99mTc-Sestamibi SPECT/CT examinations. A third expert Urologic Pathologist was subsequently asked to blindly evaluate all chRCCs included in this study to further help assess the observed in situ metabolomic differences in the Sestamibi-positive versus Sestamibi-negative chRCCs, as demonstrated in our previous study [Citation22].
Statistical analysis
Intra Class Correlation (ICC) was calculated to assess the intra-reader reliability of SUVmean and SUVmax SPECT measurements. We calculated the average value of SUVmean measurements from the two readers per case. The same was done for SUVmax. The ratio of the 99mTc- Sestamibi uptake (the uptake in the renal tumour/the uptake in the ipsilateral non-tumoral renal parenchyma) was also calculated for each case. To illustrate the trade-off in sensitivity and specificity in detecting RO, we analysed the area under the receiver operating characteristic curve (AUC) to identify an optimal cut-off value based on the relative 99mTc- Sestamibi uptake. To illustrate the results, each tumour category was designated with a letter (A-J, Supplementary Figure 1).
Results
In total, 58 renal masses from 52 patients were included in the study between 2015 and 2019; of note, four patients had multiple and/or bilateral renal lesions. Thirty-seven out of 58 (64%) renal tumours were resected with radical or partial nephrectomy, while the remaining 21 (36%) were biopsied (7 ccRCCs, 4 chRCCs, 3 ccpRCCs, 3 RO, 2 pRCCs and 2 HOCTs).
Histopathological & immunohistochemical assessment
Five renal tumours were re-classified upon re-evaluation by consensus, as follows: 4 RO were re-classified as chRCC and HOCT (2 cases each), while one pRCC (that in our previous pilot study [Citation18] exhibited slightly increased 99mTc- Sestamibi uptake) was re-classified as chRCC. One tumour initially characterised as HOCT in our previous pilot study [Citation18] actually corresponded to normal renal parenchyma. It was excluded from further analysis, thus reducing the final tumour cohort to 57. The patient and tumour characteristics are presented in . All amended diagnoses were immediately communicated to the referral urologists for appropriate clinical management and follow-up, whenever necessary. Accordingly, the final diagnoses were established (in decreasing order of frequency) as follows: 13 ccRCCs, 11 chRCCs, 11 RO (one with adjacent) papillary adenoma, 9 pRCCs, 5 HOCTs, 4 clear cells papillary RCCs (1 with adjacent papillary adenoma), 1 “collision tumour” comprising chRCC and pRCC, 1 B cell non-Hodgkin’s (follicular) lymphoma, 1 metanephric adenoma and 1 angiomyolipoma.
Table 1. Patient and renal tumour characteristics.
Visual evaluation of 99mTc-Sestamibi SPECT/CT examinations
Agreement on the visual evaluation of 99mTc-Sestamibi uptake was observed in 51 out of 57 (90%) solid renal tumours. In 6 (10%) cases, the two readers disagreed; however, a complete agreement and a final consensus were reached in all 6 cases when a third reader was included in the assessment (as previously explained). The results of the visual assessment are presented in , showing 82% sensitivity and 76% specificity in the detection of RO. When clustering RO and HOCT together, the above-mentioned performance of 99mTc-Sestamibi SPECT/CT reached 88% sensitivity and 85% specificity.
Table 2. Visual evaluation of 99mTc-Sestamibi uptake on 57 solid renal tumours.
Quantitative SUV SPECT measurements
Similar to our previous study [Citation21], the ICC for SUVmax and SUVmean measurements of the examined tumours showed a high agreement between the two readers of 88% and 94%, respectively. Likewise, the ICC between the readers, when measuring SUV SPECT parameters in the ipsilateral non-tumoral renal parenchyma showed moderate agreement, as shown in .
Table 3. Intra Class Correlation (ICC) for SUV SPECT measurements between readers on the renal tumour and the non-tumoral renal parenchyma, with a Confidence Interval of 95%.
Analysis of AUC based on the ratio of the relative 99mTc-Sestamibi uptake was performed by sub-clustering RO as a Sestamibi-positive subgroup. At the same time, all remaining tumour types were considered as a Sestamibi-negative subgroup. Optimal cut-off values for the characterisation of tumours as Sestamibi-positive vs Sestamibi-negative were obtained using the closest-top left criterion. The ratio of relative 99mTc-Sestamibi uptake based on SUVmax measurements of the tumour (T) vs non-tumoral renal parenchyma (N) demonstrated better performance (AUC: 0.787 [95% CI: 0.644–0.903]) (), when compared to the ratio based on SUVmean measurements (AUC: 0.686 [95% CI: 0.524–0.830]) (Supplementary Figure 2). The estimated cut-off 0.739 of relative ratio 99mTc-Sestamibi uptake, based on SUVmax measurements of the renal tumour and the non-tumoral parenchyma, resulted in 64% sensitivity and 83% specificity in detecting RO (AUC: 0.787 [95% CI: 0.644–0.903]).
Figure 1. The ratio of relative 99mTc-Sestamibi uptake based on SUVmax measurements on the renal tumour (T) and the non-tumoral renal parenchyma (N). The estimated cut-off value of 0.739 of relative ratio 99mTc-Sestamibi uptake, based on SUVmax measurements of the renal tumour and the non-tumoral parenchyma, resulted in 64% sensitivity and 83% specificity in detecting Renal Oncocytoma (Group A) (AUC: 0.787 [95% CI: 0.644–0.903]). The different colours and sizes of the markers on the right graph represent the different tumour categories and tumour sizes, respectively.
![Figure 1. The ratio of relative 99mTc-Sestamibi uptake based on SUVmax measurements on the renal tumour (T) and the non-tumoral renal parenchyma (N). The estimated cut-off value of 0.739 of relative ratio 99mTc-Sestamibi uptake, based on SUVmax measurements of the renal tumour and the non-tumoral parenchyma, resulted in 64% sensitivity and 83% specificity in detecting Renal Oncocytoma (Group A) (AUC: 0.787 [95% CI: 0.644–0.903]). The different colours and sizes of the markers on the right graph represent the different tumour categories and tumour sizes, respectively.](/cms/asset/f54f6999-392c-4d92-9f0f-d00c3c50ba03/isju_a_2119273_f0001_c.jpg)
When RO and HOCT are clustered together, the semiquantitative performance of 99mTc-Sestamibi SPECT/CT reaches 87,5% in sensitivity and 71% in specificity, with an estimated cut-off of 0.61, the relative ratio of 99mTc-Sestamibi uptake, based on SUVmax measurements of the renal tumour and the non-tumoral parenchyma. The last mentioned cut-off value is comparable with the results from other groups active in this specific field [Citation23] (Supplementary Figure 3).
Re-evaluation of all chRCCs
An expert Urological Pathologist re-evaluated all chRCCs, resulting in the reclassification of 5 Sestamibi-negative tumours as chRCCs classic type (n = 4) () and an eosinophilic variant of chRCC (n = 1). Of the 6 Sestamibi-positive tumours, 3 were re-classified as LOTs (), 2 as an eosinophilic variant of chRCC, and 1 as chRCC classic type. However, all these tumours were included in the chRCC subgroup because LOT represents a recently proposed and provisional renal entity recently included in the latest 2022 WHO classification of renal neoplasia [Citation13].
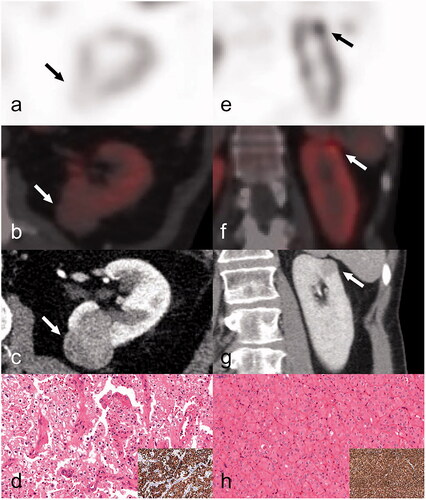
Figure 2. A Sestamibi-negative and a Sestamibi-positive chRCC from 2 different patients. First column, a–d: (case 42) SPECT axial image (a) indicates the absence of 99mTc-Sestamibi in a classic chromophobe RCC located to the dorsal aspect of the left kidney (indicated by arrow). Fused axial SPECT/CT image (b), white arrow indicates the tumour. Preoperative axial CT image (c) in the venous phase, white arrow indicates the tumour. The tumour was characterised as a chRCC (d) upon expert review and was diffusely positive for CK7 (inset; d). Second column, e–h: (case 31) SPECT coronal image (e) indicates focal 99mTc-Sestamibi uptake in a chromophobe RCC located to the upper pole of the left kidney (indicated by arrow). Fused axial SPECT/CT image (f), white arrow indicates the tumour. Preoperative axial CT image (g) in the venous phase (white arrow indicates the tumour). The tumour (h) was re-characterised as a LOT upon expert review (LOT: low-grade oncocytic tumour) displaying a CK7 positive immunoprofile (inset; h).
Discussion
Quantitative evaluation with SUV SPECT measurements performed in HERMES Hybrid Viewer PDR v2.5 did not improve the performance of the 99mTc-Sestamibi SPECT/CT examination in differentiating RO, a benign renal tumour, from RCC. The well-known intrinsic limitations of SPET/CT [Citation24], for example, the limited spatial resolution and the resulting partial volume effect, may play a crucial role in SUV SPECT measurements due to spill out and/or spill in of the signal from adjacent voxels [Citation25]. Smaller lesions are more affected by the partial volume effect than larger lesions. Uncertainties in SUV SPECT measurements performed on smaller tumours can lead to inaccurate evaluations and the moderate agreement between readers on SUV SPECT measurements performed on non-tumoral renal parenchyma. The implementation of such semiquantitative tools is rather difficult in clinical practice. The aforementioned factors that affect the SUV SPECT measurements can partly explain that no reliable absolute cut-off values in the detection of RO could be found based exclusively on SUV SPECT measurements performed on the different renal tumours. Instead, the relative 99mTc- Sestamibi uptake between renal tumour and the non-tumoral renal parenchyma is preferred. Another factor contributing to the uncertain SUV SPECT measurements is the interval from the time of 99mTc- Sestamibi injection and the time of SPECT/CT scan. In this study, this time varied between 60 and 90 min [Citation21], and it has not been studied how much this time difference affects the SUV. This time dependence is well studied as it concerns fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT), but experience in 99mTc- Sestamibi is limited [Citation24]. The risk that smaller lesions with slight 99mTc- Sestamibi uptake could be potentially misclassified as Sestamibi-negative due to the above-mentioned spill out of the signal in the adjacent tissues is sustainable also for the visual evaluation of 99mTc-Sestamibi SPECT/CT examination.
In decreasing order of frequency, increased 99mTc-Sestamibi uptake compared to the ipsilateral non-tumoral renal parenchyma was visually evident in 5 out of 5 HOCTs (100%), 9 out of 11 RO (82%) (), and 6 out of 11 chRCCs (55%). All HOCTs and approximately half of the chRCC cases were classified as Sestamibi-positive, raising the question of the clinical importance of misclassifying tumour entities with an indolent clinical course [Citation26]. This finding could be eventually incorporated into modern active surveillance programs [Citation27], resulting in a multimodality imaging approach concerning the non-invasive characterisation of renal neoplasia [Citation28] accompanied by a confirmatory renal biopsy.
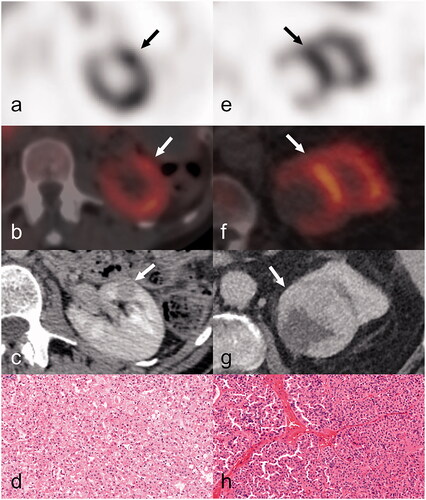
Figure 3. A RO and a HOCT, both positive on 99mTc-Sestamibi SPECT/CT examination. First column, a–d: (case n.9) SPECT axial view (a) of focal 99mTc-Sestamibi uptake in the anterior aspect of the left kidney (indicated by arrow). Anatomic correlation of the previous uptake (c) in the CT study, axial view-venous phase. The axial SPECT/CT fusion image (b) with 99mTc-Sestamibi uptake in the same solid renal neoplasm (12mm in maximum diameter) of the left kidney. This was subsequently diagnosed as HOCT on histopathology (d). Second column, e–h: (case n.13) SPECT axial view (e) displays focal 99mTc-Sestamibi uptake in the upper pole of the left kidney (indicated by arrow). Anatomic correlation of the previous uptake (g) in the CT study, axial view-venous phase. The axial SPECT/CT fusion image (f) with 99mTc-Sestamibi uptake in the same solid renal neoplasm (69 mm in maximum diameter) of the left kidney. This was subsequently diagnosed as RO on histopathology (h).
The limitations of the imaging methods for accurate differentiation of Sestamibi-positive renal tumours using hybrid molecular and conventional imaging mirror the recognised diagnostic complexities of the histopathologic evaluation of this group of tumours. The expanding spectrum of oncocytic neoplasia containing mostly indolent renal entities is a grey zone that molecular imaging cannot depict. Still, mounting evidence suggests unique and distinct histopathological features of those renal tumours [Citation15,Citation16,Citation29,Citation30]. As an example of a distinct tumour entity with an indolent course, LOT molecular pathogenesis has been recently unravelled [Citation15,Citation16], encompassing mTOR pathway activation accompanied by converging mTORC1 pathway mutations and a distinctive gene expression signature. To extend, the morphological spectrum of LOT is now expanded with oncocytic renal tumours with oncocytoma-like morphological traits displaying diffuse cytokeratin 7 immunoexpression and frequent alterations in the TSC/mTOR pathway [Citation31].
A noteworthy observation is that 3 of 11 chRCCs were re-classified as LOTs upon expert review, and these 3 tumours were all classified as Sestamibi-positive. Our findings suggest that Sestamibi-positive renal tumours are of low malignant potential and thus, could be considered in active surveillance programs, utilising renal biopsy and longer-term imaging follow-up. The emerging question, however, concerns the diagnostic accuracy and the inherent limitations of the biopsy obtained from Sestamibi-positive renal tumours. In the current study, 4 cases diagnosed initially as RO were reclassified either as HOCT or chRCC, following a combined morphological and immunohistochemical assessment of three biopsies and one resection. These reclassifications are consistent with the findings from a systematic review and meta-analysis, suggesting that core biopsy can often be unreliable for diagnosing RO [Citation32]. The diagnosis on renal biopsy can be problematic also due to possible tumour heterogeneity, which is another limitation of our study since 21 tumour diagnosis of our material was based on biopsies. For this reason, many pathologists do not even issue a definitive diagnosis of RO on biopsy. Patel et al. demonstrated that 1 in 4 RO cases was misdiagnosed, with 12.5% and 6.3% of tumours re-classified following excision, either as chRCC or HOCT [Citation32]. One of the most challenging areas in the routine renal pathology practice is the diagnosis of tumours with overlapping or equivocal features between RO and chRCC, as well as differentiating RO and chRCC from other oncocytic renal tumours that do not completely fulfil the diagnostic criteria. However, a current consensus is that these difficult-to-classify “hybrid” oncocytic tumours have an exceedingly low risk of metastatic disease [Citation12].
Immunohistochemistry plays a major role in renal tumour diagnostics due to its widespread availability; a panel comprising cytokeratin 7 (CK7)/carbonic anhydrase IX (CAIX)/alpha-methyl acyl-CoA racemase (AMACR)/KIT (CD117) may be used for screening [Citation33]. Other emerging markers such as FOXI1, RHCG, and LINC01187 that appear lineage-specific for renal epithelial neoplasms arising from intercalated cells in the distal nephron segment, may also play a role in the future [Citation34]. Nevertheless, immunohistochemistry limitations are well known in routine practice, and slight differences in the immunohistochemical profiles have also been identified among HOCT subtypes (i.e. Birt-Hogg-Dubé syndrome, renal oncocytosis/oncocytomatosis and sporadic tumours) [Citation35]. Other approaches to potentially identify unique oncocytic tumour-specific features include (i) whole scale approach of computer-assisted morphometry [Citation36], (ii) molecular genetic approaches, including gene expression, microRNA, single-nucleotide polymorphism (SNP), array comparative genomic hybridisation (array-CGH) profiling analyses [Citation37,Citation38] as well as the recently proposed oncocytic nine gene classifier by McGillivray et al. [Citation39] and (iii) an in-situ metabolomic approach [Citation40].
These limitations, both on imaging and histopathology grounds, might explain the performance differences between the visual and the semiquantitative evaluation of the 99mTc-Sestamibi SPECT/CT examination. Our work, however, adds value to a growing number of publications indicating that most ROs are Sestamibi-positive [Citation23]. Patients with Sestamibi-positive renal tumours could avoid or postpone surgical treatment. A renal biopsy could be preferred in this patient group, as well as a longer follow-up period to track the dynamics of the examined renal neoplasms. The information derived from 99mTc-Sestamibi SPECT/CT, followed by a core tissue renal biopsy, can reduce not only the overtreatment of benign renal tumours but can also reduce the proportion of untreated malignant renal tumours. The 99mTc-Sestamibi SPECT/CT accompanied by a minimally invasive renal biopsy may be useful in clinical practice as a diagnostic tool to identify several indolent or low-malignant potential renal tumours, such as RO, LOT, HOCT and a subset of chRCC [Citation41].
In conclusion, semiquantitative evaluation with SUV SPECT measurements on the renal tumour and the non-tumoral renal parenchyma did not improve the visual assessment of 99mTc-Sestamibi SPECT/CT examination in characterising benign RO and differentiating it from malignant renal tumours. Visual evaluation of 99mTc- Sestamibi SPECT/CT identified a group of mostly indolent Sestamibi-positive tumours containing RO, LOT (i.e. a new, emerged renal entity with no proven recurrence or metastatic potential), HOCT and a subset of chRCC. Our results suggest that patients with Sestamibi-negative renal tumours should be considered for surgery. In contrast, patients with Sestamibi-positive renal tumours could be better suited for biopsy and follow-up, according to the current active surveillance protocols.
Ethics approval and consent to participate
This study was approved by the Regional Ethical Review Board and by the local Radiation Safety Committee (Reference no. 2015/923-31/4). Written informed consent was required from all study participants.
Authors contributions
AT, RA initiated and designed the study. AT, TP, OG, SG, KT, LE-E, AA, MH, MK, RA evaluated data. AT, AA, GK organised data. AT wrote the first draft, and all authors revised the manuscript critically.
Email addresses to co-authors:
Thomas Papathomas: [email protected]
Ove Gustafsson: [email protected]
Stefan Gabrielson: [email protected]
Linnea Ekström-Ehn: [email protected]
Kiril Trpkov: [email protected]
Alexandros Arvanitis: [email protected]
Maria Holstensson: [email protected]
Mattias Karlsson: [email protected]
Georgia Kokaraki: [email protected]
Rimma Axelsson: [email protected]
Supplemental Material
Download MS Word (267.9 KB)Disclosure statement
The authors declare no competing interests.
Additional information
Funding
References
- van Oostenbrugge TJ, Fütterer JJ, Mulders PFA. Diagnostic imaging for solid renal tumors: a pictorial review. Kidney Cancer. 2018;2(2):79–93.
- Kim JH, Li S, Khandwala Y, et al. Association of prevalence of benign pathologic findings after partial nephrectomy with preoperative imaging patterns in the United States from 2007 to 2014. JAMA Surg. 2019;154(3):225–231.
- Morshid A, Duran ES, Choi WJ, et al. A concise review of the multimodality imaging features of renal cell carcinoma. Cureus. 2021;13(2):e13231.
- Zhang GMY, Shi B, Xue HD, et al. Can quantitative CT texture analysis be used to differentiate subtypes of renal cell carcinoma? Clin Radiol. 2019;74(4):287–294.
- Wilson MP, Katlariwala P, Murad MH, et al. Diagnostic accuracy of 99mTc-sestamibi SPECT/CT for detecting renal oncocytomas and other benign renal lesions: a systematic review and meta-analysis. Abdom Radiol. 2020;45(8):2532–2541.
- Abdessater M, Kanbar A, Comperat E, et al. Renal oncocytoma: an algorithm for diagnosis and management. Urology. 2020;143:173–180.
- Su ZT, Patel HD, Huang MM, et al. Cost-effectiveness analysis of 99mTc-sestamibi SPECT/CT to guide management of small renal masses. Eur Urol Focus. 2021;7(4):827–834.
- Udager AM, Mehra R. Morphologic, molecular, and taxonomic evolution of renal cell carcinoma a conceptual perspective with emphasis on updates to the 2016 world health organization classification. Arch Pathol Lab Med. 2016;140(10):1026–1037.
- Trpkov K, Williamson SR, Gill AJ, et al. Novel, emerging and provisional renal entities: the genitourinary pathology society (GUPS) update on renal neoplasia. Mod Pathol. 2021;34(6):1167–1184.
- Gill AJ, Moch H, Amin MB, et al. The 2022 World Health Organization Classification of Tumours of the Urinary System and Male Genital Organs — Part A: Renal, Penile, and Testicular Tumours; 2022.
- Moch H, Cubilla AL, Humphrey PA, et al. The 2016 WHO classification of tumours of the urinary system and male genital organs—part A: renal, penile, and testicular tumours. Eur Urol. 2016;70(1):93–105.
- Ruiz-Cordero R, Rao P, Li L, et al. Hybrid oncocytic/chromophobe renal tumors are molecularly distinct from oncocytoma and chromophobe renal cell carcinoma. Mod Pathol. 2019;32(11):1698–1707.
- Hes O, Trpkov K. Do we need an updated classification of oncocytic renal tumors? Mod Pathol. 2022;35(9):1140–1150.
- Trpkov K, Williamson SR, Gao Y, et al. Low-grade oncocytic tumour of kidney (CD117-negative, cytokeratin 7-positive): a distinct entity? Histopathology. 2019;75(2):174–184.
- Kapur P, Gao M, Zhong H, et al. Germline and sporadic mTOR pathway mutations in low-grade oncocytic tumor of the kidney. Mod Pathol. 2022;35(3):333–343.
- Morini A, Drossart T, Timsit MO, et al. Low-grade oncocytic renal tumor (LOT): mutations in mTOR pathway genes and low expression of FOXI1. Mod Pathol. 2022;35(3):352–360.
- Mansoor M, Siadat F, Trpkov K. Low-grade oncocytic tumor (LOT)-a new renal entity ready for a prime time: an updated review. Histol Histopathol. 2022;37(5):405–413.
- Tzortzakakis A, Gustafsson O, Karlsson M, et al. Visual evaluation and differentiation of renal oncocytomas from renal cell carcinomas by means of 99mTc-sestamibi SPECT/CT. EJNMMI Res. 2017;7(1):29.
- Gorin MA, Rowe SP, Baras AS, et al. Prospective evaluation of 99mTc-sestamibi SPECT/CT for the diagnosis of renal oncocytomas and hybrid oncocytic/chromophobe tumors. Eur Urol. 2016;69(3):413–416.
- Rowe SP, Gorin MA, Gordetsky J, et al. Initial experience using 99mTc-MIBI SPECT/CT for the differentiation of oncocytoma from renal cell carcinoma. Clin Nucl Med. 2015;40(4):309–313.
- Tzortzakakis A, Holstensson M, Hagel E, et al. Intra- and interobserver agreement of SUV SPECT quantitative SPECT/CT processing software, applied in clinical settings for patients with solid renal tumors. J Nucl Med Technol. 2019;47(3):258–262.
- Papathomas T, Tzortzakakis A, Sun N, et al. In situ metabolomics expands the spectrum of renal tumours positive on 99m Tc-sestamibi single photon emission computed tomography/computed tomography examination. Eur Urol Open Sci. 2020;22:88–96.
- Wilson MP, Katlariwala P, Abele J, et al. A review of 99mTc-sestamibi SPECT/CT for renal oncocytomas: a modified diagnostic algorithm. Intractable Rare Dis Res. 2022;11(2):46–51.
- Dickson J, Ross J, Vöö S. Quantitative SPECT: the time is now. EJNMMI Phys. 2019;6(1):4–7.
- Chan C, Liu H, Grobshtein Y, et al. Noise suppressed partial volume correction for cardiac SPECT/CT. Med Phys. 2016;43(9):5225–5239.
- Finelli A, Ismaila N, Bro B, et al. Management of small renal masses: American Society of clinical oncology clinical practice guideline. J Clin Oncol. 2017;35(6):668–680.
- McIntosh AG, Ristau BT, Ruth K, et al. Active surveillance for localized renal masses: tumor growth, delayed intervention rates, and >5-yr clinical outcomes. Eur Urol. 2018;74(2):157–164.
- England RW, Sheikhbahaei S, Solomon AJ, et al. When more is better: underused advanced imaging exams that can improve outcomes and reduce cost of care. Am J Med. 2021;134(7):848–853.e1.
- Akgul M, Al-Obaidy KI, Cheng L, et al. Low-grade oncocytic tumour expands the spectrum of renal oncocytic tumours and deserves separate classification: a review of 23 cases from a single tertiary institute. J Clin Pathol. 2021:jclinpath-2021-207478.
- Ishikawa N, Kimura N, Yoshida T, et al. A case of low-grade oncocytic tumor/chromophobe renal cell carcinoma (oncocytic variant) of the kidney. Case Rep Pathol. 2021;2021:6684774–6684777.
- Mohanty SK, Satapathy A, Aggarwal A, et al. Oncocytic renal neoplasms with diffuse keratin 7 immunohistochemistry harbor frequent alterations in the mammalian target of rapamycin pathway. Mod Pathol. 2022;35(3):361–375.
- Patel HD, Druskin SC, Rowe SP, et al. Surgical histopathology for suspected oncocytoma on renal mass biopsy: a systematic review and meta-analysis. BJU Int. 2017;119(5):661–666.
- Kim M, Joo JW, Lee SJ, et al. Comprehensive immunoprofiles of renal cell carcinoma subtypes. Cancers. 2020;12(3):602–616.
- Skala SL, Wang X, Zhang Y, et al. Next-generation RNA sequencing–based biomarker characterization of chromophobe renal cell carcinoma and related oncocytic neoplasms. Eur Urol. 2020;78(1):63–74.
- Montironi R, Gasparrini S, Cimadamore A, et al. Variants and variations in epithelial renal cell tumors in adults: the pathologist’s point of view. Eur Urol Suppl. 2017;16(12):232–240.
- Erlmeier F, Feuchtinger A, Borgmann D, et al. Supremacy of modern morphometry in typing renal oncocytoma and malignant look-alikes. Histochem Cell Biol. 2015;144(2):147–156.
- Poté N, Vieillefond A, Couturier J, et al. Hybrid oncocytic/chromophobe renal cell tumours do not display genomic features of chromophobe renal cell carcinomas. Virchows Arch. 2013;462(6):633–638.
- Andeen NK, Qu X, Antic T, et al. Clinical utility of chromosome genomic array testing for unclassified and advanced-stage renal cell carcinomas. Arch Pathol Lab Med. 2019;143(4):494–504.
- McGillivray PD, Ueno D, Pooli A, et al. Distinguishing benign renal tumors with an oncocytic gene expression (ONEX) classifier. Eur Urol. 2021;79(1):107–111.
- Buck A, Ly A, Balluff B, et al. High-resolution MALDI-FT-ICR MS imaging for the analysis of metabolites from formalin-fixed, paraffin-embedded clinical tissue samples. J Pathol. 2015;237(1):123–132.
- Kravtsov O, Gupta S, Cheville JC, et al. Low-Grade oncocytic tumor of kidney (CK7-Positive, CD117-Negative): incidence in a single institutional experience with clinicopathological and molecular characteristics. Hum Pathol. 2021;114:9–18.
