ABSTRACT
The kidney is a complex organ that performs essential functions such as blood filtration and fluid homeostasis, among others. Recent years have heralded significant advancements in our knowledge of the mechanisms that control kidney formation. Here, we provide an overview of vertebrate renal development with a focus on nephrogenesis, the process of generating the epithelialized functional units of the kidney. These steps begin with intermediate mesoderm specification and proceed all the way to the terminally differentiated nephron cell, with many detailed stages in between. The establishment of nephron architecture with proper cellular barriers is vital throughout these processes. Continuously striving to gain further insights into nephrogenesis can ultimately lead to a better understanding and potential treatments for developmental maladies such as Congenital Anomalies of the Kidney and Urinary Tract (CAKUT).
Introduction: emergence of the kidney from the intermediate mesoderm
Vertebrate development entails the formation of three germ layers, the ectoderm, mesoderm, and endoderm, which provide cellular blueprints for embryonic organogenesis. Ectoderm gives rise to the central nervous system and skin cells, and endoderm derivatives encompass cells that line the respiratory and digestive tracts. The mesoderm, or middle layer, produces cells that are most abundant in the human body constituting skeletal muscle, cartilage, heart, gonads, and blood, among other tissue types.Citation1 This review will focus on a member of the mesoderm lineage: the kidney. Much of our understanding about kidney development stems from rodent models, but also has benefited from studies in other vertebrates such as fish, frogs, and birds.Citation2The inception of mesoderm development begins with the differentiation of pluripotent epiblast cells into a transient ‘primitive streak’ zone.Citation1Position along the anterior-posterior embryonic axis and other instructive signals regulate the regionalization of paraxial, intermediate, and lateral plate mesoderm.Citation3
The urogenital system derives from the aforementioned intermediate mesoderm (IM), which is a narrow section of tissue situated between the paraxial and the lateral plate mesoderm. Early developmental studies are hampered by the limited number of molecular markers that label the emerging IM population. The first IM indicators to appear during embryogenesis are LIM-type homeobox (Lhx1) and the zinc-finger DNA-binding protein odd-skipped related (Osr1).Citation4–7 The expression domains of these two factors intersect indicating the prospective IM and lateral plate mesoderm fields, as Osr1 is expressed across the entire length of the expanding somite tissue. Specific markers solely expressed in the IM do not turn on until about the 4–8 somite stage. The activation of Pax2 and Pax8 within a narrow band early in the IM is speculated to signify that the LPM and IM have assumed separate lineage trajectories. Complementary to these early expression pattern observations, functional assays in mice have demonstrated Lhx1, Osr1, Pax2, and Pax8 are all critical regulators of IM specification.Citation4,Citation6,Citation8–10 For example, mice lacking either Lhx1 or Pax2/Pax8 fail to form the nephric duct, which is a pair of tubes required for assembly of the urinary system.Citation7,Citation11 Interestingly, experiments in chick embryos indicate that the competence to respond to these IM patterning factors is conferred by retinoic acid (RA) and Hox gene expression.Citation12
It is important to think about developing organisms in a three-dimensional context. This is especially true for understanding kidney ontogeny, as various signal gradients are radiating through the animal in a morphogenic fashion. For example, developmental studies performed in chick embryos found that bone morphogenic protein (BMP) signaling affects IM cell fate in a concentration-dependent manner.Citation5,Citation13,Citation14 A variety of studies have revealed RA and activin signaling gradients promote IM marker expression during ontogeny in various vertebrate species.Citation15–21 Originally, the addition of these factors to culture systems including animal caps, embryoid bodies, and embryonic stem cells supplemented growth and cellular differentiation of kidney fates. A decade later, similar experiments have been refined to generate IM from induced pluripotent stem cells to generate kidney organoids.Citation22
Following IM specification, the progression of vertebrate renal development involves the stepwise generation and degeneration of several kidney forms: the pronephros, mesonephros, and metanephros. Each kidney iteration develops along the anterior-posterior embryonic axis, where each subsequent version becomes more structurally complex than the previous structure. The pronephros emerges first, and while it is vestigial/nonfunctional in mammals, it is functional in other vertebrates such as fish and frogs.Citation23 The mesonephros is further developed and partially functional in mammals, while serving as the final kidney form in amphibians and fish.Citation24 However, the fully formed and functional version of this vital organ in mammals is the metanephros, which develops through branching morphogenesis events that result in an arborized structure essential for fluid homeostasis. Importantly, all three vertebrate kidney forms share the overall structure of the kidney’s functional unit: the nephron. Broadly, the nephron is composed of a blood filter, a segmented tubule, and a collecting duct system to shuttle urine to the bladder.
Mesenchyme induction
The focal point of the remaining sections of this review will cover mechanisms driving the development of nephrons, since these are unifying structures across vertebrate kidney forms. While our primary focus will be centered on recent insights on mechanisms in mammals, we will also highlight some conserved genetic regulators revealed from studies in other vertebrates.
The mammalian metanephros contains two well-characterized renal progenitor populations: the metanephric mesenchyme (MM) and the ureteric bud (UB) (). The UB derives from the nephric duct and gives rise to the collecting duct system, and the MM is the source of all nephron lineages and contains vascular, stromal, and nephron progenitor cells (NPC). The UB initiates nephron induction by invading the MM and undergoes progressive branching after receiving reciprocal signals from the MM. Occurring simultaneously, UB signals cause the MM to condense around the ureteric tips forming a structure termed the cap mesenchyme (CM), which retains the Six2+ Cited1+ NPC population. The NPCs border the ureteric epithelium and other cell populations are more distant from this site. Recent lineage-tracing studies have begun to appreciate how position, movement, and spatial exposure to differentiation cues can determine self-renewal or differentiation status within NPC pools. For example, a subset of Wnt4-expressing cells was discovered to migrate back to nephron progenitor zone and exhibit plasticity regarding nephron commitmentCitation25
Figure 1. Summary schematic of key nephrogenesis steps
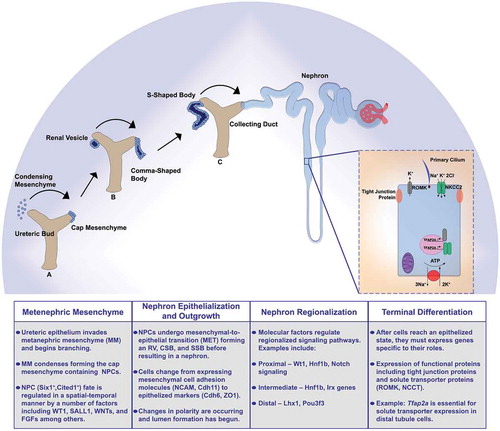
In addition to physical location, the reciprocal crosstalk between the CM and UB is essential for nephron formation. The ability to form several thousand to over one million nephrons in mammalian kidneys requires maintaining both a delicate balance of self-renewing NPCs whilst making a sufficient endowment of differentiating nephrons to support renal function. The Six2+ Cited1+ CM possesses the ability to self-renew and sustain ample progenitor cells capable of making the correct number of nephrons. Molecular signals from the UB are responsible for NPC self-renewal or nephron commitment. Factors that are required for maintenance of the progenitor pool include: WT1, SALL1, FGFR1/2, FGF8, WNT11, FGF9, and WNT9B.Citation25–38 Without the proper signals the kidney exhausts the NPC pool, which in turn yields insufficient nephron number and can lead to kidney agenesis or predisposition to chronic kidney disease.
The spatial location of NPCs is becoming increasingly appreciated as a major determinant of cell-fate decisions. Previously, it was well-accepted that the least committed NPCs were located at the top of the CM, and the most committed NPCs were positioned beneath the ureteric tip and undergo nephron induction and renal vesicle formation.Citation39 It was thought that the derivation of nephron cells from NPCs was a linear progression from the Six2+ Cited1+ self-renewing population, to the Six2+ primed cellular state, and finally the committed state demarcated by Wnt4.Citation39–43 However, results from a recent study that employed lineage tracing and computational modeling support an alternative hypothesis.Citation25 found that NPCs moved randomly around the cap mesenchyme. The authors found that an NPCs can initiate Wnt4 expression, previously believed to indicate ‘nephron commitment,’ migrate back to the progenitor domain, and regain a self-renewing progenitor status.Citation25 The authors hypothesize that random cell movements influenced by ureteric epithelium result in differing signals that then determine the fate of the NPCs.
A particularly important transitional step during nephrogenesis is when CM cells form a pre-tubular aggregate (PTA) beneath the ureteric tip. One of the most well-known factors that signals within MM to commence the differentiation process is Wnt9b. The re-expression of Osr1 and Six2 have been documented to halt PTA formation.Citation6,Citation44,Citation45 Six2 activity within the CM is believed to antagonize the function of Wnt9b by affecting the stabilization of β-catenin.Citation34,Citation42,Citation43,Citation45–49 Thus, it is essential for PTAs to downregulate Six2 while the Wnt9b/Wnt4 signaling axis prompts a mesenchymal to epithelial transition (MET) and renal vesicle (RV) polarization.
Nephron epithelization and growth
Renal vesicle (RV) formation is the beginning of many important changes that result in nephron formation (). The RV undergoes MET and becomes polarized as it transitions into more developed stages: the comma-shaped body (CSB) and the S-shaped body (SSB). The origins of epithelization are dependent on UB WNT9B signals to initiate WNT4 expression in the PTA.Citation42 This expression leads to β-catenin stabilization and polarity establishment within the RV.Citation43 Overall MET consists of changing from mesenchymal cell adhesion molecules NCAM, Cdh11, Cdh2, and Cdh4 to a more epithelized status expressing Cdh6, Cdh1, and ZO1, among others.Citation50–56
Cellular polarity is defined as specific proteins localizing to particular regions of the cell boundary denoting certain areas as apical, basal, and lateral. Cellular polarity is particularly important for kidney development as it coincides with lumen formation in the maturing nephron.Citation57 The process of apicobasal polarity establishment relies on afadin as without it, kidney tubule cells fail to form correct Par and nectin complexes and do not recruit R-cadherin.Citation57 Another factor found to be necessary for polarity in the developing nephron is the Rho GTPase Cdc42. Knock-out studies found severe defects in polarity and lumen formation.Citation58 Eventually, the RV lengthens and connects to the UB to form the collecting system.Citation59,Citation60 This is now the continuous lumen of the nephron. Nephron lumen formation is largely conserved as results from these studies align with findings from a zebrafish lumen formation study focusing on atypical protein kinase C (aPKC).Citation61 Using the various model organisms available will be beneficial to continue to understand the process of lumen formation. As we currently understand it, the establishment of apical and basolateral polarity is necessary as collectively the cells need to form the lumen on the apical side of RV cells. As previously mentioned, there are a number of molecular cues that dictate proper polarity and thus lumen formation. One of the main concepts to keep in mind is that there are cell–cell interactions as well as cell-matrix interactions taking place, which have been covered in detail in another review.Citation62 Moving forward, a better understanding of these interactions and the molecular cues initiating them is needed.
Additional aspects of nephron growth include planar cell polarity, mechanical stretch, cell migration and proliferation. It is important to understand that many of the events discussed in this review are occurring simultaneously. For example, the events in this section are taking place while transcription factors direct regionalization of nephron tubules, which will be discussed in the following section. Continuing to think of nephrogenesis in a three-dimensional context, planar cell polarity (PCP) is necessary for proper kidney development. PCP is collective tissue polarity or, polarity as it functions perpendicular to the cellular apical-basal polarity. There are numerous examples of the role PCP plays in developmental biology as it controls convergent extension and oriented cell division.Citation63 One elegant example is found as research discovered the PCP-dependent convergent extension resulting in kidney tubule formation.Citation64 Additionally, PCP controls oriented-cell division, another process that has been linked to kidney tubule elongation.Citation65 While the core PCP components are known to be needed for proper tissue development, other factors and their downstream consequences are being discovered.Citation65–70 One known factor that regulates kidney PCP is Wnt9b acting via Rho-kinase.Citation71–73 This is an area of intense research as dramatically increased proliferation can result in disease states such as polycystic kidney disease (PKD).Citation62 Hippo signaling has been linked closely with PKD cell proliferation via fat4 a negative regulator of hippo signaling providing one avenue for potential molecular exploration.Citation74–77 As we continue to learn about PCP and the other effects involved downstream, we can gain better insight to disease states. Specifically, cell proliferation and its relationship with PCP requires a better understanding as it pertains to kidney development.
Interestingly, in zebrafish, there is a concentrated cell proliferation event in the distal portion of the pronephros as cells migrate rostrally to provide the increased cell number necessary for the proximal tubule to undergo coiling morphogenesis.Citation78 This nicely supports a study that found distal proliferation of the RV occurs to join the UB as previously mentioned to make the collecting duct system.Citation59 Further, collective cell migration occurs in developing and regenerating zebrafish nephrons.Citation78–81 Combining these data with recent studies illustrating the unique cellular movement during mammalian kidney development,Citation25 future studies could be focused on cellular migratory events and their role in nephron development.
Nephron regionalization
As the RV continues to develop, its transitions into the CSB and SSB where regionalization becomes an important concept as this will be vital for a properly segmented, terminally differentiated nephron (). Though studies suggest proximo-distal regionalization can be seen in the RV it is especially evident in the later stages of nephrogenesis.Citation59,Citation82,Citation83 This is an area of research that has greatly benefitted from the many advantages of the Xenopus and zebrafish model organisms as complements to the single-cell RNA sequencing (scRNA seq) in murine and human kidneys.Citation84–89 During this time of RV elongation into CSB and SSB a number of signals dictate proximal and distal cell fates. Here, we will discuss some of these signaling factors, but not an exhaustive list.
While the RV is usually subdivided into proximal and distal domains, the SSB gains a medial (or intermediate) region. Expression of unique factors in combination with loss of function experiments have determined much of what we understand to be segmentation of the nephron during development. Recently, mostly due to the advances in scRNA seq, there has been an approach at understanding the role timing and location play in combination with gene expression for the development of individual nephron cells.Citation25,Citation90
Beginning with the proximal portion of the SSB, Wilm’s Tumor 1 (Wt1) continues its proximal expression observed in the RV, and this expression continues into mature podocytes.Citation91,Citation92 Next, there have been a number of studies to better understand the role Notch signaling plays in proximal segmentation. Losing components of Notch signaling results in loss of proximal segments. These changes appear first during the SSB stage, coinciding with expression of Notch signaling components.Citation83,Citation93–96 Interesting evidence has shown that members of the Iroquois (Irx) transcription factor family play roles in the medial SSB development. Studies in Xenopus and zebrafish have shown that loss of Irx genes have consequences for the development of intermediate segments of the nephron.Citation97–99 The transcription factor Hnf1b is expressed in mouse SSB and disruption of its expression has drastic phenotypes in Xenopus and zebrafish. Xenopus deficient in hnf1b do not properly form proximal or intermediate sections of the nephron,Citation100 while zebrafish deficient in hnf1b do not form any nephron segments.Citation101 The previously mentioned transcription factor Lhx1 is needed for proper distal formation.Citation102 Additionally, downstream of Lhx1 the POU-domain containing transcription factor POU3F3 (also called Brn1) is needed for distal tubule and Loop of Henle formation.Citation103
Recently, there have been several large-scale efforts to catalog factors that are expressed during nephron segmentation, such as scRNA sequencing of mice and human kidneys. These data repositories provide a wealth of information that will be useful to design future genetic studies. Additionally, genetic studies such as forward and chemical screens using other vertebrate models have generated new insights about mechanisms that control nephron segmentation in different species.Citation86 One premiere system has been the zebrafish embryonic kidney, or pronephros, where a growing list of genes and signaling pathways has been identified.Citation104–114 Recent evidence from human kidneys strongly suggests potential conservation in gene expression and function with their zebrafish counterpart.Citation104,Citation115 Combining the large data sets of mice and human scRNA sequencing with the relative ease of loss of function studies in zebrafish could streamline an evolutionarily conserved pipeline of essential factors for nephrogenesis.
Nephron terminal differentiation
By the time the nephron is fully developed, it will contain a number of unique cell-types that each need to have the appropriate gene expression to complete their vital functions (). The nephron begins with the blood filter, or renal corpuscle encompassing the glomerulus and Bowman’s capsule. This contains a number of cell types including capillaries, mesangium, podocytes, and parietal cells. Next, the tubule contains the proximal convoluted tubule, proximal straight tubule, the Loop of Henle (including descending limb, thin ascending limb, thick ascending limb), distal convoluted tubule, and connecting tubule. The proximal tubule functions in absorption and secretion in an effort to regulate pH of the filtrate. Largely, the Loop of Henle functions to concentrate the filtrate by reabsorbing water. The distal tubule ensures proper ion transport occurs to fine-tune the filtrate by regulating potassium, sodium, and calcium levels. Each unique segment is needed to maintain blood homeostasis by completing these functions. Nephron cells must acquire a number of features to be generally considered terminally differentiated, including proper epithelization, cilia formation, and expression of functional proteins such as tight junctions and solute transporters.
We have previously discussed nephron epithelization during the RV to SSB stages so in this section we will focus on the remaining steps of terminal differentiation beginning with cilia. Cilia are hair-like structures projecting from the apical surface of cells that have essential roles in various organs including signal transduction.Citation110 Cilia play a unique role in kidney development and function as ciliogenesis is an essential step as kidney tubule cells differentiate while also playing a role in signaling to properly form the kidney. The earliest observed cilia formation is at the RV stage as the developing nephron undergoes MET, establishes apical-basal polarity, and begins to form a lumen.Citation116 Cilia length are largely dynamic as they respond to stimuli to carry out their tasks. Cilium length increases as nephrons mature, this is speculated to indicate that they may be playing important roles that are currently not understood.Citation110,Citation116 Without proper cilia form and/or function disease states, termed ciliopathies, occur. One of the most common ciliopathies is polycystic kidney disease which is the result of mutations in cilia localized proteins such as polycystin-1 and −2.Citation110,Citation117 This is an area of nephrogenesis that is also greatly complemented by the advantages of zebrafish and Xenopus as model organisms. Several studies have found cilia-related genes affecting kidney development including Wnt/PCP genes, hnf1b, and PKD2, among others.Citation118–120 One potential interesting avenue of future research could focus on multiciliated cells, or cells with multiple cilia projecting from the cell surface that function in fluid propulsion in zebrafish and Xenopus. Though currently not believed to be present in normal adult healthy kidneys, there have been reports of multiciliated cells present in kidneys during certain disease states.Citation121
Tight junction proteins can be found along the entire nephron structure from the slit diaphragm in the glomerulus and along the tubule in various segments.Citation122–124 The function of the numerous tight junction proteins differs depending on the spatial expression, but overall tight junctions provide a controlled blockade to paracellular transfers of water and ions.Citation122 Overall, tight junctions comprise proteins including occludins, claudins, and junctional adhesion molecules.Citation123,Citation125–127 These proteins vary in the function and thus their expression along the nephron. Tight junction proteins are conserved across vertebrate species, further illustrating their importance to the general role of the kidney.Citation124
Another suite of proteins essential to nephron function are the solute transporters found along the nephron tubule. To ensure they are able to modify the filtrate, each segment will express a suite of genes that act specific to their location along the tubule. Many of these genes that specify segments are solute transporters. Interestingly, many of these segment-specific genes are largely conserved from, zebrafish, Xenopus, rodents, and humans.Citation2,Citation21,Citation86,Citation99,Citation128 This enables a robust host of opportunities to study segmentation and terminal differentiation of the nephron. Several factors previously discussed, including Hnf1b, are needed to reach epithelial status while others have been identified to push the epithelial fate to terminal differentiation of the unique segment cell types.Citation101,Citation129 A number of specific examples can be tracked across species, such as the decrease in the distal solute transporter NCCT or Slc12a3 in mice and zebrafish deficient in ppargc1a.Citation106,Citation130 One recent study from our laboratory identified the transcription factor AP-2 (tfap2a) as the key determinant in the terminal differentiation of the zebrafish distal early segment, analogous to the mammalian thick ascending limb.Citation104 In the absence of Tfap2a, the nephron cells were found to reach an epithelialized point of development in which they were primed to be distal tubule cells but failed to express the solute transporter suite of genes necessary for proper function of this particular segment.Citation104 Further, tight regulation of Tfap2a expression was found to be essential, and controlled by the paralogs potassium channel tetramerization domain containing 15a and 15b (kctd15a, 15b).Citation105 Interestingly, a study found similar localizations of TFAP2A and KCTD15 expression during human nephrogenesis suggesting possible conserved roles in development.Citation115,Citation131,Citation132
Conclusions and future directions
While there have been substantial advances in understanding the molecular mechanisms dictating the intricate events resulting in kidney development, there remain a number of challenges. Clinically, patients resort to dialysis or transplantation when faced with kidney ailments. Neither of these treatments are particularly reassuring to the many people facing kidney dysfunction. Two areas of research that are blossoming and resulting in the hope of newer treatments are single-cell RNA sequencing and organoids. Both fields are advancing quickly and can be combined with high throughput loss of function studies to continue to identify necessary genes to enhance kidney development and function.
Author Contributions
Writing—original draft preparation, J.M.C.; writing—review and editing, R.A.W.; funding acquisition, J.M.C. and R.A.W. Both authors have read and agreed to the published version of the manuscript.
Abbreviations
Disclosure of potential conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
We thank the members of our lab for discussions and insights about this work.
Additional information
Funding
References
- Loh KM, Chen A, Koh PW, Deng TZ, Sinha R, Tsai JM, Barkal AA, Shen KY, Jain R, Morganti RM, et al. Mapping the pairwise choices leading from pluripotency to human bone, heart, and other mesoderm cell types. Cell. 2016;166(2):1–13. doi:10.1016/j.cell.2016.06.011.
- Wingert RA, Davidson AJ. The zebrafish pronephros: a model to study nephron segmentation. Kidney Int. 2008;73:1120-1127. doi: 10.1038/ki.2008.37
- Ferretti E, Hadjantonakis AK. Mesoderm specification and diversification: from single cells to emergent tissues. Curr Opin Cell Biol. 2019;61:110–116. doi:10.1016/j.ceb.2019.07.012.
- Dressler GR. Advances in early kidney specification, development and patterning. Development. 2009;136(23):3863–3874. doi:10.1242/dev.034876.
- James RG, Schultheiss TM. Bmp signaling promotes intermediate mesoderm gene expression in a dose-dependent, cell-autonomous and translation-dependent manner. Dev Biol. 2005;288(1):113–125. doi:10.1016/j.ydbio.2005.09.025.
- James RG, Kamei CN, Wang Q, Jiang R, Schultheiss TM. Odd-skipped related 1 is required for development of the metanephric kidney and regulates formation and differentiation of kidney precursor cells. Development. 2006;133(15):2995–3004. doi:10.1242/dev.02442.
- Tsang TE, Shawlot W, Kinder SJ, Kobayashi A, Kwan KM, Schughart K, Kania A, Jessell TM, Behringer RR, Tam PP. Lim1 activity is required for intermediate mesoderm differentiation in the mouse embryo. Dev Biol. 2000;223(1):77–90. doi:10.1006/dbio.2000.9733.
- Brophy PD, Ostrom L, Lang KM, Dressler GR. Regulation of ureteric bud outgrowth by Pax2-dependent activation of the glial derived neurotrophic factor gene. Development. 2001;128:4747–4756.
- Torres M, Gómez-Pardo E, Dressler GR, Gruss P. Pax-2 controls multiple steps of urogenital development. Development. 1995;121:4057–4065.
- Wang Q, Lan Y, Cho ES, Maltby KM, Jiang R. Odd-skipped related 1 (Odd1) is an essential regulator of heart and urogenital development. Dev Biol. 2005;288(2):582–594. doi:10.1016/j.ydbio.2005.09.024.
- Bouchard M, Souabni A, Mandler M, Neubüser A, Busslinger M. Nephric lineage specification by Pax2 and Pax8. Genes Dev. 2002;16(22):2958–2970. doi:10.1101/gad.240102.
- Noon EPB, Barak H, Guttmann-Raviv N, Reshef R. Interplay between activin and Hox genes determines the formation of the kidney morphogenetic field. Development. 2009;136(12):1995–2004. doi:10.1242/dev.035592.
- Dudley AT, Lyons KM, Robertson EJ. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 1995;9(22):2795–2807. doi:10.1101/gad.9.22.2795.
- James RG, Schultheiss TM. Patterning of the avian intermediate mesoderm by lateral plate and axial tissues. Dev Biol. 2003;253(1):109–124. doi:10.1006/dbio.2002.0863.
- Cartry J, Nichane M, Ribes V, Colas A, Riou JF, Pieler T, Dolle E, Bellefroid EJ, Umbhauer M. Retinoic acid signaling is required for specification of pronephric cell fate. Dev Biol. 2006;299:35–51. doi:10.1016/j.ydbio.2006.06.047.
- Kim D, Dressler GR. PTEN modulates GDNF/RET mediated chemotaxis and branching morphogenesis in the developing kidney. Dev Biol. 2007;307(2):290–299. doi:10.1016/j.ydbio.2007.04.051.
- Moriya N, Uchiyama H, Asashima M. Induction of pronephric tubules by activin and retinoic acid in presumptive ectoderm of Xenopus laevis. Dev Grow Differ. 1993;35:123–128. doi:10.1111/j.1440-169X.1993.00123.x.
- Osafune K, Nishinakamura R, Komazaki S, Asashima M. In vitro induction of the pronephric duct in Xenopus explants. Dev Growth Differ. 2002;44(2):161–167. doi:10.1046/j.1440-169x.2002.00631.x.
- Taira M, Jamrich M, Good PJ, Dawid IB. The LIM domain-containing homeo box gene Xlim-1 is expressed specifically in the organizer region of Xenopus gastrula embryos. Genes Dev. 1992;6(3):356–366. doi:10.1101/gad.6.3.356.
- Vigneau C, Polgar K, Striker G, Elliott J, Hyink D, Weber O, Fehling HJ, Keller G, Burrow C, Wilson P. Mouse embryonic stem cell–derived embryoid bodies generate progenitors that integrate long term into renal proximal tubules in vivo. J Am Soc Nephrol. 2007;18(6):1709–1720. doi:10.1681/ASN.2006101078.
- Wingert RA, Selleck R, Yu J, Song HD, Chen Z, Song A, Zhou Y, Thisse B, Thisse C, McMahon AP, et al. The cdx genes and retinoic acid control the positioning and segmentation of the zebrafish pronephros. PLoS Genet. 2007;3(10):1922–1938. doi:10.1371/journal.pgen.0030189.
- Takasato M, Pei XE, Chiu HS, Maier B, Baillie GJ, Ferguson C, Parton RG, Wolvetang EJ, Roost MS, de Sousa Lopes SMC, et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature. 2015;526(7574):564–568. doi:10.1038/nature15695.
- Romagnani P, Lasagni L, Remuzzi G. Renal progenitors: an evolutionary conserved strategy for kidney regeneration. Nat Rev Nephrol. 2013;9:137–146. doi:10.1038/nrneph.2012.290.
- Wessely O, Tran U. Xenopus pronephros development—past, present, and future. Pediatr Nephrol. 2011;26(9):1545–1551. doi:10.1007/s00467-011-1881-2.
- Lawlor KT, Zappia L, Lefevre J, Park JS, Hamilton NA, Oshlack A, Little MH, Combes AN. Nephron progenitor commitment is a stochastic process influenced by cell migration. Elife. 2019;8:e41156. doi:10.7554/eLife.41156.
- Barak H, Huh SH, Chen S, Jeanpierre C, Martinovic J, Parisot M, Bole-Feysot C, Nitschké P, Salomon R, Antignac C, et al. FGF9 and FGF20 maintain the stemness of nephron progenitors in mice and man. Dev Cell. 2012;22(6):1191–1207. doi:10.1016/j.devcel.2012.04.018.
- Brown AC, Adams D, de Caestecker M, Yang X, Friesel R, Oxburgh L. FGF/EGF signaling regulates the renewal of early nephron progenitors during embryonic development. Development. 2011;138(23):5099–5112. doi:10.1242/dev.065995.
- Dudley AT, Godin RE, Robertson EJ. Interaction between FGF and BMP signaling pathways regulates development of metanephric mesenchyme. Genes Dev. 1999;13(12):1601–1613. doi:10.1101/gad.13.12.1601.
- Grieshammer U, Cebrián C, Ilagan R, Meyers E, Herzlinger D, Martin GR. FGF8 is required for cell survival at distinct stages of nephrogenesis and for regulation of gene expression in nascent nephrons. Development. 2005;132(17):3847–3857. doi:10.1242/dev.01944.
- Hartwig S, Ho J, Pandey P, MacIsaac K, Taglienti M, Xiang M, Alterovitz G, Ramoni M, Fraenkel E, Kreidberg JA. Genomic characterization of Wilms’ tumor suppressor 1 targets in nephron progenitor cells during kidney development. Development. 2010;137(7):1189–1203. doi:10.1242/dev.045732.
- Karner CM, Das A, Ma Z, Self M, Chen C, Lum L, Oliver G, Carroll TJ. Canonical Wnt9b signaling balances progenitor cell expansion and differentiation during kidney development. Development. 2011;138(7):1247–1257. doi:10.1242/dev.057646.
- Kazama I, Mahoney Z, Miner JH, Graf D, Economides AN, Kreidberg JA. Podocyte-derived BMP7 is critical for nephron development. J Am Soc Nephrol. 2008;19(11):2181–2191. doi:10.1681/ASN.2007111212.
- Luo G, Hofmann C, Bronckers AL, Sohocki M, Bradley A, Karsenty G. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev. 1995;9(22):2808–2820. doi:10.1101/gad.9.22.2808.
- Majumdar A, Vainio S, Kispert A, McMahon J, McMahon AP. Wnt11 and Ret/Gdnf pathways cooperate in regulating ureteric branching during metanephric kidney development. Development. 2003;130(14):3175–3185. doi:10.1242/dev.00520.
- Nishinakamura R, Osafune K. Essential roles of Sall family genes in kidney development. J Physiol Sci. 2006;56(2):131–136. doi:10.2170/physiolsci.M95.
- O’Brien LL, Combes AN, Short KM, Lindström NO, Whitney PH, Cullen-McEwen LA, Ju A, Abdelhalim A, Michos O, Bertram JF, et al. Wnt11 directs nephron progenitor polarity and motile behavior ultimately determining nephron endowment. Elife. 2018;7:e40392. doi:10.7554/eLife.40392.
- Perantoni AO, Timofeeva O, Naillat F, Richman C, Pajni-Underwood S, Wilson C, Vainio S, Dove LF, Lewandoski M. Inactivation of FGF8 in early mesoderm reveals an essential role in kidney development. Development. 2005;132(17):3859–3871. doi:10.1242/dev.01945.
- Poladia DP, Kish K, Kutay B, Hains D, Kegg H, Zhao H, Bates CM. Role of fibroblast growth factor receptors 1 and 2 in the metanephric mesenchyme. Dev Biol. 2006;291(2):325–339. doi:10.1016/j.ydbio.2005.12.034.
- Mugford JW, Yu J, Kobayashi A, McMahon AP. High-resolution gene expression analysis of the developing mouse kidney defines novel cellular compartments within the nephron progenitor population. Dev Biol. 2009;333(2):312–323. doi:10.1016/j.ydbio.2009.06.043.
- Brown AC, Muthukrishnan SD, Oxburgh L. A synthetic niche for nephron progenitor cells. Dev Cell. 2015;34(2):229–241. doi:10.1016/j.devcel.2015.06.021.
- Brown AC, Muthukrishnan SD, Guay JA, Adams DC, Schafer DA, Fetting JL, Oxburgh L. Role for compartmentalization in nephron progenitor differentiation. Proc Natl Acad Sci USA. 2013;110(12):4640–4645. doi:10.1073/pnas.1213971110.
- Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell. 2005;9(2):283–292. doi:10.1016/j.devcel.2005.05.016.
- Park JS, Valerius MT, McMahon AP. Wnt/β-catenin signaling regulates nephron induction during mouse kidney development. Development. 2007;134(13):2533–2539. doi:10.1242/dev.006155.
- Hartman HA, Lai HL, Patterson LT. Cessation of renal morphogenesis in mice. Dev Biol. 2007;310(2):379–387. doi:10.1016/j.ydbio.2007.08.021.
- Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, McMahon AP. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell. 2008;3(2):169–181. doi:10.1016/j.stem.2008.05.020.
- Costantini F, Kopan R. Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Dev Cell. 2010;18(5):698–712. doi:10.1016/j.devcel.2010.04.008.
- Kispert A, Vainio S, McMahon AP. Wnt-4 is a mesenchymal signal for epithelial transformation of metanephric mesenchyme in the developing kidney. Development. 2008;125:4225–4234.
- Kuure S, Popsueva A, Jakobson M, Sainio K, Sariola H. Glycogen synthase kinase-3 inactivation and stabilization of β-catenin induce nephron differentiation in isolated mouse and rat kidney mesenchymes. J Am Soc Nephrol. 2007;18(4):1130–1139. doi:10.1681/ASN.2006111206.
- Self M, Lagutin OV, Bowling B, Hendrix J, Cai Y, Dressler GR, Oliver G. Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. Embo J. 2006;25(21):5214–5228. doi:10.1038/sj.emboj.7601381.
- Cho EA, Patterson LT, Brookhiser WT, Mah S, Kintner C, Dressler GR. Differential expression and function of cadherin-6 during renal epithelium development. Development. 1998;125:803–812.
- Combes AN, Davies JA, Little MH. Cell–cell interactions driving kidney morphogenesis. Curr Top Dev Biol. 2015;112:467–508.
- Goto S, Yaoita E, Matsunami H, Kondo D, Yamamoto T, Kawasaki K, Arakawa M, Kihara I. Involvement of R-cadherin in the early stage of glomerulogenesis. J Am Soc Nephrol. 1998;9:1234–1241.
- Kimura Y, Matsunami H, Inoue T, Shimamura K, Uchida N, Ueno T, Miyazaki T, Takeichi M. Cadherin-11 expressed in association with mesenchymal morphogenesis in the head, somite, and limb bud of early mouse embryos. Dev Biol. 1995;169(1):347–358. doi:10.1006/dbio.1995.1149.
- Klein G, Langegger M, Goridis C, Ekblom P. Neural cell adhesion molecules during embryonic induction and development of the kidney. Development. 1998;102:749–761.
- Mah SP, Saueressig H, Goulding M, Kintner C, Dressler GR. Kidney development in cadherin-6 mutants: delayed mesenchyme-to-epithelial conversion and loss of nephrons. Dev Biol. 2000;223(1):38–53. doi:10.1006/dbio.2000.9738.
- Vestweber D, Kemler R, Ekblom P. Cell-adhesion molecule uvomorulin during kidney development. Dev Biol. 1985;112(1):213–221. doi:10.1016/0012-1606(85)90135-6.
- Yang Z, Zimmerman S, Brakeman PR, Beaudoin GM, Reichardt LF, Marciano DK. De novo lumen formation and elongation in the developing nephron: a central role for afadin in apical polarity. Development. 2013;140(8):1774–1784. doi:10.1242/dev.087957.
- Elias BC, Das A, Parekh DV, Mernaugh G, Adams R, Yang Z, Brakebusch C, Pozzi A, Marciano DK, Carroll TJ, et al. Cdc42 regulates epithelial cell polarity and cytoskeletal function during kidney tubule development. J Cell Sci. 2015;128(23):4293–4305. doi:10.1242/jcs.164509.
- Georgas K, Rumballe B, Valerius MT, Chiu HS, Thiagarajan RD, Lesieur E, Aronow BJ, Brunskill EW, Combes AN, Tang D, et al. Analysis of early nephron patterning reveals a role for distal RV proliferation in fusion to the ureteric tip via a cap mesenchyme-derived connecting segment. Dev Biol. 2009;332(2):273–286. doi:10.1016/j.ydbio.2009.05.578.
- Little MH, Brennan J, Georgas K, Davies JA, Davidson DR, Baldock RA, Beverdam A, Bertram JF, Capel B, Chiu HS. Clements DA high-resolution anatomical ontology of the developing murine genitourinary tract. Gene Expr Patterns. 2007;7(6):680–699. doi:10.1016/j.modgep.2007.03.002.
- Gerlach GF, Wingert RA. Zebrafish pronephros tubulogenesis and epithelial identity maintenance are reliant on the polarity proteins Prkc iota and zeta. Dev Biol. 2014;396(2):183–200. doi:10.1016/j.ydbio.2014.08.038.
- Marciano DK. A holey pursuit: lumen formation in the developing kidney. Pediatr Nephrol. 2017;32(1):7–20. doi:10.1007/s00467-016-3326-4.
- Goodrich LV, Strutt D. Principles of planar polarity in animal development. Development. 2011;138(10):1877–1892. doi:10.1242/dev.054080.
- Lienkamp SS, Liu K, Karner CM, Carroll TJ, Ronneberger O, Wallingford JB, Walz G. Vertebrate kidney tubules elongate using a planar cell polarity–dependent, rosette-based mechanism of convergent extension. Nat Genet. 2012;44(12):1382–1387. doi:10.1038/ng.2452.
- Kunimoto K, Bayly RD, Vladar EK, Vonderfecht T, Gallagher AR, Axelrod JD. Disruption of core planar cell polarity signaling regulates renal tubule morphogenesis but is not cystogenic. Curr Biol. 2017;27(20):3120–3131. doi:10.1016/j.cub.2017.09.011.
- Adler PN. The frizzled/stan pathway and planar cell polarity in the Drosophila wing. Curr Top Dev Biol. 2012;101:1–31.
- Dong B, Vold S, Olvera-Jaramillo C, Chang H. Functional redundancy of Frizzled 3 and Frizzled 6 in planar cell polarity control of mouse hair follicles. Development. 2018;145(19):dev168468. doi:10.1242/dev.168468.
- He CW, Liao CP, Chen CK, Teulière J, Chen CH, Pan CL. The polarity protein VANG-1 antagonizes Wnt signaling by facilitating Frizzled endocytosis. Development. 2018;145(24):dev168666. doi:10.1242/dev.168666.
- Seifert JR, Mlodzik M. Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat Rev Genet. 2007;8(2):126–138. doi:10.1038/nrg2042.
- Yang Y, Mlodzik M. Wnt-Frizzled/planar cell polarity signaling: cellular orientation by facing the wind (Wnt). Annu Rev Cell Dev Biol. 2015;31:623–646. doi:10.1146/annurev-cellbio-100814-125315.
- Karner CM, Chirumamilla R, Aoki S, Igarashi P, Wallingford JB, Carroll TJ. Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat Genet. 2009;41(7):793–799. doi:10.1038/ng.400.
- Lindström NO, Hohenstein P, Davies JA. Nephrons require Rho-kinase for proximal-distal polarity development. Sci Rep. 2013;3(1):1–8. doi:10.1038/srep02692.
- Meyer TN, Schwesinger C, Sampogna RV, Vaughn DA, Stuart RO, Steer DL, Bush KT, Nigam SK. Rho kinase acts at separate steps in ureteric bud and metanephric mesenchyme morphogenesis during kidney development. Differentiation. 2006;74(9‐10):638–647. doi:10.1111/j.1432-0436.2006.00102.x.
- Bagherie-Lachidan M, Reginensi A, Pan Q, Zaveri HP, Scott DA, Blencowe BJ, Helmbacher F, McNeill H. Stromal Fat4 acts non-autonomously with Dchs1/2 to restrict the nephron progenitor pool. Development. 2015;142(15):2564–2573. doi:10.1242/dev.122648.
- Happé H, Leonhard WN, van der Wal A, van de Water B, Lantinga-van Leeuwen IS, Breuning MH, de Heer E, Peters DJ. Toxic tubular injury in kidneys from Pkd1-deletion mice accelerates cystogenesis accompanied by dysregulated planar cell polarity and canonical Wnt signaling pathways. Hum Mol Genet. 2009;18(14):2532–2542. doi:10.1093/hmg/ddp190.
- Mao Y, Francis-West P, Irvine KD. Fat4/Dchs1 signaling between stromal and cap mesenchyme cells influences nephrogenesis and ureteric bud branching. Development. 2015;142(15):2574–2585. doi:10.1242/dev.122630.
- Saburi S, Hester I, Fischer E, Pontoglio M, Eremina V, Gessler M, Quaggin SE, Harrison R, Mount R, McNeill H. Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet. 2008;40(8):1010. doi:10.1038/ng.179.
- Vasilyev A, Liu Y, Mudumana S, Mangos S, Lam PY, Majumdar A, Zhao J, Poon KL, Kondrychyn I, Korzh V, et al. Collective cell migration drives morphogenesis of the kidney nephron. PLoS Biol. 2009;7(1):e9. doi:10.1371/journal.pbio.1000009.
- Naylor RW, Dodd RC, Davidson AJ. Caudal migration and proliferation of renal progenitors regulates early nephron segment size in zebrafish. Sci Rep. 2016;6(1):1–14. doi:10.1038/srep35647.
- Palmyre A, Lee J, Ryklin G, Camarata T, Selig MK, Duchemin AL, Nowak P, Arnaout MA, Drummond IA, Vasilyev A. Collective epithelial migration drives kidney repair after acute injury. PLoS One. 2014;9(7):e101304. doi:10.1371/journal.pone.0101304.
- Yakulov TA, Todkar AP, Slanchev K, Wiegel J, Bona A, Groß M, Scholz A, Hess I, Wurditsch A, Grahammer F, et al. CXCL12 and MYC control energy metabolism to support adaptive responses after kidney injury. Nat Commun. 2018;9(1):1–15. doi:10.1038/s41467-018-06094-4.
- Dressler GR. The cellular basis of kidney development. Annu Rev Cell Dev Biol. 2006;22:509–529. doi:10.1146/annurev.cellbio.22.010305.104340.
- Kopan R, Cheng HT, Surendran K. Molecular insights into segmentation along the proximal–distal axis of the nephron. J Am Soc Nephrol. 2007;18(7):2014–2020. doi:10.1681/ASN.2007040453.
- Combes AN, Phipson B, Lawlor KT, Dorison A, Patrick R, Zappia L, Harvey RP, Oshlack A, Little MH. Single cell analysis of the developing mouse kidney provides deeper insight into marker gene expression and ligand-receptor crosstalk. Development. 2019;146(12):dev178673. doi:10.1242/dev.178673.
- DeLay BD, Baldwin TA, Miller RK. Dynamin binding protein is required for Xenopus laevis kidney development. Front Physiol. 2019;10:143. doi:10.3389/fphys.2019.00143.
- Desgrange A, Cereghini S. Nephron patterning: lessons from xenopus, zebrafish, and mouse studies. Cells. 2015;4(3):483–499. doi:10.3390/cells4030483.
- Gerlach GF, Wingert RA. Kidney organogenesis in the zebrafish: insights into vertebrate nephrogenesis and regeneration. Wiley Interdiscip Rev Dev Biol. 2013;2(5):559–585. doi:10.1002/wdev.92.
- Kroeger JPT, Wingert RA. Using zebrafish to study podocyte genesis during kidney development and regeneration. Genesis. 2014;52(9):771–792. doi:10.1002/dvg.22798.
- Ransick A, Lindström NO, Liu J, Zhu Q, Guo JJ, Alvarado GF, Kim AD, Black HG, Kim J, McMahon AP. Single-cell profiling reveals sex, lineage, and regional diversity in the mouse kidney. Dev Cell. 2019;51(3):399–413. doi:10.1016/j.devcel.2019.10.005.
- Lindström NO, Brandine GDS, Tran T, Ransick A, Suh G, Guo J, Kim AD, Parvez RK, Ruffins SW, Rutledge EA, et al. Progressive recruitment of mesenchymal progenitors reveals a time-dependent process of cell fate acquisition in mouse and human nephrogenesis. Dev Cell. 2018b;45(5):s651–660. doi:10.1016/j.devcel.2018.05.010.
- Georgas K, Rumballe B, Wilkinson L, Chiu HS, Lesieur E, Gilbert T, Little MH. Use of dual section mRNA in situ hybridisation/immunohistochemistry to clarify gene expression patterns during the early stages of nephron development in the embryo and in the mature nephron of the adult mouse kidney. Histochem Cell Biol. 2008;130:927.
- Guo JK, Menke AL, Gubler MC, Clarke AR, Harrison D, Hammes A, Hastie ND, Schedl A. WT1 is a key regulator of podocyte function: reduced expression levels cause crescentic glomerulonephritis and mesangial sclerosis. Hum Mol Genet. 2002;11(6):651–659. doi:10.1093/hmg/11.6.651.
- Chen L, Al-Awqati Q. Segmental expression of Notch and Hairy genes in nephrogenesis. Am J Physiol Renal Physiol. 2005;288(5):F939–F952. doi:10.1152/ajprenal.00369.2004.
- Cheng HT, Kopan R. The role of Notch signaling in specification of podocyte and proximal tubules within the developing mouse kidney. Kidney Int. 2005;68(5):1951–1952. doi:10.1111/j.1523-1755.2005.00627.x.
- Cheng HT, Kim M, Valerius MT, Surendran K, Schuster-Gossler K, Gossler A, McMahon AP, Kopan R. Notch2, but not Notch1, is required for proximal fate acquisition in the mammalian nephron. Development. 2007;134(4):801–811. doi:10.1242/dev.02773.
- McCright B, Lozier J, Gridley T. A mouse model of Alagille syndrome: Notch2 as a genetic modifier of Jag1 haploinsufficiency. Development. 2002;129:1075–1082.
- Marra AN, Wingert RA. Roles of iroquois transcription factors in kidney development. Cell Dev Biol. 2014;3(1):1000131. doi:10.4172/2168-9296.1000131.
- Reggiani L, Raciti D, Airik R, Kispert A, Brändli AW. The prepattern transcription factor Irx3 directs nephron segment identity. Genes Dev. 2007;21(18):2358–2370. doi:10.1101/gad.450707.
- Wingert RA, Davidson AJ. Zebrafish nephrogenesis involves dynamic spatiotemporal expression changes in renal progenitors and essential signals from retinoic acid and irx3b. Dev Dyn. 2011;240(8):2011–2027. doi:10.1002/dvdy.22691.
- Heliot C, Desgrange A, Buisson I, Prunskaite-Hyyryläinen R, Shan J, Vainio S, Umbhauer M, Cereghini S. HNF1B controls proximal-intermediate nephron segment identity in vertebrates by regulating Notch signalling components and Irx1/2. Development. 2013;140(4):873–885. doi:10.1242/dev.086538.
- Naylor RW, Przepiorski A, Ren Q, Yu J, Davidson AJ. HNF1β is essential for nephron segmentation during nephrogenesis. J Am Soc Nephrol. 2013;24(1):77–87. doi:10.1681/ASN.2012070756.
- Kobayashi A, Kwan KM, Carroll TJ, McMahon AP, Mendelsohn CL, Behringer RR. Distinct and sequential tissue-specific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development. Development. 2005;132(12):2809–2823. doi:10.1242/dev.01858.
- Nakai S, Sugitani Y, Sato H, Ito S, Miura Y, Ogawa M, Nishi M, Jishage KI, Minowa O, Noda T. Crucial roles of Brn1 in distal tubule formation and function in mouse kidney. Development. 2003;130(19):4751–4759. doi:10.1242/dev.00666.
- Chambers BE, Gerlach GF, Clark EG, Chen KH, Levesque AE, Leshchiner I, Goessling W, Wingert RA. Tfap2a is a novel gatekeeper of nephron differentiation during kidney development. Development. 2019;146(13):dev172387. doi:10.1242/dev.172387.
- Chambers BE, Clark EG, Gatz AE, Wingert RA. Kctd15 regulates nephron segment development by repressing Tfap2a activity. Development. 2020: dev.191973 doi: 10.1242/dev.191973.
- Chambers JM, Poureetezadi SJ, Addiego A, Lahne M, Wingert RA. ppargc1a controls nephron segmentation during zebrafish embryonic kidney ontogeny. Elife. 2018;7:e40266. doi:10.7554/eLife.40266.
- Cheng CN, Wingert RA. Nephron proximal tubule patterning and corpuscles of Stannius formation are regulated by the sim1a transcription factor and retinoic acid in zebrafish. Dev Biol. 2015;399:100–116. doi:10.1016/j.ydbio.2014.12.020.
- Drummond BE, Li Y, Marra AN, Cheng CN, Wingert RA. The tbx2a/b transcription factors direct pronephros segmentation and corpuscle of Stannius formation in zebrafish. Dev Biol. 2016;421:52–66. doi:10.1016/j.ydbio.2016.10.019.
- Kroeger PT Jr, Drummond BE, Miceli R, McKernan M, Gerlach GF, Marra AN, Fox A, McCampbell KK, Leshchiner I, Rodriguez-Mari A, et al. The zebrafish kidney mutant zeppelin reveals that brca2/fancd1 is essential for pronephros development. Dev Biol. 2017;428(1):148–163. doi:10.1016/j.ydbio.2017.05.025.
- Marra AN, Wingert RA. Epithelial cell fate in the nephron tubule is mediated by the ETS transcription factors etv5a and etv4 during zebrafish kidney development. Dev Biol. 2016;411:231–245. doi:10.1016/j.ydbio.2016.01.035.
- Marra AN, Adeeb BD, Chambers BE, Drummond BE, Ulrich M, Addiego A, Springer M, Poureetezadi SJ, Chambers JM, Ronshaugen M, et al. Prostaglandin signaling regulates renal multiciliated cell specification and maturation. Proc Natl Acad Sci USA. 2019a;116(17):8409–8418. doi:10.1073/pnas.1813492116.
- Marra AN, Cheng CN, Adeeb B, Addiego A, Wesselman HM, Chambers BE, Chambers JM, Wingert RA. Iroquois transcription factor irx2a is required for multiciliated and transporter cell fate decisions during zebrafish pronephros development. Sci Rep. 2019b;9:6454. doi:10.1038/s41598-019-42943-y.
- Morales EM, Handa N, Drummond BE, Chambers JM, Marra AN, Addiego A, Wingert RA. Homeogene emx1 is required for nephron distal segment development in zebrafish. Sci Rep. 2018;8:18038. doi:10.1038/s41598-018-36061-4.
- Poureetezadi SJ, Cheng CN, Chambers JM, Drummond BE, Wingert RA. Prostaglandin signaling regulates nephron segment patterning of renal progenitors during zebrafish kidney development. Elife. 2016;5:e17551.
- Lindström NO, Sealfon R, Chen X, Parvez R, Ransick A, Brandine GDS, Guo J, Hill B, Tran T, Kim AD, et al. 2020. Spatial transcriptional mapping of the human nephrogenic program. bioRxiv 2020.04.27.060749; doi: 10.1101/2020.04.27.060749.
- Saraga-Babić M, Vukojević K, Bočina I, Drnašin K, Saraga M. Ciliogenesis in normal human kidney development and post-natal life. Pediatr Nephrol. 2012;27(1):55–63. doi:10.1007/s00467-011-1941-7.
- Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol. 2002;13(10):2508–2516. doi:10.1097/01.ASN.0000029587.47950.25.
- Corkins ME, Krneta-Stankic V, Kloc M, McCrea PD, Gladden AB, Miller RK. Divergent roles of the Wnt/PCP Formin Daam1 in renal ciliogenesis. PLoS One. 2019;14(8):e0221698. doi:10.1371/journal.pone.0221698.
- Obara T, Mangos S, Liu Y, Zhao J, Wiessner S, Kramer-Zucker AG, Olale F, Schier AF, Drummond IA. Polycystin-2 immunolocalization and function in zebrafish. J Am Soc Nephrol. 2006;17(10):2706–2718. doi:10.1681/ASN.2006040412.
- Sun Z, Hopkins N. vhnf1, the MODY5 and familial GCKD-associated gene, regulates regional specification of the zebrafish gut, pronephros, and hindbrain. Genes Dev. 2001;15(23):3217–3229. doi:10.1101/gad946701.
- Marra AN, Li Y, Wingert RA. Antennas of organ morphogenesis: the roles of cilia in vertebrate kidney development. Genesis. 2016;54(9):457–469. doi:10.1002/dvg.22957
- Denker BM, Sabath E. The biology of epithelial cell tight junctions in the kidney. J Am Soc Nephrol. 2011;22(4):622–625. doi:10.1681/ASN.2010090922.
- Hou J. The kidney tight junction. Int J Mol Med. 2014;34(6):1451–1457. doi:10.3892/ijmm.2014.1955.
- McKee R, Gerlach GF, Jou J, Cheng CN, Wingert RA. Temporal and spatial expression of tight junction genes during zebrafish pronephros development. Gene Expr Patterns. 2014;16(2):104–113. doi:10.1016/j.gep.2014.11.001.
- Ebnet K, Suzuki A, Ohno S, Vestweber D. Junctional adhesion molecules (JAMs): more molecules with dual functions? J Cell Sci. 2004;117(1):19–29. doi:10.1242/jcs.00930.
- Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123(6):1777–1788. doi:10.1083/jcb.123.6.1777.
- Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and-2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141(7):1539–1550. doi:10.1083/jcb.141.7.1539.
- Lindström NO, Guo J, Kim AD, Tran T, Guo Q, Brandine GDS, Ransick A, Parvez RK, Thornton ME, Basking L, et al. Conserved and divergent features of mesenchymal progenitor cell types within the cortical nephrogenic niche of the human and mouse kidney. J Am Soc Nephrol. 2018a;29:806–824.
- El-Dahr SS, Aboudehen K, Saifudeen Z. Transcriptional control of terminal nephron differentiation. Am J Physiol Renal Physiol. 2008;294(6):F1273–F1278. doi:10.1152/ajprenal.00562.2007.
- Svensson K, Schnyder S, Cardel B, Handschin C. Loss of renal tubular PGC-1α exacerbates diet-induced renal steatosis and age-related urinary sodium excretion in mice. PLoS One. 2016;11(7):e0158716. doi:10.1371/journal.pone.0158716.
- Harding SD, Armit C, Armstrong J, Brennan J, Cheng Y, Haggarty B, Houghton D, Lloyd-MacGilp S, Pi X, Roochun Y, et al. The GUDMAP database – an online resource for genitourinary research. Development. 2001;138:2845–2853. doi:10.1242/dev.063594.
- McMahon AP, Aronow BJ, Davidson DR, Davies JA, Gaido KW, Grimmond S, Lessard JL, Little MH, Potter SS, Wilder EL, et al. GUDMAP project. GUDMAP: the genitourinary developmental molecular anatomy project. J Am Soc Nephrol. 2008;19:667–671. doi:10.1681/ASN.2007101078.
