Abstract
The gastro-intestinal tract is an ecosystem containing trillions of commensal bacteria living in symbiosis with the host. These microbiota modulate a variety of our physiological processes, including production of vitamins, absorption of nutrients and development of the immune system. One of their major functions is to fortify the intestinal barrier, thereby helping to prevent pathogens and harmful substances from crossing into the general circulation. Recently, effects of these microbiota on other blood-tissue barriers have also been reported. Here, we review the evidence indicating that gut bacteria play a role in regulating the blood-brain and blood-testis barriers. The underlying mechanisms include control of the expression of tight junction proteins by fermentation products such as butyrate, which also influences the activity of histone deacetylase.
Introduction
In specialized compartments of the body, movements of molecules and cells between the blood and tissues are hindered by so-called gatekeepers or barriers. Such blood-tissue barriers were first described about 100 years ago in pioneer experiments showing that dyes administered to laboratory animals failed to stain the testis and the brain,Citation1–3 leading to the concepts of the blood-brain barrier (BBB) and the blood-testis barrier (BTB). These two barriers are considered the tightest in the body, but differ in structure and function. Here, we will review recent findings concerning the role of the gut microbiota in maintaining these 2 barriers.
The blood-brain barrier (BBB)
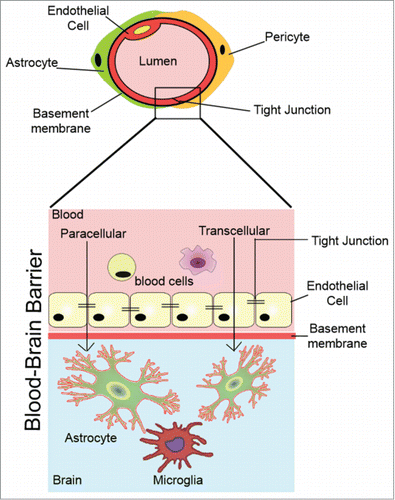
The BBB is formed by tight junctions (TJ) between endothelial cells that line cerebral microvessels (). Small gaseous molecules (O2 and CO2) and small lipophilic agents, including drugs such as ethanol, caffeine, nicotine, heroin and methadone, can diffuse freely through the lipid membranes of the BBB; whereas hydrophilic molecules such as glucose, several amino acids and neurotransmitters must be carried across by specific transporters. Two major groups of transporters are involved, i.e., the solute carriers (SLCs) and active efflux carriers (ABC transporters), both expressed on the luminal and/or adluminal surface of the BBB. Large hydrophilic molecules such as proteins can only be translocated across membranes via endocytosis (receptor-mediated transcytosis or adsorptive-mediated),Citation4 which is however, uncommon in brain endothelium. The high metabolic demands placed on cerebral endothelial cells by active transport are reflected in higher abundance of mitochondria than in systemic endothelial cells.Citation5 In addition, another route of transport and passage is the paracellular pathway that is specifically regulated by the tight junction (TJ) proteins.
Figure 1. The blood–brain barrier (BBB). Molecules cross the BBB either transcellulary or paracellularly between the cells through the junctions.
Development of the BBB
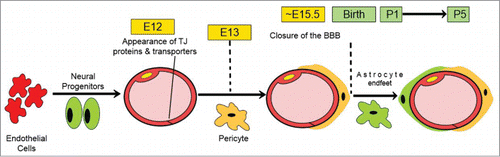
During embryonic angiogenesis, neural progenitors induce endothelial cells to express BBB-specific proteins such as TJ proteins and nutrient transporters. At E13, pericytes then strengthen the barrier properties by sealing the interendothelial TJ proteins, limiting the rate of transcytosis, down-regulating the expression of leukocyte adhesion molecules and inducing the expression of efflux transporters. A functional BBB that excludes tracers administered intravenously from the CNS parenchyma is present between E15-E16.Citation6,7 Normally, astrocytes appear postnatally to provide additional support to the functional BBB during adulthood, as well as in connection with injury and disease ().Citation6,8
Figure 2. Schematic illustration for the time-course of BBB development. Modified from ref. Citation6.
Functions of the BBB
The many vital roles played by the BBB include supplying the brain with important nutrients, mediating the efflux of numerous waste and toxic substances, e.g. misfolded proteins, and regulating ion trafficking between the blood and brain via specific ion transporters and channels to produce a brain interstitial fluid (ISF) of optimal composition for neuronal function. This composition is similar to that of blood plasma except that the protein content is much lower, the K+ and Ca2+ concentrations are also lower and the level of Mg2+ is higher. Importantly, the BBB protects the brain from fluctuations in ionic composition that can occur following exercise or after a meal. Furthermore, since immune surveillance in the CNS is limited, the BBB acts as a shield against infection and foreign materials.Citation4,9
Cell types associated with the BBB
The brain endothelial cells that form the BBB are surrounded by or closely associated with several types of cells, including the perivascular end-feet of astrocytic glia, pericytes, microglia and neuronsCitation4,9 (). The close association between such cells and brain capillaries suggests that they are involved in specific features of the BBB and, indeed, transplantation studies have demonstrated that formation of the BBB is induced by interactions between endothelial cells and the neural cells.Citation10 There is now strong evidence, particularly from cell cultures, that astrocytes can up-regulate many features of the BBB involved in creating effective tight junctions.Citation11 In addition, pericytes are required for the integrity of this barrier both during embryogenesisCitation6 and in adulthood.Citation12 Thus, the BBB of adult mice lacking pericytes is leaky to water and a range of low and high-molecular-weight tracers. During development, pericyte–endothelial cell interactions are crucial for the formation of TJPs in the BBB, as well as for vesicle trafficking by CNS endothelial cells.
The possible influence of other cell types on the BBB is less well characterized. Some investigations suggest an inductive role for microglia and macrophages derived from blood monocytes that are resident in the CNS. Accordingly, co-culture of brain endothelial cells with blood macrophages enhanced barrier tightness.Citation13 Some indirect evidence indicates that smooth muscle cells may also influence BBB functions.Citation14
The blood-testis barrier (BTB)
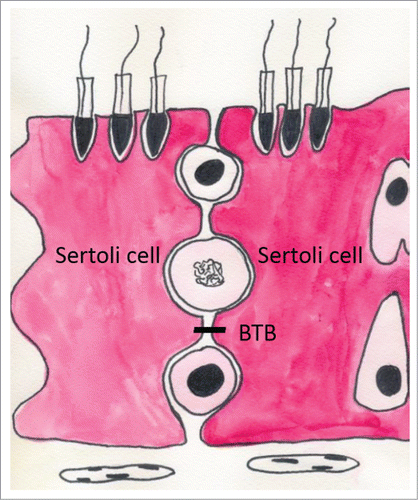
The BTB is formed by TJPs between 2 adjacent Sertoli cells at the seminiferous tubules (), with the peritubular layer of myoid cells that encircle the seminiferous tubules and the testis endothelial cells in the interstitium also making a significant contribution.Citation15,16 The primary functions of this barrier are to segregate the haploid male germ cells from the immune system, create polarity and help to create a unique environment for germ cell differentiation. At the same time, the BTB poses an obstacle to the development of non-hormonal male contraceptives by sequestering drugs (e.g., adjudin) in the apical compartment.Citation17
The BTB forms during puberty in human (at ˜12–14 years of age), while in mice a functional BTB is established ˜15–16 days after birth,Citation18,19 which coincides with the time-point at which the testis cords are transformed into seminiferous tubules with a lumen,Citation20,21 as well as when the Sertoli cells cease to divide and become terminally differentiated.Citation22 Thus, in adult mammals the number of Sertoli cells is thought to remain relatively constant,Citation19,22 although there are reports that these cells can proliferate and divide in adult rodents under experimental conditionsCitation23 and even under physiological conditions in humans.Citation24 The number of Sertoli cells determines the number of germ cells that can be supported during spermatogenesis and thus sperm production and the sperm count in adulthood.Citation21,22
Sertoli cells are highly dynamic, changing their 3-dimensional structure during the course of spermatogenesis and spermiogenesis.Citation25,26 This causes the BTB to change as well: the TJPs undergo remodeling (opening and closing) to allow the passage of preleptotene spermatocytes from the basal to the adluminal compartment, where they undergo meiosis.Citation25 This structural disassembly and reassembly of the TJPs occurs at stage VIII in the rat and is tightly regulated by testosterone and cytokines.Citation27-29
Comparison of the BBB and BTB
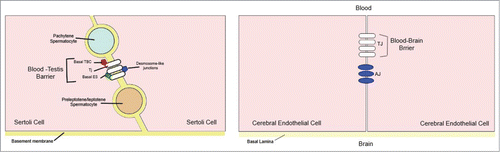
The BBB is formed by TJ proteins between endothelial cells lining the blood vessels, whereas the BTB is the result of TJ proteins between epithelial cells referred to as Sertoli cellsCitation30 and consequently Russell and Peterson (1985) coined the term ‘Sertoli blood barrier’ for the BTB.Citation31 Another fundamental difference is the organization of the TJ proteins: In the brain, these junctions are localized only at the apical surface of the endothelium, sealing the intercellular space with adherens junctions (AJ) immediately below the tight junction fibrils. In the BTB, on the other hand, TJ proteins coexist with basal ectoplasmic specializations (basal ES) and basal tubulobulbar complexes (basal TBC) (of which both are testis-specific, actin-based adherens junctions) and the desmosome-like junctions ().Citation19,30
Figure 4. A simplified diagram illustrating the morphological differences between the blood‐testis barrier (BTB) and the blood‐brain barrier (BBB). (A) In the BTB, tight junctions (TJs) coexist with basal ectoplasmic specializations (ES), basal tubulobulbar complexes (TBC), and desmosome‐like junctions. (B) In the BBB TJs are restricted to the apical surface of the endothelium, sealing the intercellular space, with adherens junctions (AJ) located immediately below. Modified from ref. Citation30.
Nevertheless, the TJ proteins that form these 2 barriers display remarkable molecular similarities, both being formed by strands of occludin, JAM, and claudin molecules linked to the cytoskeleton through the zonula occludens (ZO-1, ZO-2 and ZO-3).Citation19 In knock-out mice, loss of claudin-5 leads to disruption of the BBBCitation32 and lack of occludin is associated with calcification of the brain, with no change in the permeability of the BBB.Citation33 In the case of the testis, loss of occludin, claudin-3 and 11, the dominant forms expressed in this tissue, interferes with spermatogenesis and causes sterility.Citation3,34,35 Moreover, under pathological conditions such as stroke,Citation36 multiple sclerosisCitation37 and Alzheimer diseaseCitation38 (where the BBB is disrupted), orchitisCitation39 and male infertilityCitation3 (with disrupted BTB) the TJ proteins are down-regulated or redistributed. Interestingly, diabetes mellitus affects the paracellular and transcelluar permeability of the BBB and BTB negatively.Citation40
Both barriers physically separate their respective organs into apical and basal compartments exerting strict control over the transfer of ions and molecules. The BTB develops postnatally in rodents and during puberty in humans,Citation19 while the BBB is formed prenatally in both rodentsCitation6,7 and humans.Citation41 Both barriers create ‘immune-privileged sites’, i.e., tissue transplanted into the brainCitation42 or the interstitial space of the testisCitation39 is not rejected.
This ‘privilege’ is due to the absence of draining lymphatic vessels and an almost complete lack of circulating immune cells, which are prevented from entering by the BTB and BBB. In the case of the BTB, mature sperm cells (spermatozoa) expressing new antigens that can be recognized as ‘foreign’ arise after puberty but these new autoantigens are tolerated and do not normally evoke an immune response by the testis.Citation39 From an evolutionary perspective, immune privilege is regarded as a protection for vulnerable organs with limited capacity to regenerate.
Gut Microbiota
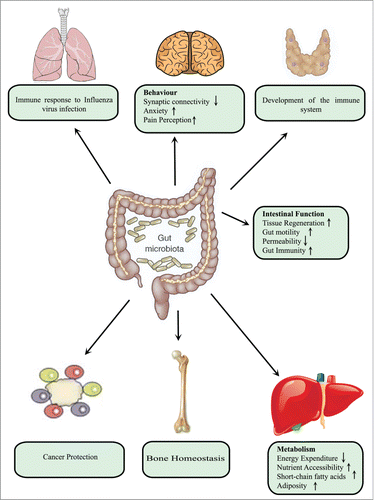
The largest microbial component is present in the large intestine of the GI tract, where it confers many benefits for the host such as pathogen displacement, development of the immune system, vitamin production and absorption of nutrients.Citation43 Microbiota are key to maintaining homeostasis and its functions extend beyond the GI tract affecting almost every organ of the bodyCitation44,45 (). In the intestine itself, microbiota influence angiogenesisCitation46 and enhance gut immunity and motility, as well as decreasing the permeability of the intestinal barrier. In the case of distant organs such as the lungs, microbiota regulate immunological defense against viral infection.Citation47 In the brain, microbiota affect behavior by reducing synaptic connectively and elevating anxietyCitation48 and perception of pain.Citation49 Moreover, microbiota modulate hepatic metabolism in such a way as to decrease energy expenditure and promote adiposity.Citation50 In addition, absence of gut microbiota leads to more bone mass in association with fewer osteoclasts surface area of bone.Citation51
Figure 5. Microbial impact on host physiology. Microbiota in the intestinal tract exerts profound effects on host physiology, both locally and at distant sites. Locally, these bacteria enhance gut immunity and motility as well as reducing intestinal permeability. At distant sites, such as the lungs, they regulate immune defense against viral infection. In the case of the brain, they may influence behavior by decreasing synaptic connectively and increasing anxiety and perception of pain. Moreover, they modulate hepatic metabolism in a manner that decreases energy expenditure and promote adiposity. In addition, absence of gut microbiota leads to more bone mass in association with fewer osteoclasts per surface area of bone.
Recent studies also point to the involvement of the microbiota in the development of personalized medicineCitation52 and in xenobiotic metabolism.Citation53 Certain environmental toxins and drugs are metabolized by the gut microbiota into less or more harmful substances. Several biological active compounds are also produced by the gut microbiota such as short chain fatty acids (SCFAs), conjugated linoleic acid, phenoles, indoles, or trimethylamine.Citation44
Short chain fatty acids
SCFAs are 1–6 carbons in length produced by fermentation of dietary fibers by the gut microbiota to butyrate, acetate and propionate.Citation54 Bacteria of the Bacteroidetes phylum such as Bacteroides thetaiotaomicron produce high levels of acetate and propionate, whereas bacteria of the Firmicutes phylum such as Clostridium Tyrobutyricum produce high amounts of butyrate. The most abundant SCFA is acetate (C2) followed by propionate (C3) and butyrate (C4). Butyrate, in particular, is the major fuel for colonocytes and is suggested to participate in the regulation of intestinal cell growth and differentiation. Butyrate also increases the expression of TJ proteins in vitroCitation55 and induces angiogenesis in the small intestine in vivo.Citation54 Acetate and propionate are transported to the liver and peripheral organs, where they act as substrates for gluconeogenesis and lipogenesis. Apart from providing energy sources for the host, SCFAs also regulate several cellular processes. SCFAs are implicated in the regulation of the gut immune system by affecting oxidative burst, degranulation, and phagocytic functions.Citation56 SCFAs also promote mineral absorption, mucin production, and expression of antimicrobial peptides.Citation57 Furthermore, certain SCFAs may lower the risk of developing gastrointestinal disorders, cancer and cardiovascular disease.Citation58
SCFAs enter cells both by simple diffusion and through the action of transporters of monocarboxylates and other solutes. Several investigations in the gut of rodents have demonstrated that the SCFAs act as messengers between the gut microbiome and the hostCitation59 promoting the formation of regulatory T-cells (Treg) and regulating immune functions.Citation60 Butyrate and propionate, but not acetate control gene expression by inhibiting histone deacetylase (HDAC), resulting in hyperacetylation of both histones and non-histone proteins.Citation61 All 3 of these SCFAs can also activate cells through G-protein-coupled receptors (GPCRs), such as GPR41 or GPR43,Citation62 with differing ligand specificities and potencies. Propionate is the most potent activator of both GPR41 and GPR43; acetate has higher affinity for GPR43; whereas butyrate activates GPR41 more potently.
Activation of GPR41 and GPR43 by SCFAs stimulates secretion of peptide YY, which reduces gastrointestinal transit. Stimulation of GPR43 by SCFAs is crucial for the regulation of energy balance and adiposity and in adipocytes, signaling via GPR41 induces lepitn secretion and elevates adipogenesis.Citation63 Moreover, signaling via GPR43 has anti-inflammatory effects as reflected in the observation that GPR43-kockout (Gpr43−/−) murine models of colitis, arthritis and asthma display exacerbated or unresolving inflammation.Citation64
Influence of Microbiota on the Intestinal Barrier
By helping to maintain the integrity of the intestinal epithelial barrier through regulation of cell-cell junctions, the gut microbiota provide their host with a physical barrier to pathogens and also aid in the preservation of homeostasis. In addition to its physical nature, this barrier contains a chemical component consisting of e.g., mucins, trefoil peptides and surfactant peptides.Citation65 The primary physical barrier is formed by a single layer of epithelial cells with the paracellular space being sealed by TJ.Citation66
The TJ regulate the flow of water ions and small molecules, as well as preventing antigens and pathogens from entering mucosal tissues. The status of these junctions provides an indicator of the functionality of the paracellular barrier, to which claudins are the primary contributors.Citation67 TJ are highly dynamic structures, constantly being remodeled in response to external stimuli such as food residues and pathogenic and commensal bacteria. Below the TJ lie the adherence junctions (AJ) involved in intracellular signaling, cell-cell adhesion and restitution of the epithelium.Citation68
Before birth, the intestine is essentially sterile, but soon after birth it is colonized by a variety of ingested and maternal microorganisms. The dense communities of bacteria in the intestine are separated from body tissues by a single layer of epithelial cells. Establishment and maintenance of a barrier that protects surrounding tissues from bacteria is also achieved by stimulation of the mucosal immune system.Citation69
This stimulation involves several mechanisms, e.g, activation of Toll-like receptors that recognize molecules derived from microbes and secretion of mucins, trefoil factors and secretory immunoglobulin A, all of which reinforce the barrier.Citation70 Maintenance of the TJ and AJ also makes an important contribution. While this role of gut microbiota in fortifying the intestinal barrier is well-documented, the influence of these same bacteria on other blood-tissue barriers requires further examination. Here, we discuss our recent findings concerning this influence on the integrity and permeability of both the BBB and BTB.
The Influence of Gut Microbiota on the BBB and BTB
In germ-free (GF) pregnant mouse dams, maturation of the fetal BBB was delayedCitation71 as was tubules formation in the postnatal testis.Citation72 These defects in permeability persisted into adulthood, as demonstrated by leakage of Evans blue dye into the brain parenchymaCitation71 and seminiferous tubules of the testis of mature mice.Citation72 This leakiness was associated with reduced expression of TJ proteinsCitation71,72: in the BBB, expression of claudin-5 and occludin was lower, while that of ZO-1 was unchanged; and in the BTB, expression of occludin and ZO-2 was lower.Citation72 Moreover, electron microscopy revealed that TJ in the brain of GF adult mice were disorganized.
Could the BBB and BTB in these GF mice be restored? Remarkably, these barriers could be “sealed” by recolonizing their intestines with fecal material from the control mice. Colonization with bacterial strains that produce only butyrate (Clostridium Tyrobutyricum) or acetate/probionate (Bacteroides thetaiotaomicron) restored the integrity of the brain parenchymaCitation71 and seminiferous tubules of the testisCitation72 as shown by exclusion of Evans blue dye. Furthermore, the protein components of the TJ complexes in these barriers were restored to control levels by either such colonization or butyrate alone.Citation72,71
Short-chain fatty acids (SCFA), provided either as pure sodium butyrate or generated by SCFA-producing bacteria, re-established barrier functions in previously GF mice, but the underlying mechanism(s) remains unclear. One possibility we explored in the case of the BBB involves epigenetic modification in the form of enhanced histone acetylation stimulated by butyrate. However, this needs to be further confirmed and the potential relationship between histone acetylation and the expression of TJ protein elucidated.
In addition, whether these changes in barrier permeability caused by SCFAs that enter the blood stream involve direct effects on brain endothelial or indirect signaling by the enteroendocrine cells of the gut warrant furthers investigation. Mercado and coworkersCitation73 have demonstrated direct effects of butyrate and other micronutrients in vitro on individual components of TJ, the transepithelial electrical resistance and transepithelial mannitol permeability of LLC-PK cells (renal epithelial cells). In this system, butyrate elevated the levels of claudin-1, 3, 4, and 5, the latter by almost 300%. Furthermore, butyrate can enhance histone acetylation in the BBB in vivoCitation71 as well as in dendritic cells in vitro.Citation74 However, butyrate acts not only on the cells that form the barrier. In the case of the testis, recolonization of GF mice with Clositridium Tyrobutyricum also restored testosterone levels to that of control mice without altering the levels of gonadotrophins, suggesting an effect on the Leydig cells.Citation72
Conclusions and Future Perspectives
The major role played by the blood-intestinal barrier in preventing pathogenic bacteria from entering the circulation is well established. We propose that the internal barriers formed by the BBB and BTB provide a second line of defense against potentially pathogenic organisms, as well as protection from our own immune system. An evolutionary perpective on this type of protection is that it appears to be localized around organs that are particularly vulnerable.
The potential effects of modulating the composition of the gut microbiota through the use of antibiotics, probiotic and prebiotics on barrier permeability has not been addressed here and require further investigation. Understanding possible sides-effect of antibiotic use on the BBB and BTB and thereby mental and reproductive health is of considerable importance. Future research should focus on unraveling the signaling pathways and identifying the metabolites involved in the establishment of these peripheral barriers, thereby improving our understanding of host-microbe crosstalk and paving the way for novel clinical interventions. Thus, detailed characterization of bacterial metabolites (e.g., SCFAs) and identification of their target tissues can provide a basis for new therapies.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank Dr. Fahad Al-Zadjali for his valuable comments.
References
- Ribbert H. Die Abscheidung intravenos injizierten gelosten Karmins in den Geweben. Z Allgem Physiol 1904; 4:201-14
- Goldmann EE. [Die aüßere und innere Sekretion des gesunden und kranken Organismus im Lichte der “vitalen Farbung”]. Beitr Klin Chir 1909; 64:192-265
- Cheng CY, Mruk DD. The blood-testis barrier and its implications for male contraception. Pharmacol Rev 2012; 64:16-64; PMID:22039149; http://dx.doi.org/10.1124/pr.110.002790
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 2006; 7:41-53; PMID:16371949; http://dx.doi.org/10.1038/nrn1824
- Ransom BR. The neuronal microenvironment. In: Boron WF, Boulpaep, E., ed. Medical Physiology (BORON). Philadelphia: Saunders, 2009:298-301
- Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 2010; 468:562-6; PMID:20944625; http://dx.doi.org/10.1038/nature09513
- Ben-Zvi A, Lacoste B, Kur E, Andreone BJ, Mayshar Y, Yan H, Gu C. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 2014; 509:507-11; PMID:24828040; http://dx.doi.org/10.1038/nature13324
- Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med 2013; 19:1584-96; PMID:24309662; http://dx.doi.org/10.1038/nm.3407
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis 2010; 37:13-25; PMID:19664713; http://dx.doi.org/10.1016/j.nbd.2009.07.030
- Stewart PA, Wiley MJ. Developing nervous tissue induces formation of blood-brain barrier characteristics in invading endothelial cells: a study using quail–chick transplantation chimeras. Dev Biol 1981; 84:183-92; PMID:7250491; http://dx.doi.org/10.1016/0012-1606(81)90382-1
- Igarashi Y, Utsumi H, Chiba H, Yamada-Sasamori Y, Tobioka H, Kamimura Y, Furuuchi K, Kokai Y, Nakagawa T, Mori M, et al. Glial cell line-derived neurotrophic factor induces barrier function of endothelial cells forming the blood-brain barrier. Biochem Biophy Res Commun 1999; 261:108-12; PMID:10405331; http://dx.doi.org/10.1006/bbrc.1999.0992
- Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, et al. Pericytes regulate the blood-brain barrier. Nature 2010; 468:557-61; PMID:20944627; http://dx.doi.org/10.1038/nature09522
- Zenker D, Begley D, Bratzke H, Rubsamen-Waigmann H, von Briesen H. Human blood-derived macrophages enhance barrier function of cultured primary bovine and human brain capillary endothelial cells. J Physiol 2003; 551:1023-32; PMID:12829721; http://dx.doi.org/10.1113/jphysiol.2003.045880
- Abbott NJ. Dynamics of CNS barriers: evolution, differentiation, and modulation. Cell Mol Neurobiol 2005; 25:5-23; PMID:15962506; http://dx.doi.org/10.1007/s10571-004-1374-y
- Ploen L, Setchell BP. Blood-testis barriers revisited. A homage to Lennart Nicander. Int J Androl 1992; 15:1-4; PMID:1544694; http://dx.doi.org/10.1111/j.1365-2605.1992.tb01108.x
- Holash JA, Harik SI, Perry G, Stewart PA. Barrier properties of testis microvessels. Proc Natl Acad Sci U S A 1993; 90:11069-73; PMID:7902579; http://dx.doi.org/10.1073/pnas.90.23.11069
- Mok KW, Lie PP, Mruk DD, Mannu J, Mathur PP, Silvestrini B, Cheng CY. The apical ectoplasmic specialization-blood-testis barrier functional axis is a novel target for male contraception. Adv Exp Med Biol 2012; 763:334-55; PMID:23397633
- Cyr DG, Hermo L, Egenberger N, Mertineit C, Trasler JM, Laird DW. Cellular immunolocalization of occludin during embryonic and postnatal development of the mouse testis and epididymis. Endocrinology 1999; 140:3815-25; PMID:10433243
- Cheng CY, Wong EW, Lie PP, Li MW, Mruk DD, Yan HH, Mok KW, Mannu J, Mathur PP, Lui WY, et al. Regulation of blood-testis barrier dynamics by desmosome, gap junction, hemidesmosome and polarity proteins: An unexpected turn of events. Spermatogenesis 2011; 1:105-15; PMID:22319658; http://dx.doi.org/10.4161/spmg.1.2.15745
- Orth JM. Proliferation of Sertoli cells in fetal and postnatal rats: a quantitative autoradiographic study. Anatom Rec 1982; 203:485-92; PMID:7137603; http://dx.doi.org/10.1002/ar.1092030408
- Petersen C, Soder O. The sertoli cell–a hormonal target and ‘super’ nurse for germ cells that determines testicular size. Horm Res 2006; 66:153-61; PMID:16804315; http://dx.doi.org/10.1159/000094142
- Sharpe RM, McKinnell C, Kivlin C, Fisher JS. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction 2003; 125:769-84; PMID:12773099; http://dx.doi.org/10.1530/rep.0.1250769
- Tarulli GA, Stanton PG, Meachem SJ. Is the adult Sertoli cell terminally differentiated? Biol Reprod 2012; 87:13, 1-1; http://dx.doi.org/10.1095/biolreprod.111.095091
- Ahmed EA, Barten-van Rijbroek AD, Kal HB, Sadri-Ardekani H, Mizrak SC, van Pelt AM, de Rooij DG. Proliferative activity in vitro and DNA repair indicate that adult mouse and human Sertoli cells are not terminally differentiated, quiescent cells. Biol Reprod 2009; 80:1084-91; PMID:19164176; http://dx.doi.org/10.1095/biolreprod.108.071662
- Mital P, Hinton BT, Dufour JM. The blood-testis and blood-epididymis barriers are more than just their tight junctions. Biol Reprod 2011; 84:851-8; PMID:21209417; http://dx.doi.org/10.1095/biolreprod.110.087452
- Wong V, Russell LD. Three-dimensional reconstruction of a rat stage V Sertoli cell: I. Methods, basic configuration, and dimensions. Am J Anat 1983; 167:143-61; PMID:6351582; http://dx.doi.org/10.1002/aja.1001670202
- Xia W, Mruk DD, Lee WM, Cheng CY. Cytokines and junction restructuring during spermatogenesis–a lesson to learn from the testis. Cytokine Growth Factor Rev 2005; 16:469-93; PMID:16023885; http://dx.doi.org/10.1016/j.cytogfr.2005.05.007
- Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nat Rev Endocrinol 2010; 6:380-95; PMID:20571538; http://dx.doi.org/10.1038/nrendo.2010.71
- Li MW, Mruk DD, Lee WM, Cheng CY. Cytokines and junction restructuring events during spermatogenesis in the testis: an emerging concept of regulation. Cytokine Growth Factor Rev 2009; 20:329-38; PMID:19651533; http://dx.doi.org/10.1016/j.cytogfr.2009.07.007
- Wong CH, Cheng CY. The blood-testis barrier: its biology, regulation, and physiological role in spermatogenesis. Curr Top Dev Biol 2005; 71:263-96; PMID:16344108; http://dx.doi.org/10.1016/S0070-2153(05)71008-5
- Russell LD, Peterson RN. Sertoli cell junctions: morphological and functional correlates. Int Rev Cytol 1985; 94:177-211; PMID:3894273; http://dx.doi.org/10.1016/S0074-7696(08)60397-6
- Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol 2003; 161:653-60; PMID:12743111; http://dx.doi.org/10.1083/jcb.200302070
- Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell 2000; 11:4131-42; PMID:11102513; http://dx.doi.org/10.1091/mbc.11.12.4131
- Mazaud-Guittot S, Meugnier E, Pesenti S, Wu X, Vidal H, Gow A, Le Magueresse-Battistoni B. Claudin 11 deficiency in mice results in loss of the Sertoli cell epithelial phenotype in the testis. Biol Reprod 2010; 82:202-13; PMID:19741204; http://dx.doi.org/10.1095/biolreprod.109.078907
- Chihara M, Ikebuchi R, Otsuka S, Ichii O, Hashimoto Y, Suzuki A, Saga Y, Kon Y. Mice stage-specific claudin 3 expression regulates progression of meiosis in early stage spermatocytes. Biol Reprod 2013; 89:3; PMID:23677978; http://dx.doi.org/10.1095/biolreprod.113.107847
- Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis 2008; 32:200-19; PMID:18790057; http://dx.doi.org/10.1016/j.nbd.2008.08.005
- Correale J, Villa A. The blood-brain-barrier in multiple sclerosis: functional roles and therapeutic targeting. Autoimmunity 2007; 40:148-60; PMID:17453713; http://dx.doi.org/10.1080/08916930601183522
- Erickson MA, Banks WA. Blood-brain barrier dysfunction as a cause and consequence of Alzheimer's disease. J Cereb Blood Flow Metab 2013; 33:1500-13; PMID:23921899; http://dx.doi.org/10.1038/jcbfm.2013.135
- Fijak M, Meinhardt A. The testis in immune privilege. Immunol Rev 2006; 213:66-81; PMID:16972897; http://dx.doi.org/10.1111/j.1600-065X.2006.00438.x
- Alves MG, Oliveira PF, Socorro S, Moreira PI. Impact of diabetes in blood-testis and blood-brain barriers: resemblances and differences. Curr Diab Rev 2012; 8:401-12; PMID:22934551; http://dx.doi.org/10.2174/157339912803529896
- Lee JY, Huerta PT, Zhang J, Kowal C, Bertini E, Volpe BT, Diamond B. Neurotoxic autoantibodies mediate congenital cortical impairment of offspring in maternal lupus. Nat Med 2009; 15:91-6; PMID:19079257; http://dx.doi.org/10.1038/nm.1892
- Carson MJ, Doose JM, Melchior B, Schmid CD, Ploix CC. CNS immune privilege: hiding in plain sight. Immunol Rev 2006; 213:48-65; PMID:16972896; http://dx.doi.org/10.1111/j.1600-065X.2006.00441.x
- Moens E, Veldhoen M. Epithelial barrier biology: good fences make good neighbours. Immunology 2012; 135:1-8; PMID:22044254; http://dx.doi.org/10.1111/j.1365-2567.2011.03506.x
- Korecka A, Arulampalam V. The gut microbiome: scourge, sentinel or spectator? J Oral Microbiol 2012; 4:9367-80; PMID:22368769; http://dx.doi.org/10.3402/jom.v4i0.9367
- Al-Asmakh M, Anuar F, Zadjali F, Rafter J, Pettersson S. Gut microbial communities modulating brain development and function. Gut Microbes 2012; 3:366-73; PMID:22743758; http://dx.doi.org/10.4161/gmic.21287
- Stappenbeck TS, Hooper LV, Gordon JI. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci U S A 2002; 99:15451-5; PMID:12432102; http://dx.doi.org/10.1073/pnas.202604299
- Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, Iwasaki A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci U S A 2011; 108:5354-9; PMID:21402903; http://dx.doi.org/10.1073/pnas.1019378108
- Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S. Normal gut microbiota modulates brain development and behavior. Proceed Natl Acad Sci U S A 2011; 108:3047-52; PMID:21282636; http://dx.doi.org/10.1073/pnas.1010529108
- Amaral FA, Sachs D, Costa VV, Fagundes CT, Cisalpino D, Cunha TM, Ferreira SH, Cunha FQ, Silva TA, Nicoli JR, et al. Commensal microbiota is fundamental for the development of inflammatory pain. Proc Natl Acad Sci U S A 2008; 105:2193-7; PMID:18268332; http://dx.doi.org/10.1073/pnas.0711891105
- Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science 2012; 336:1262-7; PMID:22674330; http://dx.doi.org/10.1126/science.1223813
- Sjogren K, Engdahl C, Henning P, Lerner UH, Tremaroli V, Lagerquist MK, Backhed F, Ohlsson C. The gut microbiota regulates bone mass in mice. J Bone Miner Res 2012; 27:1357-67; PMID:22407806; http://dx.doi.org/10.1002/jbmr.1588
- Wilson ID. Drugs, bugs, and personalized medicine: pharmacometabonomics enters the ring. Proc Natl Acad Sci U S A 2009; 106:14187-8; PMID:19706501; http://dx.doi.org/10.1073/pnas.0907721106
- Bjorkholm B, Bok CM, Lundin A, Rafter J, Hibberd ML, Pettersson S. Intestinal microbiota regulate xenobiotic metabolism in the liver. PloS One 2009; 4:e6958; PMID:19742318; http://dx.doi.org/10.1371/journal.pone.0006958
- Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol Rev 2001; 81:1031-64; PMID:11427691
- Bordin M, D'Atri F, Guillemot L, Citi S. Histone deacetylase inhibitors up-regulate the expression of tight junction proteins. Mol Cancer Res 2004; 2:692-701; PMID:15634758
- Meijer K, de Vos P, Priebe MG. Butyrate and other short-chain fatty acids as modulators of immunity: what relevance for health? Curr Opin Clin Nutr Metab Care 2010; 13:715-21; PMID:20823773; http://dx.doi.org/10.1097/MCO.0b013e32833eebe5
- Kles KA, Chang EB. Short-chain fatty acids impact on intestinal adaptation, inflammation, carcinoma, and failure. Gastroenterology 2006; 130:S100-5; PMID:16473056; http://dx.doi.org/10.1053/j.gastro.2005.11.048
- Cook SI, Sellin JH. Review article: short chain fatty acids in health and disease. Aliment Pharmacol Ther 1998; 12:499-507; PMID:9678808; http://dx.doi.org/10.1046/j.1365-2036.1998.00337.x
- Ganapathy V, Thangaraju M, Prasad PD, Martin PM, Singh N. Transporters and receptors for short-chain fatty acids as the molecular link between colonic bacteria and the host. Curr Opin Pharmacol 2013; 13:869-74; PMID:23978504; http://dx.doi.org/10.1016/j.coph.2013.08.006
- Bollrath J, Powrie F. Immunology. Feed your Tregs more fiber. Science 2013; 341:463-4; PMID:23908210; http://dx.doi.org/10.1126/science.1242674
- Grunstein M. Histone acetylation in chromatin structure and transcription. Nature 1997; 389:349-52; PMID:9311776; http://dx.doi.org/10.1038/38664
- Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem 2003; 278:11312-9; PMID:12496283; http://dx.doi.org/10.1074/jbc.M211609200
- Ichimura A, Hirasawa A, Hara T, Tsujimoto G. Free fatty acid receptors act as nutrient sensors to regulate energy homeostasis. Prostaglandins Other Lipid Mediat 2009; 89:82-8; PMID:19460454; http://dx.doi.org/10.1016/j.prostaglandins.2009.05.003
- Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009; 461:1282-6; PMID:19865172; http://dx.doi.org/10.1038/nature08530
- Lievin-Le Moal V, Servin AL. The front line of enteric host defense against unwelcome intrusion of harmful microorganisms: mucins, antimicrobial peptides, and microbiota. Clin Microbiol Rev 2006; 19:315-37; PMID:16614252; http://dx.doi.org/10.1128/CMR.19.2.315-337.2006
- Turner JR, Buschmann MM, Romero-Calvo I, Sailer A, Shen L. The role of molecular remodeling in differential regulation of tight junction permeability. Semin Cell Dev Biol 2014; 36:204-12; PMID:25263012; http://dx.doi.org/10.1016/j.semcdb.2014.09.022
- Goncalves A, Ambrosio AF, Fernandes R. Regulation of claudins in blood-tissue barriers under physiological and pathological states. Tissue Barriers 2013; 1:e24782; PMID:24665399; http://dx.doi.org/10.4161/tisb.24782
- Groschwitz KR, Hogan SP. Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol 2009; 124:3-20; quiz 1–2; PMID:19560575; http://dx.doi.org/10.1016/j.jaci.2009.05.038
- Huang XZ, Zhu LB, Li ZR, Lin J. Bacterial colonization and intestinal mucosal barrier development. World J Clin Pediatr 2013; 2:46-53; PMID:25254174; http://dx.doi.org/10.5409/wjcp.v2.i4.46
- Sharma R, Young C, Neu J. Molecular modulation of intestinal epithelial barrier: contribution of microbiota. J Biomed Biotechnol 2010; 2010:305879; PMID:20150966
- Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Toth M, Korecka A, Bakocevic N, Guan NL, Kundu P, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med 2014; 6:263ra158; http://dx.doi.org/10.1126/scitranslmed.3009759
- Al-Asmakh M, Stukenborg JB, Reda A, Anuar F, Strand ML, Hedin L, Pettersson S, Soder O. The gut microbiota and developmental programming of the testis in mice. PloS One 2014; 9:e103809; PMID:25118984; http://dx.doi.org/10.1371/journal.pone.0103809
- Mercado J, Valenzano MC, Jeffers C, Sedlak J, Cugliari MK, Papanikolaou E, Clouse J, Miao J, Wertan NE, Mullin JM. Enhancement of tight junctional barrier function by micronutrients: compound-specific effects on permeability and claudin composition. PloS One 2013; 8:e78775; PMID:24236048; http://dx.doi.org/10.1371/journal.pone.0078775
- Andrade-Oliveira V, Amano MT, Correa-Costa M, Castoldi A, Felizardo RJ, de Almeida DC, Bassi EJ, Moraes-Vieira PM, Hiyane MI, Rodas AC, et al. Gut Bacteria Products Prevent AKI Induced by Ischemia-Reperfusion. J A Soc Nephrol 2015; PMID:25589612