Abstract
Purpose
To understand the potential impact of new treatment options for urinary tract cancer, recent population trends in incidence, mortality and survival should be elucidated. This study estimated changes in the incidence, mortality and relative survival of urinary tract cancer in the Nordic countries (Denmark, Finland, Iceland, Norway and Sweden) between 1990 and 2019.
Methods
Annual counts of incident cases and deaths due to urinary tract cancer (International Classification of Diseases, Tenth Revision, Clinical Modification codes C65–C68, D09.0–D09.1, D30.1–D30.9 and D41.1–D41.9) in Nordic countries were retrieved in 5-year age categories by sex during the study period. Country-specific time trends (annual rate ratios [RRs]) were estimated using Poisson regression, and RRs were compared between sexes.
Results
The incidence rate of bladder and upper urothelial tract cancer was >3-times lower in women than men in all countries across all age groups (incidence RR for women to men ranging from 0.219 [95% CI = 0.213–0.224] in Finland to 0.291 [95% CI = 0.286–0.296] in Denmark). Incidence rates were lowest in Finland and highest in Norway and Denmark. Age-adjusted mortality decreased in Finland, Denmark and Norway and in Swedish men, with the greatest decrease seen in Danish men (annual RR = 0.976; 95% CI = 0.975–0.978). In all countries and age groups, women had a lower relative survival rate than men.
Conclusion
Between 1990 and 2019, the incidence of urinary tract cancer was stable in the Nordic countries, while mortality rates declined and relative survival increased. This could be due to earlier diagnosis and better treatment.
Introduction
Urinary tract cancer refers to tumors found in the bladder and in upper parts of the urothelial tract, including the renal pelvis, ureter and urethra. Histological subtypes include urothelial carcinoma, squamous cell carcinoma and adenocarcinoma, however, the vast majority are urothelial carcinomas, found in the epithelial walls of the urinary tract. Approximately 90% of urinary tract cancers are found in the bladder [Citation1]. In 2018, bladder cancer was the 10th most common cancer type worldwide, accounting for 3% of cases [Citation2]. In addition, bladder cancer was the 13th most deadly cancer, causing almost 200,000 deaths worldwide [Citation2]. In the Nordic countries, bladder and urinary tract cancers were the 7th deadliest cancer in men and the 12th in women, causing 2,176 deaths in total [Citation3].
Bladder and upper urothelial tract cancers have high recurrence rates in patients diagnosed with early-stage disease. Localized invasive tumors are commonly treated with radical surgery with or without neoadjuvant chemotherapy or radiotherapy, whereas noninvasive tumors are treated with transurethral resection of bladder tumor (TURBT) and adjuvant intravesical therapy. Platinum-based chemotherapy is the established standard-of-care first-line (1L) treatment for advanced bladder or urinary tract cancer, and eligibility for treatment with specific platinum agents is determined by overall health status [Citation4]. More recently, immunotherapies have become available and are now included in the National Comprehensive Cancer Network and European Society of Medical Oncology guidelines as 1L, 1L maintenance and second-line options for specified subgroups [Citation1,Citation5].
Given the high burden of bladder and urinary tract cancers, including the impact on patients’ morbidity and quality-of-life and costs to society, it is important to better understand the disease and work toward preventive measures, earlier detection and better treatment options. The aim of the current study was to assess the incidence, mortality and relative survival of bladder and urinary tract cancers from 1990 through 2019 in the Nordic countries: Denmark, Finland, Iceland, Norway and Sweden. Most of this period occurred before immunotherapies were introduced in this patient group (Sweden, 2017; other Nordic countries, 2018), therefore, this study assesses trends in this disease population in the pre-immunotherapy era, facilitating future comparisons. Differences between countries may be informative when discussing etiologic factors, delays in diagnosis and the impact of new treatments.
Materials and methods
Data were obtained from the Nordic database of cancer statistics (NORDCAN) [Citation3], which includes data from Denmark, Finland, Norway, Sweden, Iceland, the Faroe Islands and Greenland (latter two not included in this analysis). NORDCAN is a publicly available, annually updated database that provides annual counts of cancer incident cases, deaths and corresponding population size [Citation6]. Nordic cancer registries use each country’s personal identification number systems, enabling complete, population-based registry data linked across various local registries. Each registry reports almost 100% completeness on solid tumor incidence data. With few exceptions, the Nordic registries include data retrieved from hospitals, laboratories, private clinics and death certificates [Citation6].
Cancer types included in this analysis were urinary tract cancer (excluding kidney), consisting of International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes C65–C68, D09.0–D09.1, D30.1–D30.9 and D41.1–D41.9. Data were collected on incidence, mortality and relative survival from 1990 through 2019. Annual counts of incident cases and deaths were obtained by sex in age groups (5-year groups from 0–4 to 80–84 years, plus ≥ 85 years) based on age at diagnosis (incidence) or at death (mortality). Corresponding population sizes were obtained as a proxy for person-years at risk in the underlying population. Last, 5-year relative survival was estimated based on the cohort approach and stratified by broad age group (20–49, 50–59, 60–69, 70–79 and ≥ 80 years), as estimated by the NORDCAN group [Citation3]. The methods used in this analysis are in accordance with the 1964 Helsinki declaration, its later amendments and comparable ethical guidelines. Formal patient consent is not needed for this type of study.
Statistics
Incidence and mortality rates with 95% CIs were estimated using Poisson regression by country, sex and broad age group. Rates by sex in each country were compared using Poisson regression adjusted for 5-year age groups and a natural cubic spline with 4 knots for year of diagnosis or death, respectively (indirect standardization). Similarly, sex- and country-specific annual trends in incidence and mortality rates, respectively, were estimated using Poisson regression with adjustment for age category. p-values were considered statistically significant at a 5% level. All analyses were conducted using Stata 17.0 [Citation7].
Results
Overall data
Incident cases and deaths due to urinary tract cancers, together with population sizes, were collected by country (Denmark, Finland, Norway, Sweden and Iceland), sex and age group (). The number of incident cases and deaths was highest in Sweden (country with the largest population), followed by Denmark, and lowest in Iceland (country with the smallest population). In all countries, incident cases were markedly higher in men than in women in all age groups.
Table 1. Incident cases and deaths due to bladder and urinary tract cancers and person-years per 100,000 in Nordic countries, 1990–2019.
Incidence
The age-standardized incidence of urinary tract cancers over time in Nordic countries was mostly flat in men and women, with a slight increase seen in women in Sweden and Norway, and the strongest declining trend seen in Danish men (). Throughout the 30-year period, Finland had the lowest incidence rates in both sexes, whereas Norway and Denmark had the highest. Incidence rates in Iceland fluctuated over time, most likely due to its relatively small population. The same patterns in age- and sex-specific rates were seen in each country (Supplementary Table S1), with Denmark and Finland having the highest and lowest rates, respectively, across all age categories for both sexes. Rates increased markedly with age in all countries in both sexes.
Figure 1. Age-standardized incidence rate for bladder and urinary tract cancers in Nordic countries per 100,000 (adjusted for the World Standard Population) in (A) men and (B) women, 1990–2019.
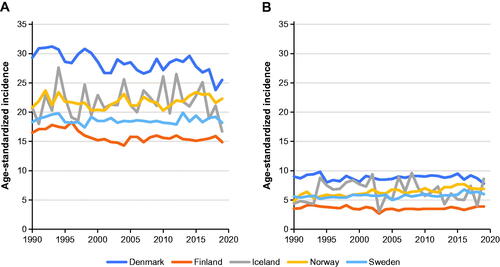
Age- and period-adjusted incidence rate ratios (IRRs) comparing women with men were similar in all countries, with IRRs ranging from 0.219 (95% CI = 0.213–0.224) in Finland to 0.291 (95% CI = 0.286–0.296) in Denmark (). Values for sex- and country-specific estimates of trends were all close to 1, with annual changes ranging from a decrease of 0.4% in Icelandic women (0.996; 95% CI = 0.986–1.006) to an increase of 1.1% in Norwegian women (1.011; 95% CI = 1.009–1.013). In women, a significant increase was seen in Denmark, Norway and Sweden, whereas trends were not statistically significant in Finland and Iceland. In men, trends observed were a decrease in Denmark and Finland, almost no change in Iceland and an increase in Norway and Sweden (). Unadjusted trends were generally larger, reflecting the aging of the populations over the study period. Trends for changes in incidence were generally smaller in younger versus older age groups; for example, in Norwegian men, the trend increased from 1.001 (95% CI = 0.994–1.007) in the 20–49-years category to 1.012 (95% CI = 1.009–1.014) in the ≥80-years category (Supplementary Table S2).
Table 2. Rate ratios comparing incidence and mortality rates for bladder and urinary tract cancers in Nordic countries in men (reference) and women, 1990–2019.
Mortality
Overall mortality from bladder and urinary tract cancer declined in all countries in both sexes, except in Swedish women, where age-standardized mortality remained stable (). The decrease was greatest in Danish men, in whom age-standardized mortality rates decreased from 9.7 in 1990 through 1994 to 5.3 in 2015 through 2019, with similar but less-pronounced decreases in Swedish and Norwegian men. Similar to incidence rates, mortality rates fluctuated over time in Icelandic men and women, most likely due to Iceland’s relatively small population.
Figure 2. Age-standardized mortality rate for bladder and urinary tract cancers in Nordic countries per 100,000 (adjusted for the World Standard Population) in (A) men and (B) women, 1990–2019.
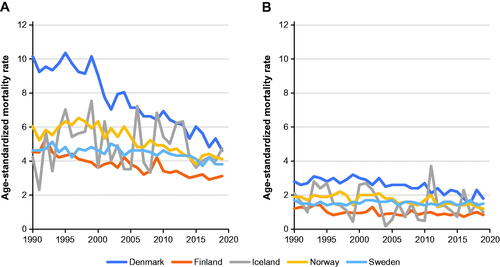
Patterns in age- and sex-specific mortality rates between countries were identical to overall patterns (Supplementary Table S3), with Denmark having the highest rates in all age categories for both sexes. Rates increased markedly with age in all countries in both sexes and were highest in Danish and Norwegian men aged ≥ 80 years (178.59; 95% CI = 170.48–187.09 and 174.10; 95% CI = 165.70–182.94 deaths per 100,000 person-years, respectively; Supplementary Table S3). In younger age groups (< 50 years), mortality rates were lowest in Sweden, whereas in older age groups (≥ 50 years) and across all age groups combined, mortality rates were lowest in Finland.
Age- and period-adjusted mortality rate ratios comparing women with men showed patterns similar to those observed for incidence, that is, lower mortality in women, with mortality rate ratios ranging from 0.261 (95% CI = 0.249–0.274) in Finland to 0.326 (95% CI = 0.317–0.335) in Sweden (). Age-adjusted mortality rate ratios declined over time for both sexes in all five countries, except in Swedish women (1.001; 95% CI = 0.999–1.004). In Iceland, trends were not statistically significant (95% CI overlapped 1), but the apparent decline was similar to that seen in other countries. Declines were larger for adjusted versus unadjusted trends. Sex- and age-specific trends were generally similar in each country, although there was a statistically significant increase in mortality among elderly (> 80 years) Swedes and Icelandic men (Supplementary Table S4).
Relative survival
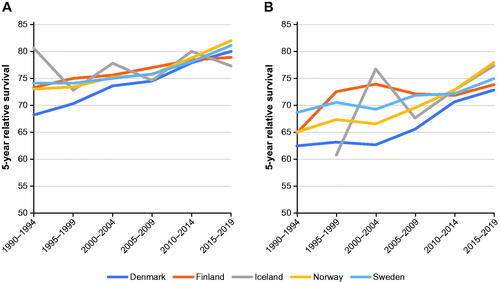
Women had lower relative survival than men in all five Nordic countries and in all age groups (). For example, the 5-year relative survival rate in the last period studied (2015 through 2019) in Denmark was 80% in men and 73% in women. Five-year relative survival rates improved over time in all countries for both sexes and across all age groups and were highest in Norway (82% in men and 78% in women in 2015 through 2019). Age was also a determining factor for relative survival in all countries (data for relative survival by age were available until 2016 and were assessed from 1987 through 2016; Supplementary Table S5). For example, men had a 5-year relative survival of ≈ 80% in the youngest age group and ≈ 55% in the oldest.
Discussion
This study provides a comprehensive overview of incidence, survival and mortality data for bladder cancer and cancers of the upper urothelial tract in the Nordic countries (Denmark, Finland, Iceland, Norway and Sweden) in the period from 1990 through 2019. Data were obtained from Nordic cancer registries, which have complete records for incidence and outcome data [Citation6]. In total, 41,914 men and 19,292 women in the five Nordic countries died of bladder and urinary tract cancer during the study period. The age-standardized incidence of bladder and urinary tract cancers was stable throughout the study period except in Norway, where a modest but statistically significant increase was seen in women. This increase was driven by an increasing incidence in the older age groups (≥ 70 years), although the explanation remains unclear. The highest incidence rates throughout the study period were seen in Denmark, although a decreasing trend was observed during the last period studied (2015 through 2019). Changes in reporting and recording may have contributed to the initially higher figures and apparent decrease [Citation8]. Another striking and well-known finding was the difference in incidence rates between sexes in this Nordic population. Overall, men had a ≥ 3-fold higher incidence rate than women and the lowest incidence was seen in Finnish women (incidence rate, 3.6 per 100,000 per year in the last period studied).
Differences between sexes in causal factors of bladder and urinary tract cancers are not fully understood, with the possible exception of smoking [Citation9]. Historically, Danes have higher smoking rates than people in other Nordic countries, and a higher proportion of men than women smoke in the Nordic countries, except Sweden [Citation10–13]. Thus, differences in smoking rates could partly explain the relatively high Danish incidence rates and contribute to the differences between sexes seen in all countries. However, the causal relationship between smoking and urinary tract cancers is less clear than between smoking and lung cancer. In the Nordic countries, lung cancer has shown a marked decline in incidence in men but an increasing trend in women [Citation14], corresponding to a greater decrease in smoking in men than in women [Citation10–12]. It is difficult to observe a similar causal relation for urinary tract cancers, although several studies and a meta-analysis have reported a strong association between smoking and urinary tract cancers, including bladder cancer [Citation15,Citation16]. Other lifestyle factors, such as high body mass index, low physical activity and related metabolic disorders, are associated with increased risk of several cancer types, including bladder cancer [Citation9]. These findings are also related to duration of these lifestyle factors, explaining the rising incidence of bladder and urinary tract cancers by age, although differences in these risk factors between sexes are unclear.
Five-year relative survival improved in men in all countries, from ≈ 70% in 1990 through 1994 to ≈ 80% in 2015 through 2019. Women had lower 5-year relative survival rates than men across all age groups. Previous studies containing detailed data from Norway and Sweden have challenged the commonly held view of bladder cancer prognosis being worse in women than men [Citation17,Citation18]. Their analyses found that both Norwegian and Swedish women had a worse prognosis than men only in the first 2 years after diagnosis, particularly when diagnosed with a muscle-invasive tumor. This discrepancy might be attributable to advanced disease being diagnosed more often in women than men, suggesting delayed diagnosis in women. Blood in urine is often the first sign of bladder cancer, and men may see this symptom earlier than women because of physiological differences.
Clinical stage is a major factor associated with prognosis, and the medical need for improved treatment is most pronounced in advanced disease [Citation19]. The increase in relative survival in this heterogenous group of Nordic patients could be due to both earlier diagnosis and better treatment. Several Nordic countries implemented national clinical guidelines and standardized care processes during the study period, including monitoring the time from onset of symptoms to first clinical contact and surgical intervention [Citation20–22]. Older age was also a negative prognostic factor in all countries, including in age-corrected data; for example, the relative survival rate in Swedish men was 84% in those aged 50–59 years versus 62% in those aged ≥ 80 years. This finding could be related to delayed diagnosis in elderly people due to fewer diagnostic procedures being performed in them [Citation23].
The corresponding mortality rates reflect both incidence and survival rates. All statistically significant mortality trends over time were negative, indicating a decline in age-standardized mortality rates in most age groups in all countries. Elderly (≥ 80 years) Swedes and Icelandic men were an exception, in whom a modest, but significantly increasing trend was observed over time. Icelandic data is especially susceptible to changes in reporting and coding, due to the relatively small sample size, which could provide an explanation. In addition, contrary to the other overall trends, a stable age-standardized mortality trend was seen in Swedish women. There were no apparent differences in risk factors between the Swedish female population and the other studied groups, which leaves the causal explanations for these trends unknown and to be investigated further.
The strength of this study was that it provides a comprehensive overview of incidence, relative survival and mortality for cancers of interest in the five Nordic countries over a 30-year period, made possible by the national cancer registries being population-based and close to complete for all cancer types. Although the analyses reported were based on aggregated data in 5-year age intervals, use of Poisson regression analysis allowed robust identification of long-term time trends, with adjustment for changes in age and sex composition in each country. Except for Iceland, the nationwide numbers had narrow CIs, reflecting the high precision of estimates. Our findings should encourage more epidemiological studies based on these registries.
The main limitation of this study was that muscle-invasive (T2–4) and non-muscle invasive (T1) cancers could not be distinguished, because these tumors are classified within the same morphology group in the registries. Furthermore, Nordic registries may differ over time with regard to coding of carcinoma in situ (CIS; ICD-10-CM codes D09.0-D09.1) and non-invasive papillary (Ta) tumors. For example, CIS and Ta tumors are coded and registered separately and not as bladder cancer in Denmark but are registered as bladder cancer in other Nordic countries. Survival rates in patients with CIS and Ta tumors are high, which may be an explanation for worse survival observed in Denmark compared with the other Nordic countries. Further coding differences may be present in relation to benign tumors (ICD-10-CM codes D30.1–30.9), however the effect of this is unclear and unlikely to significantly influence the results. The dataset does not allow for separation of these tumor types.
In addition, as a registry-based study, the current analysis does not include some types of data that can be obtained in a clinical non-interventional study, such as staging, histology and treatment. It is possible to use national Nordic clinical cancer registries (e.g. SNRUBC, Sweden; DaBlaCa, Denmark) to collect more information on treatment, follow-up and relapses, but this may require additional linkage to prescription, hospital and primary care registries, for example. Specific studies utilizing national registries have covered the impact of holiday periods on radical cystectomies [Citation24] and differences in survival in age- and tumor-stratified populations [Citation25]. Comparative studies resulting from registry linkages are an interesting topic for further research.
Overall, the data presented in this study show how the incidence, mortality and relative survival for bladder and urinary tract cancers have changed from 1990 through 2019, although there were some limitations when comparing results between countries. Observed trends were largely similar between the countries, with some interesting exceptions. Notable findings include differences in age-standardized mortality rates, where the rate in Danish males has declined rapidly, and the rate in Swedish females has remained stable, whereas other observed trends consistently point to a slight but significant decline over time. A corresponding decline is not seen in age-standardized incidence, which points to improved relative survival over time.
Supplemental Material
Download MS Word (31.7 KB)Acknowledgments
The authors thank Solveig Borkenhagen (employee at Oslo Economics) for performing significant work in data collection and analysis. Editorial support was provided by Clinical Thinking and was funded by Merck (CrossRef Funder ID: 10.13039/100009945) and Pfizer.
Disclosure statement
Eemil Karttunen is an employee at Merck OY, Espoo, Finland, an affiliate of Merck KGaA. Petteri Hervonen was affiliated with Helsinki University Hospital at the time the research was conducted and is now an employee of Janssen-Cilag Oy, Espoo, Finland, an affiliate of Johnson & Johnson, and reports advisory roles at Bristol Myers Squibb, MSD, Pfizer and Roche, and participation in clinical studies funded by MSD. Jan Oldenburg reports receiving grant support from MSD, consulting fees from Astellas, AstraZeneca, Bayer, Eisai, Ipsen and Janssen-Cilag, grant support and consulting fees from Bristol Myers Squibb, Merck and Roche, and participation in speakers bureaus sponsored by Astellas, AstraZeneca, Bayer and Bristol Myers Squibb. Helle Pappot reports receiving grant support from MSD, Pfizer and Roche. Henrik Støvring reports receiving consulting fees from AstraZeneca, Bristol Myers Squibb, Merck and Pfizer. Juan Luiz Vázquez reports advisory board participation for Photocure and a pending patent for the OPFIELD electrode. Suzanne Bergman is an employee at Merck AB, Solna, Sweden, an affiliate of Merck KGaA. Gry Magnussen is an employee at Merck AB NUF, Oslo, Norway, an affiliate of Merck KGaA. Steinar Thoresen is a consultant at Merck AB NUF, Oslo, Norway, an affiliate of Merck KGaA. Pernille Norremark is an employee at Merck A/S, Søborg, Denmark, an affiliate of Merck KGaA. Anders Ullén has received speaker honoraria or served on an advisory board for Astellas, Janssen-Cilag, Merck, MSD, Pierre-Fabre and Roche. Abolfazl Hosseini and Jukka Sairanen report no conflicts of interest.
Data availability statement
A public database was used as the source of data and all data are available at the website (https://nordcan.iarc.fr/en). Please see the Methods section for details.
Additional information
Funding
References
- Powles T, Bellmunt J, Comperat E, et al. ESMO Guidelines Committee. Bladder cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(3):244–258.
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- NORDCAN: Cancer Incidence, Mortality, Prevalence and Survival in the Nordic Countries [Internet]. Lyon, France: International Agency for Research on Cancer; 2021; [December 18, 2021]. Available from: https://nordcan.iarc.fr/en/about.
- Kamat AM, Hahn NM, Efstathiou JA, et al. Bladder cancer. Lancet. 2016;388(10061):2796–2810.
- Flaig TW, Spiess PE, Agarwal N, et al. Bladder Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2020;18(3):329–354.
- Engholm G, Ferlay J, Christensen N, et al. NORDCAN–a Nordic tool for cancer information, planning, quality control and research. Acta Oncol. 2010;49(5):725–736.
- StataCorp. Stata statistical software: Release 17. College Station, TX: StataCorp LLC; 2021.
- Pukkala E, Engholm G, Højsgaard Schmidt LK, et al. Nordic Cancer Registries – an overview of their procedures and data comparability. Acta Oncol. 2018;57(4):440–455.
- Al-Zalabani AH, Stewart KF, Wesselius A, et al. Modifiable risk factors for the prevention of bladder cancer: a systematic review of meta-analyses. Eur J Epidemiol. 2016;31(9):811–851.
- Official Statistics of Finland (OSF). Tobacco statistics [e-publication] [Internet]. Helsinki, Finland: National Institute for Health and Welfare (THL). 2021; [cited 27.06.2021]. Available from: http://www.stat.fi/til/tupk/index_en.html.
- Hoffman SJ, Christensen AI. Danskernes rygevaner, Udviklingen fra 1994 til 2017. Statens Institut for Folkesundhed, SDU. 2018. Danish.
- Røyk, alkohol og andre rusmidler [Internet]. 2021; [cited 27.06.2021]. Available from: https://www.ssb.no/helse/helseforhold-og-levevaner/statistikk/royk-alkohol-og-andre-rusmidler. (in Norwegian).
- Folkhälsömyndigheten: Daglig tobaksrökning. [Internet]. 2021; [cited 03.10.2021]. Available from: https://www.folkhalsomyndigheten.se/folkhalsorapportering-statistik/tolkad-rapportering/folkhalsans-utveckling/resultat/levnadsvanor/tobaksrokning-daglig/. (in Swedish).
- Grimsrud TK, Skaug HK, Larsen IK. Lung cancer—changes in incidence by gender, age and county of residence 1984–2013. Tidsskr nor Laegeforen. 2015;135(20):1844–1849.
- Freedman ND, Silverman DT, Hollenbeck AR, et al. Association between smoking and risk of bladder cancer among men and women. JAMA. 2011;306(7):737–745.
- Cumberbatch MG, Rota M, Catto JW, et al. The role of tobacco smoke in bladder and kidney carcinogenesis: a comparison of exposures and meta-analysis of incidence and mortality risks. Eur Urol. 2016;70(3):458–466.
- Andreassen BK, Grimsrud TK, Haug ES. Bladder cancer survival: women better off in the long run. Eur J Cancer. 2018;95:52–58.
- Radkiewicz C, Edgren G, Johansson ALV, et al. Sex differences in urothelial bladder cancer survival. Clin Genitourin Cancer. 2020;18(1):26–34.e6.
- Pasin E, Josephson DY, Mitra AP, et al. Superficial bladder cancer: an update on etiology, molecular development, classification, and natural history. Rev Urol. 2008;10(1):31–43.
- Sundhedsstyrelsen. Pakkeforløb for kraeft i urinvejene. [Internet]. Sundhedsstyrelsen; 2020; [September 23, 2021]. Available at: https://www.sst.dk/da/Udgivelser/2020/Pakkeforloeb-for-kraeft-i-urinvejene (in Danish).
- Helsedirektoratet. Nasjonalt handlingsprogram med retningslinjer for diagnostikk, behandling og oppfølging av blaere- og urotelkreft. Helsedirektoratet; 2021.
- Cancercentrum. Nationellt vårdprogram Cancer i urinblåsa, njurbäcken, urinledare och urinrör. Regionalt cancercentrum syd; 2019.
- Gallagher DJ, Milowsky MI. Bladder cancer. Curr Treat Options Oncol. 2009;10(3–4):205–215.
- Liedberg F, Hagberg O, Aljabery F, et al. Survival after radical cystectomy during holiday periods. Scand J Urol. 2021;55(4):276–280.
- Russell B, Liedberg F, Hagberg O, et al. Risk of bladder cancer death in patients younger than 50 with non-muscle-invasive and muscle-invasive bladder cancer. Scand J Urol. 2022;56(1):27–33.