ABSTRACT
The pig is a powerful model for intestinal barrier studies, and it is important to carefully plan animal care and handling for optimal study design as psychological and physiological stressors significantly impact intestinal mucosal barrier function. Here, we report the effects of a period of environmental acclimation versus acute transport stress on mucosal barrier repair after intestinal ischemic injury. Jejunal ischemia was induced in young pigs which had been allowed to acclimate to a biomedical research housing environment or had been transported immediately prior to experimental injury (non-acclimated). Mucosa was then incubated ex vivo on Ussing chambers. In uninjured mucosa, there was no difference in transepithelial electrical resistance (TEER) or epithelial integrity between groups. However, acclimated pigs had increased macromolecular flux as compared to non-acclimated pigs during the first hour of ex vivo incubation. Ischemia induced greater epithelial loss in non-acclimated pigs as compared to acclimated pigs, yet this group achieved greater wound healing during recovery. Non-acclimated pigs had more robust TEER recovery ex vivo following injury versus acclimated pigs. The expression pattern of the tight junction protein claudin-4 was disrupted in acclimated pigs following recovery but showed enhanced localization to the apical membrane in non-acclimated pigs following recovery. Acute transport stress increases mucosal susceptibility to epithelial loss but also primes the tissue for a more robust barrier repair response. Alternatively, environmental acclimation increases leak pathway and diminishes barrier repair responses after ischemic injury.
Introduction
The pig is a powerful model for intestinal barrier studies, and it is important to carefully plan animal care and handling for optimal study design as psychological and physiological stressors significantly impact mucosal barrier function. The mucosal surface of the intestine is lined by a single layer of columnar epithelial cells, which form the principal physiologic barrier against luminal bacteria and bacterial products while simultaneously facilitating selective absorption of electrolytes, water and nutrients. Due to the high energetic demand of these epithelial cells, loss of perfusion to the intestine during certain disease processes causes rapid breakdown of the intestinal epithelial barrier and onset of sepsis within a short period of time.Citation1 Fortunately, efficient epithelial restitution following ischemia and mucosal epithelial loss is a response to injury, and this restoration of the epithelial barrier and their tight junctions is critical to supporting patient survival and recovery following an ischemic event. To better understand the mechanisms which drive efficient subacute mucosal repair following ischemia in order to improve support of patients experiencing ischemic intestinal disease, our lab uses a porcine model. The pig shares many fundamental anatomical, physiological, and nutritional similarities with humans, and therefore provides a powerful translational model of human digestive biology and disease, including ischemia.Citation2–10 In our juvenile pig model, we have observed rapid repair of ischemia-injured mucosa in the small intestine, which initially involves contraction of denuded villi, followed by rapid epithelial cell migration across the denuded basement membrane (restitution), and finally re-assembly of tight junctions, resulting in swift recovery of intestinal barrier function and protection against sepsis.Citation1,Citation10
Like humans, pigs experience gastrointestinal derangements in response to physiologic and pathophysiologic stress, which includes notable effects on intestinal barrier function.Citation11,Citation12 Pig models of intestinal stress, such as early weaning stress and heat stress due to physical and social pressures, induce marked impairment of intestinal barrier function and closely mimic the effects of acute stress on the human gastrointestinal tract.Citation13–15 It is proposed that the pig is an excellent model of the effects of stress on the gut due to advanced gyrencephalic neuroanatomy in both human beings and pigs.Citation16 This may mean that pigs have similar higher centers related to interpretation of social and physical stress as humans.Citation17 While these striking similarities make for a very powerful model for the study of gastrointestinal physiology and disease, these sensitivities to stress mean that intestinal barrier studies utilizing pigs must be carefully planned to minimize unintended effects of animal transport and handling stresses on experimental outcomes. In the context of utilizing pigs as a lab animal model, the effects of varied pig handling, housing and transportation protocols on intestinal barrier function and response to injury have not been previously described. Here, we report the effects of a period of environmental acclimation versus acute transport stress on mucosal barrier function and repair after intestinal ischemic injury.
Materials and methods
Animals
All procedures were approved by NC State University Institutional Animal Care and Use Committee. Yorkshire cross pigs 8–10-weeks-of-age of either sex were either transported and allowed to acclimate to the NC State Lab Animal Resources biomedical research housing environment for 3 d prior to experimental initiation (acclimated) or were transported by passenger vehicle for approximately 30 min duration immediately prior to experiment initiation (non-acclimated).
Experimental surgery
Pigs were sedated using xylazine (1.5 mg/kg) and ketamine (11 mg/kg). Anesthesia was induced with isoflurane vaporized in 100% oxygen via face mask, after which pigs were orotracheally intubated for continued delivery of isoflurane to maintain general anesthesia. Pigs were placed on a water-circulated heating pad and intravenous fluids were administered at a maintenance rate of 15 ml・kg−1・h−1 throughout the surgery. The distal jejunum was accessed via midline or paralumbar incision and 8–10 cm loops were ligated in segments and subjected to 30 min of ischemia via ligation of local mesenteric blood vessels with 2–0 braided silk suture. Adjacent loops not subjected to ischemia were used as control tissue. At the time of tissue harvest, pigs were euthanized with an overdose of pentobarbital. Intestinal loops were excised and opened longitudinally along the antimesenteric border and placed in cold, oxygenated Ringer solution.
Ussing chamber studies
The outer seromuscular layers were removed by blunt dissection in cool, oxygenated Ringer solution. Jejunal mucosa was mounted in 1.12 cm2 aperture Ussing chambers. The tissues were bathed in 10 ml warmed, oxygenated (95% O2/5% CO2) Ringer solution on the serosal and mucosal sides. Serosal Ringer solution also contained 10 mM glucose while the mucosal Ringers solution was osmotically balanced with 10 mM mannitol. Bathing solutions were circulated in water-jacketed reservoirs and maintained at 37°C. The spontaneous potential difference (PD) was measured using Ringer-agar bridges connected to calomel electrodes, and the PD was short-circuited through Ag-AgCl electrodes with a voltage clamp that corrected for fluid resistance. Resistance (Ω・cm2) was calculated from spontaneous PD and short-circuit current (Isc). If the spontaneous PD was between −1 and 1 mV, the tissues were current-clamped at ± 100 µA and the PD re-recorded. Isc and PD were recorded every 15 min for 120 min. From these measurements, TEER was calculated.
Isotopic mannitol flux studies
All fluxes were conducted under short-circuit conditions (tissue clamped to 0 mV). 3H-mannitol (0.2 µCi/ml diluted in 10 mM mannitol) was placed on the mucosal side of tissues. Two 60-min fluxes from 0 to 60 min and from 60 to 120 min of the experimental recovery period by taking samples from the opposite side of that of isotope addition and counted for 3H β-emission in a scintillation counter. Mucosal-to-serosal fluxes (Jms) of mannitol were calculated using standard equations.Citation18
Light microscopy and histomorphometry
Tissues were fixed for 18 hours in 10% formalin at room temperature immediately following ischemic injury or after 120-min ex vivo recovery period. Formalin-fixed tissues were transferred to 70% ethanol and then paraffin-embedded, sectioned (5 µm) and stained with hematoxylin and eosin for morphological and morphometric analyses. For morphometric analysis of villus injury, the base of the villus was defined as the opening of the neck of the crypts and height of epithelialization, total height and width of villus were measured using NIH Image J® Software. The surface area of the villus was calculated as previously described using the formula for the surface area of a cylinder modified by subtracting the base of the cylinder and adding a factor that accounted for the hemispherical shape of the villus tip.Citation18 The percentage of villus epithelialization was used as an index of epithelial injury and restitution.
Immunofluorescence histology
Paraffin sections were deparaffinized and washed 3 times in PBS to rehydrate the tissue, were treated for antigen retrieval by in a decloaking chamber for 30 seconds at 120ºC followed by 90ºC for 10 seconds in a reveal citrate decloaker solution (Biocare Medical, Concord, CA, USA). Tissues were cooled for 20 min at room temperature then placed in PBS-0.3% Triton −100 solution for 20 min to permeabilize the tissues. Tissues were washed twice in PBS and incubated in blocking solution (Dako, Carpinteria, CA, USA) for 1 h at room temperature. To assess the tight junctions, tissues were incubated in Mouse anti-Claudin-4 antibody (Invitrogen, Catalog #329400) at a dilution of 1:100 in antibody diluent (Dako, Carpinteria, CA, USA) overnight at 4ºC. Tissues were placed in goat anti-mouse IgG conjugated to Alexa Fluor® 488 (Invitrogen catalog #A11029) at a dilution of 1:500 in antibody diluent for 1 h at room temperature. Tissues were counterstained with nuclear stain 4ʹ,6-Diamidino-2-Phenylindol (DAPI, Invitrogen, catalog #D1306) diluted 1:1000 in antibody diluent for 5 minutes at room temperature. Images were captured using an inverted fluorescence microscope (Olympus IX81, Tokyo, Japan) with a digital camera (ORCA-flash 4.0, Hamamatsu, Japan) using 10X objective lens with numerical aperture of 0.3 (LUC Plan FLN, Olympus, Tokyo, Japan). Specificity of primary antibodies and lack of nonspecific secondary antibody binding were confirmed by secondary only negative controls.
Statistical analysis
All data were analyzed using Prism® (GraphPad®; La Jolla, California, USA) statistical software. Data were reported as means ± SEM for a given number (n) of animals for each experiment. Results were analyzed by unpaired Mann–Whitney test for nonparametric data, two-way ANOVA (or mixed-model on datasets with missing data points) or two-way ANOVA on repeated measures. For analyses where significance was detected by ANOVA, Sidak’s test was utilized for post hoc pairwise multiple comparisons. The ⍺-level for statistical significance was set at P < .05.
Results
Environmental acclimation increases macromolecular permeability in jejunal mucosa
At baseline, there was no difference in mucosal transepithelial electrical resistance (TEER) whether or not the pigs were acclimated or used immediately following transportation (, P = .8801). However, two-way ANOVA of mucosal-to-serosal 3H-mannitol flux in control tissues identified an effect of acclimation on macromolecular flux (, P = .0452). Post hoc analysis revealed acclimation induced increased flux during the first hour of ex vivo incubation as compared to non-acclimated pigs (P = .0186). This difference between groups was abrogated by the second hour of ex vivo recovery due to trends toward decreasing flux in the acclimated pigs and increasing flux in the non-acclimated pigs. These changes in flux from the first hour to the second hour were not statistically significant within groups (P = .275 within acclimated pigs and P = .608 within non-acclimated pigs). Histology showed similar microscopic appearance to the normal intestinal mucosal architecture between the two groups (). When quantified by histomorphometry, these data confirmed that there was no difference in the integrity of the mucosal epithelium between groups (, P = .5686).
Figure 1. Effects of animal handling practices on baseline barrier function in uninjured small intestinal mucosa
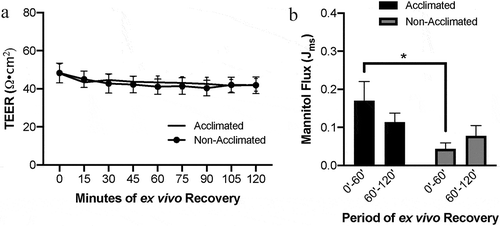
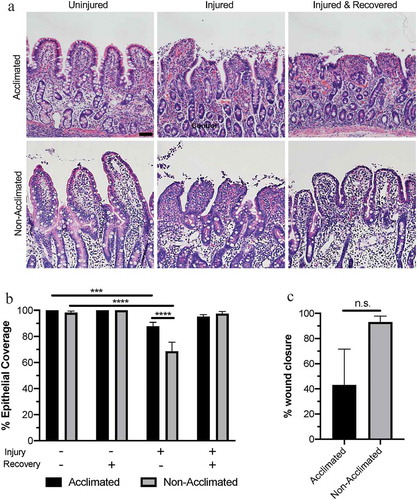
Figure 2. Combined effects of animal handing practices and acute ischemic injury on microscopic mucosal integrity in the small intestine
Environmental acclimation protects against epithelial loss during ischemic injury but impairs restitution during the recovery phase
While there was no difference in epithelial coverage in the absence of ischemic intestinal injury, 30 min of experimental ischemia induced greater epithelial injury in non-acclimated pigs as compared to acclimated pigs (). Two-way ANOVA revealed significant effects of both acclimation status (P = .0126) and experimental ischemia (P < .0001) on measured villus epithelialization, as well as an interaction between these two variables (P = .0003). In the mucosa of non-acclimated pigs transported the day of experimental injury, 68.5 ± 6.03% epithelial coverage (corresponding to 31.5 ± 6.03% epithelial loss) was measured after acute ischemic injury. However, in the mucosa of pigs acclimated to their environment prior to experimental injury, 87.8 ± 3.01% epithelial coverage (corresponding to just 12.2 ± 3.01% epithelial loss) was measured after acute ischemia. Post hoc analyses showed that these values are significantly lower than uninjured tissue epithelialization within each group (P = .0008 and P < .0001 for acclimated and non-acclimated pigs, respectively).
Both groups of pigs restituted epithelial injury after 120-min ex vivo recovery to a level which was not significantly different than uninjured tissue within groups (, P = .338 and P = .998 for acclimated and non-acclimated pigs, respectively). However, when calculating the percent wound healing within individual pigs, non-acclimated pigs achieved 93.1±4.73% wound healing while acclimated pigs achieved a mean of only 43.0% with a standard error of 28.52% indicating a highly variable ability to restitute mucosal epithelium from one pig to the next in the acclimated group (). The difference of the percent wound healing between groups was not statistically significant, however (P = .0927).
Transport stress enhances barrier recovery responses in ischemia-injured jejunum
To further assess the changes in barrier function due to combined effects of acclimation status and acute ischemic injury, transepithelial electrical resistance (TEER) was assessed as a measure of mucosal barrier tight junction permeability during the ex vivo recovery period. In comparing raw TEER data, there was a significant effect of acclimation on TEER detected by repeated measured two-way ANOVA (, P = .023). In these raw data, ischemia-injured mucosa of acclimated pigs had lower initial TEER of 27±3.5 Ω•cm2 as compared to non-acclimated pigs which had an initial TEER of 36±6.3 Ω•cm2. However, this difference was not significantly different upon post hoc analysis (P = .576). After 120 minutes of ex vivo recovery, ischemia-injured mucosa of acclimated pigs had lower final TEER of 44±6.1 Ω•cm2 as compared to non-acclimated pigs which had a final TEER of 66±10.8 Ω•cm2. However, this difference also was not significantly different upon post hoc analysis (P = .236). To control for pig-to-pig variability, TEER was then normalized relative to each individual pigs’ own uninjured baseline TEER for each recovery time point (). In these normalized data, repeated measures two-way ANOVA uncovered a similar effect of acclimation status on TEER (P = .010). However, post hoc analysis revealed significant increases in relative TEER during ex vivo recovery within the non-acclimated pig group when compared to the initial relative TEER (P < .05 at 30, 75, and 120 min; P < .01 at 90 and 105 min). These changes were not detected in the acclimated pig group (P > .05 at all recovery time points when compared to initial relative TEER).
Figure 3. Combined effects of animal handing practices and acute ischemic injury on small intestinal barrier function
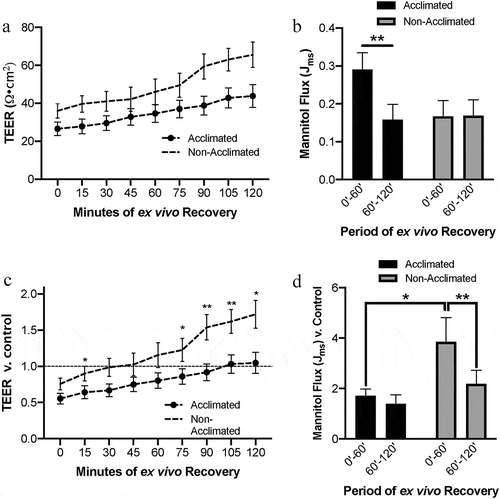
To measure the effects of acclimation status on barrier permeability to macromolecular flux in the face of acute ischemia, recovering injured tissues were incubated with 3H-mannitol added to the mucosal reservoir and leak of this molecule to the serosal reservoir was measured after the first and second hour of ex vivo recovery (). Two-way ANOVA determined there was no overall effect of acclimation on macromolecular flux (P = .317) but there was an interaction between the recovery period and acclimation (P = .015). Post hoc analysis determined that within the acclimated pig group, there was a significant decrease in flux over the recovery period (P = .003), while there was no change in flux in the non-acclimated pigs. Testing also determined there was a significant effect of the individual pig on the outcome (P = .001), so to better control for individual pig variability, flux data were normalized to each pig’s own control flux data (). When controlling for pig-to-pig variability in macromolecular permeability, two-way ANOVA determined that there was again no overall effect of acclimation (P = .066) but did show an interaction between recovery period and acclimation on the permeability (P = .038). Post hoc analysis of these normalized data showed a higher initial flux in the non-acclimated pigs as compared to acclimated pigs (P = .024), an increase which lowered significantly in the second half of the recovery period (P = .003).
Tight junction protein claudin-4 localization to the apical membrane is disrupted by environmental acclimation
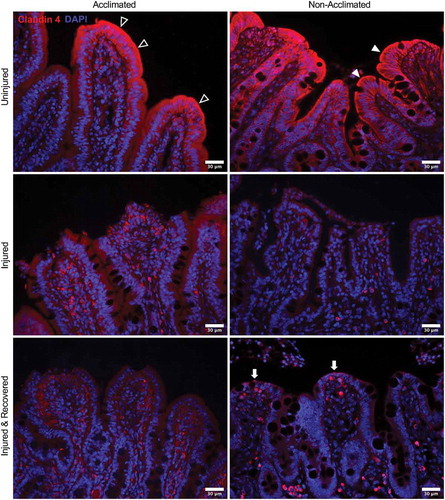
As a marker of tight junction integrity, claudin-4 expression and localization in the intestinal mucosa were assessed by immunofluorescent histology to further visualize the combined effects of animal handling practices and acute ischemic injury on the intestinal barrier (). In uninjured mucosa, claudin-4 staining is localized to the majority of epithelial cells, with particular concentration within the enterocytes at the villus tips. However, in the non-acclimated pigs, there is a more distinct localization at the cell borders, particularly at the apical membrane (, top right panel, solid arrowheads). In the acclimated pigs, the expression is more diffuse throughout the cytoplasm and less defined at the apical membrane, indicating the claudin-4 protein is internalized rather than distributed to its functional location in the membrane tight junctions (, top left panel, open arrowheads). In acutely ischemia-injured mucosa, the claudin-4 expression appears to be broadly reduced with a lack of localization to the cell membranes (, middle panels). In mucosa recovered from ischemic injury, a small amount of returning claudin-4 expression was appreciated in the apical membranes of the newly restituted mucosal enterocytes of non-acclimated pigs localizing to the restoring tight junctions (, bottom right panel, solid arrows). This pattern of staining was not visible in the acclimated pigs, whose mucosal expression of claudin-4 after ex vivo recovery appeared unchanged when comparing to the injured mucosa before recovery (, bottom left panel).
Figure 4. Combined effects of animal handing practices and acute ischemic injury on expression and localization of tight junction protein claudin-4 in the small intestinal mucosa
Discussion
Pigs are an excellent model for the study of human intestinal biology and disease due to high degrees of similarities between the gastrointestinal tract anatomy and physiology, diet and even physiologic responses to physical and psychological stress.Citation3,Citation4,Citation10,Citation17 Pigs have been shown to experience gastrointestinal barrier disturbances in response to early weaning stress and heat stress as models of social and physical pressures.Citation14 These similarities make the pig a superb model for comparative and translational studies, but the sensitivity of the pig gastrointestinal tract to exogenous stressors, as shown by Moeser et al., means that gastrointestinal studies must be designed thoughtfully to prevent confounding experimental outcomes and interpretation.Citation13,Citation14,Citation16,Citation19–21 Our lab uses the pig to study intestinal repair mechanisms following injury. Most commonly, we have used a surgical ischemia model to induce repeatable and controlled epithelial injury in order to closely observe and define the mechanisms of mucosal repair after discrete epithelial sloughing.Citation1 We have shown that the small intestinal mucosa is able to repair epithelial wounds very swiftly, characterized by contraction of injured villi to reduce the denuded surface area,Citation22,Citation23 restitution of the denuded areas by migrating wound-adjacent epithelial cells, and restoration of the tight junctions between the newly restituted epithelial cell membranes to seal the paracellular spaces and effectively restore barrier function to prevent sepsis.Citation24 Utilizing large animal models requires specialized facilities and staff in order to handle animals appropriately and provide the correct environment to support their physical and mental welfare.Citation10 While these practices are overseen by institutional animal care and use committees in the United States to ensure adequate animal care is met from a welfare standpoint, even minor changes in a pigs environment may have important impact on digestive disease studies due to the pig’s higher order of cognition and highly stress-responsive gastrointestinal tract.Citation17,Citation25 The effects of minor changes in environmental housing, pig sorting, transportation or other handling practices in the context of translational gastrointestinal research have not been previously reported. In this study, we sought to investigate the effects of two common pig handling scenarios utilized at our institution for the study of gastrointestinal barrier function and response to injury in our ischemia model: brief environmental acclimation or direct transportation from the production farm.
For these experiments, pigs in the “acclimated” group were transported several miles across campus to our research housing facilities and allowed to acclimate to their new environment for a period of 3 d prior to initiation of experimental surgery. In the “non-acclimated” group, pigs were transported the day of experiment initiation, delivered directly from the farm to the surgical facilities immediately prior to the induction of anesthesia. Both groups would have experienced degrees and durations of stress from social (animal sorting) and physical (transportation and novel environment) changes, which are important to consider. We found that these two groups did not experience variation in baseline TEER in the small intestine. However, the increase in macromolecular flux correlating with claudin-4 distribution to the epithelial cytoplasm in the acclimation group indicates that these pigs were experiencing an increase in the leak pathway of the epithelial barrier in the absence of injury.Citation26 The acclimation group appeared to be protected against epithelial sloughing, as after 30 min of experimental ischemia, there was only about 12% epithelial loss as compared to the non-acclimated group with approximately 32% epithelial loss. However, after 120 minutes of ex vivo recovery, the acclimated pigs were unable to consistently restitute the epithelial barrier as evidenced by only 43% wound healing with very high variability pig to pig. This may suggest that individual animals experienced different degrees of stress in this group or have variable sensitivity to the same stressors. Non-acclimated pigs experienced an increased degree of epithelial sloughing at the onset of injury but were able to more consistently restitute the epithelial defect at a mean efficiency of about 93%. Similarly, tight junction restoration was diminished in the acclimated group as opposed to the non-acclimated group which showed foci of claudin-4 signal at the tight junctions in the newly restituted epithelium at the villus tips. This tight junction pattern corresponded with the greater degree of TEER increase during the recovery period in the non-acclimated pigs, confirming more efficient tight junction closure in this group.
While claudin-4 was examined as a representative tight junction protein in the present study, numerous other claudins and other tight junction proteins may play a critical role in the altered intestinal barrier function observed in these pigs. Expression of tight junction proteins and their localization at the cell membrane are actively and spatially regulated in the gastrointestinal epithelium.Citation27 This results in variable paracellular permeability among different regions of the mucosal epithelium and along with different segments of the gastrointestinal tract, and this is dynamic under physiological and pathological stimuli. Claudins, in particular, exist in numerous subtypes which impart different permeability characteristics in the intestinal mucosa.Citation28 For example, claudin-2 and claudin-15 have been characterized as a pore-forming claudins which open the paracellular pathway to sodium and water flux thus forming a “leakier” barrier.Citation29 Localization of occludin, another important tight junction protein, at the apical membrane has been correlated with recovery in prior porcine studies of post-ischemic intestinal barrier repair.Citation30,Citation31 These additional claudin proteins and occludin are important in modulating intestinal permeability and therefore may be of interest in future studies.
Our group and others have shown that local tissue production of prostaglandins is required for all three phases of subacute epithelial repair following ischemic injury.Citation32,Citation33 Local prostaglandin release from the cells within the lamina propria following tissue damage is critical to efficient repair responses.Citation34 Pharmacologic inhibition of endogenous prostaglandin production reduces the efficiency of barrier recovery after ischemic injury in the small intestine.Citation24 Villus contraction is induced by 16,16-Dimethyl prostaglandin E2 by stimulating contraction of smooth muscle cells in the villus cores.Citation22,Citation23 In vitro study has shown that growth-factor-driven epithelial restitution is mediated by prostaglandin metabolites.Citation35 Finally, recruitment of tight junction proteins to the membrane to close the paracellular spaces and reestablish the barrier is driven by prostaglandins E2 and I2 via the phosphatidylinositol 3-kinase signaling pathway.Citation36 In pigs transported the day of experimental surgery, the increased tissue damage resulting from brief ischemic injury seen in may induce increased endogenous prostaglandin production, promoting enhanced restitution and tight junction recovery in this group as compared to the acclimated pigs, as is seen in represented by claudin-4 distribution to the newly forming tight junctions during recovery in this group. The protective effect of a few days’ duration of environmental stress against epithelial sloughing seen in the acclimated pigs may then be detrimental if prostaglandin production is reduced, thereby slowing the efficiency of tissue repair responses such that these animals cannot repair even this smaller degree of epithelial loss. Further studies of intestinal prostaglandin signaling in this model would be informative.
When considering what is known about central and local control of stress responses in the pig, this leads to many important and interesting questions in the context of the present findings. In a series of recent studies by Moeser et al., several key mediators regulate stress responses in the gut mucosa by both paracrine and endocrine means. This research group utilizes an early weaning protocol to model early life stressors which have been linked to chronic, lifelong inflammatory intestinal disorders characterized by intestinal barrier dysfunction.Citation25 They have shown that early weaning, which induces psychological and physical stress and leads to disrupted barrier function and diarrhea, induces systemic upregulation of systemic cortisol and corticotrophin-releasing factor (CRF), upregulation of CRF receptors on mast cells within the intestinal mucosa as well as mast cell degranulation.Citation14,Citation25 Blockade of peripheral CRF receptors or treatment with mast cell stabilizers will block these effects on the intestinal barrier, confirming the role of mast cells in altering barrier permeability following physical and psychological stress.Citation14,Citation19,Citation37 Mast cell activation induced by stress could play a key role in the effects seen in the present study, and it is likely that it takes longer than a brief period of transport for these responses to take place. As these barrier effects follow the upregulation of mucosal CRF localized to mast cells, which may also need time to marginate into the tissue from circulation, mast cell responses to stress may partly explain why intestinal barrier repair responses are different in the period immediately after a brief transport stress as compared to more prolonged environmental change over several days. Further study to investigate the role of mast cell activation and peripheral cortisol and CRF signaling in this model may be of interest.
Conclusions
These results indicate that acute transport stress on the day of experimental intestinal injury modeling may increase mucosal susceptibility to epithelial loss, but also prime the tissue for a more robust barrier repair response. Brief environmental acclimation, on the other hand, appears to increase intestinal permeability to molecular flux in the absence of injury, while possibly having a protective effect on epithelial loss during injury but slowing the restitution response during the recovery phase in some individuals. Further study to uncover the underlying mechanisms would be informative, and these noteworthy effects of handling stress are important considerations for appropriate study design when utilizing highly translational pig models for intestinal barrier research.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author contributions
Conceptualization, A.Z and A.B.; Methodology, A.Z. T.P. and A.B.; Validation, A.Z. and A.B.; Formal Analysis, A.Z.; Investigation, A.Z and T.P.; Resources, A.Z. and A.B.; Data Curation, A.Z. and T.P.; Writing – Original Draft Preparation, A.Z.; Writing – Review & Editing, A.Z. and A.B.; Visualization, A.Z.; Supervision, A.Z. and A.B.; Project Administration, A.Z. and A.B.; Funding Acquisition, A.Z. and A.B.
Acknowledgments
The authors would like to thank Hannah Gardner, Vassili Kouprianov and Lilah Craig for their contributions in acquiring, processing and organizing ex vivo recovery data, and Lindsey Shapiro for her contributions to immunofluorescent histology data.
Additional information
Funding
References
- Blikslager AT, Moeser AJ, Gookin JL, Jones SL, Odle J. Restoration of barrier function in injured intestinal mucosa. Physiol Rev. 2007;87(2):1–10. doi:10.1152/physrev.00012.2006.
- Kirk AD. Crossing the bridge: large animal models in translational transplantation research. Immunol Rev. 2003;196:176–196. doi:10.1046/j.1600-065X.2003.00081.x.
- Swindle MM, Makin A, Herron AJ, Clubb FJ, Frazier KS. Swine as models in biomedical research and toxicology testing. Vet Pathol. 2012;49(2):344–356. doi:10.1177/0300985811402846.
- Roura E, Lallès KS, Le Huerou-Luron JP, de Jager N, Schuurman T, Val-Laillet D. Critical review evaluating the pig as a model for human nutritional physiology. Nutr Res Rev. 2016;29(1):60–90. doi:10.1017/S0954422416000020.
- Rothkotter HJ, Sowa E, Pabst R. The pig as a model of developmental immunology. Hum Exp Toxicol. 2002;21(9–10):533–536. doi:10.1191/0960327102ht293oa.
- Bendixen E, Danielsen M, Larsen K, Bendixen C. Advances in porcine genomics and proteomics–a toolbox for developing the pig as a model organism for molecular biomedical research. Brief Funct Genomics. 2010;9(3):208–219. doi:10.1093/bfgp/elq004.
- Lunney JK. Advances in swine biomedical model genomics. Int J Biol Sci. 2007;3(3):179–184. doi:10.7150/ijbs.3.179.
- Hart EA, Caccamo M, Harrow JL, Humphray SJ, Gilbert JG, Trevanion S, Hubbard T, Rogers J, Rothschild MF. Lessons learned from the initial sequencing of the pig genome: comparative analysis of an 8 Mb region of pig chromosome 17. Genome Biol. 2007;8(8):R168. doi:10.1186/gb-2007-8-8-r168.
- Ibrahim Z, Busch J, Awwad M, Wagner R, Wells K, Cooper DKC. Selected physiologic compatibilities and incompatibilities between human and porcine organ systems. Xenotransplantation. 2006;13(6):488–499. doi:10.1111/j.1399-3089.2006.00346.x.
- Ziegler A, Gonzalez L, Blikslager A. Large animal models: the key to translational discovery in digestive disease research. Cell Mol Gastroenterol Hepatol. 2016;2(6):716–724. doi:10.1016/j.jcmgh.2016.09.003.
- Soderholm JD, Perdue MH. Stress and gastrointestinal tract. II. Stress and intestinal barrier function. Am J Physiol Gastrointest Liver Physiol. 2001;280(1):G7–G13. doi:10.1152/ajpgi.2001.280.1.G7.
- Lambert GP. Stress-induced gastrointestinal barrier dysfunction and its inflammatory effects. J Anim Sci. 2009;87(14 Suppl):E101–8. doi:10.2527/jas.2008-1339.
- Smith F, Clark JE, Overman BL, Tozel CC, Huang JH, Rivier JEF, Blisklager AT, Moeser AJ. Early weaning stress impairs development of mucosal barrier function in the porcine intestine. Am J Physiol Gastrointest Liver Physiol. 2010;298(3):G352–63. doi:10.1152/ajpgi.00081.2009.
- Moeser AJ, Klok CV, Ryan KA, Wooten JG, Little D, Cook VL, Blikslager AT. Stress signaling pathways activated by weaning mediate intestinal dysfunction in the pig. Am J Physiol Gastrointest Liver Physiol. 2007;292(1):G173–81. doi:10.1152/ajpgi.00197.2006.
- Pearce SC, Mani V, Boddicker RL, Johnson JS, Weber TE, Ross JW, Rhoads RP, Baumgard LH, Gabler NK. Heat stress reduces intestinal barrier integrity and favors intestinal glucose transport in growing pigs. PLoS One. 2013;8(8):e70215. doi:10.1371/journal.pone.0070215.
- Ziegler AL, Blikslager AT. Impaired intestinal barrier function and relapsing digestive disease: lessons from a porcine model of early life stress. Neurogastroenterol Motil. 2017;29(11):1–4. doi:10.1111/nmo.13216.
- Gieling ET, Schuurman T, Nordquist RE, van der Staay FJ. The pig as a model animal for studying cognition and neurobehavioral disorders. Curr Top Behav Neurosci. 2011;7:359–383.
- Argenzio RA, Lecce J, Powell DW. Prostanoids inhibit intestinal NaCl absorption in experimental porcine cryptosporidiosis. Gastroenterology. 1993;104(2):440–447. doi:10.1016/0016-5085(93)90412-6.
- Moeser AJ, Ryan KA, Nighot PK, Blikslager AT. Gastrointestinal dysfunction induced by early weaning is attenuated by delayed weaning and mast cell blockade in pigs. Am J Physiol Gastrointest Liver Physiol. 2007;293(2):G413–21. doi:10.1152/ajpgi.00304.2006.
- Overman EL, Rivier JE, Moeser AJ. CRF induces intestinal epithelial barrier injury via the release of mast cell proteases and TNF-alpha. PLoS One. 2012;7(6):e39935. doi:10.1371/journal.pone.0039935.
- Pohl CS, Medland JE, Moeser AJ. Early-life stress origins of gastrointestinal disease: animal models, intestinal pathophysiology, and translational implications. Am J Physiol Gastrointest Liver Physiol. 2015;309(12):G927–41. doi:10.1152/ajpgi.00206.2015.
- Moore R, Carlson S, Madara JL. Villus contraction aids repair of intestinal epithelium after injury. Am J Physiol. 1989;257:G274–83.
- Erickson RA, Tarnawski A, Dines G, Stachura J. 16,16-Dimethyl prostaglandin E2 induces villus contraction in rats without affecting intestinal restitution. Gastroenterology. 1990;99(3):708–716. doi:10.1016/0016-5085(90)90959-5.
- Blikslager AT, Roberts MC, Rhoads JM, Argenzio RA. Prostaglandins I2 and E2 have a synergistic role in rescuing epithelial barrier function in porcine ileum. J Clin Invest. 1997;100(8):1928–1933. doi:10.1172/JCI119723.
- Pohl CS, Medland JE, Mackey E, Edwards LL, Bagley KD, DeWilde MP, Williams KJ, Moeser AJ. Early weaning stress induces chronic functional diarrhea, intestinal barrier defects, and increased mast cell activity in a porcine model of early life adversity. Neurogastroenterol Motil. 2017;29(11):e13118. doi:10.1111/nmo.13118.
- Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol. 2011;73:283–309. doi:10.1146/annurev-physiol-012110-142150.
- Pasternak JA, Kent-Dennis C, Van Kessel AG, Wilson HL. Claudin-4 undergoes age-dependent change in cellular localization on pig jejunal villous epithelial cells, independent of bacterial colonization. Mediators Inflamm. 2015;2015:263629. doi:10.1155/2015/263629.
- Garcia-Hernandez V, Quiros M, Nusrat A. Intestinal epithelial claudins: expression and regulation in homeostasis and inflammation. Ann N Y Acad Sci. 2017;1397(1):66–79. doi:10.1111/nyas.13360.
- Ong M, Yeruva S, Sailer A, Nilsen SP, Turner JR. Differential regulation of claudin-2 and claudin-15 expression in children and adults with malabsorptive disease. Lab Invest. 2020;100(3):483–490. doi:10.1038/s41374-019-0324-8.
- Moeser AJ, Haskell MM, Shifflett DE, Little D, Schultz BD, Blikslager AT. ClC-2 chloride secretion mediates prostaglandin-induced recovery of barrier function in ischemia-injured porcine ileum. Gastroenterology. 2004;127(3):802–815.
- Kuo WT, Shen L, Zuo L, Shashikanth N, Ong MLDM, Wu L, Zha J, Edelblum KL, Wang Y, Wang Y, et al. Inflammation-induced occludin downregulation limits epithelial apoptosis by suppressing caspase 3 expression. Gastroenterology. 2019;157:1323–1337. doi:10.1053/j.gastro.2019.07.058.
- Blikslager AT, Pell SM, Young KM. PGE2 triggers recovery of transmucosal resistance via EP receptor cross talk in porcine ischemia-injured ileum. Am J Physiol Gastrointest Liver Physiol. 2001;281(2):G375–81. doi:10.1152/ajpgi.2001.281.2.G375.
- Moeser AJ, Nighot PK, Ryan KA, Wooten JG, Blikslager. AT. Prostaglandin-mediated inhibition of Na+/H+ exchanger isoform 2 stimulates recovery of barrier function in ischemia-injured intestine. Am J Physiol Gastrointest Liver Physiol. 2006;291:G885–G894. doi:10.1152/ajpgi.00380.2005.
- Blikslager AT, Roberts MC, Argenzio RA. Prostaglandin-induced recovery of barrier function in porcine ileum is triggered by chloride secretion. Am J Physiol. 1999;276:G28–G36.
- Zushi S, Shinomura Y, Kiyohara T, Minami T, Sugimachi M, Higashimoto Y, Kanayama S, Matsuzawa Y. Role of prostaglandins in intestinal epithelial restitution stimulated by growth factors. Am J Physiol. 1996;270(5 Pt 1):G757–62.
- Little D, Dean RA, Young KM, McKane SA, Martin LD, Jones SL, Blikslager. AT. PI3K signaling is required for prostaglandin-induced mucosal recovery in ischemia-injured porcine ileum. Am J Physiol Gastrointest Liver Physiol. 2003;284:G46–G56. doi:10.1152/ajpgi.00121.2002.
- D’Costa S, Ayyadurai S, Gibson AJ, Mackey E, Rajput M, Sommerville LJ, Wilson N, Li Y, Kubat E, Kumar A, et al. Mast cell corticotropin-releasing factor subtype 2 suppresses mast cell degranulation and limits the severity of anaphylaxis and stress-induced intestinal permeability. J Allergy Clin Immunol. 2019;143(5):1865–1877. e4. doi:10.1016/j.jaci.2018.08.053.
