Abstract
Background
Bladder cancer is molecularly one of the most heterogenous malignancies characterized by equally heterogenous clinical outcomes. Standard morphological assessment with pathology and added immunohistochemical analyses is unable to fully address the heterogeneity, but up to now treatment decisions have been made based on such information only. Bladder cancer molecular subtypes will likely provide means for a more personalized bladder cancer care.
Methods
To facilitate further development of bladder cancer molecular subtypes and clinical translation, the UROSCAN-biobank was initiated in 2013 to achieve systematic biobanking of preoperative blood and fresh frozen tumor tissue in a population-based setting. In a second phase, we established in 2018 a parallel logistic pipeline for molecular profiling by RNA-sequencing, to develop and validate clinical implementation of molecular subtyping and actionable molecular target identification in real-time.
Results
Until June 2021, 1825 individuals were included in the UROSCAN-biobank, of which 1650 (90%) had primary bladder cancer, 127 (7%) recurrent tumors, and 48 (3%) unknown tumor status. In 159 patients, multiple tumors were sampled, and metachronous tumors were collected in 83 patients. Between 2016 and 2020 the UROSCAN-biobanking included 1122/2999 (37%) of all primary bladder cancer patients in the Southern Healthcare Region. Until June 2021, the corresponding numbers subjected to RNA-sequencing and molecular subtyping was 605 (UROSCANSEQ), of which 52 (9%) samples were not sequenced due to inadequate RNA-quality (n = 47) or technical failure/lost sample (n = 5).
Conclusions
The UROSCAN-biobanking and UROSCANSEQ-infrastructure for molecular subtyping by real-time RNA-sequencing represents, to our knowledge, the largest effort of evaluating population-wide molecular classification of bladder cancer.
Background
Bladder cancer is a common malignancy that worldwide accounts for 550000 new cases and 200000 deaths each year worldwide [Citation1]. The costs of bladder cancer treatment and management per patient is among the highest of all cancers [Citation2,Citation3]. Today bladder cancer treatment is stratified based on pathological stage. Non-muscle invasive bladder cancer (NMIBC) of stage Ta is only growing above the basal membrane, whereas muscle-invasive bladder cancer (MIBC) are aggressive tumors growing into the underlying bladder muscle. Stage Ta tumors rarely progress but frequently recur, and therefore adjuvant instillations are applied after transurethral resection (TURB). Despite such additional treatment almost 50% of patients with NMIBC suffer from local recurrence [Citation4]. From a clinical perspective, NMIBC stage T1 tumors invading the lamina propria represent a clinical dilemma with their propensity to recur but also to progress. T1 tumors are commonly treated with TURB, a subsequent re-resection (re-TURB) within 4 − 6 weeks, followed by adjuvant instillations with BCG. Even though such an organ-sparing strategy is preferred and will result in a long-time progression-free survival (PFS) of 50%, a large proportion of patients will relapse with more aggressive tumors. On the other hand, radical cystectomy (RC) in the setting of stage T1-disease results in excellent outcome, but at the cost of morbidity and mortality related to RC.
For MIBC the standard treatment is neoadjuvant chemotherapy with cisplatin-based combinations followed by RC, however despite the 5–8% increased absolute survival from neoadjuvant therapy [Citation5–7], a large proportion of patients succumb to bladder cancer. Consequently, bladder cancer mortality has been unchanged for decades [Citation8].
With the description of bladder cancer subtypes in 2012 [Citation9], the knowledge about bladder cancer biology increased significantly [Citation10]. A recent British initiative, including both health care professionals and bladder cancer patients, aiming to prioritize unanswered bladder cancer research questions stated that the most important research issue was molecular profiling of MIBC to select and stratify patients for treatments in respect to risk of relapse and/or prognosis [Citation11].
In the wake of the description of molecular subtypes in bladder cancer and the unmet need to individualize bladder cancer treatment, a biobank initiative was launched in the Southern Healthcare Region in 2013 (UROSCAN) that in 2018 was further developed to facilitate real-time molecular classification through RNA-sequencing (UROSCANSEQ). The current cohort profile aims to describe the structure and progress of this translational effort and outline future putative developments.
Methods
Ethics statement
The study was approved by the Regional Ethical Review Board of Lund (2012/74 and 2013/452 (UROSCAN)) and (2017/34, 2017/269, 2018/963, 2020-05559, and 2021-00296 (UROSCANSEQ)). All patients signed an informed consent after receiving verbal information about study inclusion.
Infrastructure
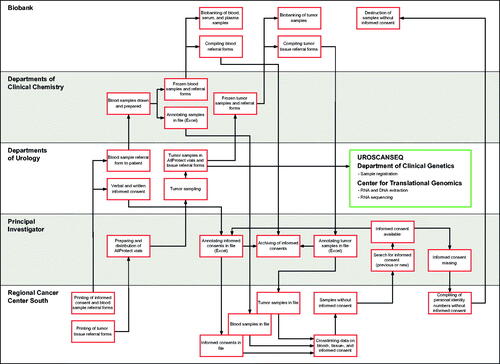
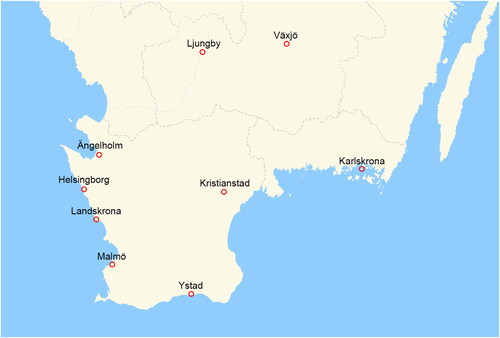
The biobanking in UROSCAN involves healthcare professionals in the Southern Healthcare Region in Sweden, corresponding to all nine units (Karlskrona, Växjö, Ljungby, Ängelholm, Helsingborg, Kristianstad, Landskrona, Ystad and Malmö) performing transurethral bladder cancer resections (TURB) (). The biobanking engages clinicians and researchers in the same geographic area, where urologists and bladder cancer nurses (patient navigators) in the Southern Sweden Urothelial Carcinoma Group collect samples under the auspices of Regional Cancer Center South biobank facility in Lund (Region Skåne). Departments of clinical chemistry locally freeze the samples before the specimens are transported to the biobank in batches (). Using the same logistic pipeline as for UROSCAN, real-time prospective RNA-sequencing and tumor classification was initiated in November 2018 (UROSCANSEQ) (ISRCTN15459149) in two of the units (Malmö and Landskrona), successively expanding over time to include six out of nine units in the Southern Healthcare Region and one additional hospital outside the UROSCAN-biobanking area (Southern Health Care Region) in the Southeastern Healthcare Region (Eksjö) from 2021 and onwards.
Figure 1. Geographical distribution of UROSCAN-sites in the Southern Healthcare Region in Sweden (Red indicators = Urologic unit with both outpatient clinic and where TURB are performed).
In Sweden histologically, cytologically, or clinically newly diagnosed tumors are by law reported to the national Swedish Cancer Registry [Citation12]. Since January 1, 1997 all newly diagnosed bladder cancers in individuals 18 years or older are requested to be reported into their respective Regional Cancer Center, which forward this information to the Swedish National Register for Urinary Bladder Cancer (SNRUBC) [Citation13] that once a year report to the Swedish Cancer Registry. In the SNRUBC, registration of tumor characteristics and primary treatment at diagnosis is performed. Subsequently, patients with non-muscle invasive bladder cancer are followed with information on time to local recurrence, progression, and death five years after date of diagnosis, and a radical cystectomy complication form with perioperative information is collected for individuals subjected to RC. From January 1, 2017, detailed data on oncological treatment with details on neoadjuvant, adjuvant, induction or palliative systemic treatment is also registered in SNRUBC.
Patients and samples
On January 29th 2013 six out of the nine units started the collection of preoperative blood, serum, plasma, and tumor samples during TURB to the UROSCAN-biobank, with the final expansion to the ninth and last unit performing TURB in the Southern Healthcare Region in 2020 (Ystad) (). Eligible patients are those with primary or recurrent bladder cancer subjected to TURB. Information on clinical tumor characteristics and treatments for the patients in the UROSCAN-biobank were retrieved from SNRUBC in September 2021.
Tumor and blood sample collection, logistics and processing
At diagnosis of primary or recurrent bladder cancer, usually by cystoscopy or after CT-urography with strong indication of bladder cancer, patients are informed about biobanking and the molecular diagnostics research project verbally and through written information in order to give informed consent. Tumor biopsy samples are obtained from single- or multiple tumors, with sampling preferably performed by cold-cup biopsies (to avoid diathermia cauterization artifacts) and from the exophytic tumor portion from a non-necrotic location. The specimens are collected in pre-aliquoted 2 ml cryotubes with 1 ml of Qiagen AllProtect Tissue Reagent storage solution and kept below 8 °C. Within five days the biobank samples are transported to the respective department of clinical chemistry for −80 °C storage until transferred in batches to the biobank. For each tumor, one sample is transferred to the Department of Clinical Genetics, Pathology and Molecular Diagnostics, Office for Medical Services, Region Skåne, for registration and subsequent RNA and DNA extraction. The venous blood samples are drawn into two EDTA-vials (7 ml each) and one serum vial (7 ml), where one of the EDTA-vials and the serum-vial are centrifugated. The whole blood sample and aliquots of serum and plasma are then transferred and stored in −80 °C within two hours of collection until transferred in batches to the biobank. An overview of blood sample and tumor biobank sample collection and logistics in the corresponding departments of clinical chemistry and departments of urology is illustrated in . The Regional Cancer Center South prints and archives forms, assesses that an informed consent is available for every patient, and maintains a central registry of patients and samples.
RNA/DNA extraction and sequencing
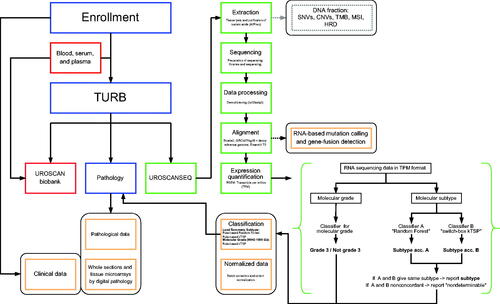
RNA and DNA extraction and RNA-sequencing analyses were performed at the Center for Translational Genomics (CTG), Lund University in Lund. Biopsies were washed from excess AllProtect solution, after which RNA and DNA was extracted using Qiagen QIAshredder and AllPrep RNA/DNA using the QIAcube system. Sequencing libraries were prepared with Illumina TruSeq Stranded mRNA Library Prep Kit following protocols established at CTG. All samples yielding viable libraries were sequenced with the Illumina NextSeq 500 System or with the Illumina NovaSeq 6000 System (from 04/2021) using a 150-8-8-150 read configuration. Demultiplexed (bcl2fast2 v2.20) fastq files were aligned using bowtie2 (v2.3.3) with expression calculated with RSEM (v1.3.0) using the GRCh37/hg19 genome build and Ensembl v75.
Molecular subtyping and grading
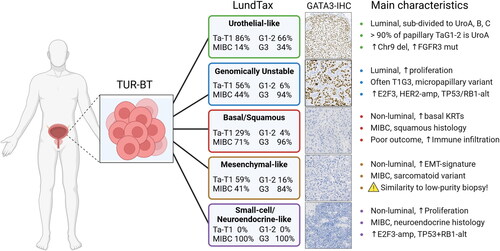
Molecular subtype classification according to the Lund Taxonomy [Citation9] () was created using two separate rule-based single-sample classifiers applied to transcripts per million (TPM) data [Citation14]. Both classifiers were built on a training dataset composed of in-house microarray data and TCGA RNA-sequencing data [Citation14–17], to design the single-sample classifiers [Citation18]. Both methods operate on the principle of binary gene-pair rules to perform single-sample transcriptomic class prediction. Each sample was also scored according to a rule-based single-sample molecular grade classifier trained on the WHO 1999 grading system (G1, G2, and G3) to identify G3 cases. The training data for this classifier consisted of 314 samples of the Urothelial-like subtype from two previous microarray studies [Citation9,Citation14]. The molecular results were reported to the clinician (without molecular subtype affecting treatment decisions at the multidisciplinary tumor board) using the same system as for the pathology report ().
Results
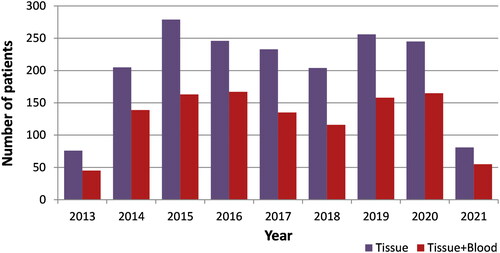
Between January 29th 2013 and June 21st 2021, 1825 individuals were included in the UROSCAN-biobank, of which 682 (37%) had a tissue sample only (). Tumor samples from 1650 individuals represented newly diagnosed bladder cancer, 127 (7%) samples were from locally recurrent tumors, and 48 samples lacked information about primary or recurrent tumor. In 159 patients with multiple tumors, two or more tumor samples were collected to the biobank. For 83 of the patients, additional tissue specimens were also collected from subsequently recurring metachronous tumors. The stage and grade distribution among the 1650 individuals with primary bladder cancer are given in . The majority were urothelial carcinomas (1630), and 20 tumors were of non-urothelial histology. Between 2016 and 2020, 2999 patients were diagnosed with bladder cancer in the Southern Healthcare region [Citation13], and the coverage for the UROSCAN-biobanking during these years were 1122/2999 (37%).
Table 1. Primary stage group distribution for the 1650 primary tumors and corresponding nodal stage and M-stage in the UROSCAN-biobank with grading according to WHO 1999 and staging according to TNM 2017.
The treatment received (in addition to TURB) in the 1650 newly diagnosed patients are given in . According to the Swedish National Guidelines, treatment discussion in a multidisciplinary tumor board (MDT) is recommended for patients with tumor stages T1-T4 (https://kunskapsbanken.cancercentrum.se/diagnoser/urinblaseochurinvagscancer/vardprogram/), and 594/726 (82%) of the patients within these tumor categories were subjected to such MDT-discussion.
Table 2. Primary treatment in addition to TURB in the UROSCAN-biobank.
RNA-sequencing of bladder cancer transcriptomes
Until June 21st 2021, the number of patients registered in UROSCANSEQ for RNA-sequencing and molecular subtyping was 605, of which 52 samples (9%) could not be sequenced due to inadequate RNA-quality after extraction (n = 47), technical failure (n = 3), no tube with sample arrived to the laboratory (n = 1), and no biopsy in the vial (n = 1).
Molecular subtyping
The two independent subtype classification algorithms were found to be highly concordant. On a 5-subtype level, the classifiers agreed in 510/553 (92%) of the cases, with 381 cases classified as Urothelial-like (UroA/UroB/UroC), 51 as Genomically Unstable (GU), 27 as Basal/Squamous-like (Ba/Sq), 10 as Small Cell/Neuroendocrine-like (Sc/NE), and 41 as Mesenchymal-like (Mes-like). Among samples with discordant classification, 21/43 (49%) involved the stroma-rich Mesenchymal-like subtype, with the remainder occurring between subtypes with similar transcriptional features, most commonly Genomically Unstable (GU) and Urothelial-like C (UroC) (n = 13). Examination of cohort-normalized expression data showed that tumor classification results strongly recapitulated previously described subtype-associated expression pattens. The preliminary grade prediction results closely mirrored recent observations from two independent NMIBC cohorts in terms of G3 frequencies across subtypes as well as associations with cell cycle and HOX-gene expression patterns [Citation19]. A full cohort tissue microarray (TMA) is currently under construction and whole section IHC is planned for cases with discordant classification or with a strong stromal expression signature component. This resource will be used for further classification algorithm optimization and refinements, as no separate fractions for histology routinely were obtained from the area around the UROSCANSEQ-samples during the TURB-procedures. Planned data expansions include FGFR3 hotspot mutation and fusion detection from RNA-sequencing data, genotyping on the Illumina Global Screening Array-Multiple Disease (GSA-MD) platform, and DNA sequencing using a clinical solid tumor panel currently under development by Genomic Medicine Sweden designed for mutation screening of approximately 560 genes as well as assessing tumor mutation burden (TMB), microsatellite instability (MSI), and homologous recombination deficiency (HRD) [Citation20].
Discussion
We have developed an infrastructure for prospective multicenter population-based bladder cancer biobanking in all nine units performing TURB in the Southern Healthcare Region in Sweden. Using the same logistic pipeline, we have additionally coupled transcriptomic profiling through real-time RNA-sequencing since 2018. Applying the latter with a prospective observational study-design, biomarker discovery projects and translation of bladder cancer molecular subtypes into clinical practice will be feasible i.e. by both validating previous findings in independent population-based series, as well as by testing of gene expression signatures from the literature. Thus, the biobanking (UROSCAN) and sequencing (UROSCANSEQ) will together shorten the time from discovery to clinical implementation of translational bladder cancer research-findings.
The prospective nature of biobanking and molecular subtyping will with time enable testing of a number of clinically relevant hypotheses in a population-based and adequately powered setting, depending on the research questions raised. For example, in the current imprecise assessment of progression risk in stage T1-disease based on clinical risk factors and pathological characteristics only [Citation21], the additional and independent prognostic value of molecular subtypes can be validated [Citation19,Citation22]. Thus, the treatment recommendation of either a bladder-sparing approach or radical cystectomy (RC) can be further supported by current hypothesis-generating findings that the Ba/Sq-like subtype has an independently increased risk of progression to muscle-invasive disease supporting RC upfront [Citation19,Citation22,Citation23]. Similarly, for patients with muscle-invasive disease without radiological signs of metastatic disease, three courses of neoadjuvant cisplatin-based combination chemotherapy increase survival by approximately 6% compared to RC only. This implies that the numbers needed to treat is above 16 to cure one additional patient, and that the neoadjuvant chemotherapy in the other individuals either was unnecessary or that the tumor was insensitive to chemotherapy. Recent findings supporting that Ba/Sq-like tumors are less likely to respond to cisplatin-based combination therapy [Citation24,Citation25], suggesting that the numbers needed to treat can decrease by including such treatment predictive information when deciding on applying neoadjuvant chemotherapy or not. Furthermore, in the wake of new third line treatments for bladder cancer emerging in the metastatic setting after chemotherapy and checkpoint inhibitors with enfortumab vedotin and erdafitinib [Citation26,Citation27], of which one (erdafitinib) is based on treatment-predictive FGFR3-mutations, consideration of molecular subtypes and treatment-impacting genomic alterations are also likely to be of importance when selecting sequence of such systemic treatments. The WHO 2022 classification of genitourinary tumors also highlights the potential impact of the novel molecular classification of bladder cancer on the diagnosis and future management of the disease [Citation28]. Additionally, the WHO 2022 state that the overlap between the different classification systems including the Lund Taxonomy is significant, which is illustrated by the recent consensus classification of muscle-invasive tumors [Citation28].
The lack of complete coverage of biobanking and RNA-sequencing are limitations of the current effort, needing improvement during the coming years. We additionally plan to expand the infrastructure to include blood samples at routine intervals to test hypotheses related to circulating tumor cells and presence of ctDNA to identify individuals suitable for adjuvant checkpoint-inhibition after radical cystectomy [Citation29], despite that the intention-to-treat analysis was negative in the original phase III trial [Citation30]. To further strengthen the current research effort, we also extend an invitation to other hospitals in Sweden and the Nordic countries to join the UROSCANSEQ network. Additionally, to include sampling and subtyping of metastatic lesions would also increase the knowledge about bladder cancer biology. Finally, the single-sample classifiers for molecular subtyping need to comply with the recent in vitro diagnostic medical devices regulation (IVDR) (2017/746/EU) before broader clinical implementation is launched.
Conclusions
We herein report initial experiences with the implementation of a large population-based infrastructure for prospective RNA-sequencing and molecular subtyping by applying previously established infrastructure for biobanking in the same population. To our knowledge this effort is currently the largest of its kind, although expansion would further increase the value of the project.
Acknowledgements
The authors thank the patients who have chosen to be part of the current effort and the Center for Translational Genomics, Lund University and Clinical Genomics Lund, SciLifeLab for providing sequencing service. The authors also thank the collaborators within urology, pathology, and clinical chemistry at the hospitals in Eksjö, Karlskrona, Växjö, Ljungby, Ängelholm, Helsingborg, Kristianstad, Landskrona, Ystad and Malmö for including patients and sampling tissue and blood and the Region Skåne Biobank for storage of samples and the Regional Cancer Center South for INCA data.
Disclosure satatement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Richters A, Aben KKH, Kiemeney LALM. The global burden of urinary bladder cancer: an update. World J Urol. 2020;38(8):1895–1904.
- Botteman MF, Pashos CL, Redaelli A, et al. The health economics of bladder cancer: a comprehensive review of the published literature. Pharmacoeconomics. 2003;21(18):1315–1330.
- Sievert KD, Amend B, Nagele U, et al. Economic aspects of bladder cancer: what are the benefits and costs? World J Urol. 2009;27(3):295–300.
- Liedberg F, Hagberg O, Holmäng S, et al. Local recurrence and progression of non-muscle-invasive bladder cancer in Sweden: a population-based follow-up study. Scand J Urol. 2015;49(4):290–295.
- Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349(9):859–866.
- International collaboration of trialists, medical research council advanced bladder cancer working party (now the national cancer research institute bladder cancer clinical studies group), european organisation for research and treatment of cancer Genito-Urinary tract cancer group, et al. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol. 2011;29:2171–2177.
- Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 2005;48:202–205. discussion 205–206.
- Malmström P-U, Gårdmark T, Sherif A, et al. Incidence, survival and mortality trends of bladder cancer in Sweden 1997-2016. Scand J Urol. 2019;53(4):193–199.
- Sjödahl G, Lauss M, Lövgren K, et al. A molecular taxonomy for urothelial carcinoma. Clin Cancer Res. 2012;18(12):3377–3386.
- Black PC. Seeking the molecular truth in bladder cancer: biology = genome×(transcriptome)2. Eur Urol. 2017;72(3):366–367.
- Bessa A, Maclennan S, Enting D, et al. Consensus in bladder cancer research priorities Between patients and healthcare professionals using a four-stage modified delphi method. Eur Urol. 2019;76(2):258–259.
- National Cancer Register [Internet]. Socialstyrelsen. 2022. https://www.socialstyrelsen.se/en/statistics-and-data/registers/national-cancer-register/
- Nationellt kvalitetsregister för urinblåsecancer - RCC [Internet]. 2022. https://www.cancercentrum.se/samverkan/cancerdiagnoser/urinblasa-urinvagar/kvalitetsregister/
- Sjödahl G, Eriksson P, Liedberg F, et al. Molecular classification of urothelial carcinoma: global mRNA classification versus tumour-cell phenotype classification. J Pathol. 2017;242(1):113–125.
- Robertson AG, Kim J, Al-Ahmadie H, TCGA Research Network, et al. Comprehensive molecular characterization of Muscle-Invasive bladder cancer. Cell. 2017;171(3):540–556.e25.
- Marzouka N-A-D, Eriksson P, Rovira C, et al. A validation and extended description of the lund taxonomy for urothelial carcinoma using the TCGA cohort. Sci Rep. 2018;8(1):3737.
- Marzouka N-A-D, Eriksson P. multiclassPairs: an R package to train multiclass pair-based classifier. Bioinformatics. 2021;37(18):3043–3044.
- Eriksson P, Marzouka N-A-D, Sjödahl G, et al. A comparison of rule-based and centroid single-sample multiclass predictors for transcriptomic classification. Bioinformatics. 2022;38(4):1022–1029.
- Marzouka N-A-D, Eriksson P, Bernardo C, et al. The lund molecular taxonomy applied to non-muscle–invasive urothelial carcinoma. J Mol Diagnostics. 2022;24(9):992–1008. https://www.sciencedirect.com/science/article/pii/S1525157822001908.
- Solid tumours | Genomic Medicine Sweden [Internet]. 2022. https://genomicmedicine.se/en/solid-tumours/.
- Sylvester RJ, Rodríguez O, Hernández V, et al. European association of urology (EAU) prognostic factor risk groups for non-muscle-invasive bladder cancer (NMIBC) incorporating the WHO 2004/2016 and WHO 1973 classification systems for grade: an update from the EAU NMIBC guidelines panel. Eur Urol. 2021;79(4):480–488.
- Patschan O, Sjödahl G, Chebil G, et al. A molecular pathologic framework for risk stratification of stage T1 urothelial carcinoma. Eur Urol. 2015;68(5):824–832. discussion 835–836.
- Jackson CL, Chen L, Hardy CS, et al. Diagnostic and prognostic implications of a three-antibody molecular subtyping algorithm for non-muscle invasive bladder cancer. J Pathol Clin Res. 2022;8(2):143–154.
- Taber A, Christensen E, Lamy P, et al. Molecular correlates of cisplatin-based chemotherapy response in muscle invasive bladder cancer by integrated multi-omics analysis. Nat Commun. 2020;11(1):4858.
- Sjödahl G, Abrahamsson J, Holmsten K, et al. Different responses to neoadjuvant chemotherapy in urothelial carcinoma molecular subtypes. Eur Urol. 2021;S0302-2838(21):02138–02132.
- Powles T, Rosenberg JE, Sonpavde GP, et al. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N Engl J Med. 2021;384(12):1125–1135.
- Loriot Y, Necchi A, Park SH, BLC2001 Study Group, et al. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N Engl J Med. 2019;381(4):338–348.
- Netto GJ, Amin MB, Berney DM, et al. The 2022 world health organization classification of tumors of the urinary system and male genital Organs-Part B: prostate and urinary tract tumors. Eur Urol. 2022;82(5):469–482.
- Powles T, Assaf ZJ, Davarpanah N, et al. ctDNA guiding adjuvant immunotherapy in urothelial carcinoma. Nature. 2021;595(7867):432–437.
- Bellmunt J, Hussain M, Gschwend JE, IMvigor010 Study Group, et al. Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22(4):525–537.