Abstract
Objectives
To assess the adverse impact of the first 5 months of androgen deprivation therapy on body composition, physical performance, cardiometabolic health and health-related quality-of-life in prostate cancer patients.
Materials and Methods
Thirty-four prostate cancer patients (70 ± 7 years) were assessed shortly after initiation of androgen deprivation therapy and again 5 months thereafter. Measurements consisted of whole-body dual-energy x-ray absorptiometry (body composition), computed tomography scanning of the upper leg (muscle mass), one-repetition maximum leg press (muscle strength), cardiopulmonary exercise testing (aerobic capacity), blood draws (metabolic parameters), accelerometry (habitual physical activity) and questionnaires (health-related quality-of-life). Data were analyzed with Student’s paired t-tests.
Results
Over time, whole-body fat mass (from 26.2 ± 7.7 to 28.4 ± 8.3 kg, p < 0.001) and fasting insulin (from 9.5 ± 5.8 to 11.3 ± 6.9 mU/L, p < 0.001) increased. Declines were observed for quadriceps cross-sectional area (from 66.3 ± 9.1 to 65.0 ± 8.5 cm2, p < 0.01), one-repetition maximum leg press (from 107 ± 27 to 100 ± 27 kg, p < 0.01), peak oxygen uptake (from 23.2 ± 3.7 to 20.3 ± 3.4 mL/min/kg body weight, p < 0.001), step count (from 7,048 ± 2,277 to 5,842 ± 1,749 steps/day, p < 0.01) and health-related quality-of-life (from 84.6 ± 13.5 to 77.0 ± 14.6, p < 0.001).
Conclusions
Androgen deprivation therapy induces adverse changes in body composition, muscle strength, cardiometabolic health and health-related quality-of-life already within 5 months after the start of treatment, possibly largely contributed by diminished habitual physical activity. Prostate cancer patients should, therefore, be stimulated to increase their habitual physical activity immediately after initiation of androgen deprivation therapy, to limit adverse side-effects and to improve health-related quality-of-life.
Introduction
Androgen Deprivation Therapy (ADT) forms the cornerstone of treatment for (locally) advanced and metastatic prostate cancer (PCa) [Citation1]. While being effective in (temporarily) stopping disease progression, ADT can lead to adverse health effects. PCa patients lose muscle mass (∼2%) [Citation2] and gain fat mass (∼11%) [Citation3] in the first year of ADT. In addition, cross-sectional studies indicate that PCa patients undergoing ADT have decreased aerobic capacity [Citation4], unfavorable blood lipid profiles and impaired blood glucose homeostasis [Citation5], resulting in a higher risk of developing cardiovascular comorbidities [Citation6].
In contrast to the evident changes in body composition and cardiovascular risk profile, the adverse effects of ADT on muscle strength and physical performance are less conclusive. Studies show that PCa patients either preserve [Citation7] or slightly lose [Citation8] muscle strength, with physical performance remaining unchanged [Citation8] or decreasing [Citation9]. Equally uncertain is the impact of ADT on health-related quality-of-life (HRQoL), which has been shown to decrease among PCa patients in some [Citation10] but not all studies [Citation11].
Discrepancies in literature are likely attributed to the inclusion of PCa patients who were assessed at a variety of stages during their treatment with ADT. To obtain more insight in the early development of these adverse side-effects, longitudinal research directly upon ADT initiation is required. Furthermore, previous studies often chose to exclude PCa patients with comorbidities and bone metastases. However, it is important to include these vulnerable patients in order to obtain results that are generalizable to the entire PCa patient population. In addition, longitudinal data on changes in lifestyle factors (e.g. habitual nutritional intake and physical activity) which are likely to contribute to the adverse effects of ADT are generally lacking. Therefore, assessing the wide variety of adverse effects and modifiable lifestyle factors, following the onset of ADT initiation, will paint a better picture on the magnitude and coherence of the ADT-induced adverse effects. Moreover, this information is crucial to obtain more insight in the type and preferred timing of interventions aiming to counteract the adverse effects of ADT.
Forty patients with diagnosed PCa were recruited to participate in this study that assessed the full spectrum of ADT-induced adverse side-effects, including modifiable lifestyle factors, during the first 5 months of treatment. This study provides an extensive overview of the impact of commencing ADT on body composition, physical performance, cardiometabolic health and HRQoL in PCa patients.
Methods
Patients
From June 2018 to February 2021, 40 PCa patients were included in the study. The inclusion criterium was that patients had to be started with gonadotropin-releasing hormone (GnRH) agonists or antagonists for at least 6 months. Exclusion criteria were: any contraindications for maximal exercise testing, an estimated life expectancy < 1 year, cognitive disorders, severe emotional instability, or the inability to speak, understand or read the Dutch language. The study was approved by the Medical Ethical Committee of the Maastricht University Medical Center+ (MUMC+), The Netherlands (METC 16-3-040), and all patients signed written informed consent. This study was part of a greater project investigating the impact of resistance exercise and protein supplementation on counteracting the adverse effects of ADT in PCa patients and registered at the Dutch Trial Register (International Clinical Trial Registry Platform: NTR6432).
Study design
Potential participants were referred to the investigators by their treating urologist or urology nurse. The investigators provided full oral and written study information. After ≥ 1 week, interested patients were invited for a screening visit to confirm eligibility, obtain informed consent, evaluate medical history and in- and exclusion criteria, measure blood pressure and perform a cardiopulmonary exercise test (CPET).
At ADT initiation, baseline anthropometric measurements (height, body weight, waist circumference), computed tomography (CT) of the dominant leg, whole-body dual-energy X-ray absorptiometry (DXA) were performed and fasting blood samples were collected. After lunch, physical performance tests and maximal strength assessments were done. The screening visit and baseline measurements were preferably separated by at least 7 days. During this time period, patients were instructed to wear an accelerometer and to fill out a 3-day food diary and several questionnaires. All baseline measurements were repeated 5 months later at the second assessment day. The CPET was performed at least 48 h before or after the 5 month measurements, to prevent any influence on the other outcome measurements. Throughout the manuscript, causal terminology is used with the awareness that other factors than ADT (e.g. additional treatment) also contributed to the observed changes.
Dietary intake and physical activity
Patients were instructed to refrain from any exhaustive physical activity 48 h before the experimental test days and to arrive in a rested and fasted state. In the week prior to the experimental test days, patients reported their dietary intake on two weekdays and one weekend day. Average daily dietary intake was calculated using web-based software Eetmeter (Voedingscentrum, Den Haag, The Netherlands). Patients were instructed to wear a triaxial accelerometer (wGT3X-BT; ActiGraph, Pensacola, FL) on their waist during wakefulness for 7 days prior to the experimental test days. Data were extracted with ActiLife (version 6.13.4; ActiGraph) and included in the analyses if patients wore the accelerometer for a minimum of 5 days and at least 10 h per day.
Body composition
Body weight, height and waist circumference were measured after voiding. Quadriceps muscle cross-sectional area (CSA) of the dominant leg was assessed by CT scanning (SOMATOM Definition Flash; Siemens, München, Germany) as described previously [Citation12] and calculated by manual tracing using ImageJ software (version 1.52p, National Institute of Health, Bethesda, MD). Lean mass and fat mass were assessed by whole-body DXA (Discovery A; Hologic, Marlborough, MA [MUMC + and JBZ] and LUNAR iDXA; GE Healthcare, Chicago, IL [JBZ]).
Physical performance and muscle strength
Physical performance was assessed by the timed up and go test [Citation13], the 30-second chair stand test [Citation14] and the stair climb test [Citation15]; always performed in the same order. Maximal strength was assessed by one-repetition maximum (1RM) tests on the leg press (Technogym, Milan, Italy) and leg extension machines (Technogym, Milan, Italy [MUMC+] and Keiser Corporation, Fresno, CA [JBZ]). Patients started with a warm-up on a cycle ergometer followed by a warm-up of 10 and 5 repetitions at a selected load on the specific exercise machines. Subsequently, the 1RM was determined by increasing the load after each successful single lift until failure [Citation16].
Cardiopulmonary exercise testing
Patients’ maximal workload and aerobic capacity was tested with a CPET to exhaustion with continuous electrocardiogram monitoring and respiratory gas analysis. Patients performed an individualized ramp protocol [Citation17] on a cycle ergometer (Lode Corival, Groningen, The Netherlands [MUMC+] or Ergoline; Bitz, Germany [JBZ]). Ventilatory parameters were measured breath-by-breath (Carefusion; San Diego, CA [MUMC+] or Geratherm Respiratory; Bad Kissingen, Germany [JBZ]). Maximal workload (Wmax) was defined as the final registered workload. Peak oxygen uptake (VO2peak), peak respiratory exchange ratio (RERpeak) and peak heart rate were recorded as the final 30-second averaged value of the test.
Blood parameters
Plasma insulin concentrations were determined by commercially available radioimmunoassay kits (Human Insulin specific RIA, Millipore Corporation, MA). Plasma glucose, free fatty acids (FFAs), total cholesterol and high-density lipoproteins were measured with enzymatic assays on an automated spectrophotometer (ABX Pentra 400 autoanalyzer, Horiba ABX, Montpellier, France). Low-density lipoprotein cholesterol was calculated using the Friedewald formula [Citation18]. All blood analyses were performed at MUMC+.
Quality-of-life and fatigue
HRQoL was questioned with the European Organization for Research and Treatment of Cancer Quality-of-Life Questionnaire (EORTC QLQ-C30) [Citation19] and the Pca-specific EORTC QLQ-PR25 [Citation20]. Self-reported levels of fatigue were questioned with the Multidimensional Fatigue Inventory (MFI) [Citation21].
Statistical analysis
Data are expressed as means ± standard deviations (SDs) (normally distributed continuous variables), as median and interquartile range (non-normally distributed continuous variables) and frequency and percentages (categorical variables). Student’s paired t-tests were performed to assess changes over time for all continuous variables. If the differences between pairs were not normally distributed, the Wilcoxon signed-rank test was performed. Significance was set at p < 0.05. All analyses were performed with SPSS (version 27.0; IBM Corp., Armonk, NY).
Results
Patients
Of the 40 patients initially included, data of 34 patients were analyzed. Six patients dropped out due to: medical reasons (n = 2) or the COVID-19 lockdown (n = 4). The mean age of the study population was 70 ± 7 years at baseline. Patients were slightly overweight (BMI: 26.4 ± 3.6 kg/m2) and most patients suffered from two or more comorbidities (). Patients were on average 26 ± 18 days on ADT (all GnRH agonists) and 56% of patients received either radiotherapy or chemotherapy (6 cycles of docetaxel) during the study period.
Table 1. Patients’ baseline characteristics (n = 34).
Body composition
BMI (+0.6 ± 1.1 kg/m2), waist circumference (+2 ± 3 cm), whole-body fat mass (+2.1 ± 1.8 kg, ) and fat percentage (+1.9 ± 1.8%) had significantly increased following 5 months ADT (). A significant decrease over time was found for appendicular lean mass (−0.5 ± 1.3 kg) and quadriceps CSA (−1.3 ± 2.5 cm2, ).
Figure 1. Individual values of quadriceps cross-sectional area (CSA, a) and whole-body fat mass (b) before and after 5 months androgen deprivation therapy in prostate cancer patients. *Significantly different from baseline (a, p < 0.001; b, p = 0.006).
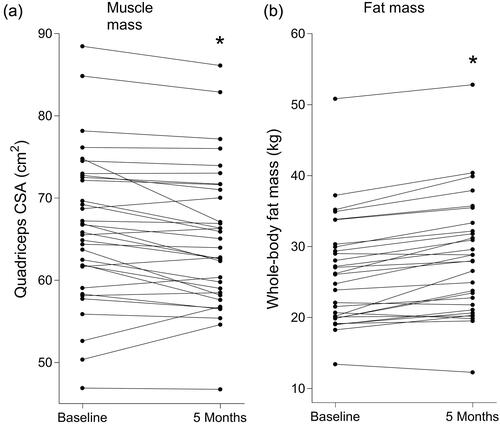
Table 2. Changes in body composition in prostate cancer patients following 5 months androgen deprivation therapy.
Muscle strength, physical performance and cardiopulmonary outcomes
Both leg press (−6 ± 11 kg, ) and leg extension (−3 ± 8 kg) 1RM had significantly decreased following 5 months ADT. Patients needed significantly more time to complete the stair climb test (+0.61 ± 1.60 s). Wmax (−0.23 ± 0.18 W/kg body weight) and VO2peak (−2.9 ± 2.6 mL/min/kg body weight, ) significantly decreased during the 5 month period ().
Figure 2. Individual values of one-repetition maximum (1RM) leg press (a) and peak oxygen uptake (VO2peak, b) before and after 5 months androgen deprivation therapy in prostate cancer patients. * Significantly different from baseline (a, p = 0.003; b, p < 0.001).
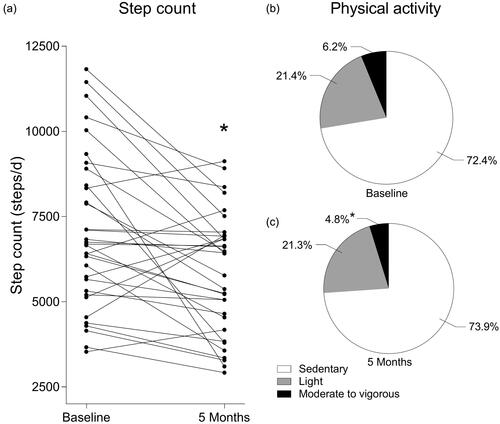
Table 3. Changes in muscle strength, physical performance, aerobic capacity and habitual physical activity in prostate cancer patients following 5 months androgen deprivation therapy.
Physical activity and nutritional intake
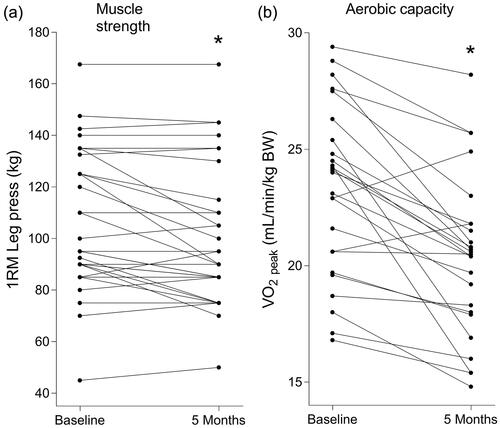
After 5 months ADT, step count had significantly decreased (−1,207 ± 1,942 steps/day, ) and patients were spending significantly less time performing moderate-to-vigorous activities (−1.4 ± 2.5%, ). Nutritional intake had not changed over time and patients were consuming ample amounts of protein (>1.0 g/kg body weight/d) both at baseline and after 5 months ADT (Supplementary Table S1).
Figure 3. Individual values of step count before and after 5 months androgen deprivation therapy in prostate cancer patients (a). Percentage of the day spent sedentary, performing light activities or performing moderate to vigorous activities before (b) and after (c) 5 months androgen deprivation therapy in prostate cancer patients. * Significantly different from baseline (a, p = 0.001; c, p = 0.003).
Blood parameters
Fasting plasma insulin (+1.8 ± 2.5 mU/L) and triacylglycerol (+286 ± 489 µmol/L) levels had significantly increased after 5 months ADT. No significant changes over time were found for plasma glucose, cholesterol, or FFAs. Homeostatic model assessment as a proxy for whole-body insulin resistance (HOMA-IR) index had significantly increased following the 5 month period (+0.6 ± 0.8, Supplementary Table S2).
Quality-of-life and fatigue
Global health status (−7.6 ± 10.3) decreased and fatigue (+12.5 ± 18.6) had increased significantly following 5 months of ADT. Looking more specifically at fatigue (MFI), both general (+7.3 ± 4.1) and physical (+7.5 ± 3.9) fatigue had significantly increased. In addition, patients had significantly increased negative feelings about their weight gain, more hot flushes and decreased sexual activity and functioning following 5 months ADT (Supplementary Table S3).
Discussion
In the present study, we showed that 5 months of ADT strongly decreased muscle mass and increased fat mass in prostate cancer patients. This was accompanied by a decrease in muscle strength, cardiometabolic health, physical activity levels and quality-of-life. These unfavorable changes occurred rapidly and were observed within 5 months after initiating ADT.
Patients had lost as much as 2 ± 5% appendicular lean mass following merely 5 months of ADT (). This decline is in line with the literature but our data extend on previous work by showing that the decline is already achieved well within the previous observed assessment period of 12 months of ADT [Citation2]. Therefore, our data imply that changes in body composition occur rapidly following onset of ADT. We complemented DXA measurements with measurements of quadriceps CSA by using CT and confirmed a 2 ± 4% decline in quadriceps CSA within the 5 month assessment period (). The combination of DXA and CT measurements in the present study clearly shows that ADT leads to considerable lean mass loss, which is accompanied by a substantial reduction in leg muscle mass. The latter is of importance as a decline in leg muscle mass is generally associated with compromised (metabolic) health and impaired ambulation. In fact, loss of leg muscle mass generally represents a good indicator of diminished physical performance and the loss of independence in older patient populations [Citation22].
Previous work in PCa patients on ADT remains inconclusive with regards to changes in strength and physical performance. Hand-grip strength testing is often applied and studies find either no or only a slight adverse impact of ADT on hand-grip strength [Citation7,Citation8]. Because leg strength is a more important indicator for daily functioning and independent living in older adults, we assessed leg strength. We observed a significant decrease in both leg press (−5 ± 10%, ) and leg extension (−7 ± 14%) strength following 5 months of ADT. Furthermore, previous cross-sectional studies indicate that there likely is an unwanted effect of ADT on aerobic capacity [Citation4,Citation23]. We, however, are the first to assess longitudinal changes. Peak oxygen uptake had declined by 12 ± 10% following the 5 months treatment (). It is evident that it is of clinical relevance to attenuate or even prevent such declines in muscle strength and aerobic capacity, as both are directly responsible for compromised daily functioning, independent living and self-reliance [Citation22,Citation24] and strongly modulate perceived HRQoL, health care consumption and societal participation.
The declines in lean mass and physical performance in our patient group were accompanied by a significant increase in fat mass (). This is in line with previous research showing that ADT increases fat mass with ∼11% during the first year of treatment [Citation3]. Again, we observed an 8 ± 8% increase in fat mass following merely 5 months of treatment, indicating that these changes in body composition occur rapidly following onset of ADT. In line, patients experienced a significant increase in waist circumference, which supports the contention that fat mass gain during ADT predominantly occurs in the abdominal region. Increased abdominal fat mass forms a risk factor for the development of insulin resistance and can eventually lead to type II diabetes. In accordance, patients in the present study had a significantly increased HOMA-IR index after 5 months of treatment. These metabolic derangements need to be diminished to prevent the development of type II diabetes or other cardiometabolic diseases and its impact on survival in PCa patients on ADT [Citation25].
Lifestyle changes in diet and physical activity can strongly affect body composition, muscle strength, aerobic capacity and cardiometabolic risk profile. Therefore, it is of importance to assess possible changes in lifestyle factors following onset of ADT as these may contribute substantially to our observed changes in metabolic health and performance. Even though total daily energy intake did not seem to change over time (Supplementary Table S1), we observed a significant decline in habitual physical activity. Following ADT initiation, our patients spent over 70% of their day being sedentary (). More specifically, daily step count significantly decreased over time with a substantial 13 ± 25% decline in total steps taken per day (). Therefore, we cannot exclude that the adverse health effects experienced during treatment are at least partly attributed to the concomitant changes in lifestyle. Also, the impact of the burden of diagnosis and/or disease on habitual physical activity remains unknown in our population, but could be substantial. In an exploratory analysis, we divided patients in two groups; patients below and patients above the mean change in step count of the total study population. Indeed, patients in the group with the large decrease in step count (−3,286 ± 1,344 steps/day) seem to be more prone to losing muscle strength compared to the group with no decrease in step count (−18 ± 983 steps/day). Physical activity and exercise are modifiable factors that can strongly improve metabolic health and physical performance in these patients [Citation26]. Physical activity has also been shown to improve HRQoL in PCa patients [Citation27]. In addition, a modest amount of vigorous activity may even substantially improve PCa-specific survival [Citation28]. Therefore, it is obvious that PCa patients should be stimulated to adopt a more active lifestyle immediately following the onset of ADT. Awareness amongst urologists about the importance of increasing habitual activity and the application of exercise training is prerequisite. Involved healthcare professionals will be fundamental to prescribe and support lifestyle interventions upon the onset of ADT. Sports physicians, rehabilitation physicians and/or physical therapists may offer support on how to successfully embed physical activity and exercise in daily life and as such improve HRQoL and survival of patients with (metastasized) PCa. The present study is the first to show this broad spectrum of ADT-induced side-effects all within one single population of PCa patients. Preferably, patients only on ADT monotherapy were included, but in order to increase inclusion rates we broadened our inclusion criteria. In a sub analysis, however, patients who were also treated with additional therapies seem to have a similar decrease in muscle mass and strength compared to patients only treated with ADT (data not shown). Also, comorbidities and metastatic disease might have influenced the outcomes, and should be taken into account when considering the results. However, combining ADT with other therapies is more often used in PCa patients and comorbidities and metastatic disease are common in this population. Consequently, the generalizability of our results to the entire PCa population receiving ADT is higher.
In conclusion, onset of androgen deprivation therapy leads to a rapid decline in muscle mass, muscle strength, cardiometabolic health and quality-of-life in prostate cancer patients. These adverse effects are accompanied by diminished habitual physical activity, which should be corrected during the early stages of treatment.
Author contributions
Study design: Luc J. C. van Loon, Milou Beelen, Sandra Beijer; Data analysis: Maarten Overkamp, Lisanne H. P. Houben, Luc J. C. van Loon, Milou Beelen, Sandra Beijer; Article draft: Maarten Overkamp, Lisanne H. P. Houben, Luc J. C. van Loon, Milou Beelen, Sandra Beijer; Critical revision: all authors. Publication approval: all authors.
Supplemental Material
Download MS Word (19.7 KB)Acknowledgments
The authors greatly appreciate all patients who volunteered to participate in the study. We thank the staff of the urologic departments of the participating hospitals, the department of physiotherapy of JBZ, and the Sports Medical Center of JBZ for their collaboration and assistance. Also, we thank the staff of the radiology departments of the participating hospitals.
Disclosure statement
M.S. Larsen is an employee of Arla Foods Ingredients Group. L. J. C. van Loon has received research grants, consulting fees, speaking honoraria, or a combination of these for research on the impact of exercise and nutrition on muscle metabolism, which include funding from companies such as Friesland Campina and Arla Foods Ingredients Group. A full overview on research funding is provided at: https://www.maastrichtuniversity.nl/l.vanloon. M. Beelen has received a research grant from Arla Foods Ingredients Group. S. Beijer has received a research grant, consulting fees, speaking honoraria, or a combination of these from Baxter, Nutricia, and Arla Foods Ingredients Group. All other authors declare no competing interests.
Additional information
Funding
References
- Cornford P, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer. Part II-2020 update: treatment of relapsing and metastatic prostate cancer. Eur Urol. 2021;79(2):263–282.
- Smith MR, Saad F, Egerdie B, et al. Sarcopenia during androgen-deprivation therapy for prostate cancer. J Clin Oncol. 2012;30(26):3271–3276.
- van Londen GJ, Levy ME, Perera S, et al. Body composition changes during androgen deprivation therapy for prostate cancer: a 2-year prospective study. Crit Rev Oncol Hematol. 2008;68(2):172–177.
- Wall BA, Galvão DA, Fatehee N, et al. Reduced cardiovascular capacity and resting metabolic rate in men with prostate cancer undergoing androgen deprivation: a comprehensive Cross-Sectional investigation. Adv Urol. 2015;2015:976235.
- Basaria S, Muller DC, Carducci MA, et al. Hyperglycemia and insulin resistance in men with prostate carcinoma who receive androgen-deprivation therapy. Cancer. 2006;106(3):581–588.
- Kintzel PE, Chase SL, Schultz LM, et al. Increased risk of metabolic syndrome, diabetes mellitus, and cardiovascular disease in men receiving androgen deprivation therapy for prostate cancer. Pharmacotherapy. 2008;28(12):1511–1522.
- Stone P, Hardy J, Huddart R, et al. Fatigue in patients with prostate cancer receiving hormone therapy. European Journal of Cancer (Oxford, England: 1990). 2000;36(9):1134–1141.
- Gonzalez BD, Jim HSL, Small BJ, et al. Changes in physical functioning and muscle strength in men receiving androgen deprivation therapy for prostate cancer: a controlled comparison. Support Care Cancer. 2016;24(5):2201–2207.
- Levy ME, Perera S, van Londen GJ, et al. Physical function changes in prostate cancer patients on androgen deprivation therapy: a 2-year prospective study. Urology. 2008;71(4):735–739.
- Huang YT, Li CC, Chou YH, et al. Health-related quality of life of exposed versus non-exposed androgen deprivation therapy patients with prostate cancer: a cross-sectional study. Int J Clin Pharm. 2019;41(4):993–1003.
- Boevé L, Hulshof M, Verhagen P, et al. Patient-reported quality of life in patients with primary metastatic prostate cancer treated with androgen deprivation therapy with and Without concurrent radiation therapy to the prostate in a prospective randomised clinical trial; data from the HORRAD trial. Eur Urol. 2021;79(2):188–197.
- Backx EMP, Hangelbroek R, Snijders T, et al. Creatine loading does not preserve muscle mass or strength During leg immobilization in healthy, young males: a randomized controlled trial. Sports Med. 2017; Aug47(8):1661–1671.
- Podsiadlo D, Richardson S. The timed "Up & go": a test of basic functional mobility for frail elderly persons. Journal of the American Geriatrics Society. 1991;39(2):142–148.
- Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport. 1999;70(2):113–119.
- Nightingale EJ, Pourkazemi F, Hiller CE. Systematic review of timed stair tests. J Rehabil Res Dev. 2014;51(3):335–350.
- Verdijk LB, Koopman R, Schaart G, et al. Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am J Physiol Endocrinol Metab. 2007;292(1):E151–7.
- Mezzani A, Agostoni P, Cohen-Solal A, et al. Standards for the use of cardiopulmonary exercise testing for the functional evaluation of cardiac patients: a report from the exercise physiology section of the european association for cardiovascular prevention and rehabilitation. Eur J Cardiovasc Prev Rehabil. 2009;16(3):249–267.
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502.
- Aaronson NK, Ahmedzai S, Bergman B, et al. The european organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. 3
- van Andel G, Bottomley A, Fosså SD, et al. An international field study of the EORTC QLQ-PR25: a questionnaire for assessing the health-related quality of life of patients with prostate cancer. Eur J Cancer. 2008;44(16):2418–2424.
- Smets EM, Garssen B, Bonke B, et al. The multidimensional fatigue inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39(3):315–325.
- Wang DXM, Yao J, Zirek Y, et al. Muscle mass, strength, and physical performance predicting activities of daily living: a meta-analysis. J Cachexia Sarcopenia Muscle. 2020;11(1):3–25.
- Gong J, Payne D, Caron J, et al. Reduced cardiorespiratory fitness and increased cardiovascular mortality After prolonged androgen deprivation therapy for prostate cancer. JACC CardioOncol. 2020;2(4):553–563.
- Shephard RJ. Maximal oxygen intake and independence in old age. Br J Sports Med. 2009 May;43(5):342–346.
- Shevach J, Gallagher EJ, Kochukoshy T, et al. Concurrent diabetes mellitus may negatively influence clinical progression and response to androgen deprivation therapy in patients with advanced prostate cancer. Front Oncol. 2015;5:129.
- Andersen MF, Midtgaard J, Bjerre ED. Do patients with prostate cancer benefit from exercise interventions? A systematic review and Meta-Analysis. IJERPH. 2022;19(2):972.
- Chipperfield K, Brooker J, Fletcher J, et al. The impact of physical activity on psychosocial outcomes in men receiving androgen deprivation therapy for prostate cancer: a systematic review. Health Psychol. 2014;33(11):1288–1297.
- Kenfield SA, Stampfer MJ, Giovannucci E, et al. Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J Clin Oncol. 2011;29(6):726–732.
- Sangha O, Stucki G, Liang MH, et al. The Self-Administered comorbidity questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49(2):156–163.

