ABSTRACT
Angulin-1/LSR is a tricellular tight junction molecule, that plays an important role in maintaining the epithelial and endothelial barriers. The actin cytoskeleton at tricellular contacts also contributes to the maintenance of the epithelial barrier. Loss of angulin-1/LSR enhances the migration of various cancer cells. Angubindin-1 is a novel binder to angulin-1/LSR and angulin-3. It is a peptide generated from the angulin-1 binding site of Clostridium perfringens iota toxin, which affects the actin cytoskeleton and decreases the epithelial and endothelial barrier functions. However, its regulatory mechanisms are not well understood. To investigate the regulatory mechanisms of the epithelial barrier dysfunction and cell migration induction by angubindin-1, we used human endometrial cancer cell line Sawano, which has high LSR expression and the epithelial barrier function. Angubindin-1 decreased LSR expression and the epithelial barrier function and increased cell migration. It inhibited the recovery of the epithelial barrier function in a Ca-switch model. At tricellular contacts, sinking of the membrane and an increase of actin fibers near the junctions were caused by angubindin-1. It dynamically changed F-actin from lines to dot-like structures at tricellular contacts. Angubindin-1 transiently increased the phosphorylation of cofilin and JNK, which are involved in the regulation of the intracellular actin cytoskeleton. Furthermore, knockdown of JNK and the JNK inhibitor SP600125 prevented the decrease of the epithelial barrier function and the increase of cell migration induced by angubindin-1. These findings suggest that angubindin-1 might reversibly regulate the epithelial barrier and cell migration at tricellular contacts via JNK/cofilin/actin cytoskeleton dynamics.
Introduction
Cell-cell junctions are essential for maintaining the epithelial barrier function of epithelial cells and for maintaining epithelial homeostasis via adherens junctions (AJs), tight junctions (TJs) and the actin cytoskeleton.Citation1 TJs, which consist of claudin-mediated bicellular TJs (bTJs) and tricellular TJs (tTJs), seal the paracellular spaces between adjacent cells and form a selective barrier.Citation2
tTJs form at the convergence of bTJs where three epithelial cells meet in polarized epithelia.Citation3,Citation4 The family of angulins consists of angulin-1 (also termed lipolysis-stimulated lipoprotein receptor [LSR]), angulin-2, (immunoglobulin-like domain-containing receptor 1 [ILDR1]) and angulin-3 (ILDR2).Citation5–Citation7 Angulin-1/LSR recruits tricellulin (TRIC), which was the first tTJ molecule discovered. Angulin-1/LSR has a barrier function,Citation3,Citation5 and is involved in invasion and migration of cancer cells via YAP.Citation8
To maintain the epithelial barrier, the presence of actin, which is one component of the cytoskeleton, is also important, and tricellular contacts are thought to have a specific mechanism in the regulation of the actin skeleton.Citation9,Citation10 Regulation of the epithelial barrier is one of the important functions of the actin cytoskeleton.Citation11,Citation12 Actin filaments are associated with the regulation of assembly and function of AJs and TJs.Citation13–Citation15 Cofilin is a member of the actin-depolymerizing factor (ADF)/cofilin family, which is a group of proteins that directly regulate actin dynamics.Citation16 Dephosphorylation of phosphorylated cofilin activates its direct regulation of actin dynamics.Citation16 It is reported that cofilin mediates tight junction opening by redistributing actin and tight junction proteins.Citation17 It is also reported that the reversible increase in tight junction permeability induced by capsaicin, which is a pungent ingredient found in chili peppers, is mediated via cofilin-actin cytoskeletal dynamics.Citation18
It is known that extracellular Ca2+ is essential for the maintenance of intercellular junctions, including adherens and tight junctions.Citation19,Citation20 Activation of c-Jun N-terminal kinase (JNK) is induced by Ca2+ depletion and mediates osmotic stress-induced tight junction disruption.Citation21 JNK, a stress-activated protein kinase, belongs to the mitogen-activated protein kinase (MAPK) group of serine threonine protein kinases, and its pathway plays crucial roles in cell proliferation, differentiation and apoptosis.Citation22 JNK activation is also essential for disassembly of adherens and tight junctions in human epithelial cells.Citation23 SP600125 is an ATP-competitive JNK inhibitor that enhances epithelial barrier function through differential modulation of claudin expression in human epithelial cells.Citation24,Citation25 JNK downregulates actin-reorganizing Rho-dependent kinase (ROCK) and upregulates F-actin-membrane linker proteins of the ERM family.Citation26
Angubindin-1 is a novel binder to angulin-1 and -3 that was recently developed from Clostridium perfringens iota toxin,Citation27 which is a binary toxin composed of an enzymatic component (Ia) and a receptor binding component (Ib).Citation28 The Ib domain contains 664 amino acids and it is cytotoxic.Citation29 However, a fragment of the Ib domain corresponding to amino acids 421–664, which comprise angubindin-1, modulates tTJs without causing cytotoxicity and increases intestinal absorption of large molecules via a paracellular route by disrupting tricellulin recruitment.Citation27 Therefore, angubindin-1 is a novel paracellular absorption enhancer acting at tTJs.Citation27 It is also reported that angubindin-1 opens the blood-brain barrier in vivo for delivery of antisense oligonucleotides to the central nervous system.Citation30
In this study, we found that angubindin-1 reversibly regulated the epithelial barrier and cell migration at tricellular contacts via JNK/cofilin/actin cytoskeleton dynamics.
Materials and methods
Ethics statement
The protocol for human study was reviewed and approved by the ethics committee of the Sapporo Medical University School of Medicine. All experiments were carried out in accordance with the approved guidelines and the Declaration of Helsinki.
Antibodies and reagents
A rabbit polyclonal anti-tricellulin (TRIC) antibody was obtained from Zymed Laboratories (San Francisco, CA, USA). A rabbit polyclonal anti-lipolysis-stimulated lipoprotein receptor (LSR) antibody was from Novus Biologicals (Littleton, CO, USA). A mouse monoclonal anti-LSR antibody was from Abnova (Taipei, Taiwan). A rabbit polyclonal anti-PAR3 antibody was from Millipore Co. (Bedford, UK). Rabbit polyclonal anti-YAP, anti-phosphorylated YAP (pYAP), anti-JNK, anti-phosphorylated JNK (pJNK), anti-cofiin and anti-phosphorylated cofilin (p-cofilin) antibodies were from Cell Signaling Technology (Danvers, MA, USA). A rabbit polyclonal anti-actin antibody was from Sigma-Aldrich (St. Louis, MO, USA). Alexa 488 (green)-conjugated anti-rabbit IgG and Alexa 594 (red)-conjugated anti-mouse IgG antibodies and Alexa 594 (red)-conjugated Phalloidin were from Molecular Probes, Inc. (Eugene, OR). Capsaicin was obtained from Sigma-Aldrich (St. Louis, MO, USA). The JNK inhibitor (SP600125) was purchased from Calbiochem-Novabiochem Corporation (San Diego, CA). HRP-conjugated polyclonal goat anti-rabbit IgG was from Dako A/S (Glostrup, Denmark). The ECL Western blot system was from GE Healthcare UK, Ltd. (Buckinghamshire, UK).
Preparation of angubindin-1
Angubindin-1 was a kind gift from the Drug Discovery Center, Graduate School of Pharmaceutical Sciences, Osaka University, Osaka, Japan as part of our joint research.Citation27,Citation30 The plasmid encoding angubindin-1 (pGEX-Ib421–664) was expressed as a fusion protein with glutathione S-transferase (GST) in Escherichia coli strain BL21 (DE3). After growth at 37°C and induction with isopropyl β-D-thiogalactopyranoside (Nacalai Tesque, Kyoto, Japan) of a 1000-mL culture, cells were harvested, resuspended in buffer F (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 2 mM CaCl2), and then lysed by sonication. The lysates were applied to Glutathione Sepharose 4B beads (GE Healthcare, Buckinghamshire, UK) and purified GST-tagged angubindin-1 was cleaved with thrombin. After the removal of thrombin using Benzamidine Sepharose 4 Fast Flow (GE Healthcare), the solvent for angubindin-1 was changed to phosphate-buffered saline (PBS) by gel filtration using a PD-10 column (GE Healthcare), and the purified proteins were stored at −80°C until use. The concentration of the purified proteins was determined using a BCA protein assay kit (Thermo Fisher Scientific, Waltham, MA) with bovine serum albumin (BSA) as the standard. Purification of the recombinant proteins was confirmed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). To investigate the distribution of angubindin-1 in the brain, a DyLight 550 antibody labeling kit (Thermo Scientific) was used to label angubindin-1 with DyLight 550.
Cell line culture and treatment
The human endometrial cancer cell line Sawano (RCB1152) was purchased from RIKEN BioResource Center (Tsukuba, Japan). Sawano cells were maintained in minimum essential medium (MEM: Sigma-Aldrich; Merck Millipore) supplemented with 10% dialyzed fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.). The medium contained 100U/ml penicillin, 100 μg/ml streptomycin and 50 μg/ml amphotericin-B. The cells were plated on 35 and 60-mm culture dishes, which were coated with rat tail collagen (500 μg dried tendon/ml in 0.1% acetic acid) and incubated in a humidified 5% CO2 incubator at 37°C. They were treated with 2.5 or 5.0 μg/ml angubindin-1, 10 μM capsaicin or 10 μM JNK inhibitor SP600125.
Ca-switch model
For the calcium-switch model, Sawano cells were cultured in Ca2+-free MEM medium (Sigma-Aldrich) with 10% FBS for 16 h and then the medium was replaced by normal medium containing 1.8 mM Ca2+. Some cells were treated with 2.5 μg/ml angubindin-1 in the calcium-switch.
siRNA experiment
StealthTM Select RNAi against JNK was synthesized by Invitrogen (Carlsbad, CA). The sequences were as follows: siRNA of JNK (sense: 5ʹ- GGGCCTACAGAGAGCTAGTTCTTAT-3ʹ; antisense: 5ʹ- TUTTGTTCUTGCUCUCUGUTGGCCC-3ʹ). At 24 h after plating, the Sawano cells were transfected with 100 nM siRNA of JNK using LipofectamineTM RNAiMAX Reagent (Invitrogen) for 48 h. A scrambled siRNA sequence (BLOCK-iT Alexa Fluor Fluorescent, Invitrogen) was employed as control siRNA. Some cells were transfected with 100 nM siRNA of JNK with or without 2.5 μg/ml angubindin-1.
Western blot analysis
The Sawano cells were scraped from a 35 mm dish containing 300 μl of buffer (1 mM NaHCO3 and 2 mM phenylmethylsulfonyl fluoride), collected in microcentrifuge tubes, and then sonicated for 10s. The protein concentrations of the samples were determined using a BCA protein assay reagent kit (Pierce Chemical Co.; Rockford, IL). Aliquots of 15 μl of protein/lane for each sample were separated by electrophoresis in 5–20% SDS polyacrylamide gels (Wako, Osaka, Japan), and electrophoretically transferred to a nitrocellulose membrane (Immobilon; Millipore Co.; Bedford, UK). The membrane was saturated for 30 min at room temperature with blocking buffer (25 mM Tris, pH 8.0, 125 mM NaCl, 0.1% Tween 20, and 4% skim milk) and incubated with anti-LSR, anti-TRIC, anti-phosphorylated-YAP, anti-YAP, anti-phosphorylated-JNK, anti-JNK, anti-phosphorylated-cofilin, anti-cofilin and anti-actin antibodies (1:1000) at room temperature overnight. Then it was incubated with HRP-conjugated anti-mouse and anti-rabbit IgG antibodies at room temperature for 1 h. The immunoreactive bands were detected using an ECL Western blot system.
Immunocytochemistry
Sawano cells in 35-mm glass-coated wells (Iwaki, Chiba, Japan), were fixed with cold acetone and ethanol (1:1) at – 20°C for 10 min. After rinsing in PBS, the cells were incubated with anti-LSR, anti-TRIC, anti-PAR3 antibodies (1:100) and Alexa 594-phalloidin (1:200) overnight at 4°C. Alexa Fluor 488 (green)-conjugated anti-rabbit IgG and Alexa Fluor 592 (red)-conjugated anti-mouse IgG (Invitrogen) were used as secondary antibodies. The specimens were examined and photographed with an Olympus IX 71 inverted microscope (Olympus Co.; Tokyo, Japan) and a confocal laser scanning microscope (LSM510; Carl Zeiss, Jena, Germany).
Transmission electron microscopy (TEM) analysis
Sawano cells were cultured to confluence in 8 chambers of CultureSlides (FALCON). For transmission electron microscopy (TEM), the cultured cells were fixed in 2.5% glutaraldehyde in PBS overnight at 4°C, followed by post-fixing in 2% osmium tetroxide in the same buffer. Then the cells were dehydrated with a graded ethanol series and embedded in Epon 812. Ultrathin sections were cut on a Sovall Ultramicrotoe MT-5000. The sections were stained with uranyl acetate followed by lead citrate and examined at 80 kV with a transmission electron microscope (H7500; Hitachi, Tokyo, Japan).
Measurement of transepithelial electrical resistance (TEER)
Sawano cells were cultured to confluence in the inner chambers of 12-mm Transwell inserts with 0.4-µm pore-size filters (Corning Life Sciences). TEER was measured using an EVOM voltameter with an ENDOHM-12 (World Precision Instruments, Sarasota, FL) on a heating plate (Fine, Tokyo, Japan) adjusted to 37°C. The values were expressed in standard units of ohms per square centimeter and presented as the mean ± S.D. For calculation, the resistance of blank filters was subtracted from that of filters covered with cells.
Matrigel invasion assay
For the invasion assay, Matrigel (BD Biosciences, Bedford, MA, USA) and Cell Culture Inserts (pore size 8 µm; BD Biosciences) were used. Sawano cells were plated using MEM without FBS onto the upper Matrigel-coated chamber (354480; Corning Incorporated, Corning, NY, USA), and the lower chamber of the Transwell was filled with human fibroblast-conditioned medium containing 10 nM epidermal growth factor (EGF) as the chemoattractant. The cells were then incubated for 36 h. Subsequent to this, the medium was removed, and the Matrigel-coated upper chamber was fixed with 100% methanol for 10 min and stained with Giemsa for 20 min. The areas of invading cells were measured in 1,000,000 µm2 areas by automatic binarization using a microscope imaging system (Olympus Corporation).
Migration assay
After the cells were plated onto 35 mm dishes, they were cultured to confluence. At 24 h we wounded the cell layer using a plastic pipette tip (P100), and measured the length of the wound using a microscope imaging system (Olympus, Tokyo, Japan).
Proliferation assay
Cells were seeded onto 96 well culture plates (Corning, NY, USA). After 24 hours, the absorbance of three wells were measured using a Cell Counting Kit-8 (Wako, Osaka, Japan) according to the manufacturer’s instructions. The absorption at 450 nm was measured using an iMark Microplate Reader (Bio-Rad, Hercules, CA).
Data analysis
Signals were quantified using Scion Image Beta 4.02 Win (Scion Co.; Frederick, MA). Each set of results shown is representative of at least three separate experiments. Results are given as means ± SEM. Differences between groups were tested by ANOVA followed by a post hoc test and an unpaired two-tailed Student’s t test and considered to be significant when p < .05.
Results
Effects of angubindin-1 on the epithelial barrier and proteins of tTJ, YAP and JNK in Sawano cells
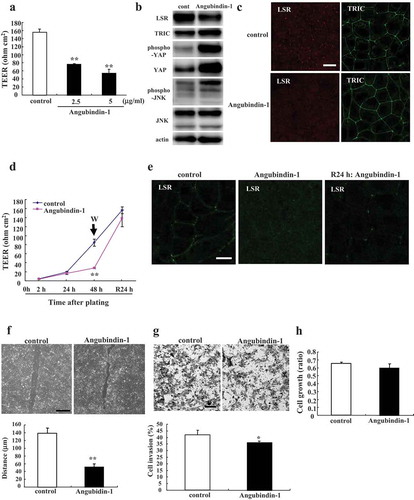
To investigate the effects of angubindin-1 on the epithelial barrier and proteins of tTJ and signaling, cells were treated with 2.5 or 5 μg/ml angubindin-1 and subjected to epithelial barrier analysis, immunocytochemical analysis for LSR and tricellulin, and western blot analysis for LSR, tricellulin, pYAP, YAP, pJNK and JNK. The values of TEER were decreased by treatment with angubindin-1 in a dose-dependent manner ()). Western blot analysis revealed that the treatment with 2.5 μg/ml angubindin-1 decreased LSR expression and increased the expression of pYAP, YAP and pJNK ()). Immunocytochemical analysis showed that the treatment with 2.5 μg/ml angubindin-1 decreased LSR expression at the membranes of tricellular contacts ()). When angubindin-1 was removed from the medium after the treatment with it at 2.5 μg/ml for 48 h, the values of TEER and LSR expression recovered until 24 h after the removal ().
Figure 1. Effects of angubindin-1 on the epithelial barrier, the proteins of tTJ, YAP and JNK, cell migration, invasion and proliferation in Sawano cells.
(a) Bar graph of TEER values representing barrier function in Sawano cells treated with 2.5 μg/ml or 5 μg/ml angubindin-1 for 24 h. **p < .01, vs control. (b) Western blot analysis for LSR, TRIC, pYAP, YAP, pJNK and JNK in Sawano cells treated with 2.5 μg/ml angubindin-1 for 24 h. (c) Immunocytochemical analysis for LSR (red) and TRIC (green) in Sawano cells treated with 2.5 μg/ml angubindin-1 for 24 h. Bar: 5 μm. (d) TEER values representing barrier function of Sawano cells treated with 2.5 μg/ml angubindin-1 from 2 h to 48 h and then after washing out angubindin-1 for 24h. **p < .01, vs control. (e) Immunocytochemical staining for LSR (green) and F-actin (Alexa 594-Phalloidin, red) in Sawano cells treated with 2.5 μg/ml angubindin-1 for 24 h and then after washing out angubindin-1 for 24 h. Bar: 5 μm. (f) Migration assay of Sawano cells treated with 2.5 μg/ml angubindin-1 for 6 h. Bar: 100 μm. **p < .01, vs control. (g) Matrigel invasion assay of Sawano cells treated with 2.5 μg/ml angubindin-1 for 24 h. Bar: 400 μm. *p < .05, vs control. (h) Proliferation assay of Sawano cells treated with 2.5 μg/ml angubindin-1 for 24 h.
Effects of angubindin-1 on cell migration, invasion and proliferation in Sawano cells
To investigate the effects of angubindin-1 on migration, invasion and cell proliferation, cells were treated with it at 2.5 μg/ml and analyzed. Treatment with angubindin-1 significantly increased cell migration, whereas it did not affect cell invasion or proliferation (–h)).
Effects of angubindin-1 on the membrane and actin cytoskeleton at tricellular contacts in Sawano cells
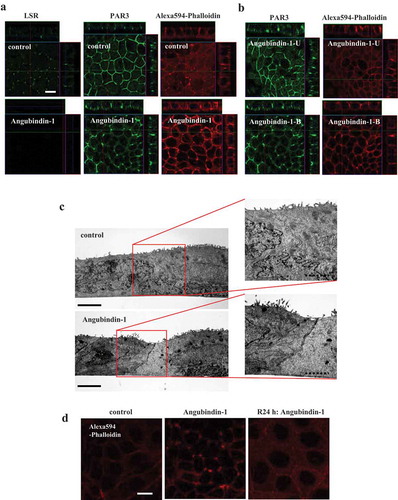
LSR is also a membrane receptor for the binary toxin Clostridium difficile transferase (CDT), which induces the restructuring of the actin cytoskeleton.Citation31 To investigate the effects of angubindin-1 on the membrane and actin cytoskeleton at tricellular contacts, cells were treated with it at 2.5 μg/ml and subjected to immunocytochemical analysis for cell polarity molecule PAR3 and F-actin, and TEM analysis. Immunocytochemical analysis revealed that PAR3 and F-actin dynamics were changed from lines to dot-like structures at tricellular contacts by treatment with angubindin-1 ()). The changes of PAR3 and F-actin were observed on the basal side of tricellular contacts, while PAR3 and F-actin were observed as lines on the apical side of the bicellular contacts ()). In TEM analysis, sinking of the membrane and an increase of actin fibers near the junctions were found to be induced at tricellular contacts by treatment with angubindin-1 ()). When angubindin-1 was removed from the medium after the treatment with it at 2.5 μg/ml for 48 h, the F-actin change recovered at 24 h after the removal ()).
Figure 2. Effects of angubindin-1 on the membrane and actin cytoskeleton at tricellular contacts in Sawano cells.
(a) Immunocytochemical analysis for LSR (green), PAR3 (green) and F-actin (Alexa 594-Phalloidin, red) in Sawano cells treated with or without 2.5 μg/ml angubindin-1 for 24 h. Bars: 5 μm. (b) Immunocytochemical analysis for PAR3 (green) and F-actin (Alexa 594-Phalloidin, red) in Sawano cells treated with 2.5 μg/ml angubindin-1 for 24 h. angubindin-1-L: Basal side. Angubindin-1-U: Apical side. Bars: 5 μm. (c) Transmission electron microscopic (TEM) analysis of Sawano cells treated with or without 2.5 μg/ml angubindin-1 for 24 h. Bars: 5 μm, Dotted bars: 2 μm. (d) Immunocytochemical analysis for F-actin (Alexa 594-Phalloidin, red) in Sawano cells at 24 h after washing out angubindin-1 after treatment with 2.5 μg/ml angubindin-1 for 24 h. Bar: 5 μm.
Effects of angubindin-1 and capsaicin on cofilin and JNK in Sawano cells
Cofilin mediates tight junction opening by redistributing actin and tight junction proteins.Citation17 Capsaicin decreases the epithelial barrier and the level of F-actin at bicellular junctions but increases it at tricellular junctions with its concentration on the apical side of the lateral membrane via cofilin-actin cytoskeletal dynamics.Citation18
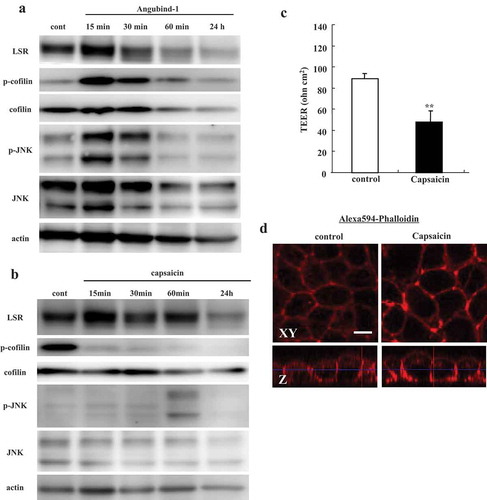
To investigate whether angubindin-1 affected cofilin like capsaicin, cells were treated with 2.5 μg/ml angubindin-1 or 10 μM capsaicin for 15 min, 30 min, 60 min and 24 h and subjected to western blot analysis, barrier function analysis and immunocytochemical analysis. Western blotting showed that angubindin-1 transiently increased the expression of phosphorylated cofilin and phosphorylated JNK from 15 to 30 min after treatment, and decreased the expression of cofilin from 60 min after treatment ()). Capsaicin decreased the expression of phosphorylated cofilin from 15 min after treatment, and increased the expression of phosphorylated JNK from 60 min after treatment ()). The values of TEER were decreased by treatment with capsaicin ()). Immunocytochemical analysis revealed that F-actin was concentrated in a dot-like manner at tricellular contacts by treatment with capsaicin ()).
Figure 3. Effects of angubindin-1 and capsaicin on cofilin and JNK in Sawano cells.
(a) Western blot analysis for LSR, p-cofilin, cofilin, pJNK and JNK in Sawano cells treated with 2.5 μg/ml angubindin-1 for 24 h. (b) Western blot analysis for LSR, p-cofilin, cofilin, pJNK and JNK in Sawano cells treated with 10 μM capsaicin for 24 h. (c) Bar graph TEER values representing barrier function of Sawano cells treated with 10 μM capsaicin for 24 h. **p < .01, vs control. (d) Immunocytochemical analysis for F-actin (Alexa 594-Phalloidin, red) in Sawano cells treated with 10 μM capsaicin for 24 h. Bar: 5 μm.
Effects of angubindin-1 on Ca-switch model of Sawano cells
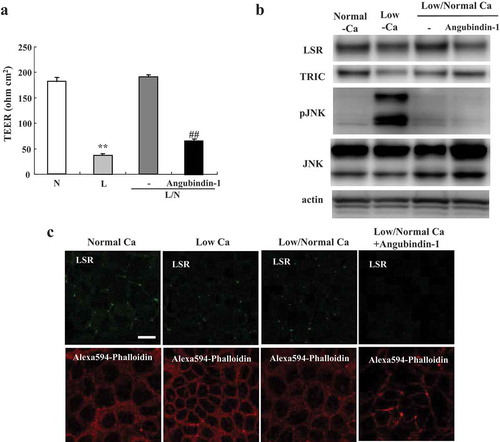
Activation of JNK is induced by Ca2+ depletion and mediates osmotic stress-induced tight junction disruption.Citation21,Citation25 To investigate whether angubindin-1 affected the epithelial barrier via JNK, Sawano cells were treated in a Ca2+ switch model and subjected to barrier function analysis, western blot analysis and immunocytochemical analysis. In non-treated cells, the values of TEER were decreased in Ca2+-free medium for 16 h and then recovered at 8 h after replacement by normal Ca2+ medium ()). After treatment with 2.5 μg/ml angubindin-1, the values of TEER were not recovered by replacement by normal Ca2+ medium ()). In western blot analysis, the expression of phosphorylated JNK was found to be increased in Ca2+-free medium for 16 h and then its expression was recovered at 8 h after replacement by normal Ca2+ medium, whereas treatment with angubindin-1 did not affect the expression of phosphorylated JNK in the Ca-switch model ()). In immunocytochemical analysis, LSR decreased and F-actin was concentrated dot-like at tricellular contacts by treatment with angubindin-1 in the Ca-switch model ()).
Figure 4. Effects of angubindin-1 in Ca-switch model of Sawano cells.
(a) Bar graph of TEER values representing barrier function of Sawano cells treated with or without 2.5 μg/ml angubindin-1 in a Ca-switch model. L: Low Ca2+. N: Normal Ca2+. L/N: Low Ca2+/Normal Ca2+. **p < .01, vs normal. ##p < .01, vs non-treatment. (b) Western blot analysis for LSR, TRIC, pJNK and JNK in Sawano cells treated with or without 2.5 μg/ml angubindin-1 in a Ca-switch model. (c) Immunocytochemical staining for LSR (green) and F-actin (Alexa 594-Phalloidin, red) in Sawano cells treated with and without 2.5 μg/ml angubindin-1 in Ca-switch model. Bar: 5 μm.
JNK inhibitor SP600125 prevented effects of angubindin-1 in Sawano cells
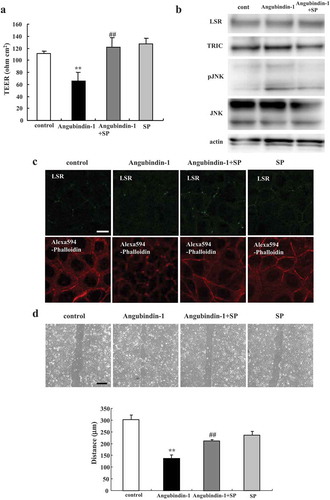
To investigate whether a JNK inhibitor could prevent the effects of angubindin-1, Sawano cells were pretreated with 10 μM JNK inhibitor SP600125 with or without 2.5 μg/ml angubindin-1 and subjected to barrier function analysis, western blot analysis, immunocytochemical analysis and migration assay. The decrease of TEER values caused by treatment with angubindin-1 was inhibited by the treatment with SP600125 ()). Western blot analysis revealed that SP600125 prevented the slight increase of the expression of phosphorylated JNK induced by treatment with angubindin-1 ()). Immunocytochemical analysis showed that SP600125 prevented the change of F-actin to a dot-like pattern at tricellular contacts induced by treatment with angubindin-1 ()). In the migration assay, SP600125 was found to inhibit the induction of cell migration caused by treatment with angubindin-1 ()).
Figure 5. JNK inhibitor SP600125 prevents effects of angubindin-1 in Sawano cells (a) Bar graph of TEER values representing barrier function of Sawano cells pretreated with 10 μM JNK inhibitor SP600125 with or without 2.5 μg/ml angubindin-1. **p < .01, vs control. ##p < .01, vs angubindin-1. (b) Western blot analysis for LSR, TRIC, pJNK and JNK in Sawano cells pretreated with 10 μM SP600125 with or without 2.5 μg/ml angubindin-1. (c) Immunocytochemical analysis for LSR (green) and F-actin (Alexa 594-Phalloidin, red) in Sawano cells treated with 10 μM SP600125 with or without 2.5 μg/ml angubindin-1. Bar: 5 μm. (d) Migration assay of Sawano cells pretreated with 10 μM SP600125 with or without 2.5 μg/ml angubindin-1. Bars: 100 μm. **p < .01, vs control. ##p < .01, vs angubindin-1.
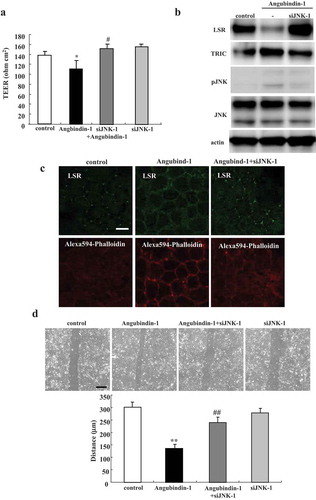
Knockdown of JNK prevented effects of angubindin-1 in Sawano cells
Furthermore, to investigate the relation between angubindin-1 and JNK, Sawano cells were pretreated with transfection of siRNA of JNK before treatment with or without angubindin-1 and subjected to barrier function analysis, western blot analysis, immunocytochemical analysis and migration assay. The decrease of TEER values induced by treatment with angubindin-1 was inhibited by knockdown of JNK ()). Western blot analysis revealed that knockdown of JNK prevented the slight increase of phosphorylated JNK induced by treatment with angubindin-1 ()). Immunocytochemical analysis showed that knockdown of JNK prevented the change of F-actin to a dot-like structure at tricellular contacts induced by treatment with angubindin-1 ()). In the migration assay, knockdown of JNK was found to inhibit the induction of cell migration caused by treatment with angubindin-1 ()).
Figure 6. Knockdown of JNK prevents effects of angubindin-1 in Sawano cells.
(a) Bar graph of TEER values representing barrier function of Sawano cells pretreated with transfection of siRNA of JNK before with or without 2.5 μg/ml angubindin-1. *p < .05, vs control. #p < .05, vs angubindin-1. (b) Western blot analysis for LSR, TRIC, pJNK and JNK in Sawano cells pretreated with transfection of siRNA of JNK with or without 2.5 μg/ml angubindin-1. (c) Immunocytochemical analysis for LSR (green) and F-actin (Alexa 594-Phalloidin, red) in Sawano cells pretreated with transfection of siRNA of JNK with or without 2.5 μg/ml angubindin-1. Bar: 5 μm. (d) Migration assay of Sawano cells pretreated with transfection of siRNA of JNK with or without 2.5 μg/ml angubindin-1. Bar: 100 μm. **p < .01, vs control. ##p < .01, vs angubindin-1.
Discussion
In this study, we found that a novel binder to angulin-1/LSR, angubindin-1, regulated the epithelial barrier and cell migration via JNK/cofilin/actin cytoskeleton dynamics at tricellular contacts.
Angubindin-1 enhances the absorption of macromolecular substances in vitro and in vivo via the tTJ and reversibly decreases blood-brain-barrier (BBB) integrity in vitro.Citation27,Citation30 It also decreases LSR expression and the epithelial barrier, and they are recovered after its removal.Citation30 In the present study, angubindin-1 decreased LSR expression and the epithelial barrier function in human endometrial cancer cell line Sawano. Furthermore, it dynamically changed F-actin from lines to dot-like structures at tricellular contacts and sinking of the membrane and an increase of actin fibers near the junctions were observed at tricellular contacts. Angubindin-1 affected not only LSR expression but also cell shape via F-actin at tricellular contacts.
Cofilin mediates tight junction opening by redistributing actin and tight junction proteins.Citation17 On the other hand, capsaicin decreases the epithelial barrier and the level of F-actin at bicellular junctions but increases them at tricellular junctions with its concentration on the apical side of the lateral membrane via cofilin-actin cytoskeletal dynamics.Citation18 In the present study, angubindin-1 increased the expression of phosphorylated cofilin with the decrease of the epithelial barrier function in Sawano cells. Capsaicin decreased the expression of phosphorylated cofilin and the epithelial barrier function. F-actin was concentrated as dot-like structures at tricellular contacts by capsaicin. These results suggested that angubindin-1 regulated the epithelial barrier function via not only the decrease of LSR but also cofilin-actin dynamics at tricellular contacts.
On the other hand, angubindin-1 also increased the expression of phosphorylated c-Jun N-terminal kinase (JNK) in Sawano cells. Knockdown of JNK and JNK inhibitor SP600125 prevented the decrease of the epithelial barrier function by angubindin-1. The JNK pathway regulates gene expression, regeneration, apoptosis and metabolic adaptation in response to both extrinsic and intrinsic stressors.Citation32 JNK activation is essential for disassembly of adherens and tight junctions in human epithelial cells.Citation23 It is also largely involved in the regulation of tricellular tight junctions via tricellulin in human pancreatic duct epithelial cells.Citation33 The activities of JNK1 and JNK2 are required for the exclusive localization of LSR at tTJs.Citation34 The inhibition of JNK activity promotes the formation of adherens junctions in low-Ca2+ medium.Citation35 JNK inhibitor SP600125 enhances the barrier function of human pancreatic cancer cell line HPAC in a Ca-switch model.Citation36 Our findings suggested that Angubindin-1 regulated the expression of LSR and the epithelial barrier via the JNK pathway.
Angubindin-1 increased cell migration with the decrease of LSR in Sawano cells. Furthermore, knockdown of JNK and JNK inhibitor SP600125 prevented the increase of cell migration by angubindin-1. We previously reported that LSR was colocalized with TRIC, AMOT and Merlin at tricellular contacts and that the loss of LSR promoted cell invasion and migration via upregulation of AREG dependent on YAP/pYAP and AMOT/Merlin in Sawano cells.Citation8 Hippo signaling promotes JNK-dependent cell migration.Citation37 It is possible that angubindin-1 may affect cell migration via the Hippo/YAP/JNK signaling pathway.
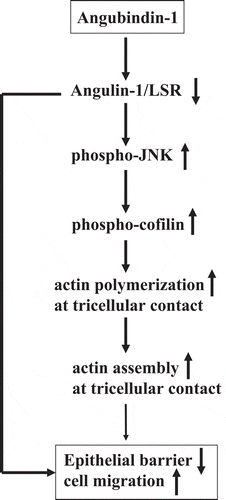
In conclusion, we suggest that a ligand of angulin-1/LSR angubindin-1, reversibly regulates the epithelial barrier and cell migration via JNK/cofilin/actin cytoskeleton dynamics (). Our findings may lead to the development of new drug delivery systems for normal and cancer cells, although further study of angubindin-1 in the regulation of the epithelial barrier and cell migration is necessary.
Figure 7. Overview in the regulation of epithelial barrier and cell migration via JNK/cofilin/actin by angubindin-1.
(1) Angubindin-1 downregulates angulin-1/LSR. (2) Downregulation of angulin-1/LSR induces cell migration and activation of phospho-JNK and phospho-cofilin and decreases epithelial barrier. (3) Activation of phospho-cofilin induces actin polymerization at tricellular contacts. (4) Induction of actin polymerization at tricellular contacts may induce cell migration and decrease epithelial barrier.
Authors contributions statements
T. Konno, T Kohno, K.T. and T. Kojima designed and coordinated the study and wrote the main manuscript text. T. Konno, S.K. and H.S. analyzed the data. M. Kondoh, S.S. and T.S. contributed reagents/materials. All authors reviewed the manuscript.
Conflict of interest
The authors declare no competing financial interests.
Additional information
Funding
References
- Campbell HK, Maiers JL, DeMali KA. Interplay between tight junctions & adherens junctions. Exp Cell Res. 2017:358:39–44. PMID: 28372972. doi:10.1016/j.yexcr.2017.03.061
- Guillot C, Lecuit T. Mechanics of epithelial tissue homeostasis and morphogenesis. Science. 2013;340:1–15. PMID: 23744939. doi:10.1126/science.1235249.
- Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S, Tsukita S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol. 2005;171:939–945. PMID: 1636516. doi:10.1083/jcb.200510043.
- Furuse M, Izumi Y, Oda Y, Higashi T, Iwamoto N. Molecular organization of tricellular tight junctions. Tissue Barriers. 2014;2:e28960. PMID: 25097825. doi:10.4161/tisb.28960.
- Masuda S, Oda Y, Sasaki H, Ikenouchi J, Higashi T, Akashi M, Nishi E, Furuse M. LSR defines cell corners for tricellular tight junction formation in epithelial cells. J Cell Sci. 2011;124:548–555. PMID: 21245199. doi:10.1242/jcs.072058.
- Furuse M, Oda Y, Higashi T, Iwamoto N, Masuda S. Lipolysis-stimulated lipoprotein receptor: a novel membrane protein of tricellular tight junctions. Ann N Y Acad Sci. 2012;1257:54–58. PMID: 22671589. doi:10.1111/j.1749-6632.2012.06486.x.
- Higashi T, Tokuda S, Kitajiri S, Masuda S, Nakamura H, Oda Y, Furuse M. Analysis of the ‘angulin’ proteins LSR, ILDR1 and ILDR2–tricellulin recruitment, epithelial barrier function and implication in deafness pathogenesis. J Cell Sci. 2013;126:966–977. PMID: 23239027. doi:10.1242/jcs.116442.
- Shimada H, Abe S, Kohno T, Satohisa S, Konno T, Takahashi S, Hatakeyama T, Arimoto C, Kakuki T, Kaneko Y, et al. Loss of tricellular tight junction protein LSR promotes cell invasion and migration via upregulation of TEAD1/AREG in human endometrial cancer. Sci Rep. 2017;7:37049. PMID: 28071680. doi:10.1038/srep37049.
- Turner JR. ‘Putting the squeeze’ on the tight junction: understanding cytoskeletal regulation. Semin Cell Dev Biol. 2000;11:301–308. PMID: 10966864. doi:10.1006/scdb.2000.0180.
- Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. PMID: 11283726. doi:10.1038/35067088.
- Ivanov AI. Actin motors that drive formation and disassembly of epithelial apical junctions. Front Biosci. 2008;13:6662e6681. PMID: 18508686.
- Rodgers LS, Fanning AS. Regulation of epithelial permeability by the actin cytoskeleton. Cytoskeleton (Hoboken). 2011;68:653–660. PMID: 22083950. doi:10.1002/cm.20547.
- Mege RM, Gavard J, Lambert M. Regulation of cell-cell junctions by the cytoskeleton. Curr Opin Cell Biol. 2006;18:541–548. PMID: 16905303. doi:10.1016/j.ceb.2006.08.004.
- Chhabra ES, Higgs HN. The many faces of actin: matching assembly factors with cellular structures. Nat Cell Biol. 2007;9:1110–1121. PMID: 17909522. doi:10.1038/ncb1007–1110.
- Pollard TD, Blanchoin L, Mullins RD. Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu Rev Biophys Biomol Struct. 2000;29:545–576. PMID: 10940259. doi:10.1146/annurev.biophys.29.1.545.
- Bamburg JR. Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu Rev Cell Dev Biol. 1999;15:185–230. PMID: 10611961. doi:10.1146/annurev.cellbio.15.1.185.
- Nagumo Y, Han J, Bellila A, Isoda H, Tanaka T. Cofilin mediates tight-junction opening by redistributing actin and tight-junction proteins. Biochem Biophys Res Commun. 2008;377:921–925. PMID: 18952063. doi:10.1016/j.bbrc.2008.10.071.
- Shiobara T, Usui T, Han J, Isoda H, Nagumo Y. The reversible increase in tight junction permeability induced by capsaicin is mediated via cofilin-actin cytoskeletal dynamics and decreased level of occludin. PLoS One. 2013;8:e79954. PMID: 24260326. doi:10.1371/journal.pone.0079954.
- Volberg T, Geiger B, Kartenbeck J, Franke WW. Changes in membrane-microfilament interaction in intercellular adherens junctions upon removal of extracellular Ca2+ ions. J Cell Biol. 1986;102:1832–1842. PMID: 3084500. doi:10.1083/jcb.102.5.1832.
- Chen YH, Lu Q, Goodenough DA, Jeansonne B. Nonreceptor tyrosine kinase c-Yes interacts with occludin during tight junction formation in canine kidney epithelial cells. Mol Biol Cell. 2002;13:1227–1237. PMID: 11950934. doi:10.1091/mbc.01–08–0423.
- Samak G, Suzuki T, Bhargava A, Rao RK. c-Jun NH2-terminal kinase-2 mediates osmotic stress-induced tight junction disruption in the intestinal epithelium. Am J Physiol Gastrointest Liver Physiol. 2010;299:G572–84. PMID: 20595622. doi:10.1152/ajpgi.00265.2010.
- Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007;19:142–149. PMID: 17303404. doi:10.1016/j.ceb.2007.02.001.
- You H, Lei P, Andreadis ST. JNK is a novel regulator of intercellular adhesion. Tissue Barriers. 2013;1:e26845. PMID: 24868495. doi:10.4161/tisb.26845.
- Carrozzino F, Pugnale P, Féraille E, Montesano R. Inhibition of basal p38 or JNK activity enhances epithelial barrier function through differential modulation of claudin expression. Am J Physiol Cell Physiol. 2009;297:C775–87. PMID: 19605737. doi:10.1152/ajpcell.00084.2009.
- Kojima T, Yamaguchi H, Ito T, Kyuno D, Kono T, Konno T, Sawada N. Tight junctions in human pancreatic duct epithelial cells. Tissue Barriers. 2013;1:e24894. PMID: 24665406. doi:10.4161/tisb.24894.
- Naydenov NG, Hopkins AM, Ivanov AI. c-Jun N-terminal kinase mediates disassembly of apical junctions in model intestinal epithelia. Cell Cycle. 2009;8:2110–2121. PMID: 19502798. doi:10.4161/cc.8.13.8928.
- Krug SM, Hayaishi T, Iguchi D, Watari TA, Fromm M, Nagahama M, Takeda H, Okada Y, Sawasaki T, Doi T, et al. Angubindin-1, a novel paracellular absorption enhancer acting at the tricellular tight junction. J Control Release. 2017;260:1–11. PMID: 28528740. doi:10.1016/j.jconrel.2017.05.024.
- Nagahama M, Yamaguchi A, Hagiyama T, Ohkubo N, Kobayashi K, Sakurai J. Binding and internalization of Clostridium perfringens iota-toxin in lipid rafts. Infect Immun. 2004;72:3267–3275. PMID: 15155629. doi:10.1128/IAI.72.6.3267–3275.2004.
- Nagahama M, Umezaki M, Oda M, Kobayashi K, Tone S, Suda T, Ishidoh K, Sakurai J, Bliska JB. Clostridium perfringens iota-toxin b induces rapid cell necrosis. Infect Immun. 2011;79:4353–4360. PMID: 21911469. doi:10.1128/IAI.05677–11.
- Zeniya S, Kuwahara H, Daizo K, Watari A, Kondoh M, Yoshida-Tanaka K, Kaburagi H, Asada K, Nagata T, Nagahama M, et al. Angubindin-1 opens the blood-brain barrier in vivo for delivery of antisense oligonucleotide to the central nervous system. J Control Release. 2018;283:126–134. PMID: 29753959. doi:10.1016/j.jconrel.2018.05.010.
- Papatheodorou P, Carette JE, Bell GW, Schwan C, Guttenberg G, Brummelkamp TR, Aktories K. Lipolysis-stimulated lipoprotein receptor (LSR) is the host receptor for the binary toxin Clostridium difficile transferase (CDT). Proc Natl Acad Sci U S A. 2011;108:16422–16427. PMID: 21930894. doi:10.1073/pnas.1109772108.
- Biteau B, Karpac J, Hwangbo D, Jasper H. Regulation of Drosophila lifespan by JNK signaling. Exp Gerontol. 2011;46:349–354. PMID: 21111799. doi:10.1016/j.exger.2010.11.003.
- Kojima T, Fuchimoto J, Yamaguchi H, Ito T, Takasawa A, Ninomiya T, Kikuchi S, Ogasawara N, Ohkuni T, Masaki T, et al. c-Jun N-terminal kinase is largely involved in the regulation of tricellular tight junctions via tricellulin in human pancreatic duct epithelial cells. J Cell Physiol. 2010;225:720–733. PMID: 20533305. doi:10.1002/jcp.22273.
- Nakatsu D, Kano F, Taguchi Y, Sugawara T, Nishizono T, Nishikawa K, Oda Y, Furuse M, Murata M. JNK1/2-dependent phosphorylation of angulin-1/LSR is required for the exclusive localization of angulin-1/LSR and tricellulin at tricellular contacts in EpH4 epithelial sheet. Genes Cells. 2014;19:565–581. PMID: 24889144. doi:10.1111/gtc.12158.
- Lee MH, Koria P, Qu J, Andreadis ST. JNK phosphorylates beta-catenin and regulates adherens junctions. Faseb J. 2009;23:3874–3883. PMID: 19667122. doi:10.1096/fj.08–117804.
- Konno T, Ninomiya T, Kohno T, Kikuchi S, Sawada N, Kojima T. c-Jun N-terminal kinase inhibitor SP600125 enhances barrier function and elongation of human pancreatic cancer cell line HPAC in a Ca-switch model. Histochem Cell Biol. 2015;143:471–479. PMID: 25511417. doi:10.1007/s00418-014-1300-4.
- Ma X, Wang H, Ji J, Xu W, Sun Y, Li W, Zhang X, Chen J, Xue L. Hippo signaling promotes JNK-dependent cell migration. Proc Natl Acad Sci U S A. 2017;114:1934–1939. PMID: 28174264. doi:10.1073/pnas.1621359114.
