ABSTRACT
Gut epithelium covers the inner layer of the gastrointestinal tract and provides a physical barrier to separate the host from its external environment, and its barrier function is critical for maintaining host health. AMP-activated protein kinase (AMPK) as a master regulator of energy metabolism plays a critical role in epithelial barrier function. AMPK activation promotes epithelial differentiation and facilitates cell polarity establishment, both of which strengthen epithelial barrier. In addition, AMPK promotes the assembly of tight junctions and adherens junctions by direct phosphorylation of proteins composing apical junctions, junctional anchors, and cytoskeletons. Pharmacological and nutraceutical compounds, as well as physiological states triggering AMPK activation strengthen epithelial barrier function. This review summarized recent progress in delineating the regulatory roles of AMPK in apical junction formation and barrier function of intestinal epithelium.
Introduction
Epithelium forms a physical barrier to perform both fence and gate functions, which protect against the penetration of noxious substances and allow the passage of nutrients, ions and small solutes.Citation1,Citation2 Intestinal epithelium constitutes a single layer of epithelial cells that are constantly renewed.Citation3 Epithelial cells are tightly linked through apical junctions, including tight junctions (TJs) and adherens junctions (AJs).Citation4 The functionality of epithelial barrier is orchestrated by delicate balances among epithelial proliferation, differentiation and apoptosis, as well as apical junction formation and assembly.Citation1 The disturbance of junctional organization leads to increased permeability of the intestinal epithelium, or “leaky gut”.Citation5–9 Subsequently, dysfunctional barrier function could cause various diseases, such as inflammatory bowel diseases (IBD),Citation10,Citation11 hypercalciuria and hypomagnesemia kidney diseases,Citation12 and autoimmune diseases.Citation13 In addition, endotoxemia due to the leaky gut barrier is associated with metabolic diseases,Citation14 Parkinson’s,Citation15 Alzheimer’s,Citation16 and multiple sclerosis.Citation17
AMP-activated protein kinase (AMPK), a master regulator of energy metabolism,Citation18,Citation19 is increasingly known for its vital role in regulating myogenesis,Citation20 adipogenesisCitation21 and cardiac differentiation.Citation22 We recently reported that AMPK plays an important role in regulating gut epithelial differentiation and barrier function.Citation8,Citation23 Pharmacological, nutraceutical and physiological activation of AMPK strengthens epithelial apical junctions and protects epithelial barrier against environmental stresses,Citation24–Citation28 while AMPK inhibition due to metabolic disorders is concurrent with impaired epithelial barrier function.Citation18,Citation29 This review summarizes the recent literature on favorable effects of AMPK on epithelial apical junction assembly and intestinal barrier function.
Epithelial apical junction overview
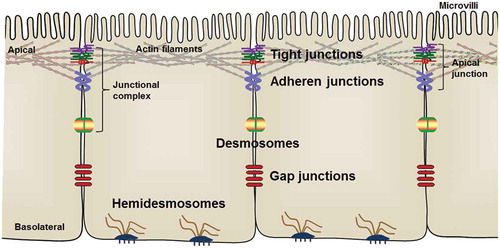
Epithelial cells are joined by a series of intercellular junctions and polarized into the apical and the basolateral domains.Citation30,Citation31 The apical domain of epithelial cells is linked with adjacent epithelial cells through TJs and AJs, which are also referred to as apical junctions (). The assembly of apical junctions is indispensable for the formation and maintenance of epithelial barrier integrity.Citation1,Citation32 Desmosomes are intercellular junctions located below AJs on the lateral membrane and link to intermediate filaments to stabilize the epithelial layer and provide mechanical strength to tissues.Citation33,Citation34 On the basolateral membrane, hemidesmosomes connect to intermediate filament and facilitate epithelial cell adhesion to extracellular matrix in the basal lamina.Citation35 In addition, epithelial cells communicate with surrounding cells through gap junctions () that are composed of connexins and assembled into hexameric pore-forming channels.Citation36
Figure 1. Arrangement of intestinal epithelial cells and intercellular junctions between epithelial cells. The apical junctions are composed of tight junctions and adherens junctions.
Epithelial tight junctions
Tight junctions are localized at the most apical constituent of the intestinal epithelium, regulate paracellular permeability and contribute to epithelial cell polarity.Citation37 Primary constituents of TJs are mostly transmembrane proteins, including claudins, occludin, and junctional adhesion molecules (JAMs), which are stabilized by intracellular scaffolding protein, zona occludens (ZO) () and further linked to actin filaments.Citation4,Citation37 Occludin is the first identified protein of TJs that is a tetraspan integral membrane protein with both the N- and C- terminus located on the cytoplasmic side.Citation38 Occludin is involved in signal transduction and critical in maintaining the stability of TJs, and also highly phosphorylated at serine and threonine residues.Citation39,Citation40 Occludin together with tricellulin and marvelD3 form the tight junction-associated MARVEL (MAL and related proteins for vesicle trafficking and membrane link) protein (TAMP) family. These proteins interact each other and have distinct but overlapping functions at TJs.Citation41 Claudins are the backbone of TJs and major players of epithelial TJs and paracellular barrier function. Claudins are integral membrane proteins containing four transmembrane domains with one intracellular loop, two extracellular loops, a cytoplasmic N-terminus and C-terminus.Citation40,Citation42 Claudins interact with cytoplasmic PDZ scaffolding proteins such as ZOs through PDZ domain-binding motifs in its C- terminus domain.Citation43 Up to now, 27 members have been identified in the mammalian claudin family.Citation44 Each claudin member has a unique pattern of cell and tissue-specific distribution. Most members of the claudin family have TJ-tightening or barrier-forming properties; however, some claudin members, such as claudin 2, confer a pore-forming activity that is commonly associated with leaky epithelium.Citation45,Citation46 The expression of claudin 2 is increased under different pathologic states such as IBD.Citation5,Citation47,Citation48 The structure, distribution and function of claudin-2 and other claudin family members have been comprehensively reviewed.Citation40,Citation49
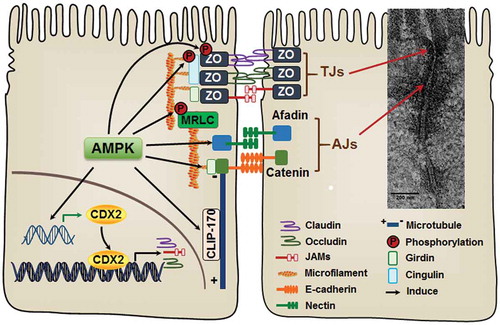
Figure 2. AMP-activated protein kinase (AMPK) activation accelerates the assembly of epithelial apical junctions. The apical side of epithelial cells is linked with adjacent epithelial cells through tight junctions (TJs) and adherens junctions (AJs). The primary constituents of TJs include transmembrane proteins, claudins, occludin, and junctional adhesion molecules (JAMs), which are stabilized by intracellular scaffolding protein zona occludens (ZO). AJs consist of two groups of transmembrane proteins, cadherins and nectins. Cadherins and nectins bind to the cytoskeleton by catenin-anchor proteins and afadin, respectively. AMPK activation accelerates TJ assembly stabilizes and maintains apical junctions, most likely through phosphorylation of TJ proteins and their associated proteins. In addition, AMPK enhances the expression of CDX2, which promotes epithelial differentiation and TJ protein expression.
JAMs are glycosylated transmembrane proteins with a single transmembrane domain, an extracellular N-terminus and a short cytoplasmic C-terminus.Citation50 They are widely distributed in various cell types and tissues Citation50 and have pleiotropic physiological functions, including epithelial barrier function, which has been previously reviewed.Citation40,Citation51 JAMs function as cell-cell adhesion molecules through homophilic interactions with JAMs expressed by adjacent or opposing cells.Citation52 JAM-A is a member of the JAM family highly expressed in intestinal epithelium.Citation53 JAM-A-deficient mice had enhanced intestinal permeability and were more susceptible to dextran sulfate sodium (DSS)-induced colitis,Citation6,Citation7 while JAM-A content was decreased in intestinal mucosal extract of IBD patients compared to that from healthy subjects.Citation7 Overexpression of JAM-A enhanced barrier function in Caco-2 cells and ameliorated the barrier dysfunction caused by ethanol treatment.Citation53 The molecular structure, dimerization and functional motifs of JAM-A, and its interaction with scaffolding proteins, have been reviewed recently.Citation52
Tricellulin is a 64-kD tetraspan, tricellular TJ protein commonly located at the region where three or more cells are adjacent,Citation54 and is involved in maintaining epithelial barrier integrity. Tricellulin is down-regulated in colon tissue biopsied from IBD, particularly from patients with ulcerative colitis,Citation55 as well as from enteropathogenic E. coli infected human colonic epithelial cells,Citation56 which are correlated with compromised barrier integrity.
Epithelial adherens junctions
AJs, located below TJs, maintain both the physical association between epithelial cells and cell polarity, and are involved in signal transduction. AJs consist of two groups of integral membrane proteins, cadherins and nectins. Cadherins and nectins bind to the cytoskeleton via catenin anchor proteins or afadin ().Citation57
The cadherin superfamily contains of more than 20 members. Of these, epithelial cadherin (E-cadherin) is the most prominent in epithelial tissues and has a vital role in epithelial cell AJs assembly. The extracellular domain of E-cadherin contains five repetitive domains or cadherin repeats. Each cadherin repeat has a calcium-binding domain.Citation58 In the presence of calcium, E-cadherin interacts with other E-cadherins of the same or opposed cells through respective extracellular domains.Citation59 The cytoplasmic region of cadherin interacts with catenins and forms the cadherins-catenins complex, which then binds to actin microfilament ().Citation60 Similar to cadherins, nectins have a single transmembrane domain and interact with each other through an extracellular domain.Citation61 The Inter-cellular interaction of nectins is not dependent on Ca2+. Cytoplasmic tails of nectins interact with afadins through their PDZ binding motifs, which further associate with actin filaments ().Citation62 Nectins cooperate with cadherins in generating functional AJs.Citation57
TJs and AJs are associated with each other physically via interaction between cytoplasmic scaffolding protein ZOs and catenins or afadins.Citation63,Citation64 They are also linked through signaling molecules.Citation65 Ca2+ deprivation rapidly destroys and Ca2+ replenishment reinitiates TJ formation.Citation8 In addition, Ca2+ also drives AJ formation, which consolidates TJs,Citation66 suggesting that TJs and AJs both contribute to barrier integrity.
Favorite roles of AMPK in epithelial apical junctions
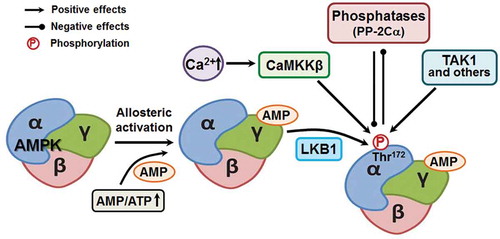
AMPK, a highly conserved serine/threonine kinase, regulates energy homeostasis to promote ATP generation and energy restoration when AMP/ATP ratio is elevated.Citation18 AMPK is composed of three subunits, including a catalytic α subunit, structural β subunit, and a regulatory γ subunit (). Under elevated AMP levels, the γ subunit binds to AMP, which renders AMPK a better phosphorylation substrate for its upstream kinase, liver kinase B (LKB1), at Thr 172,Citation67 and also inhibits its dephosphorylation.Citation68,Citation69 AMPK can also be activated in response to the elevation of cellular Ca2+, which involves its phosphorylation at Thr 172 by Ca2+/calmodulin-dependent protein kinase kinase beta (CaMKKβ),Citation70 as well as transforming growth factor beta-activated kinase 1 (TAK1).Citation71 On the other hand, the phosphorylation of AMPK at Thr 172 can be removed by protein phosphatases such as protein phosphatase 2C alpha (PP-2Cα) ().Citation69 AMPK activity is commonly subjected to change in response to numerous physiological factors, such as hormones, cytokines, and dietary nutrients as well as pathological conditions, such as aging, metabolic syndrome, cancer and chronic inflammation.Citation72 Accumulating evidence has shown that AMPK plays an important role in determining gut epithelial health. Pharmacological and nutraceutical activation of AMPK strengthens epithelial barrier function,Citation8,Citation25,Citation27,Citation28,Citation73 while AMPK inhibition due to metabolic disorders is concurrent with impaired epithelial barrier function,Citation18,Citation29 clearly showing the regulatory role of AMPK in epithelial permeability.
Figure 3. AMP-activated protein kinase (AMPK) structure and activation. AMPK is structurally composed of catalytic α subunit, docking β subunit and regulatory γ subunit. AMPK is phosphorylated/activated by LKB1, CaMKKβ, TAK1 and other kinases. CaMKKβ: calmodulin-mediated kinase kinase β; LKB1: liver kinase B1; PP-2Cα: protein phosphatase 2C alpha; TAK1: transforming growth factor-β activated kinase 1.
AMPK in tight junction formation and assembly
TJs assembly: TJ assembly of polarized epithelial cells is dynamic and critical in the formation and maintenance of the epithelial barrier. Extracellular Ca2+ is indispensable in TJs assembly, and AMPK is activated during Ca2+-induced TJ assembly in MDCK cells.Citation74,Citation75 AMPK activation by 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR) enhanced the assembly of TJs while expression of AMPK kinase-dead constructs, K45R (AMPK α2 mutant) or D157A (AMPK α1 mutant) compromised TJ assembly as indicated by accelerated or delayed ZO-1 relocation to TJs and impaired transepithelial electrical resistance (TEER) in MDCK cells.Citation74,Citation75 Furthermore, AICAR triggers and accelerates TJ assembly regardless of calcium availability,Citation74 suggesting AMPK activation promotes TJ formation independent of Ca2+. Citation76 Also, in cultured MDCK cells, microvesicles derived from mesenchymal stromal cells facilitated Ca2+-induced relocation of ZO-1 to TJ in an AMPK-dependent way.Citation77 Similarly, lymphocytes accelerated TJs assembly in MDCK cells following Ca2+ switch via AMPK activation.Citation78
Likewise, in intestinal epithelial Caco-2 cells, ZO-1 relocation to TJs was enhanced in cells treated with AICAR or those with overexpression of AMPK wild-type plasmid, but was inhibited in cells expressing K45R AMPK mutant plasmid, which was associated with enhanced or compromised TEER and barrier function, respectively.Citation8 Consistently, the ultrastructure of TJs was greatly loosened, which was associated with increased intestinal permeability in AMPK VilCre mice where AMPK was specifically knocked out in villin-expressing epithelial cells.Citation8 Treatment with butyrate, a key metabolite of gut microbiota, activated AMPK and promoted TJ assembly in Caco-2 cells as indicated by enhanced ZO-1 and occludin redistribution, which was associated with enhanced TEER and decreased paracellular inulin permeability; however, Compound C (a cell-permeable AMPK inhibitor) treatment abolished butyrate-induced AMPK activation as well as barrier strengthening effect.Citation25 We recently reported that polyphenol-rich purple potato extract enhanced TEER in an AMPK- dependent manner and promoted ZO-1 relocation to TJs in Caco-2 cells.Citation27 Either chitosan oligosaccharides or flufenamic acid can promote TJ reassembly following Ca2+ switch and increase TEER in T84 cells in an AMPK-dependent manner, since co-incubation of either chitosan oligosaccharides or flufenamic acid with Compound C abolishes their beneficial effects on TJ assembly.Citation79,Citation80
Though the mechanism leading to AMPK-induced TJ assembly remains elusive, it might be regulated partially through direct phosphorylation of TJ proteins and associated proteins. AMPK phosphorylates claudin 1 at Thr191 that promotes the formation of TJs in EpH4 breast cells.Citation81 In submandibular SMG-C6 cells, AMPK activation phosphorylates claudin 4 at Ser199 and further enhances claudin 4 and occludin interaction.Citation82 Cytoskeleton microtubules are critical for the maintenance of TJ structure and function.Citation83 The elaborate interaction between apical junctions and cytoskeleton is essential for the maintenance and stabilization of TJs. Microtubules interact with TJs via cingulin anchored to claudin and occludin by ZO-1Citation84 Upon phosphorylation by AMPK at Ser132 and Ser150, cingulin connects microtubules to ZO-1, contributing to epithelial morphogenesis. AMPK inhibition distorts epithelial morphogenesis and detaches the network between micro-tubules and TJs,Citation84 showing a regulatory role of AMPK in anchoring of TJs to cytoskeleton. Girdin (also known as GIV), a polarity scaffold protein, preserves cell polarity and stabilizes TJs.Citation85 AMPK directly phosphorylates Girdin at Ser245, which is essential for maintaining apical junctional integrity and barrier function in MDCK cells.Citation85
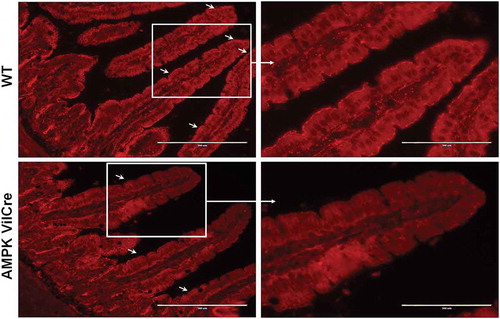
TJ protein content: In addition, AMPK can promote TJs through increasing TJ protein expression. Metformin supplementation increased barrier-promoting claudin 3 and decreased pore-forming caludin 2 content in the ileum tissue of interleukin (IL)-10-deficient mice, which was associated with improved barrier function.Citation73 In addition to strengthened TEER and decreased paracellular permeability, polyphenol-rich propolis extractCitation28 or purple potato extract Citation27 supplementation enhanced AMPK phosphorylation and the content of TJ proteins such as ZO-1, occludin and claudin1 in Caco-2 cells. Similarly, theaflavins promote barrier function in Caco-2 cells, likely through increasing TJ protein content, which was correlated with AMPK activation.Citation86 L-glutamine supplementation also promotes TEER and increases the content of TJ protein occludin, claudin 3, claudin 4, ZO-1, ZO-2 and ZO-3 as well as JAM-A in in vitro cultured intestine porcine epithelial cells, accompanied with AMPK activation.Citation87 Butyrate strengthens intestinal barrier through enhancing TJ assembly instead of increasing TJ protein expression.Citation25 Conversely, AMPK intestinal specific deletion reduced ZO-1 content and impaired ZO-1 immunofluorescence staining at the tips of villi in jejunum tissue of AMPK Vilcre mice, ()Citation8.
Figure 4. Immunofluorescent staining of ZO-1 in jejunum tissues of wild-type (WT) and AMPK VilCre knock out male mice at age of 10-week. Arrows indicate ZO-1 at the border of villus. Scale bar is 200 μm. Adopted from Sun et al., 2017 published in Cell Death & Differentiation.Citation8
AMPK in adherens junctions formation
AJs consist of two groups of transmembrane proteins, cadherins and nectins. The content of E-cadherin is upregulated in Caco−2 cells treated with AICAR, an AMPK activator, while downregulated in Caco−2 cells overexpressing K45R AMPK mutant or in jejunum tissues of mice with epithelium-specific AMPK knockout.Citation8 AMPK activation phosphorylates afadin, which induces Ca2+-independent relocalization of AJ components,Citation76 providing a possible link between AMPK activation and apical junction stabilization. Metformin supplementation increased E-cadherin content in ileum tissue of both wild-type and IL-10-deficient mice, while E-cadherin content decreased in IL-10-deficient mice compared to wild-type mice.Citation73
In addition, AMPK mediates cytoskeleton attachment of AJs. Microtubule organization is determined by proteins at both ends.Citation88 Microtubule plus-ends are dynamic, while minus-end proteins maintain stability.Citation89 CLIP-170, a microtubule plus-end binding protein, is phosphorylated by AMPK at Ser 311 to accelerate the microtubule polymerization and cell migration in renal 293T cells.Citation90 Non-muscle myosin regulatory light chain (MRLC) is an integrator driving cell adhesion and migration.Citation91,Citation92 AMPK directly phosphorylates MRLC at Thr 21 in Drosophila.Citation93 The activation of AMPK due to 2-deoxyglucose-induced energy deprivation polarizes actin cytoskeleton in epithelial LS174T cells, which depends on MRLC phosphorylation by AMPK, Citation93 suggesting MRLC is involved in AMPK-dependent epithelial polarity establishment. AMPK is also a mediator of cell responses to mechanical force. In response to pulling forces applied to E-cadherin in breast MCF10A cells, AMPK is activated wit strengthens the cadherin-cytoskeleton complex to keep epithelial integrity.Citation24
AMPK in epithelial differentiation, inflammation and barrier function
The establishment of apical junctions is positively related to epithelial differentiation.Citation94 AMPK promotes intestinal epithelial differentiation through promoting the expression of CDX2, a key transcription factor regulating epithelial differentiation.Citation8 Polyphenol-rich purple potato extract triggers AMPK activation and increases expression of CDX2 in both Caco-2 cells and ex vivo guts. It also enhances barrier function and the epithelial differentiation markers villin, as well as brush border enzymes such as alkaline phosphatase, aminopeptidase and sucrose isomerase.Citation27 These beneficial effects were absent in Caco-2 cells with AMPK knockdownCitation27, indicating the beneficial effects of purple potato polyphenol extract on epithelial cell differentiation is mediated by AMPK. Besides transcription factors, epithelial differentiation is also regulated by complex signaling pathways. Bone morphogenetic protein (BMP) is one of the key pathways promoting intestinal differentiation.Citation95 Metformin supplementation decreases intestinal permeability, stimulates AMPK phosphorylation, and induces differentiation of goblet cells and Paneth cells in IL-10-deficient mice via AMPK-mediated BMP signaling pathway.Citation73
Inflammation impairs epithelial barrier function.Citation26,Citation96 Inflammation directly disrupts the assembly of apical junctions and thus increases epithelial permeability.Citation97 In addition, inflammation activates Wingless and Int (Wnt)/β-catenin signaling, which promotes epithelial proliferation but inhibits differentiation, thus impairing epithelial barrier formation.Citation96,Citation98 AMPK activation triggers cell cycle arrest,Citation99,Citation100 consistent with the repressive roles of AMPK in epithelial proliferation and promotion of differentiation. On the other hand, AMPK activation creates a pseudo-starving state that promotes oxidative metabolism and inhibits inflammation.Citation101 Metformin-induced AMPK activation reduces macrophage abundance, which suppresses mucosal inflammation and improves epithelial barrier function in IL-10-deficient mice.Citation73 Flufenamic acid or metformin supplementation protects barrier leakage in mouse ileum loop caused by Vibrio cholera infection. Either supplementation is associated with activation of AMPK and suppression of nuclear factor kappa B (NF-κB) inflammatory signaling and related inflammatory cytokine production.Citation80 Dietary red raspberry-activated AMPK attenuated inflammation and colitis symptoms in DSS-treated mice accompanied with reduced content of pore-forming claudin 2, and increased contents of barrier-strengthening TJ proteins.Citation47 Maternal high-fat diet enhanced offspring susceptibility to DSS-induced colitis, increased inflammatory cytokine IL-1β, IL-6 and IL-17, and amplified NF-кB inflammatory signaling response in mice, which was associated with suppressed AMPK phosphorylation.Citation102 Interestingly, in IL-10-deficient mice, AMPK phosphorylation is elevated, which could be due to energy dysregulation associated with chronic inflammation and oxidative stress; consistently, grape seed extract enriched with polyphenolic compounds prevented inflammation, and restored AMPK activity and barrier function in these mice.Citation96 In summary, AMPK improves gut epithelial barrier function through promoting oxidative metabolism and suppressing inflammation.
Perspectives and conclusion
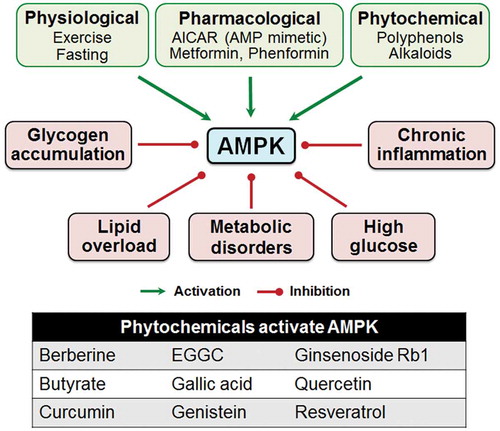
AMPK activity is commonly subjected to change in response to physiological factors including hormones and cytokines, as well as pathological conditions, such as aging, metabolic syndrome, and chronic inflammation ().Citation72 Therefore, effectively restoring AMPK activity can serve as a therapeutic target to treat various metabolic diseases Citation103,Citation104 and to improve various physiological functions, such as reinforcing apical junction assembly and gut barrier.
Metformin, a widely used medication for managing type 2 diabetes,Citation105 activates AMPK by increasing the cellular AMP level. Besides improving lipid metabolism, metformin protects intestine epithelial barrier function in mice with fructose-induced steatosisCitation106 and in IL-10-deficient mice. Citation73 In addition to pharmacological drugs, many naturally occurring phytochemicals such as polyphenols and ginsenoside Rb1, a type of alkaloid isolated from ginseng, can effectively activate AMPK ().Citation107,Citation108,Citation109 Polyphenols, a diverse and abundant group of plant-derived bioactive compounds known for their anti-oxidative and anti-inflammatory properties, have preventive or therapeutic effects against metabolic diseases such as obesity, diabetes, aging, cardiovascular diseases and IBD.Citation110,Citation111–Citation114 The growing list of these compounds, including chlorogenic acid, curcumin, quercetin, resveratrol, and polyphenol-rich purple potato extract, exert their beneficial effects via activation of AMPK.Citation27,Citation110,Citation115–Citation117 In addition, polyphenol-rich extracts and fruits Citation27,Citation28,Citation47 and gut microbial metabolite butyrateCitation25 activate AMPK and improve barrier function.
In summary, AMPK promotes the assembly and stability of apical junctions via the phosphorylation of TJ proteins and associated proteins. Additionally, AMPK promotes epithelial differentiation by upregulating epithelial transcription factors, suppressing Wnt/β-catenin signaling, and activating BMP signaling. Collectively, AMPK promotes epithelial barrier functions exerting health beneficial effects. The use of pharmacological and nutraceutical compounds, as well as the manipulation of physiological states triggering AMPK activation, are promising methods for strengthening epithelial barrier function and preventing metabolic diseases.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was reported by the authors.
Acknowledgments
We thank Michael H. Taylor for his critical reading of the review.
Additional information
Funding
References
- Matter K, Balda MS. Signalling to and from tight junctions. Nat Rev Mol Biol. 2003;4(3):225–236. doi: 10.1038/nrm1055.
- Gumbiner B. Structure, biochemistry, and assembly of epithelial tight junctions. Am J Physiol. 1987;253(6 Pt 1):C749–758. doi: 10.1152/ajpcell.1987.253.6.C749.
- Heath JK. Transcriptional networks and signaling pathways that govern vertebrate intestinal development. Curr Top Dev Biol. 2010;90:159–192. doi: 10.1016/S0070-2153(10)90004-5.
- Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2(4):285–293. doi: 10.1038/35067088.
- Zeissig S, Burgel N, Gunzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007;56(1):61–72. doi: 10.1136/gut.2006.094375.
- Laukoetter MG, Nava P, Lee WY, Severson EA, Capaldo CT, Babbin BA, Williams IR, Koval M, Peatman E, Campbell JA, et al. JAM-A regulates permeability and inflammation in the intestine in vivo. J Exp Med. 2007;204(13):3067–3076. doi: 10.1084/jem.20071416.
- Vetrano S, Rescigno M, Cera MR, Correale C, Rumio C, Doni A, Fantini M, Sturm A, Borroni E, Repici A, et al. Unique role of junctional adhesion molecule-a in maintaining mucosal homeostasis in inflammatory bowel disease. Gastroenterology. 2008;135(1):173–184. doi: 10.1053/j.gastro.2008.04.002.
- Sun X, Yang Q, Rogers CJ, Du M, Zhu MJ. AMPK improves gut epithelial differentiation and barrier function via regulating Cdx2 expression. Cell Death Differ. 2017;24(5):819–831. doi: 10.1038/cdd.2017.14.
- Yu TX, Gu BL, Yan JK, Zhu J, Yan WH, Chen J, Qian LX, Cai W. CUGBP1 and HuR regulate E-cadherin translation by altering recruitment of E-cadherin mRNA to processing bodies and modulate epithelial barrier function. Am J Physiol Cell Physiol. 2016;310(1):C54–65. doi: 10.1152/ajpcell.00112.2015.
- Kiesslich R, Duckworth CA, Moussata D, Gloeckner A, Lim LG, Goetz M, Pritchard DM, Galle PR, Neurath MF, Watson AJ. Local barrier dysfunction identified by confocal laser endomicroscopy predicts relapse in inflammatory bowel disease. Gut. 2012;61(8):1146–1153. doi: 10.1136/gutjnl-2011-300695.
- Lee SY, Lee SH, Yang EJ, Kim EK, Kim JK, Shin DY, Cho ML. Metformin ameliorates inflammatory bowel disease by suppression of the STAT3 signaling pathway and regulation of the between Th17/Treg Balance. PLoS One. 2015;10(9):e0135858. doi: 10.1371/journal.pone.0135858.
- Piepenhagen PA, Nelson WJ. Differential expression of cell-cell and cell-substratum adhesion proteins along the kidney nephron. Am J Physiology-Cell Physiol. 1995;269(6):C1433–C1449. doi: 10.1152/ajpcell.1995.269.6.C1433.
- Förster C. Tight junctions and the modulation of barrier function in disease. Histochem Cell Biol. 2008;130(1):55–70. doi: 10.1007/s00418-008-0424-9.
- Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. doi: 10.2337/db06-1491.
- Clairembault T, Leclair-Visonneau L, Coron E, Bourreille A, Le Dily S, Vavasseur F, Heymann M-F, Neunlist M, Derkinderen P. Structural alterations of the intestinal epithelial barrier in Parkinson’s disease. Acta Neuropathol Commun. 2015;3(1):12. doi: 10.1186/s40478-015-0196-0.
- Z Alam M, Alam Q, A Kamal M, M Abuzenadah A, Haque AA. possible link of gut microbiota alteration in type 2 diabetes and Alzheimer’s disease pathogenicity: an update. CNS Neurol Disord Drug Targets (Formerly Current Drug Targets-CNS Neurological Disorders). 2014;13(3):383–390.
- Buscarinu MC, Cerasoli B, Annibali V, Policano C, Lionetto L, Capi M, Mechelli R, Romano S, Fornasiero A, Mattei G. Altered intestinal permeability in patients with relapsing–remitting multiple sclerosis: A pilot study. Mult Scler J. 2017;23(3):442–446. doi: 10.1177/1352458516652498.
- Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1(1):15–25. doi: 10.1016/j.cmet.2004.12.003.
- Hardie DG. Biochemistry. Balancing cellular energy. Science. 2007;315(5819):1671–1672. doi: 10.1126/science.1140737.
- Fu X, Zhao JX, Liang J, Zhu MJ, Foretz M, Viollet B, Du M. AMP-activated protein kinase mediates myogenin expression and myogenesis via histone deacetylase 5. Am J Physiol Cell Physiol. 2013;305(8):C887–895. doi: 10.1152/ajpcell.00124.2013.
- Wang S, Liang X, Yang Q, Fu X, Zhu M, Rodgers BD, Jiang Q, Dodson MV, Du M. Resveratrol enhances brown adipocyte formation and function by activating AMP-activated protein kinase (AMPK) alpha1 in mice fed high-fat diet. Mol Nutr Food Res. 2017;61(4):1600746. doi: 10.1002/mnfr.201600746.
- Dzeja PP, Chung S, Faustino RS, Behfar A, Terzic A. Developmental enhancement of adenylate kinase-AMPK metabolic signaling axis supports stem cell cardiac differentiation. PLoS One. 2011;6(4):e19300. doi: 10.1371/journal.pone.0019300.
- Sun X, Fu X, Du M, Zhu MJ. Ex vivo gut culture for studying differentiation and migration of small intestinal epithelial cells. Open Biol. 2018;8(4):170256. doi: 10.1098/rsob.170256.
- Bays JL, Campbell HK, Heidema C, Sebbagh M, DeMali KA. Linking E-cadherin mechanotransduction to cell metabolism through force-mediated activation of AMPK. Nat Cell Biol. 2017;19(6):724–731. doi: 10.1038/ncb3537.
- Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. 2009;139(9):1619–1625. doi: 10.3945/jn.109.104638.
- Scharl M, Paul G, Barrett KE, McCole DF. AMP-activated protein kinase mediates the interferon-gamma-induced decrease in intestinal epithelial barrier function. J Biol Chem. 2009;284(41):27952–27963. doi: 10.1074/jbc.M109.046292.
- Sun X, Du M, Navarre DA, Zhu MJ. Purple potato extract promotes intestinal epithelial differentiation and barrier function by activating AMP-activated protein kinase. Mol Nutr Food Res. 2018;62(4):1700536. doi: 10.1002/mnfr.201700536.
- Wang K, Jin X, Chen Y, Song Z, Jiang X, Hu F, Conlon MA, Topping DL. Polyphenol-rich propolis extracts strengthen intestinal barrier function by activating AMPK and ERK signaling. Nutrients. 2016;8(5):272. doi: 10.3390/nu8050272.
- Meddings J. The significance of the gut barrier in disease. Gut. 2008;57(4):438–440. doi: 10.1136/gut.2007.143172.
- Denker BM, Nigam SK. Molecular structure and assembly of the tight junction. Am J Physiol. 1998;274(1 Pt 2):F1–9.
- Kimura Y, Shiozaki H, Hirao M, Maeno Y, Doki Y, Inoue M, Monden T, Ando-Akatsuka Y, Furuse M, Tsukita S, et al. Expression of occludin, tight-junction-associated protein, in human digestive tract. Am J Pathol. 1997;151(1):45–54.
- Mehta S, Nijhuis A, Kumagai T, Lindsay J, Silver A. Defects in the adherens junction complex (E-cadherin/beta-catenin) in inflammatory bowel disease. Cell Tissue Res. 2015;360(3):749–760. doi: 10.1007/s00441-014-1994-6.
- Burdett ID. Aspects of the structure and assembly of desmosomes. Micron. 1998;29(4):309–328.
- Garrod D, Chidgey M. Desmosome structure, composition and function. Biochim Biophys Acta. 2008;1778(3):572–587. doi: 10.1016/j.bbamem.2007.07.014.
- Walko G, Castanon MJ, Wiche G. Molecular architecture and function of the hemidesmosome. Cell Tissue Res. 2015;360(3):529–544. doi: 10.1007/s00441-015-2216-6.
- Goodenough DA, Paul DL. Gap junctions. Cold Spring Harb Perspect Biol. 2009;1(1):a002576. doi: 10.1101/cshperspect.a002576.
- Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778(3):660–669. doi: 10.1016/j.bbamem.2007.07.012.
- Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123(6 Pt 2):1777–1788.
- Suzuki T, Elias BC, Seth A, Shen L, Turner JR, Giorgianni F, Desiderio D, Guntaka R, Rao R. PKC eta regulates occludin phosphorylation and epithelial tight junction integrity. Proc Natl Acad Sci U S A. 2009;106(1):61–66. doi: 10.1073/pnas.0802741106.
- Chiba H, Osanai M, Murata M, Kojima T, Sawada N. Transmembrane proteins of tight junctions. Biochim Biophys Acta. 2008;1778(3):588–600. doi: 10.1016/j.bbamem.2007.08.017.
- Raleigh DR, Marchiando AM, Zhang Y, Shen L, Sasaki H, Wang Y, Long M, Turner JR. Tight junction-associated MARVEL proteins marveld3, tricellulin, and occludin have distinct but overlapping functions. Mol Biol Cell. 2010;21(7):1200–1213. doi: 10.1091/mbc.E09-08-0734.
- Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and −2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141(7):1539–1550.
- Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol. 1999;147(6):1351–1363.
- Mineta K, Yamamoto Y, Yamazaki Y, Tanaka H, Tada Y, Saito K, Tamura A, Igarashi M, Endo T, Takeuchi K, et al. Predicted expansion of the claudin multigene family. FEBS Lett. 2011;585(4):606–612. doi: 10.1016/j.febslet.2011.01.028.
- Amasheh S, Meiri N, Gitter AH, Schoneberg T, Mankertz J, Schulzke JD, Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci. 2002;115(Pt 24):4969–4976.
- Rosenthal R, Milatz S, Krug SM, Oelrich B, Schulzke JD, Amasheh S, Gunzel D, Fromm M. Claudin-2, a component of the tight junction, forms a paracellular water channel. J Cell Sci. 2010;123(Pt 11):1913–1921. doi: 10.1242/jcs.060665.
- Bibi S, Kang Y, Du M, Zhu MJ. Dietary red raspberries attenuate dextran sulfate sodium-induced acute colitis. J Nutr Biochem. 2018;51:40–46. doi: 10.1016/j.jnutbio.2017.08.017.
- Prasad S, Mingrino R, Kaukinen K, Hayes KL, Powell RM, MacDonald TT, Collins JE. Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab Invest. 2005;85(9):1139–1162. doi: 10.1038/labinvest.3700316.
- Luettig J, Rosenthal R, Barmeyer C, Schulzke JD. Claudin-2 as a mediator of leaky gut barrier during intestinal inflammation. Tissue Barriers. 2015;3(1–2):e977176. doi: 10.4161/21688370.2014.977176.
- Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A, et al. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 1998;142(1):117–127.
- Ebnet K. Junctional adhesion molecules (JAMs): cell adhesion receptors with pleiotropic functions in cell physiology and development. Physiol Rev. 2017;97(4):1529–1554. doi: 10.1152/physrev.00004.2017.
- Steinbacher T, Kummer D, Ebnet K. Junctional adhesion molecule-A: functional diversity through molecular promiscuity. Cell Mol Life Sci. 2018;75(8):1393–1409. doi: 10.1007/s00018-017-2729-0.
- Chopyk DM, Kumar P, Raeman R, Liu Y, Smith T, Anania FA. Dysregulation of junctional adhesion molecule-A contributes to ethanol-induced barrier disruption in intestinal epithelial cell monolayers. Physiol Rep. 2017;5(23):e13541. doi: 10.14814/phy2.13262.
- Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S, Tsukita S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol. 2005;171(6):939–945. doi: 10.1083/jcb.200510043.
- Krug SM, Bojarski C, Fromm A, Lee IM, Dames P, Richter JF, Turner JR, Fromm M, Schulzke JD. Tricellulin is regulated via interleukin-13-receptor alpha2, affects macromolecule uptake, and is decreased in ulcerative colitis. Mucosal Immunol. 2018. doi: 10.1038/mi.2017.1052.
- Morampudi V, Graef FA, Stahl M, Dalwadi U, Conlin VS, Huang T, Vallance BA, Yu HB, Jacobson K. Tricellular tight junction protein tricellulin is targeted by the enteropathogenic Escherichia coli effector EspG1, leading to epithelial barrier disruption. Infect Immun. 2017;85(1):e00700–00716. doi: 10.1128/IAI.00700-16.
- Meng W, Takeichi M. Adherens junction: molecular architecture and regulation. Cold Spring Harb Perspect Biol. 2009;1(6):a002899. doi: 10.1101/cshperspect.a002899.
- Overduin M, Harvey TS, Bagby S, Tong KI, Yau P, Takeichi M, Ikura M. Solution structure of the epithelial cadherin domain responsible for selective cell adhesion. Science. 1995;267(5196):386–389.
- Wu Y, Jin X, Harrison O, Shapiro L, Honig BH, Ben-Shaul A. Cooperativity between trans and cis interactions in cadherin-mediated junction formation. Proc Natl Acad Sci U S A. 2010;107(41):17592–17597. doi: 10.1073/pnas.1011247107.
- Rimm DL, Koslov ER, Kebriaei P, Cianci CD, Morrow JS. Alpha 1(E)-catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc Natl Acad Sci U S A. 1995;92(19):8813–8817.
- Takahashi K, Nakanishi H, Miyahara M, Mandai K, Satoh K, Satoh A, Nishioka H, Aoki J, Nomoto A, Mizoguchi A, et al. Nectin/PRR: an immunoglobulin-like cell adhesion molecule recruited to cadherin-based adherens junctions through interaction with Afadin, a PDZ domain-containing protein. J Cell Biol. 1999;145(3):539–549.
- Reymond N, Borg JP, Lecocq E, Adelaide J, Campadelli-Fiume G, Dubreuil P, Lopez M. Human nectin3/PRR3: a novel member of the PVR/PRR/nectin family that interacts with afadin. Gene. 2000;255(2):347–355.
- Itoh M, Nagafuchi A, Moroi S, Tsukita S. Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to alpha catenin and actin filaments. J Cell Biol. 1997;138(1):181–192.
- Yamamoto T, Harada N, Kano K, Taya S, Canaani E, Matsuura Y, Mizoguchi A, Ide C, Kaibuchi K. The Ras target AF-6 interacts with ZO-1 and serves as a peripheral component of tight junctions in epithelial cells. J Cell Biol. 1997;139(3):785–795.
- Campbell HK, Maiers JL, DeMali KA. Interplay between tight junctions & adherens junctions. Exp Cell Res. 2017;358(1):39–44. doi: 10.1016/j.yexcr.2017.03.061.
- Yoshida-Noro C, Suzuki N, Takeichi M. Molecular nature of the calcium-dependent cell-cell adhesion system in mouse teratocarcinoma and embryonic cells studied with a monoclonal antibody. Dev Biol. 1984;101(1):19–27.
- Oakhill JS, Steel R, Chen ZP, Scott JW, Ling N, Tam S, Kemp BE. AMPK is a direct adenylate charge-regulated protein kinase. Science. 2011;332(6036):1433–1435. doi: 10.1126/science.1200094.
- Ross FA, Jensen TE, Hardie DG. Differential regulation by AMP and ADP of AMPK complexes containing different gamma subunit isoforms. Biochem J. 2016;473(2):189–199. doi: 10.1042/BJ20150910.
- Davies SP, Helps NR, Cohen PT, Hardie DG. 5ʹ-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2C alpha and native bovine protein phosphatase-2AC. FEBS Lett. 1995;377(3):421–425. doi: 10.1016/0014-5793(95)01368-7.
- Fujiwara Y, Kawaguchi Y, Fujimoto T, Kanayama N, Magari M, Tokumitsu H. Differential AMP-activated protein kinase (AMPK) recognition mechanism of Ca2+/Calmodulin-dependent protein kinase kinase isoforms. J Biol Chem. 2016;291(26):13802–13808. doi: 10.1074/jbc.M116.727867.
- Momcilovic M, Hong SP, Carlson M. Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J Biol Chem. 2006;281(35):25336–25343. doi: 10.1074/jbc.M604399200.
- Steinberg GR, Kemp BE. AMPK in health and disease. Physiol Rev. 2009;89(3):1025–1078. doi: 10.1152/physrev.00011.2008.
- Xue Y, Zhang H, Sun X, Zhu MJ. Metformin improves ileal epithelial barrier function in interleukin-10 deficient mice. PLoS One. 2016;11(12):e0168670. doi: 10.1371/journal.pone.0168670.
- Zhang L, Li J, Young LH, Caplan MJ. AMP-activated protein kinase regulates the assembly of epithelial tight junctions. Proc Natl Acad Sci U S A. 2006;103(46):17272–17277. doi: 10.1073/pnas.0608531103.
- Zheng B, Cantley LC. Regulation of epithelial tight junction assembly and disassembly by AMP-activated protein kinase. Proc Natl Acad Sci U S A. 2007;104(3):819–822. doi: 10.1073/pnas.0610157104.
- Zhang L, Jouret F, Rinehart J, Sfakianos J, Mellman I, Lifton RP, Young LH, Caplan MJ. AMP-activated protein kinase (AMPK) activation and glycogen synthase kinase-3β (GSK-3β) inhibition induce Ca2+-independent deposition of tight junction components at the plasma membrane. J Biol Chem. 2011;286(19):16879–16890. doi: 10.1074/jbc.M110.186932.
- Rowart P, Erpicum P, Krzesinski JM, Sebbagh M, Jouret F. Mesenchymal stromal cells accelerate epithelial tight junction assembly via the AMP-activated protein kinase pathway, independently of liver kinase B1. Stem Cells Int. 2017;2017:9717353. doi: 10.1155/2017/9717353.
- Tang XX, Chen H, Yu S, Zhang L, Caplan MJ, Chan HC. Lymphocytes accelerate epithelial tight junction assembly: role of AMP-activated protein kinase (AMPK). PLoS One. 2010;5(8):e12343. doi: 10.1371/journal.pone.0012343.
- Muanprasat C, Wongkrasant P, Satitsri S, Moonwiriyakit A, Pongkorpsakol P, Mattaveewong T, Pichyangkura R, Chatsudthipong V. Activation of AMPK by chitosan oligosaccharide in intestinal epithelial cells: mechanism of action and potential applications in intestinal disorders. Biochem Pharmacol. 2015;96(3):225–236. doi: 10.1016/j.bcp.2015.05.016.
- Pongkorpsakol P, Satitsri S, Wongkrasant P, Chittavanich P, Kittayaruksakul S, Srimanote P, Chatsudthipong V, Muanprasat C. Flufenamic acid protects against intestinal fluid secretion and barrier leakage in a mouse model of Vibrio cholerae infection through NF-kappaB inhibition and AMPK activation. Eur J Pharmacol. 2017;798:94–104. doi: 10.1016/j.ejphar.2017.01.026.
- Shiomi R, Shigetomi K, Inai T, Sakai M, Ikenouchi J. CaMKII regulates the strength of the epithelial barrier. Sci Rep. 2015;5:13262. doi: 10.1038/srep13262.
- Xiang RL, Mei M, Cong X, Li J, Zhang Y, Ding C, Wu LL, Yu GY. Claudin-4 is required for AMPK-modulated paracellular permeability in submandibular gland cells. J Mol Cell Biol. 2014;6(6):486–497. doi: 10.1093/jmcb/mju048.
- Glotfelty LG, Zahs A, Iancu C, Shen L, Hecht GA. Microtubules are required for efficient epithelial tight junction homeostasis and restoration. Am J Physiology-Cell Physiol. 2014;307(3):C245–C254. doi: 10.1152/ajpcell.00336.2013.
- Yano T, Matsui T, Tamura A, Uji M, Tsukita S. The association of microtubules with tight junctions is promoted by cingulin phosphorylation by AMPK. J Cell Biol. 2013;203(4):605–614. doi: 10.1083/jcb.201304194.
- Aznar N, Patel A, Rohena CC, Dunkel Y, Joosen LP, Taupin V, Kufareva I, Farquhar MG, Ghosh P. AMP-activated protein kinase fortifies epithelial tight junctions during energetic stress via its effector GIV/Girdin. Elife. 2016;5:e20795. doi: 10.7554/eLife.20795.
- Park HY, Kunitake Y, Hirasaki N, Tanaka M, Matsui T. Theaflavins enhance intestinal barrier of Caco-2 Cell monolayers through the expression of AMP-activated protein kinase-mediated Occludin, Claudin-1, and ZO-1. Bioscience Biotechnology and Biochemistry. 2015;79(1):130–137. doi: 10.1080/09168451.2014.951027.
- Wang B, Wu Z, Ji Y, Sun K, Dai Z, Wu G. L-glutamine enhances tight junction integrity by activating CaMK kinase 2-AMP-activated protein kinase signaling in intestinal porcine epithelial cells. J Nutr. 2016;146(3):501–508. doi: 10.3945/jn.115.224857.
- Akhmanova A, Steinmetz MO. Control of microtubule organization and dynamics: two ends in the limelight. Nat Rev Mol Cell Biol. 2015;16(12):711. doi: 10.1038/nrm4084.
- Takeichi M. Dynamic contacts: rearranging adherens junctions to drive epithelial remodelling. Nat Rev Mol Cell Biol. 2014;15(6):397. doi: 10.1038/nrm3802.
- Nakano A, Kato H, Watanabe T, Min K-D, Yamazaki S, Asano Y, Seguchi O, Higo S, Shintani Y, Asanuma H. AMPK controls the speed of microtubule polymerization and directional cell migration through CLIP-170 phosphorylation. Nat Cell Biol. 2010;12(6):583–590. doi: 10.1038/ncb2060.
- Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol. 2009;10(11):778.
- Cunningham KE, Turner JR. Myosin light chain kinase: pulling the strings of epithelial tight junction function. Ann N Y Acad Sci. 2012;1258(1):34–42. doi: 10.1111/j.1749-6632.2012.06526.x.
- Lee JH, Koh H, Kim M, Kim Y, Lee SY, Karess RE, Lee S-H, Shong M, Kim J-M, Kim J. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature. 2007;447(7147):1017–1020. doi: 10.1038/nature05828.
- Ginzberg RD, Gilula NB. Modulation of cell junctions during differentiation of the chicken otocyst sensory epithelium. Dev Biol. 1979;68(1):110–129.
- Batts LE, Polk DB, Dubois RN, Kulessa H. Bmp signaling is required for intestinal growth and morphogenesis. Dev Dyn. 2006;235(6):1563–1570. doi: 10.1002/dvdy.20741.
- Yang G, Wang H, Kang Y, Zhu MJ. Grape seed extract improves epithelial structure and suppresses inflammation in ileum of IL-10-deficient mice. Food Funct. 2014;5(10):2558–2563. doi: 10.1039/c4fo00451e.
- Di Fusco D, Dinallo V, Monteleone I, Laudisi F, Marafini I, Franze E, Di Grazia A, Dwairi R, Colantoni A, Ortenzi A, et al. Metformin inhibits inflammatory signals in the gut by controlling AMPK and p38 MAP kinase activation. Clin Sci (Lond). 2018. doi: 10.1042/CS20180167.
- Koh SJ, Kim JM, Kim IK, Ko SH, Kim JS. Anti-inflammatory mechanism of metformin and its effects in intestinal inflammation and colitis-associated colon cancer. J Gastroenterol Hepatol. 2014;29(3):502–510.
- Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, Birnbaum MJ, Thompson CB. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18(3):283–293. doi: 10.1016/j.molcel.2005.03.027.
- Shaw RJ, Bardeesy N, Manning BD, Lopez L, Kosmatka M, DePinho RA, Cantley LC. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6(1):91–99. doi: 10.1016/j.ccr.2004.06.007.
- O’neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493(7432):346–355. doi: 10.1038/nature11862.
- Bibi S, Kang Y, Du M, Zhu MJ. Maternal high-fat diet consumption enhances offspring susceptibility to DSS-induced colitis in mice. Obesity (Silver Spring). 2017;25(5):901–908. doi: 10.1002/oby.21816.
- Brusq J-M, Ancellin N, Grondin P, Guillard R, Martin S, Saintillan Y, Issandou M. Inhibition of lipid synthesis through activation of AMP kinase: an additional mechanism for the hypolipidemic effects of berberine. J Lipid Res. 2006;47(6):1281–1288. doi: 10.1194/jlr.M600020-JLR200.
- Chen MB, McAinch AJ, Macaulay SL, Castelli LA, O’Brien PE, Dixon JB, Cameron-Smith D, Kemp BE, Steinberg GR. Impaired activation of AMP-kinase and fatty acid oxidation by globular adiponectin in cultured human skeletal muscle of obese type 2 diabetics. J Clin Endocrinol Metabolism. 2005;90(6):3665–3672. doi: 10.1210/jc.2004-1980.
- Group DPPR. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;2002(346):393–403.
- Spruss A, Kanuri G, Stahl C, Bischoff SC, Bergheim I. Metformin protects against the development of fructose-induced steatosis in mice: role of the intestinal barrier function. Lab Invest. 2012;92(7):1020–1032. doi: 10.1038/labinvest.2012.75.
- Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y, Ye JM, Lee CH, Oh WK, Kim CT, et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006;55(8):2256–2264. doi: 10.2337/db06-0006.
- Shen L, Xiong Y, Wang DQ, Howles P, Basford JE, Wang J, Xiong YQ, Hui DY, Woods SC, Liu M. Ginsenoside Rb1 reduces fatty liver by activating AMP-activated protein kinase in obese rats. J Lipid Res. 2013;54(5):1430–1438. doi: 10.1194/jlr.M035907.
- Reiter CE, Kim JA, Quon MJ. Green tea polyphenol epigallocatechin gallate reduces endothelin-1 expression and secretion in vascular endothelial cells: roles for AMP-activated protein kinase, Akt, and FOXO1. Endocrinology. 2010;151(1):103–114. doi: 10.1210/en.2009-0997.
- Wang S, Liang X, Yang Q, Fu X, Rogers CJ, Zhu M, Rodgers BD, Jiang Q, Dodson MV, Du M. Resveratrol induces brown-like adipocyte formation in white fat through activation of AMP-activated protein kinase (AMPK) alpha1. Int J Obes (Lond). 2015;39(6):967–976. doi: 10.1038/ijo.2015.23.
- Yang G, Xue Y, Zhang H, Du M, Zhu MJ. Favourable effects of grape seed extract on intestinal epithelial differentiation and barrier function in IL10-deficient mice. Br J Nutr. 2015;114(1):15–23. doi: 10.1017/S0007114515001415.
- Harikumar KB, Aggarwal BB. Resveratrol: a multitargeted agent for age-associated chronic diseases. Cell Cycle. 2008;7(8):1020–1035. doi: 10.4161/cc.7.8.5740.
- Tome-Carneiro J, Gonzalvez M, Larrosa M, Yanez-Gascon MJ, Garcia-Almagro FJ, Ruiz-Ros JA, Garcia-Conesa MT, Tomas-Barberan FA, Espin JC. One-year consumption of a grape nutraceutical containing resveratrol improves the inflammatory and fibrinolytic status of patients in primary prevention of cardiovascular disease. Am J Cardiol. 2012;110(3):356–363. doi: 10.1016/j.amjcard.2012.03.030.
- Robich MP, Osipov RM, Nezafat R, Feng J, Clements RT, Bianchi C, Boodhwani M, Coady MA, Laham RJ, Sellke FW. Resveratrol improves myocardial perfusion in a swine model of hypercholesterolemia and chronic myocardial ischemia. Circulation. 2010;122(11 Suppl):S142–149. doi: 10.1161/CIRCULATIONAHA.109.920132.
- Ong KW, Hsu A, Tan BK. Anti-diabetic and anti-lipidemic effects of chlorogenic acid are mediated by ampk activation. Biochem Pharmacol. 2013;85(9):1341–1351. doi: 10.1016/j.bcp.2013.02.008.
- Hashemzehi M, Behnam-Rassouli R, Hassanian SM, Moradi-Binabaj M, Moradi-Marjaneh R, Rahmani F, Fiuji H, Jamili M, Mirahmadi M, Boromand N, et al. Phytosomal-curcumin antagonizes cell growth and migration, induced by thrombin through AMP-Kinase in breast cancer. J Cell Biochem. 2018. doi: 10.1002/jcb.v119.7.
- Liu K, Mei F, Wang Y, Xiao N, Yang L, Wang Y, Li J, Huang F, Kou J, Liu B, et al. Quercetin oppositely regulates insulin-mediated glucose disposal in skeletal muscle under normal and inflammatory conditions: the dual roles of AMPK activation. Mol Nutr Food Res. 2016;60(3):551–565. doi: 10.1002/mnfr.201500509.