ABSTRACT
Tightly controlled communication among the various resident and recruited cells in the intestinal tissue is critical for maintaining tissue homeostasis, re-establishment of the barrier function and healing responses following injury. Emerging evidence convincingly implicates extracellular vesicles (EVs) in facilitating this important cell-to-cell crosstalk by transporting bioactive effectors and genetic information in healthy tissue and disease. While many aspects of EV biology, including release mechanisms, cargo packaging, and uptake by target cells are still not completely understood, EVs contribution to cellular signaling and function is apparent. Moreover, EV research has already sparked a clinical interest, as a potential diagnostic, prognostic and therapeutic tool. The current review will discuss the function of EVs originating from innate immune cells, namely, neutrophils, monocytes and macrophages, as well as intestinal epithelial cells in healthy tissue and inflammatory disorders of the intestinal tract. Our discussion will specifically emphasize the contribution of EVs to the regulation of vascular and epithelial barrier function in inflamed intestines, wound healing, as well as trafficking and activity of resident and recruited immune cells.
Mucosal integrity
Maintenance of the intestinal barrier integrity and rapid resealing of mucosal wounds is critical for proper function of the gastrointestinal (GI) tract. Intestinal epithelial cells (IECs) lining the intestinal lumen form a selectively permeable barrier to separate luminal contents from the underlying tissues. Through complex communication with the microbiome and the immune system, IECs maintain gut homeostasis. Dysregulation of the immune cell composition during gut injury results in impairment of the intestinal barrier and underlies a wide spectrum of inflammatory disorders of the GI tract, including Inflammatory Bowel Disease (IBD).Citation1,Citation2
Basis of epithelial healing
IECs are constantly exposed to a repertoire of dietary substances, foreign antigens, commensal and pathogenic bacteria, and thus are susceptible to injury.Citation3 Rapid resealing and repair of mucosal wounds is essential for reestablishing the intestinal barrier and limiting antigen leakage into underlying tissues. If not efficiently repaired, bacterial translocation and antigenic exposure associated with a breached barrier inevitably results in aberrant immune response and augmentation of epithelial injury.Citation3 Wound healing requires efficient tissue remodeling, where IECs proliferate and migrate into the wound bed to cover denuded surfaces.Citation4 Epithelial cells migrate as cohesive sheets and require actin cytoskeleton-driven depolarization and dynamic turnover of focal cell-matrix associations.Citation4 To reestablish barrier function and tissue homeostasis, wound healing is terminated by resolution of inflammation and removal of damaged cells, a process in which resident and recruited immune cells are key players (summarized in).Citation5
Innate immune cells are key contributors to the healing process
It is well-established that an innate immunity is a critical component of wound healing and gut homeostasis. Coordinated recruitment of leukocytes in response to chemotactic gradient generated at injury site is critical for host defense, resolution of inflammation, and tissue regeneration.Citation6 Among the immune cells, neutrophils (polymorphonuclear, PMNs) are the first to respond to insult and the ensuing chemotactic cues.Citation7,Citation8 As the predominant players during the onset of injury-induced inflammation, tissue-infiltrating PMNs elicit “in-danger” cues that amplify and sustain inflammation by promoting recruitment of other inflammatory effector cells, including monocytes/macrophages and T helper 17 (Th17) cells.Citation9 At the site of injury, PMNs produce reactive oxygen species (ROS) and lytic enzymes critical for host defense,Citation10 but harmful to surrounding tissues.Citation11 As a consequence, the presence of PMNs in tissues is often viewed as detrimental and regarded as the hallmark of many inflammatory diseases, including IBD. However, emerging evidence demonstrates increased PMN plasticity, life-span, and phenotypic heterogeneity in inflamed tissues.Citation12 As such, in addition to their phagocytic activity that protects against pathogens and removes apoptotic/necrotic cells and cellular debris, PMNs are capable of producing a milieu of pro-resolving mediators, including antibacterial peptides,Citation13 resolvins,Citation14 defensins,Citation15 and cationic peptides such as LL-37,Citation16 nitric oxide,Citation17 and transforming growth factor beta (TGFβ)Citation18 in order to promote epithelial repair.Citation19 PMNs can further physically interact with epithelial receptors such as intercellular adhesion molecule-1 (ICAM-1) to direct IEC proliferation,Citation20 repair of blood vessels,Citation21 and sequential recruitment of pro-resolving macrophages to assist wound closure.Citation22 Macrophages replace PMNs at the late phase of the healing process and provide additional protection against pathogens, contributing to wound debridement, clearance of dead and inflammatory cells, and resolution of inflammation.Citation23 In this regard, PMNs and macrophages play a context-dependent role in wound repair and tissue regeneration. It is important to note that as with the innate immune cells, adaptive immunity counterparts also contribute significantly to intestinal homeostasis, where regulatory T-cells, for example, play key roles in resolution of inflammation and wound repair as seen in ulcerative colitis and Crohn's disease.Citation24
The biogenesis and composition of extracellular vesicles (EVs)
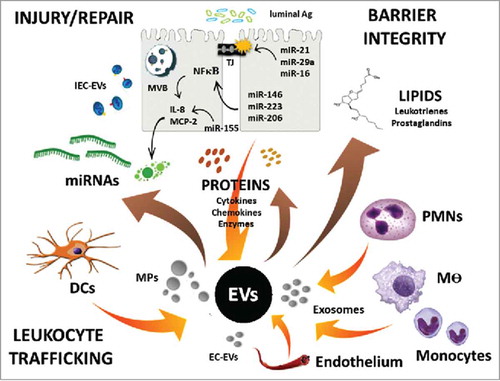
To maintain tissue homeostasis, immune cells communicate with IECs via physical interaction or in a paracrine fashion by exchanging soluble effectors such as, cytokines, chemokines, small peptides, and lipid mediators.Citation2,Citation22 Intriguingly, substantial evidence supports an emerging way of cell-to-cell crosstalk in the form of extracellular vesicles (EVs).Citation25,Citation26 EVs are secreted by almost every cell type, and serve to shuttle, and protect bioactive effectors as well as transport genetic information between cells, in healthy tissue and disease.Citation25,Citation26,Citation27 As such, EVs emerged as important contributors to the coordinated signaling events and communication between the microbiota,Citation28 IECs,Citation29 endothelial cells,Citation30 and immune cellsCitation31 during homeostasis, immune activation, and inflammation.
EVs are lipid vesicles with sizes ranging from 50 to 1000 nm in diameter.Citation32,Citation33 Based on size and biogenesis, EVs can be further subdivided into exosomes and microvesicles, or in the case of immune cells often referred to as ectosomes or microparticles (MPs).Citation33 Exosomes are vesicles with diameter of 40–150 nm and are derived during the inward budding of early endosomes to form multivesicular bodies (MVBs) and later released when these compartments fuse with the plasma membrane ().Citation33,Citation34 Recent proteomic analyses of exosomes suggested enrichment of tetraspanin proteins, as well as different classes of lipids, including cholesterol, sphingomyelin, ceramide, and phosphatidylserine.Citation34,Citation35 Similarly, endosomal proteins (ESCRT, ALIX), tetraspanins (CD9, CD63, and CD81), and heat-shock proteins (HSP-70, HSP-90) were shown to be highly concentrated in exosomes of various cell types, and are currently used as universal markers for these vesicles.Citation35,Citation36,Citation37 Possibly, one of the key functions of exosomes is to transport regulatory microRNAs (miRNAs),Citation38,Citation39,Citation40 which are otherwise extremely unstable and are rapidly degraded in the tissues.Citation41
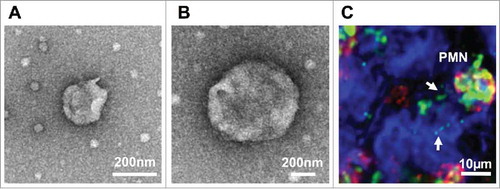
Figure 1. Characterization of PMN-derived EVs. (A-B) PMNs were stimulated with fMLF (1μM) to produce EVs. EVs were isolated by serial centrifugation and analyzed by transmission electron microscopy. (A) A representative EV with the size of exosomes (< 100 nm). (B) A representative microparticle/ectosome with the size of ∼600 nm. (C) PMNs (immunolabeled for CD11b, red and myeloperoxidase, green) release myeloperoxidase-containing EVs (shown by arrows) following adhesion to and migration across IECs (surface stain, blue).
Ectosomes or MPs, on the other hand, are larger particles with diameters ranging from 200 to 1000 nm that are generated by the outward budding of the plasma membraneCitation33,Citation34,Citation37 (). Since ectosomes are primarily membrane-derived, they contain lipids and many of the surface molecules characteristic of parental cells they were originated from.Citation34 Electron microscopy and proteomic analyses confirmed size and composition heterogeneity of ectosomesCitation42,Citation43,Citation44 and established phosphatidylserine as a reliable ectosome marker.Citation45,Citation46 Importantly, while the heterogeneous content of EVs reflects the parental cell phenotype, the composition, including, levels and the bioactivity of specific mediators is stimulus-dependent and is dramatically altered as a result of stimulatory conditions and the environmental milieu.Citation34,Citation40,Citation42 Thus not surprisingly, EV contribution to cell function is context-dependent, and has been assigned both pro- and anti-inflammatory effects.Citation47,Citation48 Finally, EVs by yet unknown mechanism can bind target cells to modulate the expression and localization of surface proteins by way of MMPsCitation49 and/or be internalized by target cells, resulting in the release of their content. The processes of EVs uptake is dependent on the recipient cell type, and involves clathrin-, cholesterol- and lipid raft-dependent endocytosis by immune cells.Citation34,Citation37
The biological activity of EVs in healthy and inflamed intestine
Proteins, lipids, mRNAs and miRNAs that are shuttled by EVs among neighboring cells serve as secondary messengers to temporally and spatially modulate and coordinate cellular responses.Citation33,Citation34 As such, EV-associated matrix metalloproteinases (MMPs), growth factors, chemokines and miRNA can rapidly and in a localized fashion help reorganize the extracellular matrix and junctional complexes, promote cell growth and migration, as well as facilitate recruitment of immune cells. The specific contribution of EV-associated miRNAs to the regulation of these key processes mediating tissue injury and repairCitation47 are summarized in . EVs can be readily isolated from bodily fluids, such as serum,Citation50 saliva,Citation51 urine,Citation52 and in the intestine from luminal aspirates.Citation53 The number of EVs and their composition can reflect both healthy and pathological states.Citation54 Specifically, in IBD an increased number of EVs in the serum and the intestinal lumen53 was correlated with disease severity and were shown to contain immune cell- and IEC-specific markers as well as many inflammatory markers associated with the diseases.Citation55 PMN-derived MPs, in particular, have been shown to be highly enriched at sites of inflammation.Citation56,Citation57,Citation58 Since EVs can be released by immune cells, endothelial, and epithelial cells, understanding the biogenesis and function of EVs in inflamed tissue will help decipher mechanisms governing the complex interplay of these cells in maintaining barrier integrity and facilitating tissue repair. In the following sections, we will discuss the contribution of EVs to intestinal homeostasis and immune cell function, specifically focusing on epithelial barrier, wound healing, and leukocyte recruitment to sites of inflammation.
Table 1. A summary of miRNAs that have been shown to be transported by immune, epithelial, and endothelial cell-derived EVs and their contribution to cellular signaling and intestinal homeostasis.
EVs regulate epithelial barrier integrity
IECs via apical junctional complexes (AJCs) form a barrier to separate luminal content from the underlying tissue. Disruption of IEC junctions leads to loss of barrier integrity, a feature that underlies intestinal injury and IBD. PMN migration across IECs is a hallmark of intestinal inflammation, and is often associated with the loss barrier function.Citation59,Citation60As such, mislocalization/loss of several key components of the AJCs including, E-cadherin, Occludin, Claudin-1, Zonula Occluden-1 (ZO-1), and Junctional Adhesion Molecule-A (JAM-A) adjacent to clusters of transmigrating neutrophils was reported in clinical samples obtained from patients with IBD and cultured IECs.Citation59,Citation61,Citation62 Importantly, while most of the pathological effects of PMNs were attributed to PMN-derived soluble mediators, recently PMN-derived EVs have been implicated in contributing to these processes.Citation56,Citation57 We recently reported abundant association of MMP-9 with PMN-derived MPs, released during transepithelial migration (TEM).Citation49 PMN-MPs were found to bind IECs and potently cleave desmoglein-2 (Dsg-2), a key desmosomal cadherin, and destabilize epithelial cell-to cell adhesions.Citation49 Loss of Dsg-2 has been previously correlated with perturbed epithelial permeabilityCitation63 and mislocalization of other IEC junctional components associated with known function in regulating epithelial permeability,Citation64 including ZO-1 and coxsackie and adenovirus receptor (CAR).Citation65 Thus, tissue-infiltrating PMNs via the release of EVs can exacerbate barrier dysfunction and drive acute inflammatory responses and tissue injury in inflamed intestines. Similarly, MMP-9 was shown to degrade/cleave key adherens junction protein, E-cadherin.Citation66,Citation67 Since assembly of adherens junctions is required for proper organization of barrier regulating tight junctional proteins,Citation68 PMNs via the release of EVs can affect the assembly of IEC junctional complexes and IEC permeability. In contrast, tissue macrophages that contribute to tissue homeostasis release EVs during differentiation that contain high levels of galectin-3.Citation69 Galectin-3 functions to stabilize Dsg-2 at cell junctions, enhancing the integrity of the IEC monolayer.Citation70 Intriguingly, PMN-derived ectosomes were found to be taken up by macrophages, inducing NFκB inhibition, and polarizing them to pro-resolving phenotype.Citation71,Citation72 Thus, during injury, tissue-infiltrating PMNs may contribute to the reestablishment of epithelial barrier through reprograming of macrophages.
As with MMPs, inflammatory cytokines can promote epithelial damage and barrier dysfunction. Granulocyte-derived MPs that were isolated from intestinal luminal aspirates of IBD patients were found to contain inflammatory cytokines, including IL-6, IL-8 and TNFα.Citation53 IL-6 and TNFα are known to increase epithelial permeability via downregulation or mislocalization of tight junction proteins, including ZO-1, Claudins, Occludins and JAM-A.Citation73,Citation74,Citation75 As we have discussed above, EVs serve to transport regulatory miRNAs,Citation38,Citation39,Citation40 which can post-transcriptionally alter protein expression in target cells. As such, EV-associated miRNAs have been implicated in targeting IEC junctional components and modulating barrier function during intestinal inflammation.Citation76 For example, increased intestinal permeability in a subset of patients with Irritable Bowel Syndrome (IBS) has been correlated with an increased number of miR-29a-rich EVs in blood and the intestinal tissue.Citation77 Increased intestinal permeability in these patients was suggested to be due to miR-29a-mediated downregulation of glutamine synthetase.Citation77 miR-29a was further shown to downregulate Claudin-1, causing increases in epithelial permeability.Citation78 miR-29a was also found in EVs released by dendritic cells (DCs) into the extracellular environment during cognate T-cell-DC interactions.Citation79 Since DCs act as sentinels in the intestinal mucosa to prime T cells activation in the case of injury or bacterial infection, EVs and miR-29a can contribute to intestinal function and barrier integrity.
miR-21 is another miRNA that is released within EVs by DCs, macrophages, and PMNs (unpublished observations) during intestinal inflammation, and can have profound effects on IEC permeability. Increases in miR21 were reported in mucosa and serum of IBD patients.Citation80,Citation81 In cultured Caco-2 IECs, miR-21 impaired intestinal permeability by targeting Ras-related small GTP-binding protein B (RhoB) and cell division control protein 42 (CDC42).Citation80 miR-21 has also been suggested to increase intestinal epithelial tight junction permeability through activation of PTEN/PI3K/Akt signaling pathway, and knockout of miR-21 in mice led to increased intestinal permeability and apoptosis of epithelial cells.Citation82,Citation83 Furthermore, miR-21 overexpression significantly downregulated Occludin and E-Cadherin, while increased IL-6 and IL-8 production,Citation83 confirming an important contribution of miR-21 to barrier integrity and immune cell recruitment.
Immune cells and IEC-derived miRNAs transported by EVs can further alter IEC function and intestinal barrier by modulating the activity of inflammatory transcription genes, such as NFκB and cytokine production. For example, miR-146a released in EVs by monocytes and macrophages can target interleukin-1 receptor-associated kinase 1 (IRAK1) and TNF receptor-associated factor 6 (TRAF6) to suppress NFκB signaling.Citation84,Citation85,Citation86 If taken up by either gut immune cells or IECs, miR-146a can potently suppress the release of barrier-altering cytokines, including TNFα and IL-6, reducing the inflammatory response and improving intestinal barrier.Citation86,Citation87 Indeed, in IECs, miR-146a protects small intestine against ischemia/reperfusion injury by downregulating Toll-like Receptor 4 (TLR4)/TRAF6/NF-κB pathway.Citation88 Epithelial cell-derived miR-146a was further found to promote IL-10 released by monocytes and limit nasal inflammation.Citation89 Similarly, miR-16 expressed by epithelial cellsCitation91 and released in EVs can facilitate rapid degradation of RNAs containing AU-rich elements within their 3′UTRs, causing downregulation of inflammatory cytokine, such as TNFα, IL-8 and IL-6.Citation90,Citation91
IEC-derived EVs help protect against pathogenic infections
Enteropathogenic infections and the resulting inflammation can present itself with symptoms similar to IBD, causing epithelial injury and barrier disruption.Citation92,Citation93 Rapid activation of the immune system is required for pathogen clearance and reestablishment of the barrier integrity.Citation3 Interestingly, emerging evidence suggest that EVs may confer the ability of static epithelial cells to act at a distance to both limit bacterial spreading and inform local innate and adaptive immune responses to luminal pathogens. Indeed, the release of exosomes from the epithelium into the intestinal lumen was increased following infection by the protozoan parasite Cryptosporidium parvum.Citation94 IEC-derived exosomes carried antimicrobial peptides, including cathelicidin-37 and beta-defensin 2, and were found to bind and help eliminate invading pathogens.Citation94 Thus, IEC-derived EVs can help protect against pathogenic infections. Intriguingly, electron microscopy examination of the luminal IEC surface revealed a layer of EVs up to 50 nm in diameter between the microvilli and mucous gel, suggesting that EV layer can act as an additional barrier to limit adherence by both commensal and pathogenic bacteria.Citation95
EVs released apically or basolaterally by IECs were also found to contain MHC class II and other accessory molecules involved in antigen presentation, suggesting that they can act as antigen-presenting vesicles to prime adaptive immune cells for immunogenic responses in the mucosa.Citation29,Citation96 Similarly, epithelial cell-derived exosomes entrapped and transported αvβ6 integrin and food antigens to DCs, resulting in production of active TGFβ by DCs and generation of antigen-specific regulatory T cells.Citation97 In contrast, EVs released by an enteric pathogen, Giardia intestinalis, can potentially promote inflammation and IEC injury by facilitating attachment of Giardia to IECs.Citation98
EVs contribution to intestinal injury and repair
Emerging evidence suggests that following injury and the ensuing inflammation, immune cell, stroma cell, and IEC-derived EVs can locally alter the production of cytokines, growth and transcription factors in wounded mucosa to regulate cell migration, proliferation, and differentiation. As such, EVs have been suggested to both impede and promote resolution of inflammation and tissue repair.
PMNs are the first immune cells to infiltrate the intestinal mucosa following injury. During TEM, PMNs were found to release microparticles (MPs) that contain abundant levels of myeloperoxidase (MPO).Citation99 The binding of PMN derived, and MPO containing EVs to IEC is shown in (). While MPO primarily functions in bacterial killing, during PMN activation, it is mobilized to the cell surface and is released in association with MPs. MP-associated MPO is enzymatically active, and when delivered to IECs, can impair actin dynamics, migration, and proliferation, significantly impeding IEC wound closure.Citation99 PMN-derived EVs were further suggested to potentiate endothelial cell injury via deposition of MPO-rich EVs,Citation100 increased production of reactive oxygen species,Citation101 and increased pro-inflammatory activity of metalloproteinase domain containing proteins 10 and 17 (ADAM10 and ADAM17).Citation102 PMN-derived EVs binding to endothelial cells facilitated leukocyte recruitment by inducing IL-6, IL-8 and MCP-1 release and upregulating adhesion molecules by inflamed endothelial cells, thus aggravating tissue injury.Citation103,Citation104
In contrast, granulocytic myeloid-derived suppressor cells (MDSC)-derived exosomes were found to attenuate dextran sulfate sodium (DSS)-induced epithelial injury by reducing the number of Th1 cells and increasing T regulatory cells in a TGFβ-dependent manner.Citation105 DC-derived EVs may similarly act to suppress inflammation and tissue damage, as they contain abundant amounts of milk fat globule EGF/factor VIII (MFG-E8),Citation106 which has been shown to promote dead cell clearance and inhibition of NFκB-dependent release of pro-inflammatory cytokines in experimental colitis.Citation107 Intriguingly, given the well-established cross-communication between various immune cells in inflammation,Citation108 PMN-derived EVs were suggested to suppress inflammatory responses and promote pro-repair function of macrophages in injured tissue. PMN-derived EVs were shown to inhibit NFκB signaling and increase the release of TGFβCitation71 and pro-resolving lipid mediators, such as Resolvin D1 and Resolvin E2.Citation109 Moreover, in addition to serving as an important source of pro-resolving mediators and their role in wound debridement, macrophages via the release of miR-223-containing EVs can potentially promote wound healing by enhancing epithelial cell migratory behavior, as has been shown for breast cancer cells.Citation110 Indeed, miR-223−/y mice presented exacerbated, myeloid cell-driven experimental colitis with heightened clinical, histopathological, and inflammatory cytokine readouts.Citation111 Injured IECs were also shown to release EVs, which served to promote resolution of inflammation and healing. A recent work elegantly demonstrated that IEC-derived exosomes containing Annexin A1 (ANXA1), promoted wound closure by binding to formyl peptide receptors (FPRs) and FPR-dependent generation ROS.Citation19 Physiological relevance of these findings was confirmed by the observation of elevated ANXA1-containing EVs in patients with active IBD.Citation19 Interestingly, in the circulation, PMNs were similarly shown to release MPs containing ANXA1, where they served to limit PMN adhesion to the endothelial cells and inhibit inflammatory recruitment of PMNs.Citation112 Because the release of MPs by PMNs is stimulus-dependent, whether this is also true in the setting of intestinal injury, and whether by function of ANXA1 PMN-MPs could promote IEC repair remains to be determined. Similarly, TGFβ-containing exosomes from injured epithelial cells were found to activate fibroblasts and promote tissue repair by increased matrix deposition and fibrosis.Citation113 TGFβ mRNA transported by exosomes induced proliferation, α-smooth muscle actin expression, and F-actin expression in fibroblasts. It is reasonable to speculate that if taken up by neighboring IECs, these exosomes could similarly promote IEC migration and proliferation; however, this would need to be experimentally confirmed. Furthermore, fibroblasts in the wound bed can promote epithelial cell motility, which is an essential component of wound healing,Citation114 via the release of CD81-containing EVs, as has been shown for breast cancer cells.Citation115 Epithelial exosomes also contain the A33 antigen, which is a transmembrane protein expressed predominantly in intestinal epithelium and is associated with the regulation of IEC migration and proliferation.Citation116 Mice lacking A33 antigen expression were compromised in their ability to resolve hapten-induced mucosal damage, exhibiting impaired IEC proliferation.Citation116 Thus, through the activity of A33, IEC-derived EVs may promote intestinal wound repair. Finally, Wnt5a, which is significantly increased at the wound bed and contributes to epithelial healing,Citation117,Citation118 has been detected in the exosomal fraction of Caco-2 IECs.Citation119
EVs contribution to leukocyte trafficking in inflamed tissue
Immune cells in the intestinal mucosa and other tissues play critical roles in host defense, and as discussed above contribute significantly to tissue injury and resolution of inflammation. Recruitment of immune cells is dependent upon the generation of local gradients of chemokines, growth factors, and cytokines produced by resident and recruited cells. EVs by way of miRNAs can regulate the expression of these chemotactic factors, or directly shuttle them to the surrounding tissue to promote/limit leukocyte recruitment. Leukocytes recruited to sites of inflammation must first cross the endothelial barrier, a process that is mediated by several classes of adhesion molecules and chemotactic cues.Citation120 In the circulation, EVs released by activated monocytes were found to induce expression of ICAM-1 (a key leukocyte adhesion molecule)Citation121 and the release of CCL2 (a potent monocyte chemoattractant)Citation122 to promote leukocyte recruitment.Citation123 These effects were attributed to the presence of pro-inflammatory miR-155 in these EVs and its delivery to endothelial cells. Indeed, endothelial miR-155 has been confirmed to positively regulate expression of ICAM-1 and VCAM-1.Citation124 miR-155 is one of several miRNAs, that were found to be highly enriched in IBD and was further suggested to regulate cytokine production.Citation125,Citation126 As such, miR-155 was found to inhibit suppressor of cytokine signaling 1 (SOCS1), an important anti-inflammatory gene,Citation127 resulting in elevated production of leukocyte agonists and chemoattractants, including IL-6 and IL-8, by intestinal myofibroblasts.Citation128 Similarly, serum EVs, presumably produced by the immune and endothelial cells in a mouse model of colitis were found to promote inflammatory activation of gut macrophages and increased production of TNFα.129 TNFα can impair both endothelialCitation130 and epithelial barriersCitation131,Citation132 and enhance leukocyte recruitment.Citation133,Citation134 Macrophage activation by EVs, leading to pro-inflammatory cytokine release and increased recruitment of inflammatory cells, was also substantiated in lung injury.Citation135 EV numbers were found to be increased in Crohn's patients, with an elevated disease score, which includes quantification of inflammatory cell infiltrate,Citation53 providing further clinical relevance of these observations.
In contrast to anticipated pro-inflammatory functions, EVs released by circulating PMNs upon adhesion to vascular endothelium were shown to be enriched with Annexin A1, an anti-inflammatory protein.Citation112 In this setting, EVs exerted an anti-inflammatory effect by inhibiting PMN adhesion and recruitment to inflamed tissue.Citation112 Similarly, ICAM-1 released in association with EVs has been suggested to competitively inhibit leukocyte adhesion to endothelium.Citation136 Endothelial and mesenchymal cell can also release EVs containing miR-206.Citation137 MiR-206 is significantly elevated in IBD,Citation138 and acts to suppress NFκB signaling and the release of leukocyte chemoattractants, IL-8, CXCL1 and CXCL2,Citation139 thus limiting immune cell infiltration. Several other miRNAs were similarly implicated in dampening inflammatory cell recruitment and the resulting tissue injury. For example, miR-141 that is released in EVs was suggested to keep inflammatory cytokine production in check.Citation140 Downregulation of miR-141 in Crohn's patients and experimental models of colitis resulted in increased CXCL12β production by IECs and enhanced leukocyte infiltration of the intestinal mucosa.Citation141 miR-146 expression in epithelial cells has been shown to decrease IL-8 and RANTES/CCL5 release,Citation142 suggesting its role in leukocyte trafficking during colonic inflammation. Following injury, IEC-derived EVsCitation143 are enriched with hypoxia-induced miRNAs, miR-221 and miR-320a, which induce upregulation and activation of matrix mettaloproteinase-9 (MMP-9), a protease known to be involved in macrophage and PMN trafficking by facilitating reorganization of junctional complexes and the extracellular matrix.Citation49
Finally, in addition to regulating cytokine expression, EVs can transport various chemokines and lipids, and can locally generate chemotactic gradients for migrating leukocytes. Indeed, in a Trans-well setup, macrophage-derived EVs were shown to induce granulocyte migration.Citation144 Moreover, IL-8Citation145 and IL-18Citation146 encapsulated in EVs can act as chemoattractants of PMNs in various disease settings. Macrophage and DC-derived EVs were found to contain biologically active enzymes for leukotrienes biosynthesis (LTA4 and LTB4) and promote granulocyte recruitment.Citation144 Granulocytic peripheral blood cells (RBL-2H3) were shown to release exosomes containing prostaglandins, such as, PGE2,Citation147 which among other functions can promote calcium and CCR7-dependent migration of monocyte-derived DCs.Citation148
Concluding remarks and future perspectives
As our knowledge of EV biogenesis and content expands, the contribution of EVs to intercellular communication and regulation of cellular processes in healthy and inflamed tissue become apparent. Many aspects of EV biology still remain unanswered, including active versus passive release by parent cells, cargo protection, uptake by target cells, content release, and importantly, pro- versus anti-inflammatory function of EVs. However, as we have outlined in this review, particularly in the gut, EVs contribute to the regulation of vascular and epithelial barrier function, wound healing, and function of resident and recruited immune cells (EVs release by various cell types, content and effects on cell function are summarized by schematic representation, ). EVs research has already sparked a clinical interest, as EVs were found to be elevated in the serum and tissues of patients with IBD and other multifactorial disorders. Moreover, given that the cargos associated with EVs often reflect diverse healthy and pathogenic states of the releasing cells and tissues, ongoing efforts are dedicated to exploring the possibility in which EVs can be used as biomarkers to diagnose and assess the therapeutic success of complex disorders, including IBD. For example, analysis of serum EV microRNA content in the clinic could be easily achieved by next-generation sequencing or digital PCR techniques, potentially yielding diseases specific gene signatures.Citation38,Citation39 Furthermore, since EVs are specifically equipped to mediate the transfer of regulatory short RNA molecules between cells, the possibility of exploiting these vesicles for therapeutic purposes is now being investigated. As such, ongoing efforts are being made to develop techniques that encapsulate therapeutic peptides, nucleic acids, and small molecule inhibitors into EVs, and protect them and increase their bio-availability and delivery to disease tissues.Citation149 This new line of therapy is a great premise for treatment of inflammatory diseases, such as IBDs.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank the Northwestern University Center for Advanced Microscopy core (supported by NCI CCSG P30 CA060553 awarded to the Robert H Lurie Comprehensive Cancer Center) for the help with electron and confocal microscopy imaging of PMN-MPs.
Additional information
Funding
References
- Xavier RJ, Podolsky DK. Unraveling the pathogenesis of inflammatory bowel disease. Nat. 2007;448(7152):427–34. doi:10.1038/nature06005. PMID:17653185.
- Xu X, Liu C, Liu Z. Dysregulation of mucosal immune response in pathogenesis of inflammatory bowel disease. World J Gastroenterol. 2014;20(12):3255–64. doi:10.3748/wjg.v20.i12.3255. PMID:24695798.
- Vrakas S, Mountzouris KC, Michalopoulos G, Karamanolis G, Papatheodoridis G, Tzathas C, Gazouli M. Intestinal bacteria composition and translocation of bacteria in inflammatory bowel disease. Plos One. 2017;12(1):e0170034. doi:10.1371/journal.pone.0170034. PMID:28099495.
- Sumagin R, Robin AZ, Nusrat A, Parkos CA. Activation of PKCβII by PMA facilitates enhanced epithelial wound repair through increased cell spreading and migration. PLoS ONE. 2013;8(2):e55775. doi:10.1371/journal.pone.0055775. PMID:23409039.
- Leoni G, Neumann P, Sumagin R, Denning TL, Nusrat A. Wound repair: role of immune–epithelial interactions. Mucosal Immunol. 2015;8(5):959–68. doi:10.1038/mi.2015.63. PMID:26174765.
- Chao LH, Murray MM. The role of inflammation and blood cells in wound healing. The ACL Handb. 2013;7(6):73–89. doi:10.1007/978-1-4614-0760-7_6.
- Hoang AN, Jones CN, Dimisko L, Hamza B, Martel J, Kojic N, Irimia D. Measuring neutrophil speed and directionality during chemotaxis, directly from a droplet of whole blood. Technology. 2013;01(01):49–57. doi:10.1142/s2339547813500040.
- Jones CN, Hoang AN, Dimisko L, Hamza B, Martel J, Irimia D. Microfluidic platform for measuring neutrophil chemotaxis from unprocessed whole blood. J Visualized Exp. 2014;88(1):1–6. doi:10.3791/51215.
- Leliefeld PH, Wessels CM, Leenen LP, Koenderman L, Pillay J. The role of neutrophils in immune dysfunction during severe inflammation. Crit Care. 2016;20(1):73. doi:10.1186/s13054-016-1250-4. PMID:27005275.
- Segal AW. How neutrophils kill microbes. Annu Rev Immunol. 2005;23(1):197–223. doi:10.1146/annurev.immunol.23.021704.115653. PMID:15771570.
- Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20(7):1126–67. doi:10.1089/ars.2012.5149. PMID:23991888.
- Perobelli S, Galvani R, Gonçalves-Silva T, Xavier C, Nóbrega A, Bonomo A. Plasticity of neutrophils reveals modulatory capacity. Brazilian J Med Biol Res. 2015;48(8):665–75. doi:10.1590/1414-431x20154524.
- Mariano F, Campanelli A, Mattos-Graner R, Gonçalves R. Antimicrobial peptides and nitric oxide production by neutrophils from periodontitis subjects. Brazilian J Med Biol Res. 2012;45(11):1017–24. doi:10.1590/s0100-879x2012007500123.
- Kebir DE, Gjorstrup P, Filep JG. Resolvin E1 promotes phagocytosis-induced neutrophil apoptosis and accelerates resolution of pulmonary inflammation. Proc Natl Acad Sci. 2012;109(37):14983–8. doi:10.1073/pnas.1206641109. PMID:22927428.
- Brook M, Tomlinson GH, Miles K, Smith RW, Rossi AG, Hiemstra PS, Gray M. Neutrophil-derived alpha defensins control inflammation by inhibiting macrophage mRNA translation. Proc Natl Acad Sci. 2016;113(16):4350–5. doi:10.1073/pnas.1601831113. PMID:27044108.
- Alalwani M, Sierigk J, Bals R. The antimicrobial peptide LL-37 modulates the inflammatory and host defense response of human neutrophils. Eur J Immunol. 2010;40(4):1118–1126. doi:10.1002/eji.200939275.
- Tsuji S, Taniuchi S, Hasui M, Yamamoto A, Kobayashi Y. Increased nitric oxide production by neutrophils from patients with chronic granulomatous disease on trimethoprim–sulfamethoxazole. Nitric Oxide. 2002;7(4):283–8. doi:10.1016/s1089-8603(02)00110-6. PMID:12446177.
- Grotendorst GR, Smale G, Pencev D. Production of transforming growth factor beta by human peripheral blood monocytes and neutrophils. J Cell Physiol. 1989;140(2):396–402. doi:10.1002/jcp.1041400226. PMID:2745570.
- Leoni G, Neumann P, Kamaly N, Quiros M, Nishio H, Jones HR, Nusrat A. Annexin A1–containing extracellular vesicles and polymeric nanoparticles promote epithelial wound repair. J Clin Invest. 2015;125(3):1215–27. doi:10.1172/jci76693. PMID:25664854.
- Sumagin R, Brazil JC, Nava P, Nishio H, Alam A, Luissint AC, Parkos CA. Neutrophil interactions with epithelial-expressed ICAM-1 enhances intestinal mucosal wound healing. Mucosal Immunol. 2016;9(5):1151–62. doi:10.1038/mi.2015.135. PMID:26732677.
- Nusrat A, Parkos C, Liang T, Carnes D, Madara J. Neutrophil migration across model intestinal epithelia: Monolayer disruption and subsequent events in epithelial repair. Gastroenterology. 1997;113(5):1489–500. doi:10.1053/gast.1997.v113.pm9352851. PMID:9352851.
- Lech M, Anders H. Macrophages and fibrosis: how resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochim Biophys Acta (BBA) – Mol Basis Dis. 2013;1832(7):989–97. doi:10.1016/j.bbadis.2012.12.001.
- Novak ML, Koh TJ. Phenotypic transitions of macrophages orchestrate tissue repair. Am J Pathol. 2013;183(5):1352–63. doi:10.1016/j.ajpath.2013.06.034 PMID:24091222.
- Yamada A, Arakaki R, Saito M, Ishimaru N. Role of regulatory T cell in the pathogenesis of inflammatory bowel disease. World J Gastroenterol. 2016;22(7):2195–205. doi:10.3748/wjg.v22.i7.2195 PMID:26900284.
- Buzas EI, György B, Nagy G, Falus A, Gay S. Emerging role of extracellular vesicles in inflammatory diseases. Nat Rev Rheumatol. 2014;10(6):356–64. doi:10.1038/nrrheum.2014.19. PMID:24535546.
- Cicero AL, Stahl PD, Raposo G. Extracellular vesicles shuffling intercellular messages: for good or for bad. Curr Opin Cell Biol. 2015;35:69–77. doi:10.1016/j.ceb.2015.04.013. PMID:26001269.
- Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30(1):255–89. doi:10.1146/annurev-cellbio-101512-122326. PMID:25288114.
- Al-Nedawi K, Mian MF, Hossain N, Karimi K, Mao Y, Forsythe P, Bienenstock J. Gut commensal microvesicles reproduce parent bacterial signals to host immune and enteric nervous systems. FASEB J. 2014;29(2):684–95. doi:10.1096/fj.14-259721. PMID:25392266.
- Niel GV, Raposo G, Candalh C, Boussac M, Hershberg R, Cerf–Bensussan N, Heyman M. Intestinal epithelial cells secrete exosome–like vesicles. Gastroenterology. 2001;121(2):337–49. doi:10.1053/gast.2001.26263. PMID:11487543.
- Yamamoto S, Niida S, Azuma E, Yanagibashi T, Muramatsu M, Huang TT, Sasahara M. Inflammation-induced endothelial cell-derived extracellular vesicles modulate the cellular status of pericytes. Sci Rep. 2015;5(1):8505. doi:10.1038/srep08505.
- Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14(3):195–208. doi:10.1038/nri3622. PMID:24566916.
- Kastelowitz N, Yin H. Exosomes and microvesicles: identification and targeting by particle size and lipid chemical probes. Chem Bio Chem. 2014;15(7):923–8. doi:10.1002/cbic.201400043. PMID:24740901.
- Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–83. doi:10.1083/jcb.201211138. PMID:23420871.
- Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2017;75(2):193–208. doi:10.1007/s00018-017-2595-9. PMID:28733901.
- Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Théry C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci. 2016;113(8):968–77. doi:10.1073/pnas.1521230113.
- Durcin M, Fleury A, Taillebois E, Hilairet G, Krupova Z, Henry C, Lay SL. Characterisation of adipocyte-derived extracellular vesicle subtypes identifies distinct protein and lipid signatures for large and small extracellular vesicles. J Extracell Vesicles. 2017;6(1):1305677. doi:10.1080/20013078.2017.1305677. PMID:28473884.
- Abels E, Breakefield X. Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cell Mol Neurobiol. 2016;36(3):301–12. doi:10.1007/s10571-016-0366-z. PMID:27053351.
- Liu Y, Lu Q. Extracellular vesicle microRNAs: biomarker discovery in various diseases based on RT-qPCR. Biomarkers Med. 2015;9(8):791–805. doi:10.2217/bmm.15.45.
- Moldovan L, Batte K, Wang Y, Wisler J, Piper M. Analyzing the circulating MicroRNAs in exosomes/extracellular vesicles from Serum or Plasma by qRT-PCR. Methods Mol Biol Circulating MicroRNAs. 2013;1024:129–45. doi:10.1007/978-1-62703-453-1_10.
- Zhang J, Li S, Li L, Li M, Guo C, Yao J, Mi S. Exosome and exosomal MicroRNA: trafficking, sorting, and function. Genomics Proteomics Bioinformatics. 2015;13(1):17–24. doi:10.1016/j.gpb.2015.02.001. PMID:25724326.
- Turchinovich A, Weiz L, Langheinz A, Burwinkel B. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 2011;39(16):7223–33. doi:10.1093/nar/gkr254. PMID:21609964.
- Dalli J, Montero-Melendez T, Norling LV, Yin X, Hinds C, Haskard D, Perretti M. Heterogeneity in neutrophil microparticles reveals distinct proteome and functional properties. Mol Cell Proteomics. 2013;12(8):2205–19. doi:10.1074/mcp.m113.028589. PMID:23660474.
- Perez-Pujol S, Marker PH, Key NS. Platelet microparticles are heterogeneous and highly dependent on the activation mechanism: Studies using a new digital flow cytometer. Cytometry Part A. 2007;71A(1):38–45. doi:10.1002/cyto.a.20354.
- Kalra H, Drummen G, Mathivanan S. Focus on extracellular vesicles: introducing the next small big thing. Int J Mol Sci. 2016;17(2):170. doi:10.3390/ijms17020170. PMID:26861301.
- Frey B, Gaipl US. The immune functions of phosphatidylserine in membranes of dying cells and microvesicles. Semin Immunopathol. 2010;33(5):497–516. doi:10.1007/s00281-010-0228-6. PMID:20941495.
- Liu M, Reilly MP, Casasanto P, Mckenzie SE, Williams KJ. Cholesterol enrichment of human monocyte/macrophages induces surface exposure of phosphatidylserine and the release of biologically-active tissue factor-positive microvesicles. Arterioscler Thromb Vasc Biol. 2006;27(2):430–5. doi:10.1161/01.atv.0000254674.47693.e8. PMID:17158353.
- Fernández-Messina L, Gutiérrez-Vázquez C, Rivas-García E, Sánchez-Madrid F, Fuente HD. Immunomodulatory role of microRNAs transferred by extracellular vesicles. Biol Cell. 2015;107(3):61–77. doi:10.1111/boc.201400081. PMID:25564937.
- Robbins PD, Dorronsoro A, Booker CN. Regulation of chronic inflammatory and immune processes by extracellular vesicles. J Clin Invest. 2016;126(4):1173–80. doi:10.1172/jci81131. PMID:27035808.
- Butin-Israeli V, Houser MC, Feng M, Thorp EB, Nusrat A, Parkos CA, Sumagin R. Deposition of microparticles by neutrophils onto inflamed epithelium: a new mechanism to disrupt epithelial intercellular adhesions and promote transepithelial migration. FASEB J. 2016;30(12):4007–20. doi:10.1096/fj.201600734r. PMID:27553226.
- Helwa I, Cai J, Drewry MD, Zimmerman A, Dinkins MB, Khaled ML, Liu Y. A comparative study of serum exosome isolation using differential ultracentrifugation and three commercial reagents. Plos One. 2017;12(1):e0170628. doi:10.1371/journal.pone.0170628. PMID:28114422.
- Palanisamy V, Sharma S, Deshpande A, Zhou H, Gimzewski J, Wong DT. Nanostructural and transcriptomic analyses of human saliva derived exosomes. PLoS ONE. 2010;5(1):e8577. doi:10.1371/journal.pone.0008577. PMID:20052414.
- Lv L, Cao Y, Liu D, Xu M, Liu H, Tang R, Liu B. Isolation and Quantification of MicroRNAs from urinary exosomes/microvesicles for biomarker discovery. Int J Biol Sci. 2013;9(10):1021–31. doi:10.7150/ijbs.6100. PMID:24250247.
- Mitsuhashi S, Feldbrügge L, Csizmadia E, Mitsuhashi M, Robson SC, Moss AC. Luminal extracellular vesicles (EVs) in inflammatory bowel disease (IBD) exhibit proinflammatory effects on epithelial cells and macrophages. Inflamm Bowel Dis. 2016;22(7):1587–95. doi:10.1097/mib.0000000000000840. PMID:27271497.
- Schwab A, Meyering SS, Lepene B, Iordanskiy S, Hoek ML, Hakami RM, Kashanchi F. Extracellular vesicles from infected cells: potential for direct pathogenesis. Front Microbiol. 2015;6:1132. doi:10.3389/fmicb.2015.01132.
- Mi S, Zhang J, Zhang W, Huang RS. Circulating MicroRNAs as biomarkers for inflammatory diseases. MicroRNA. 2013;2(1):64–72. doi:10.2174/2211536611302010007.
- Headland SE, Jones HR, Norling LV, Kim A, Souza PR, Corsiero E, Perretti M. Neutrophil-derived microvesicles enter cartilage and protect the joint in inflammatory arthritis. Sci Transl Med. 2015;7(315):e315. doi:10.1126/scitranslmed.aac5608. PMID:26606969.
- Rossaint J, Kühne K, Skupski J, Aken HV, Looney MR, Hidalgo A, Zarbock A. Directed transport of neutrophil-derived extracellular vesicles enables platelet-mediated innate immune response. Nat Commun. 2016;7:13464. doi:10.1038/ncomms13464. PMID:27845343.
- Hezel ME, Nieuwland R, Bruggen RV, Juffermans NP. The ability of extracellular vesicles to induce a pro-inflammatory host response. Int J Mol Sci. 2017;18(6):1285. doi:10.3390/ijms18061285.
- Kucharzik T, Walsh S, Chen J, Parkos C, Nusrat A. Neutrophil transmigration in inflammatory bowel disease is associated with altered expression of intercellular junction proteins. Gastroenterology. 2001;120(5):2001–9. doi:10.1016/s0016-5085(01)80927-6.
- Koelink PJ, Overbeek SA, Braber S, Morgan ME, Henricks PA, Roda MA, Kraneveld AD. Collagen degradation and neutrophilic infiltration: a vicious circle in inflammatory bowel disease. Gut. 2013;63(4):578–87. doi:10.1136/gutjnl-2012-303252. PMID:23525573.
- Michielan A, D'Incà R. Intestinal permeability in inflammatory bowel disease: pathogenesis, clinical evaluation, and therapy of Leaky Gut. Mediat Inflamm. 2015;2015:628157. doi:10.1155/2015/628157.
- Landy J, Ronde E, English N, Clark SK, Hart AL, Knight SC, Al-Hassi HO. Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. World J Gastroenterol. 2016;22(11):3117. doi:10.3748/wjg.v22.i11.3117. PMID:27003989.
- Schlegel N, Meir M, Heupel WM, Holthofer B, Leube RE, Waschke J. Desmoglein 2-mediated adhesion is required for intestinal epithelial barrier integrity. AJP Gastrointest Liver Physiol. 2010;298(5):774–83. doi:10.1152/ajpgi.00239.2009.
- Ungewiß H, Vielmuth F, Suzuki ST, Maiser A, Harz H, Leonhardt H, Waschke J. Desmoglein 2 regulates the intestinal epithelial barrier via p38 mitogen-activated protein kinase. Sci Rep. 2017;7(1):6329. doi:10.1038/s41598-017-06713-y. PMID:28740231.
- Lie PP, Cheng CY, Mruk DD. Crosstalk between desmoglein-2/desmocollin-2/Src kinase and coxsackie and adenovirus receptor/ZO-1 protein complexes, regulates blood-testis barrier dynamics. Int J Biochem Cell Biol. 2010;42(6):975–86. doi:10.1016/j.biocel.2010.02.010. PMID:20188849.
- Symowicz J, Adley BP, Gleason KJ, Johnson JJ, Ghosh S, Fishman DA, Stack MS. Engagement of collagen-binding integrins promotes matrix metalloproteinase-9-dependent E-cadherin ectodomain shedding in Ovarian Carcinoma Cells. Cancer Res. 2007;67(5):2030–39. doi:10.1158/0008-5472.can-06-2808. PMID:17332331.
- Gao H, Lan X, Li S, Xue Y. Relationships of MMP-9, E-cadherin, and VEGF expression with clinicopathological features and response to chemosensitivity in gastric cancer. Tumor Biol. 2017;39(5):1–7. doi:10.1177/1010428317698368.
- Campbell HK, Maiers JL, Demali KA. Interplay between tight junctions & adherens junctions. Exp Cell Res. 2017;358(1):39–44. doi:10.1016/j.yexcr.2017.03.061. PMID:28372972.
- Madrigal-Matute J, Lindholt JS, Fernandez-Garcia CE, Benito-Martin A, Burillo E, Zalba G, Martin-Ventura JL. Galectin-3, a biomarker linking oxidative stress and inflammation with the clinical outcomes of patients with atherothrombosis. J Am Heart Assoc. 2014;3(4):e000785. doi:10.1161/jaha.114.000785.
- Jiang K, Rankin CR, Nava P, Sumagin R, Kamekura R, Stowell SR, Nusrat A. Galectin-3 Regulates Desmoglein-2 and intestinal epithelial intercellular adhesion. J Biol Chem. 2014;289(15):10510–7. doi:10.1074/jbc.m113.538538. PMID:24567334.
- Eken C, Martin PJ, Sadallah S, Treves S, Schaller M, Schifferli JA. Ectosomes released by polymorphonuclear neutrophils induce a MerTK-dependent anti-inflammatory pathway in macrophages. J Biol Chem. 2010;285(51):39914–21. doi:10.1074/jbc.m110.126748. PMID:20959443.
- Eken C, Sadallah S, Martin PJ, Treves S, Schifferli JA. Ectosomes of polymorphonuclear neutrophils activate multiple signaling pathways in macrophages. Immunobiology. 2013;218(3):382–92. doi:10.1016/j.imbio.2012.05.021. PMID:22749214.
- Ma TY. TNF-α -induced increase in intestinal epithelial tight junction permeability requires NFκB activation. AJP Gastrointest Liver Physiol. 2004;286(3):367–76. doi:10.1152/ajpgi.00173.2003.
- Al-Sadi R, Guo S, Ye D, Ma TY. TNF-α modulation of intestinal epithelial tight junction barrier is regulated by ERK1/2 activation of Elk-1. Am J Pathol. 2013;183(6):1871–84. doi:10.1016/j.ajpath.2013.09.001. PMID:24121020.
- Mazzon E, Cuzzocrea S. Role of TNF-α in ileum tight junction alteration in mouse model of restraint stress. AJP Gastrointest Liver Physiol. 2008;294(5):1268–80. doi:10.1152/ajpgi.00014.2008.
- Cichon C, Sabharwal H, Rüter C, Schmidt MA. MicroRNAs regulate tight junction proteins and modulate epithelial/endothelial barrier functions. Tissue Barriers. 2014;2(4):e944446. doi:10.4161/21688362.2014.944446. PMID:25610754.
- Zhou Q, Croce CM, Souba WW, Verne GN. Micro-RNA-29a regulates intestinal membrane permeability in patients with irritable bowel syndrome. Gut. 2010;59(6):775–84. doi:10.1136/gut.2009.181834.
- Zhou Q, Costinean S, Croce CM, Brasier AR, Merwat S, Larson SA, Verne GN. MicroRNA 29 targets nuclear factor-κB–repressing factor and Claudin 1 to increase intestinal permeability. Gastroenterology. 2015;148(1):158–169. doi:10.1053/j.gastro.2014.09.037.
- Nolte-t H. Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood. 2009;113(9):1977–81. (2009). doi:10.1182/blood-2009-08-237313. PMID:19064723.
- Ludwig K, Fassan M, Mescoli C, Pizzi M, Balistreri M, Albertoni L, Rugge M. PDCD4/miR-21 dysregulation in inflammatory bowel disease-associated carcinogenesis. Virchows Arch. 2012;462(1):57–63. doi:10.1007/s00428-012-1345-5. PMID:23224068.
- Wu F, Zikusoka M, Trindade A, Dassopoulos T, Harris ML, Bayless TM, Kwon JH. MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2α. Gastroenterology. 2008;135(5):1624–35. doi:10.1053/j.gastro.2008.07.068.
- Shi C, Liang Y, Yang J, Xia Y, Chen H, Han H, Qin H. MicroRNA-21 knockout improve the survival rate in DSS induced fatal colitis through protecting against inflammation and tissue injury. PLoS ONE. 2013;8(6):e66814. doi:10.1371/journal.pone.0066814.
- Zhang L, Shen J, Cheng J, Fan X. MicroRNA-21 regulates intestinal epithelial tight junction permeability. Cell Biochem Funct. 2015;33(4):235–40. doi:10.1002/cbf.3109. PMID:25997617.
- Ho B, Yu I, Lu L, Rudensky A, Chen H, Tsai C, Yu S. Inhibition of miR-146a prevents enterovirus-induced death by restoring the production of type I interferon. Nat Commun. 2014;5:3344. doi:10.1038/ncomms4344.
- Mortazavi-Jahromi S, Jamshidi MM, Farazmand A, Aghazadeh Z, Yousefi M, Mirshafiey A. Pharmacological effects of β- d -mannuronic acid (M2000) on miR-146a, IRAK1, TRAF6 and NF-κB gene expression, as target molecules in inflammatory reactions. Pharmacol Rep. 2017;69(3):479–84. doi:10.1016/j.pharep.2017.01.021. PMID:28324845.
- Alexander M, Hu R, Runtsch MC, Kagele DA, Mosbruger TL, Tolmachova T, O'Connell RM. Exosome-delivered microRNAs modulate the inflammatory response to endotoxin. Nat Commun. 2015;6:7321. doi:10.1038/ncomms8321. PMID:26084661.
- Roos J, Enlund E, Funcke J, Tews D, Holzmann K, Debatin K, Fischer-Posovszky P. MiR-146a-mediated suppression of the inflammatory response in human adipocytes. Sci Rep. 2016;6(1):38339. doi:10.1038/srep38339.
- He X, Zheng Y, Liu S, Shi S, Liu Y, He Y, Zhou X. MiR-146a protects small intestine against ischemia/reperfusion injury by down-regulating TLR4/TRAF6/NF-κB pathway. J Cell Physiol. 2017;233(3):2476–88. doi:10.1002/jcp.26124.
- Luo X, Han M, Liu J, Wang Y, Luo X, Zheng J, Li H. Epithelial cell-derived micro RNA-146a generates interleukin-10-producing monocytes to inhibit nasal allergy. Sci Rep. 2015;5(1):15937. doi:10.1038/srep15937.
- Zhou R, Ohara SP, Chen X. MicroRNA regulation of innate immune responses in epithelial cells. Cell Mol Immunol. 2011;8(5):371–9. doi:10.1038/cmi.2011.19. PMID:21725335.
- Pigati L, Yaddanapudi SC, Iyengar R, Kim D, Hearn SA, Danforth D, Duelli DM. Selective release of MicroRNA species from normal and malignant mammary epithelial cells. PLoS ONE. 2010;5(10):e13515. doi:10.1371/journal.pone.0013515. PMID:20976003.
- Awad W, Hess C, Hess M. Enteric pathogens and their toxin-induced disruption of the intestinal barrier through alteration of tight junctions in chickens. Toxins. 2017;9(2):60. doi:10.3390/toxins9020060.
- Riddle MS, Porter CK. Detection bias and the association between inflammatory bowel disease and Salmonella and Campylobacter infection. Gut. 2011;61(4):635. doi:10.1136/gutjnl-2011-300617. PMID:21730102.
- Hu G, Gong A, Roth AL, Huang BQ, Ward HD, Zhu G, Chen X. Release of luminal exosomes contributes to TLR4-mediated epithelial antimicrobial defense. PLoS Pathog. 2013;9(4):e1003261. doi:10.1371/journal.ppat.1003261.
- Hill RH. Prevention of adhesion by indigenous bacteria to rabbit cecum epithelium by a barrier of microvesicles. Infect Immun. 1985;47(2):540–3. PMID:3881354.
- Niel GV. Intestinal epithelial exosomes carry MHC class II/peptides able to inform the immune system in mice. Gut. 2003;52(12):1690–7. doi:10.1136/gut.52.12.1690. PMID:14633944.
- Chen X, Song C, Feng B, Li T, Li P, Zheng P, Yang P. Intestinal epithelial cell-derived integrin 6 plays an important role in the induction of regulatory T cells and inhibits an antigen-specific Th2 response. J Leukoc Biol. 2011;90(4):751–9. doi:10.1189/jlb.1210696. PMID:21724807.
- Evans-Osses I, Mojoli A, Monguió-Tortajada M, Marcilla A, Aran V, Amorim M, Ramirez MI. Microvesicles released from Giardia intestinalis disturb host-pathogen response in vitro. Eur J Cell Biol. 2017;96(2):131–42. doi:10.1016/j.ejcb.2017.01.005. PMID:28236495.
- Slater TW, Finkielsztein A, Mascarenhas LA, Mehl LC, Butin-Israeli V, Sumagin R. Neutrophil microparticles deliver active myeloperoxidase to injured mucosa to inhibit epithelial wound healing. J Immunol. 2017;198(7):2886–97. doi:10.4049/jimmunol.1601810. PMID:28242649.
- Pitanga T, França LD, Rocha VC, Meirelles T, Borges VM, Gonçalves M, Dos-Santos WL. Neutrophil-derived microparticles induce myeloperoxidase-mediated damage of vascular endothelial cells. BMC Cell Biol. 2014;15(1):21. doi:10.1186/1471-2121-15-21. PMID:24915973.
- Hong Y, Eleftherio D, Hussain AA, Brogan PA. Anti-neutrophil cytoplasmic antibodies stimulate release of neutrophil microparticles. J Am Soc Nephrol. 2012;23(1):49–62. doi:10.1681/ASN.2011030298. PMID:22052057.
- Folkesson M, Li C, Frebelius S, Swedenborg J, Wågsäter D, Williams KJ, Liu M. Proteolytically active ADAM10 and ADAM17 carried on membrane microvesicles in human abdominal aortic aneurysms. Thromb Haemost. 2015;114(6):1165–1174. doi:10.1160/th14-10-0899. PMID:26422658.
- Mesri M, Altieri DC. Leukocyte microparticles stimulate endothelial cell cytokine release and tissue factor induction in a JNK1 signaling pathway. J Biol Chem. 1999;274(33):23111–8. doi:10.1074/jbc.274.33.23111. PMID:10438480.
- Vince RV, Chrismas B, Midgley AW, Mcnaughton LR, Madden LA. Hypoxia mediated release of endothelial microparticles and increased association of S100A12 with circulating neutrophils. Oxid Med Cell Longev. 2009;2(1):2–6. doi:10.4161/oxim.2.1.7611. PMID:20046638.
- Wang Y, Tian J, Tang X, Rui K, Tian X, Ma J, Wang S. Exosomes released by granulocytic myeloid-derived suppressor cells attenuate DSS-induced colitis in mice. Oncotarget. 2016;7(13):15356–68. doi:10.18632/oncotarget.7324. PMID:26885611.
- Véron P, Segura E, Sugano G, Amigorena S, Théry C. Accumulation of MFG-E8/lactadherin on exosomes from immature dendritic cells. Blood Cells Mol Dis. 2005;35(2):81–88. doi:10.1016/j.bcmd.2005.05.001. PMID:15982908.
- Zhang Y, Brenner M, Yang W, Wang P. Recombinant human MFG-E8 ameliorates colon damage in DSS- and TNBS-induced colitis in mice. Lab Invest. 2015;95(5):480–90. doi:10.1038/labinvest.2015.32. PMID:25751740.
- Scapini P, Cassatella MA. Social networking of human neutrophils within the immune system. Blood. 2014;124(5):710–9. doi:10.1182/blood-2014-03-453217. PMID:24923297.
- Dalli J, Serhan CN. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012;120(15):e60–72. doi:10.1182/blood-2012-04-423525. PMID:22904297.
- Yang M, Chen J, Su F, Yu B, Su F, Lin L, Song E. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol Cancer. 2011;10(1):117. doi:10.1186/1476-4598-10-117. PMID:21939504.
- Neudecker V, Haneklaus M, Jensen O, McNamee EN. Myeloid-derived miR-223 regulates intestinal inflammation via repression of the NLRP3 inflammasome. J Exp Med. 2017;214(6):1737–52. doi:10.1084/jem.20160462. PMID:28487310.
- Dalli J, Norling LV, Renshaw D, Cooper D, Leung K, Perretti M. Annexin 1 mediates the rapid anti-inflammatory effects of neutrophil-derived microparticles. Blood. 2008;112(6):2512–9. doi:10.1182/blood-2008-02-140533. PMID:18594025.
- Borges FT, Melo SA, Ozdemir BC, Kato N, Revuelta I, Miller CA, Kalluri R. TGF- 1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J Am Soc Nephrol. 2012;24(3):385–92. doi:10.1681/asn.2012101031. PMID:23274427.
- Pi B. Wound healing and the role of fibroblasts. J Wound Care. 2013;22(8):407–12. doi:10.12968/jowc.2013.22.8.407. PMID:23924840.
- Luga V, Zhang L, Viloria-Petit A, Ogunjimi A, Inanlou M, Chiu E, Wrana J. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151(7):1542–56. doi:10.1016/j.cell.2012.11.024. PMID:23260141.
- Pereira-Fantini PM, Judd LM, Kalantzis A, Peterson A, Ernst M, Heath JK, Giraud AS. A33 antigen-deficient mice have defective colonic mucosal repair. Inflamm Bowel Dis. 2010;16(4):604–12. doi:10.1002/ibd.21114. PMID:19856415.
- Nomachi A, Nishita M, Inaba D, Enomoto M, Hamasaki M, Minami Y. Receptor Tyrosine Kinase Ror2 Mediates Wnt5a-induced polarized cell migration by activating c-Jun N-terminal Kinase via actin-binding protein filamin A. J Biol Chem. 2008;283(41):27973–81. doi:10.1074/jbc.m802325200. PMID:18667433.
- Miyoshi H. Wnt-expressing cells in the intestines: guides for tissue remodeling. J Biochem. 2016;161(1):19–25. doi:10.1093/jb/mvw070. PMID:28013225.
- Harada T, Yamamoto H, Kishida S, Kishida M, Awada C, Takao T, Kikuchi A. Wnt5b-associated exosomes promote cancer cell migration and proliferation. Cancer Sci. 2016;108(1):42–52. doi:10.1111/cas.13109. PMID:27762090.
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7(9):678–89. doi:10.1038/nri2156. PMID:17717539.
- Sumagin R, Sarelius IH. Intercellular adhesion molecule-1 enrichment near tricellular endothelial junctions is preferentially associated with leukocyte transmigration and signals for reorganization of these junctions to accommodate leukocyte passage. J Immunol. 2010;184(9):5242–52. doi:10.4049/jimmunol.0903319. PMID:20363969.
- Qian B, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475(7355):222–5. doi:10.1038/nature10138. PMID:21654748.
- Tang N, Sun B, Gupta A, Rempel H, Pulliam L. Monocyte exosomes induce adhesion molecules and cytokines via activation of NF-κB in endothelial cells. FASEB J. 2016;30(9):3097–106. doi:10.1096/fj.201600368rr. PMID:27226520.
- Cerutti C, Soblechero-Martin P, Wu D, Lopez-Ramirez MA, Vries HD, Sharrack B, Romero IA. MicroRNA-155 contributes to shear-resistant leukocyte adhesion to human brain endothelium in vitro. Fluids Barriers CNS. 2016;13(1):8–15. doi:10.1186/s12987-016-0032-3. PMID:27246706.
- Béres NJ, Szabó D, Kocsis D, Szűcs D, Kiss Z, Müller KE, Veres G. Role of altered expression of miR-146a, miR-155, and miR-122 in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2016;22(2):327–35. doi:10.1097/mib.0000000000000687. PMID:26752469.
- Fasseu M, Tréton X, Guichard C, Pedruzzi E, Cazals-Hatem D, Richard C, Ogier-Denis E. Identification of Restricted Subsets of Mature microRNA abnormally expressed in inactive colonic mucosa of patients with inflammatory bowel disease. PLoS ONE. 2010;5(10):e13160. doi:10.1371/journal.pone.0013160.
- Yoshimura A, Suzuki M, Sakaguchi R, Yasukawa H. SOCS, inflammation, and autoimmunity. Front Immunol. 2012;3:20. doi:10.3389/fimmu.2012.00020. PMID:22566904.
- Pathak S, Grillo AR, Scarpa M, Brun P, Dincà R, Nai L, Castagliuolo I. MiR-155 modulates the inflammatory phenotype of intestinal myofibroblasts by targeting SOCS1 in ulcerative colitis. Exp Mol Med. 2015;47(5):e164. doi:10.1038/emm.2015.21. PMID:25998827.
- Wong W, Lee MM, Chan BD, Kam RK, Zhang G, Lu A, Tai WC. Proteomic profiling of dextran sulfate sodium induced acute ulcerative colitis mice serum exosomes and their immunomodulatory impact on macrophages. Proteomics. 2016;16(7):1131–45. doi:10.1002/pmic.201500174. PMID:26806198.
- Shinichiro H, Masaru H, Hironori K, Takumi K, Eitaro T, Ryukichi K, Michio S. Tumor necrosis factor-alpha and interferon-gamma directly impair epithelial barrier function in cultured mouse cholangiocytes. Liver Int. 2003;23(1):3–11. doi:10.1034/j.1600-0676.2003.01707.x.
- Kaiser G, Polk D. Tumor necrosis factor alpha regulates proliferation in a mouse intestinal cell line. Gastroenterology. 1997;112(4):1231–40. doi:10.1016/s0016-5085(97)70135-5. PMID:9098007.
- Schmitz H, Fromm M, Bentzel CJ, Schulzke JD. Tumor necrosis factor α (TNFα) impairs barrier function in epithelial monolayers of HT-29/B6 cells. Gastroenterology. 1999;108(4):137–46. doi:10.1016/0016-5085(95)23946-4.
- Williams MR, Luscinskas FW. Leukocyte rolling and adhesion via ICAM-1 signals to endothelial permeability. Focus on “Leukocyte rolling and adhesion both contribute to regulation of microvascular permeability to albumin via ligation of ICAM-1”. AJP Cell Physiol. 2011;301(4):777–9. doi:10.1152/ajpcell.00250.2011.
- Zhao C, Sardella A, Chun J, Poubelle PE, Fernandes MJ, Bourgoin SG. TNF-α promotes LPA1- and LPA3-mediated recruitment of leukocytes in vivo through CXCR2 ligand chemokines. J Lipid Res. 2011;52(7):1307–18. doi:10.1194/jlr.m008045. PMID:21521824.
- Kojima M, Gimenes-Junior JA, Chan TW, Eliceiri BP, Baird A, Costantini TW, Coimbra R. Exosomes in postshock mesenteric lymph are key mediators of acute lung injury triggering the macrophage activation via Toll-like receptor 4. FASEB J. 2017;32(1):97–110. doi:10.1096/fj.201700488r.
- Lee HM, Choi E, Kim JH, Kim TD, Kim Y, Kang C, Gho YS. A membranous form of ICAM-1 on exosomes efficiently blocks leukocyte adhesion to activated endothelial cells. Biochem Biophys Res Commun. 2010;397(2):251–6. doi:10.1016/j.bbrc.2010.05.094. PMID:20529672.
- Nakamura Y, Miyaki S, Ishitobi H, Matsuyama S, Nakasa T, Kamei N, Ochi M. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 2015;589(11):1257–65. doi:10.1016/j.febslet.2015.03.031. PMID:25862500.
- Lin J, Welker NC, Zhao Z, Li Y, Zhang J, Reuss SA, Bronner MP. Novel specific microRNA biomarkers in idiopathic inflammatory bowel disease unrelated to disease activity. Mod Pathol. 2013;27(4):602–8. doi:10.1038/modpathol.2013.152. PMID:24051693.
- Keklikoglou I, Hosaka K, Bender C, Bott A, Koerner C, Mitra D, Wiemann S. MicroRNA-206 functions as a pleiotropic modulator of cell proliferation, invasion and lymphangiogenesis in pancreatic adenocarcinoma by targeting ANXA2 and KRAS genes. Oncogene. 2014;34(37):4867–78. doi:10.1038/onc.2014.408. PMID:25500542.
- Hao X, Li Z, Ma Y, Wang J, Zeng X, Li R, Kang W. Exosomal microRNA-141 is upregulated in the serum of prostate cancer patients. Onco Targets Ther. 2015;9:139–148. doi:10.2147/ott.s95565.
- Huang Z, Shi T, Zhou Q, Shi S, Zhao R, Shi H, Zhang J. MiR-141 Regulates colonic leukocytic trafficking by targeting CXCL12β during murine colitis and human Crohns disease. Gut. 2013;63(8):1247–57. doi:10.1136/gutjnl-2012-304213. PMID:24000293.
- Perry MM, Moschos SA, Williams AE, Shepherd NJ, Larner-Svensson HM, Lindsay MA. Rapid Changes in MicroRNA-146a expression negatively regulate the IL-1-induced inflammatory response in human lung alveolar epithelial cells. J Immunol. 2008;180(8):5689–98. doi:10.4049/jimmunol.180.8.5689. PMID:18390754.
- Lee H, Zhang D, Zhu Z, Cruz CS, Jin Y. Epithelial cell-derived microvesicles activate macrophages and promote inflammation via microvesicle-containing microRNAs. Sci Rep. 2016;6(1):35250. doi:10.1038/srep35250.
- Esser J, Gehrmann U, Dalexandri FL, Hidalgo-Estévez AM, Wheelock CE, Scheynius A, Rådmark O. Exosomes from human macrophages and dendritic cells contain enzymes for leukotriene biosynthesis and promote granulocyte migration. J Allergy Clin Immunol. 2010;126(5):1032–40. doi:10.1016/j.jaci.2010.06.039. PMID:20728205.
- Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K. Establishment of essential involvement of IL-8 in acute inflammation. Cytokine. 1994;6(5):547. doi:10.1016/1043-4666(94)90129-5.
- Leung BP, Culshaw S, Gracie JA, Hunter D, Canetti CA, Campbell C, Mcinnes IB. A Role for IL-18 in neutrophil activation. J Immunol. 2001;167(5):2879–86. doi:10.4049/jimmunol.167.5.2879. PMID:11509635.
- Subra C, Grand D, Laulagnier K, Stella A, Lambeau G, Paillasse M, Record M. Exosomes account for vesicle-mediated transcellular transport of activatable phospholipases and prostaglandins. J Lipid Res. 2010;51(8):2105–20. doi:10.1194/jlr.m003657. PMID:20424270.
- Scandella E. Prostaglandin E2 is a key factor for CCR7 surface expression and migration of monocyte-derived dendritic cells. Blood. 2002;100(4):1354–61. doi:10.1182/blood-2001-11-0017. PMID:12149218.
- Ha D, Yang N, Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B. 2016;6(4):287–96. doi:10.1016/j.aps.2016.02.001. PMID:27471669.