ABSTRACT
Keratinization provides tolerance to desiccation and mechanical durability. Loricrin, which is an epidermal thiol-rich protein, efficiently stabilizes terminally differentiated keratinocytes and maintains redox homeostasis. The discovery of the largely asymptomatic loricrin knockout (LKO) phenotype decades ago was rather unpredicted. Nevertheless, when including redox-driven, NF-E2-related factor 2-mediated backup responses, LKO mice provide opportunities for the observation of altered or “quasi-normal” homeostasis. Specifically, given that the tissue structure, as well as the local metabolism, transmits immunological signals, we sought to dissect the consequence of truncated epidermal differentiation program from immunological perspectives. Through a review of the aggregated evidence, we have attempted to generate an integrated view of the regulation of the peripheral immune system, which possibly occurs within the squamous epithelial tissue with truncated differentiation. This synthesis might not only provide insights into keratinization but also lead to the identification of factors intrinsic to the epidermis that imprint the immune effector function.
Introduction
Facing the external environment, the epithelial tissue serves as an insulation barrier. The regulation of the paracellular permeability through cell-cell adhesion is a universal strategy for insulation. The prime example could be the intercellular adhesion complex tight junction (TJ), whose components and functions are evolutionary ancientCitation.1 Transmembrane proteins, such as claudins or occludins, are thought to represent the diffusion barriers, and decreased expression of claudin 1 is associated with ichthyosisCitation1 and atopic dermatitis (AD) in humans.Citation2 Another strategy could be active secretion of protective substances, such as mucus or lipids, to the surface area. Lamellar granules (LGs), which function as lipid storage and secretory organelles, are conserved across many cell types.Citation3 For instance, LG-derived lipid film on the cell surface optimizes lung gas exchange and protects against aggressive gastric juice.Citation3 To cope with the harsh terrestrial environment, the stratified squamous epithelium epidermis is armed with a specialized barrier, the stratum corneum (SC). Densely packed keratin intermediate filaments provide the epidermis with mechanical durability, and LG-derived, covalently bound lipids confer desiccation tolerance, both of which are compared to “the brick” and “the mortar,” respectively.Citation4 The epidermal differentiation program promotes the assembly of the macromolecular complex, the cornified cell envelopes (CE).Citation5 For the establishment of “the mortar,” the earlier CE precursors envoplakin/periplakin/involucrin participates in the formation of the lipid-based outer coat corneocyte lipid envelope (CLE),Citation6 thus serving as a scaffold for the lipid-based paracellular permeability barrier.Citation7 For the reinforcement of “the brick,” the later CE precursor loricrin (LOR) facilitates corneocyte maturation through promoting the cross-linking of cytoskeletal proteins at the cell periphery.Citation4 Following the fundamental principle that desiccation tolerance the priority in terrestrial life, recent studies on medical genetics have revealed the pathogenic importance of the “broken mortar” situation. This leads to various degrees of epidermal hyperkeratosis.Citation8 Given that keratinization is considered an example of adaptive tissue responses, the ichthyosiform hyperkeratosis may be an epitome of tissue maladaptation compensating for structural defects or metabolic disorders.Citation9
The “thiol-based sensor–effector apparatus” the Kelch-like erythroid cell-derived protein with the cap-n-collar homology-associated protein 1 (KEAP1)/NF-E2-related factor 2 (NRF2) signaling pathway mechanistically revealed its own importance as a regulator of keratinization.Citation10,Citation11 Several mouse models have also shown that aberrant activation of the stress signaling pathway not only lead to autosomal recessive congenital ichthyosis (ARCI)-like hyperkeratosis,Citation12,Citation13 but also contributes to the pathogenesis of the most common skin disorders, such as atopic dermatitis (AD),Citation14 psoriasis,Citation15 or acneCitation.16 In accordance with the “brick and mortar” model,Citation4 the major later CE precursor LOR was dispensable for the lipid-based SC permeability barrier per se.Citation4,Citation17 Although somewhat anticipated from the structural basis,Citation4 the discovery of the very mild LOR knockout (LKO) phenotype was counterintuitive and rather surprising.Citation17,Citation18 Nonetheless, LKO mice provided us opportunities to uncover the backup responses that maintain the quasi-homeostasis, both structurally and functionally.Citation17,Citation19–21
Because LOR is nonessential for the formation of the lipid-based SC permeability barrier,Citation17,Citation19–23 there is a need to revisit the fundamental principle of keratinization (i.e. sulfur metabolismCitation24,Citation25); it could be hypothesized that the LKO epidermal microenvironment, including altered metabolism of sulfur,Citation20 serves as an intrinsic microenvironmental cue that affects even the immune effector function. To be specific, as in AD,Citation26 the attenuated cell-mediated immune response in LKO miceCitation27,Citation28 possibly suggests the immunoanatomical principle of epidermal differentiation: structural maturation possibly establishes immunological competence, or conversely, desiccation tolerance can provide reinforcement of immunological tolerance. In broad terms, this may be an answer to the conundrum, “Why is dry-surfaced skin highly immunogenic as compared with the wet-surfaced inner mucosa?”Citation29,Citation30 This theoretical and hypothetical article, in an attempt to synthesize the structure and function of the epidermis from multiple points of view, focuses on the principle of epidermal differentiation with special attention paid to the cross talk between structure and metabolism.
Beyond gene hunting
The past several decades were an era of advances in medical genetics, which revealed previously uncharacterized pathomechanisms of inherited diseases. The major pillars of such discoveries were loss-of-function (LOF) mutations in transglutaminase 1 (TGM1),Citation31 ATP-binding cassette subfamily A member 12 (ABCA12),Citation32,Citation33 and filaggrin (FLG),Citation34 resulting in ARCI1 (lamellar ichthyosis, OMIM #190195), ARCI4B (harlequin ichthyosis, OMIM #2425500), and ichthyosis vulgaris (IV; a common dry skin condition, OMIM #146700), respectively. TGM1 catalyzes the ε-(γ-glutamyl) lysine cross-linkages between protein–protein or protein–lipid in the CLE, whereas ABCA12 transports ω-hydroxyceramides to the CLE.Citation4 FLG undergoes proteolytic cascades and ultimately serves as a humectant in corneocytes.Citation35 Because a major threat to fully terrestrial lifestyles is desiccation, higher levels of transepidermal water loss can evoke protective responses and manifest as pathologic keratinization.Citation9,Citation33 Thus, it is likely that clinical phenotypes depend on the degree of the breach of the epidermal paracellular barrier, ranging from mild, “flaky” retention keratosis in IVCitation34,Citation36 to severe, platelike hyperkeratosis in ARCI4B.Citation8,Citation32 It is important to note here again that the phenotypes are derived solely from the host’s tissue responseCitation10 rather than from the defects in structure or function. In particular, this could be the case with multigenic disorders because there is often a distance between disease-susceptibility genes and disease phenotypes. For instance, to manifest as AD, the LOF mutation in the FLG gene (ATOD2, OMIM #605803) requires another predisposing factor of internal (gene variants)Citation37,Citation38 or external origin (environmental factors). The endogenous factor not only depletes the gene expression product, but it can sometimes also evoke backup responses,Citation9,Citation17 often rendering the disease phenotypes invisible.Citation17 The regulation of gene expression is profoundly affected by an exogenous factor, thus evoking adaptive tissue responses.Citation14 Therefore, regardless of the consequences of gene variants (homeostasis, disease, or death), the phenotype consists of an integrated sequence of biological reactions that involve many forms of interactions between the surrounding environment and genes.Citation39
Cross talk between structure and epidermal metabolism: ARCI as an example
The epidermis is considered as a regenerative tissue, and impaired metabolism affects the permeability barrier of the SC.Citation8 To date, autosomal recessive conditions that are relatively rare have been a clear demonstration of the genotype–phenotype correlation, and we use ARCI as an example. As shown in , ARCI1 is caused by an LOF mutation in TGM,Citation31 which catalyzes the structural basis of CLE, and other forms of ARCI result from gene mutations due to impaired lipid metabolism (ARCI2–ARCI10 and ARCI13–ARCI14)Citation40,Citation41,Citation42,Citation43,Citation44,Citation45,Citation46,Citation47 or proteolytic processing of FLG (ARCI11–ARCI12).Citation48,Citation49,Citation50 The accumulated evidence suggests that either impairment in the formation of CLE (the “mortar”) or production of corneocyte humectant is the cause of the ARCI phenotypes, most of which are phenocopied by gene deletion in mouse. Reactive hyperkeratosis and accompanying inflammation constitute the universal ARCI phenotype, and these features are caused by the host’s adaptive response but not the gene mutations themselves.Citation10
Table 1. ARCI and causative mutated genes
Keratinization as sulfur metabolism
It is well known that epidermal differentiation involves the metabolism of sulfur, and thiol (–SH) groups of the proliferative basal layer are converted to covalent disulfide (–S–S–) bridges of keratin molecules.Citation25 In support of this principle, radioactive tracer experiments have revealed that the keratinocytes (KCs) in the uppermost living layer (stratum granulosum) uptake radiolabeled cystine (disulfide cross-linked cysteine) nearly twice as much as that in the basal or spinous layer.Citation24 These observations imply that KCs in the stratum granulosum undergo robust sulfur metabolism to finalize epidermal differentiation.Citation51 This notion could be supported by the clinical evidence indicating that the topical application of a cysteine antioxidant N-acetyl cysteine (NAC) improves the hyperkeratotic phenotype in ARCI1.Citation52 This suggests that NAC serves as a free cysteine donor (nucleophile), reduces disulfide (–S–S–) bridges, and attenuates the hyperkeratosis.
The KEAP1/NRF2 system in keratinization
The following question is raised: What causes the hyperkeratotic tissue response? Vertebrates are armed with the KEAP1/NRF2 signaling pathway, a thiol-based sensor-effector apparatus.Citation11 The phylogenetically conserved interaction between the thiol-rich cytoskeletal protein KEAP1 and transcription factor NRF2 regulates myriad stress responses.Citation11 KEAP1 forms a complex with cullin 3-based ubiquitin ligase in the steady state and subjects NRF2 to proteasome-mediated degradation in the cytosol, thus repressing the activity of NRF2.Citation10,Citation11 The oxidative stress that causes redox imbalance, such as reactive oxygen species or electrophiles, modifies KEAP1’s cysteine residues and results in conformational changes.Citation10,Citation11 This redox exchange unleashes NRF2’s transcriptional activity and upregulates the detoxification/antioxidant enzyme, ATP-binding cassette transporters, and other biomolecules that are associated with stress responses.Citation53 However, witnessing the consequence of the genetic deletion of Keap1 in mice would have been surprising. Although the Keap1-null mutation systemically enhanced the detoxifying responses, the mice suffered from postnatal malnutrition because of keratinized materials that filled the lumina of the esophagus and forestomach.Citation13 The concomitant deletion of NRF2 rescued the severe phenotype, which suggests that the KEAP1/NRF2 system is an important keratinization regulator.Citation13 In accordance with the inner squamous epithelium phenotype, the epidermis exhibited orthohyperkeratosis, which somewhat resembled ARCI.Citation13 In another setting, the ARCI-like hyperkeratosis was reproducible, in which mutant NRF2 incapable of interacting with KEAP1 is overexpressed in the epidermis.Citation12 Taken together, the KEAP1/NRF2 system serves as a central regulator of sulfur metabolism and thus keratinization in the epidermis.Citation10
The consequence of the absent epidermal thiol-rich protein
Despite the fact that the keratin intermediate filament constitutes the structural backbone of the epidermis, the amino acid composition of the differentiated keratins K1/K10 cannot explain the unusually high sulfur content in the differentiated epidermal layer.Citation51 Therefore, researchers hypothesized the existence of a sulfur-rich CE precursor, and it was not until the 1990s that Mehrel and Roop et al. cloned and characterized the sulfur-rich CE precursor LOR.Citation51 Subsequently, LKO mice were generated, but because CE maturation does not take place in submerged KC cultures,Citation5,Citation54 the mouse model taught us the consequence of the loss of the major thiol-rich epidermal structural protein. The most important finding from LKO mice is that LOR is nonessential for the lipid-based SC permeability barrierCitation17 but is mandatory for corneocyte maturation.Citation23,Citation55 We attribute the rather unpredicted outcome at that time to the following two causes. First, the LG system is ubiquitous and is even observed in nonepithelial tissue.Citation3 Second, the CLE forms outside corneocytes subsequent to scaffolding, and the following reinforcement process precipitates disulfide (–S–S–) at the inner periphery, but not the outside, of corneocytes.Citation4 An additional important finding is the induction of a compensatory response, which induces a massive amount of the cross-bridging CE precursor, the small proline-rich protein (SPRR)Citation17,Citation20 or the late cornified envelope (LCE).Citation19 These alternative reinforcing CE components are somewhat structurally similar to LOR but less efficient executors of ε-(γ-glutamyl) lysine or disulfide (–S–S) cross-linkages.Citation19 Consequently, the LKO epidermis suffers from structural fragility, rather than an impaired SC permeability barrier.Citation17 Nonetheless, as was the case with ARCI, which can be caused by defects in either the structure or the metabolism,Citation8 LKO affects the epidermal sulfur metabolism; KEAP1 senses the cytosolic redox imbalance, and NRF2 serves as an important effector that massively induces the SPRRCitation20 or the LCE.Citation19 Because both the SPRRCitation56,Citation57 and LCECitation58,Citation59 are induced in response to primary injury, such as ultraviolet irradiation or tape stripping, it may be likely that the LKO phenocopies an adaptive tissue response, and the superior nucleophilic property of SPRRCitation56 would prevent further oxidative stress-mediated tissue injury, alongside enhanced LG biogenesis (unpublished observation).
Metabolic control of the adaptive tissue response
Now that we know that LKO affects the epidermal metabolism rather than the lipid-based SC permeability barrier, let us take (1) the electrophilic counterattack responseCitation60 and (2) contact hypersensitivity (CHS)Citation61 as redox-mediated adaptive tissue responses.
7,12-dimethylbenz(a)anthracene (DMBA) is a prototypic environmental carcinogen. DMBA is converted into the ultimate mutagenic carcinogen DMBA-3,4-diol-1,2-epoxide (DMBADE) through the cytochrome P450-mediated metabolic conversion of the host.Citation62 DMBADE possesses a strong electrophilic property, forms adducts with genomic DNA, and exerts genotoxicity.Citation11 The host’s antioxidative response is raised by a weak electrophilic stress, which could protect against higher doses of the same or other electrophiles, including the carcinogen. Because the KEAP1/NRF2 signaling pathway plays a pivotal role in phase II detoxification and is activated in LKO mice, we questioned whether the structural protein LOR, which promotes corneocyte maturation,Citation23,Citation55 is simply chemopreventive or whether the NRF2-mediated compensatory response in LKO mice could be equivalent to the electrophilic counterattack response.Citation60 In line with the susceptibility against ultraviolet B,Citation55 LOR protected against the chemical carcinogen DMBA.Citation28
CHS is a cell-mediated adaptive tissue response in which a strong, antigen-specific cytotoxic immune response is evoked at a place distant from the primary exposure site.Citation63 Most reactive haptens are electrophilic and potentially activate the KEAP1/NRF2 signaling pathway.Citation14 Nevertheless, if the concentration is low enough or devoid of irritancy (such as the lipophilicity),Citation64 CHS does not ensue, and this phenomenon is known as low zone tolerance.Citation65 Similar to the defects in the lipid-based SC permeability barrierCitation37,Citation38 or FLG deficiency,Citation34 reduced tolerance to desiccation might increase sensitivity. However, it was found that LKO mice tolerated desiccation well, LG biosynthesis was increased (unpublished observations), and corneocytes were abundant with unlinked FLG.Citation23,Citation55 Accordingly, we recently discovered that LKO mice exhibited attenuated CHS in the effector phase.Citation28
Immunoanatomical bases of the squamous epithelium
Both CHS and tumor eradication involve cytotoxic immune responses that are strong, self-destructive, and potentially harmful.Citation61 Regardless of the antigens involved, the epidermis is more immunogenic than the mucosal epithelium.Citation66 For instance, one of the most common sites of graft-versus-host reaction and adverse drug reaction is the skin.Citation67 Likewise, percutaneous entry of allergens favors type 2 immunological memory (atopy), whereas permucosal routes lead to tolerance (regulatory T-cell memory).Citation29 It is presumed that the epidermal tissue renders immunogenicity, which might make it a more amenable target organ for topical immunotherapy or immune checkpoint inhibitionCitation68 as compared with nonkeratinizing squamous mucosa.Citation69,Citation70 We compared, from an immunoanatomical perspective, the epidermis with nonkeratinizing squamous epithelia.
The cornea covers the eye’s anterior chamber surface and allows efficient penetration of visible light. Because clear eyesight requires corneal transparency, the eye needs to tolerate foreign materials, such as microbes or viruses.Citation66,Citation71 This strategic hyporesponsiveness (attenuated cytotoxicity) – or more precisely, tailor-made immune effector function – has been simply referred to as the immune privilege or the anterior chamber-associated immune deviation.Citation71 This immunoanatomical peculiarity renders corneal transplantation one of the most successful solid organ graft procedures, with success rates as high as 90%.Citation71 In cases of severe herpetic viral infection, the stromal Fas ligand prevents the destruction of the immune-mediated tissue via the induction of apoptotic death of infiltrating effector cells.Citation72 By contrast, antigen-presenting cells deliver tissue-associated signals and maintain immune homeostasis in the steady state,Citation73 but autoimmune disorders can result from the absence of dendritic cells (DCs).Citation74,Citation75 Squamous epithelia, including the cornea, are home to Langerhans cells (LCs), which sample tissue antigens and migrate to a secondary lymphoid organ.Citation76,Citation77 By definition, LCs constitutively express major histocompatibility complex (MHC) class II antigen and C-type lectin receptor CD207/Langerin,Citation76,Citation77 corneal LCs lack MHC class IICitation78 but express CD207 and CD11c (the conventional DC marker).Citation79 Because of the importance of CD207 in maintaining tolerance in the squamous epitheliumCitation76 as well as the intestinal epithelium,Citation80 it is conceivable that the corneal epithelial LCs may endow the nonkeratinizing epithelium with a protective barrierCitation81 via tolerizing exogenous agents that invade through the paracellular route, such as bacteria or viruses.
In the same vein, permucosal exposure of foreign antigens, particularly through the intestinal epithelium, favors tolerance (regulatory T-cell memory), whereas exposure through the broken SC permeability barrier breaks tolerance.Citation66 It is worth noting, however, that the percutaneous route does not necessarily break tolerance (and thus atopy) because the induction of transcutaneous tolerance against exogenous antigens is feasible.Citation82 Instead, it would be legitimate to consider that the nature (or the class) of the immune responses depends on the status of the tissue in which the biological response arises.Citation66
Metabolic imprinting of cutaneous immune effector functions
Although myriad epithelial tissue-derived cytokines that skew immune effector classes toward type 2 immunity, such as interleukin (IL)-33, IL-25, or thymic stromal lymphopoietin,Citation83 have been characterized over the past decades, the important example of the metabolic cues mediated by the lipophilic nutrients vitamin A (VA) and vitamin D (VD).Citation84 It appears that the VA and VD signaling pathways work somewhat antagonistically in the epidermis. The former promotes the hydrolysis of polar lipids and plays a role in the maturation of lipid-based SC permeability (the “mortar”),Citation85,Citation86 whereas the latter promotes the assemblage of the cytoskeletal filament network (the “brick”).Citation87 Persistent activation of epidermal VD receptor signaling through the application of calcipotriol (a low-calcemic calcitriol analog)Citation88 or the genetic ablation of the retinoid X receptorCitation89 results in the production of AD-like phenotypes, whereas systemic retinoid treatment proves useful for the management of chronic hand eczemaCitation90 as well as ARCI,Citation91 both conditions that are closely associated with “broken mortar” situations.Citation8,Citation9
When taking into consideration the regulation of immune effector functions in the periphery, lessons from VA deficiency (VAD) may be important.Citation92,Citation93 With the aid of food-derived VA, small intestine (SI) epithelial cells produce a tissue-derived cue through the metabolic cascade, which converts retinols (VA) into retinoic acids (RAs)Citation92,Citation94,Citation95 (). SI epithelial cells express retinaldehyde dehydrogenase 1 (RALDH1) and can convert cellular VA storage (retinyl esters) into RAs, which, in turn, activate RALDH2 expressed in DCs, further amplifying the tissue-derived cue.Citation92
Figure 1. Overview of the retinoid metabolism
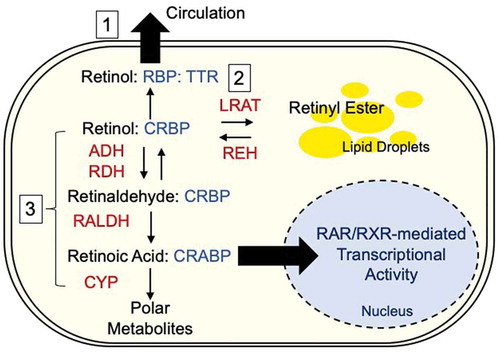
Because this positive feedback loop mediated by the RA receptor (RAR) maintains immune effector function, VAD patients with infectious diseases suffer from persistent diarrhea, which is ameliorated by VA supplementation.Citation93 Other salient features of VAD are ectopic keratinization of nonkeratinizing squamous epithelia and squamous metaplasia,Citation96 the latter of which accompanies the appearance of CD207+ cells.Citation80,Citation97 This might be analogous to the development of the epidermal LC network in mice. LC precursors that originate from the fetal liver or yolk sac are seeded into the epidermis around embryonic day 18 and expand rapidly during the neonatal periodCitation98 depending on the relatively low levels of RAR signaling in the CD11c+ lineage (conventional DC).Citation80 Similar to SI epithelium,Citation92 RALDH3-expressing epidermal KCs can augment RAR signaling,Citation99 and defective signal processing results in a dry, scaly VAD skin phenotype.Citation85,Citation86 For this reason, it is tempting to speculate that this epidermal metabolic cue profoundly affects cutaneous immune effector function and that LCs (or dermal DCs) in the vicinity of KCs deliver and/or amplify the signaling, downstream of which are cutaneous immune effector functions.Citation80
Conclusion
Because deviated cutaneous immune effector function, but not defective permeability, is unpredictable in the absence of the thiol-rich protein LOR, our interest in the immunological consequence of the absent epidermal thiol-rich protein has grown.Citation17,Citation18 However, after reviewing the evidence on this topic that has accumulated over the past decades, we acknowledge here that LOR primarily functions as the “brick” protein that establishes the epidermal sulfur metabolism, which is the la raison d’être of the epidermis, other than the lipid-based SC permeability barrier.Citation100 This important epidermal structural protein not only reinforces structural integrityCitation4 but also supports the robustness of immune effector functions, which could manifest as a strong cytotoxic immune response to CHS.Citation28 Although these preliminary findings are intriguing, the following questions persist: (1) What are the metabolic cues located downstream of LKO, other than the KEAP1/NRF2 signaling pathway? Can we define VA- or VD-mediated signaling pathways? (2) Is the LKO-mediated signaling received by LCs (or dermal DCs) merely enhanced migration or do factors intrinsic to the epidermis matter more? (3) Is DC migration merely the consequence of relative epidermal instability or any other cue (i.e. cytokines or lipid-mediated factors)? (4) Are the detuned immune effector functions analogous to anterior chamber-associated immune deviation, and if so, are deviations in the subset of LCs or other innate/adaptive immune cells induced by LKO? (5) What is the significance of having LOR in the stratified squamous epithelium, functionally, phylogenetically, and so forth?
Disclosure statement
The authors state no conflict of interest.
Additional information
Funding
References
- Zihni C, Mills C, Matter K, Balda MS. Tight junctions: from simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol. 2016;17:1–11. doi:10.1038/nrm.2016.80.
- Gruber R, Börnchen C, Rose K, Daubmann A, Volksdorf T, Wladykowski E, Vidal-Y-Sy S, Peters EM, Danso M, Bouwstra JA, et al. Diverse regulation of claudin-1 and claudin-4 in atopic dermatitis. Am J Pathol. 2015;185:2777–2789. doi:10.1016/j.ajpath.2015.06.021.
- Schmitz G, Muller G. Structure and function of lamellar bodies, lipid-protein complexes involved in storage and secretion of cellular lipids. J Lipid Res. 1991;32:1539–1570.
- Nemes Z, Steinert PM. Bricks and mortar of the epidermal barrier. Exp Mol Med. 1999;31:5–19. doi:10.1038/emm.1999.2.
- Rice RH, Green H. The cornified envelope of terminally differentiated human epidermal keratinocytes consists of cross-linked protein. Cell. 1977;11:417–422. doi:10.1016/0092-8674(77)90059-9.
- Swartzendruber DC, Wertz PW, Madison KC, Downing DT. Evidence that the corneocyte has a chemically bound lipid envelope. J Invest Dermatol. 1987;88:709–713.
- Sevilla LM, Nachat R, Groot KR, Klement JF, Uitto J, Djian P, Määttä A, Watt FM. Mice deficient in involucrin, envoplakin, and periplakin have a defective epidermal barrier. J Cell Biol. 2007;179:1599–1612. doi:10.1083/jcb.200706187.
- Takeichi T, Akiyama M. Inherited ichthyosis: non-syndromic forms. J Dermatol. 2016;43:242–251. doi:10.1111/1346-8138.13243.
- Kuramoto N, Takizawa T, Takizawa T, Matsuki M, Morioka H, Robinson JM, Yamanishi K. Development of ichthyosiform skin compensates for defective permeability barrier function in mice lacking transglutaminase 1. J Clin Invest. 2002;109:243–250. doi:10.1172/JCI0213563.
- Ishitsuka Y, Ogawa T, Roop D. The KEAP1/NRF2 signaling pathway in keratinization. Antioxidants (Basel). 2020;9.
- Yamamoto M, Kensler TW, Motohashi H. The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol Rev. 2018;98:1169–1203. doi:10.1152/physrev.00023.2017.
- Schafer M, Farwanah H, Willrodt AH, Huebner AJ, Sandhoff K, Roop D, Hohl D, Bloch W, Werner S. Nrf2 links epidermal barrier function with antioxidant defense. EMBO Mol Med. 2012;4:364–379. doi:10.1002/emmm.201200219.
- Wakabayashi N, Itoh K, Wakabayashi J, Motohashi H, Noda S, Takahashi S, Imakado S, Kotsuji T, Otsuka F, Roop DR, et al. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat Genet. 2003;35:238–245. doi:10.1038/ng1248.
- Ogawa T, Ishitsuka Y, Nakamura Y, Kubota N, Saito A, Fujisawa Y, Watanabe R, Okiyama N, Suga Y, Roop DR, et al. NRF2 augments epidermal antioxidant defenses and promotes atopy. J Immunol. 2020;205(4):907–914. doi:10.4049/jimmunol.2000274.
- Ogawa T, Ishitsuka Y, Inoue S, Nakamura Y, Saito A, Okiyama N, Fujisawa Y, Furuta J, Watanabe R, Fujimoto M, et al. Nuclear factor erythroid 2-related factor 2 (Nrf2) regulates epidermal keratinization under psoriatic skin inflammation. Am J Pathol. 2020;190(3):577–585. doi:10.1016/j.ajpath.2019.10.022.
- Schafer M, Willrodt AH, Kurinna S, Link AS, Farwanah H, Geusau A, Gruber F, Sorg O, Huebner AJ, Roop DR, et al. Activation of Nrf2 in keratinocytes causes chloracne (MADISH)-like skin disease in mice. EMBO Mol Med. 2014;6(4):442–457. doi:10.1002/emmm.201303281.
- Koch PJ, de Viragh PA, Scharer E, Bundman D, Longley MA, Bickenbach J, Kawachi Y, Suga Y, Zhou Z, Huber M, et al. Lessons from loricrin-deficient mice: compensatory mechanisms maintaining skin barrier function in the absence of a major cornified envelope protein. J Cell Biol. 2000;151(2):389–400. doi:10.1083/jcb.151.2.389.
- Steinert PM. The complexity and redundancy of epithelial barrier function. J Cell Biol. 2000;151:F5–8. doi:10.1083/jcb.151.2.F5.
- Ishitsuka Y, Huebner AJ, Rice RH, Koch PJ, Speransky VV, Steven AC, Roop DR. Lce1 family members are Nrf2-target genes that are induced to compensate for the loss of loricrin. J Invest Dermatol. 2016;136:1656–1663. doi:10.1016/j.jid.2016.04.022.
- Huebner AJ, Dai D, Morasso M, Schmidt EE, Schafer M, Werner S, Roop DR. Amniotic fluid activates the nrf2/keap1 pathway to repair an epidermal barrier defect in utero. Dev Cell. 2012;23:1238–1246. doi:10.1016/j.devcel.2012.11.002.
- Jarnik M, de Viragh PA, Scharer E, Bundman D, Simon MN, Roop DR, Steven AC. Quasi-normal cornified cell envelopes in loricrin knockout mice imply the existence of a loricrin backup system. J Invest Dermatol. 2002;118:102–109. doi:10.1046/j.0022-202x.2001.01661.x.
- Ishitsuka Y, Roop DR. Loricrin confers photoprotective function against UVB in corneocytes. J Invest Dermatol. 2018;138(12):2684–2687. doi:10.1016/j.jid.2018.06.164.
- Rice RH, Durbin-Johnson BP, Ishitsuka Y, Salemi M, Phinney BS, Rocke DM, Roop DR. Proteomic analysis of loricrin knockout mouse epidermis. J Proteome Res. 2016;15:2560–2566. doi:10.1021/acs.jproteome.6b00108.
- Fukuyama K, Epstein WL. Sulfur-containing proteins and epidermal keratinization. J Cell Biol. 1969;40:830. doi:10.1083/jcb.40.3.830.
- Van Scott E, Flesch P. Sulfhydryl and disulfide in keratinization. Science. 1954;119:70–71. doi:10.1126/science.119.3080.70.
- Guttman-Yassky E, Suarez-Farinas M, Chiricozzi A, Nograles KE, Shemer A, Fuentes-Duculan J, Cardinale I, Lin P, Bergman R, Bowcock AM, et al. Broad defects in epidermal cornification in atopic dermatitis identified through genomic analysis. J Allergy Clin Immunol. 2009;124:1235–44 e58. doi:10.1016/j.jaci.2009.09.031.
- Ong PY, Leung DY. Bacterial and viral infections in atopic dermatitis: a comprehensive review. Clin Rev Allergy Immunol. 2016;51:329–337. doi:10.1007/s12016-016-8548-5.
- Ogawa T, Ishitsuka Y, Roop D, Fujimoto M. 314 Loricrin protects against chemical carcinogenesis but affects cancer immunoediting. J Invest Dermatol. 2019;139:S54. doi:10.1016/j.jid.2019.03.390.
- Lack G. Update on risk factors for food allergy. J Allergy Clin Immunol. 2012;129:1187–1197. doi:10.1016/j.jaci.2012.02.036.
- Dalziel K, Dykes PJ, Marks R. Inflammation due to intra-cutaneous implantation of stratum corneum. Br J Exp Pathol. 1984;65:107–115.
- Huber M, Rettler I, Bernasconi K, Frenk E, Lavrijsen SP, Ponec M, Bon A, Lautenschlager S, Schorderet D, Hohl D, et al. Mutations of keratinocyte transglutaminase in lamellar ichthyosis. Science. 1995;267:525–528. doi:10.1126/science.7824952.
- Akiyama M, Sugiyama-Nakagiri Y, Sakai K, McMillan JR, Goto M, Arita K, Tsuji-Abe Y, Tabata N, Matsuoka K, Sasaki R, et al. Mutations in lipid transporter ABCA12 in harlequin ichthyosis and functional recovery by corrective gene transfer. J Clin Invest. 2005;115:1777–1784. doi:10.1172/JCI24834.
- Zuo Y, Zhuang DZ, Han R, Isaac G, Tobin JJ, McKee M, Welti R, Brissette JL, Fitzgerald ML, Freeman MW, et al. ABCA12 maintains the epidermal lipid permeability barrier by facilitating formation of ceramide linoleic esters. J Biol Chem. 2008;283:36624–36635. doi:10.1074/jbc.M807377200.
- Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, Goudie DR, Sandilands A, Campbell LE, Smith FJD, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38:441–446. doi:10.1038/ng1767.
- Harding CR, Aho S, Bosko CA. Filaggrin - revisited. Int J Cosmet Sci. 2013;35:412–423. doi:10.1111/ics.12049.
- Presland RB, Boggess D, Lewis SP, Hull C, Fleckman P, Sundberg JP. Loss of normal profilaggrin and filaggrin in flaky tail (ft/ft) mice: an animal model for the filaggrin-deficient skin disease ichthyosis vulgaris. J Invest Dermatol. 2000;115:1072–1081.
- Saunders SP, Goh CS, Brown SJ, Palmer CN, Porter RM, Cole C, Campbell LE, Gierlinski M, Barton GJ, Schneider G, et al. Tmem79/Matt is the matted mouse gene and is a predisposing gene for atopic dermatitis in human subjects. J Allergy Clin Immunol. 2013;132:1121–1129. doi:10.1016/j.jaci.2013.08.046.
- Sasaki T, Shiohama A, Kubo A, Kawasaki H, Ishida-Yamamoto A, Yamada T, Hachiya T, Shimizu A, Okano H, Kudoh J, et al. A homozygous nonsense mutation in the gene for Tmem79, a component for the lamellar granule secretory system, produces spontaneous eczema in an experimental model of atopic dermatitis. J Allergy Clin Immunol. 2013;132:1111–20 e4. doi:10.1016/j.jaci.2013.08.027.
- Dawkins R. The selfish gene. Oxford, England: Oxford university press; 2016.
- Grall A, Guaguere E, Planchais S, Grond S, Bourrat E, Hausser I, Hitte C, Le Gallo M, Derbois C, Kim G-J, et al. PNPLA1 mutations cause autosomal recessive congenital ichthyosis in golden retriever dogs and humans. Nat Genet. 2012;44:140–147. doi:10.1038/ng.1056.
- Israeli S, Khamaysi Z, Fuchs-Telem D, Nousbeck J, Bergman R, Sarig O, Sprecher E. A mutation in LIPN, encoding epidermal lipase N, causes a late-onset form of autosomal-recessive congenital ichthyosis. Am J Hum Genet. 2011;88:482–487. doi:10.1016/j.ajhg.2011.02.011.
- Jobard F, Lefevre C, Karaduman A, Blanchet-Bardon C, Emre S, Weissenbach J, Özgüc M, Lathrop M, Prud’homme J-F, Fischer J. Lipoxygenase-3 (ALOXE3) and 12(R)-lipoxygenase (ALOX12B) are mutated in non-bullous congenital ichthyosiform erythroderma (NCIE) linked to chromosome 17p13.1. Hum Mol Genet. 2002;11:107–113. doi:10.1093/hmg/11.1.107.
- Lefevre C, Audebert S, Jobard F, Bouadjar B, Lakhdar H, Boughdene-Stambouli O, Blanchet-Bardon C, Heilig R, Foglio M, Weissenbach J, et al. Mutations in the transporter ABCA12 are associated with lamellar ichthyosis type 2. Hum Mol Genet. 2003;12:2369–2378. doi:10.1093/hmg/ddg235.
- Ohno Y, Nakamichi S, Ohkuni A, Kamiyama N, Naoe A, Tsujimura H, Yokose U, Sugiura K, Ishikawa J, Akiyama M, et al. Essential role of the cytochrome P450 CYP4F22 in the production of acylceramide, the key lipid for skin permeability barrier formation. Proc Natl Acad Sci U S A. 2015;112:7707–7712.
- Radner FP, Marrakchi S, Kirchmeier P, Kim GJ, Ribierre F, Kamoun B, Abid L, Leipoldt M, Turki H, Schempp W, et al. Mutations in CERS3 cause autosomal recessive congenital ichthyosis in humans. PLoS Genet. 2013;9:e1003536. doi:10.1371/journal.pgen.1003536.
- Takeichi T, Hirabayashi T, Miyasaka Y, Kawamoto A, Okuno Y, Taguchi S, Tanahashi K, Murase C, Takama H, Tanaka K, et al. SDR9C7 catalyzes critical dehydrogenation of acylceramides for skin barrier formation. J Clin Invest. 2020;130:890–903. doi:10.1172/JCI130675.
- Wajid M, Kurban M, Shimomura Y, Christiano AM. NIPAL4/ichthyin is expressed in the granular layer of human epidermis and mutated in two Pakistani families with autosomal recessive ichthyosis. Dermatology. 2010;220:8–14. doi:10.1159/000265757.
- Heinz L, Kim GJ, Marrakchi S, Christiansen J, Turki H, Rauschendorf MA, Lathrop M, Hausser I, Zimmer AD, Fischer J, et al. Mutations in SULT2B1 cause autosomal-recessive congenital ichthyosis in humans. Am J Hum Genet. 2017;100:926–939. doi:10.1016/j.ajhg.2017.05.007.
- Kirchmeier P, Zimmer A, Bouadjar B, Rosler B, Fischer J. Whole-exome-sequencing reveals small deletions in CASP14 in patients with autosomal recessive inherited ichthyosis. Acta Derm Venereol. 2017;97:102–104. doi:10.2340/00015555-2510.
- Basel-Vanagaite L, Attia R, Ishida-Yamamoto A, Rainshtein L, Amitai DB, Lurie R, Pasmanik-Chor M, Indelman M, Zvulunov A, Saban S, et al. Autosomal recessive ichthyosis with hypotrichosis caused by a mutation in ST14, encoding type II transmembrane serine protease matriptase. Am J Human Genet. 2007;80:467–477. doi:10.1086/512487.
- Mehrel T, Hohl D, Rothnagel JA, Longley MA, Bundman D, Cheng C, Lichti U, Bisher ME, Steven AC, Steinert PM, et al. Identification of a major keratinocyte cell envelope protein, loricrin. Cell. 1990;61:1103–1112. doi:10.1016/0092-8674(90)90073-N.
- Redondo P, Bauza A. Topical N-acetylcysteine for lamellar ichthyosis. Lancet. 1999;354:1880. doi:10.1016/S0140-6736(99)04245-2.
- Suzuki T, Motohashi H, Yamamoto M. Toward clinical application of the Keap1-Nrf2 pathway. Trends Pharmacol Sci. 2013;34:340–346.
- Rice RH, Green H. Presence in human epidermal cells of a soluble protein precursor of the cross-linked envelope: activation of the cross-linking by calcium ions. Cell. 1979;18:681–694.
- Ishitsuka Y, Roop DR. Loricrin confers photoprotective function against UVB in corneocytes. J Invest Dermatol. 2018;138:2684–2687
- Vermeij WP, Alia A, Backendorf C. ROS quenching potential of the epidermal cornified cell envelope. J Invest Dermatol. 2011;131:1435–1441. doi:10.1038/jid.2010.433.
- Gibbs S, Fijneman R, Wiegant J, van Kessel AG, van De Putte P, Backendorf C. Molecular characterization and evolution of the SPRR family of keratinocyte differentiation markers encoding small proline-rich proteins. Genomics. 1993;16:630–637. doi:10.1006/geno.1993.1240.
- Jackson B, Tilli CM, Hardman MJ, Avilion AA, MacLeod MC, Ashcroft GS, Byrne C. Late cornified envelope family in differentiating epithelia--response to calcium and ultraviolet irradiation. J Invest Dermatol. 2005;124:1062–1070. doi:10.1111/j.0022-202X.2005.23699.x.
- de Cid R, Riveira-Munoz E, Zeeuwen PL, Robarge J, Liao W, Dannhauser EN, Giardina E, Stuart PE, Nair R, Helms C, et al. Deletion of the late cornified envelope LCE3B and LCE3C genes as a susceptibility factor for psoriasis. Nat Genet. 2009;41:211–215. doi:10.1038/ng.313.
- Prestera T, Zhang Y, Spencer SR, Wilczak CA, Talalay P. The electrophile counterattack response: protection against neoplasia and toxicity. Adv Enzyme Regul. 1993;33:281–296. doi:10.1016/0065-2571(93)90024-8.
- Kehren J, Desvignes C, Krasteva M, Ducluzeau MT, Assossou O, Horand F, Hahne M, Kägi D, Kaiserlian D, Nicolas J-F. Cytotoxicity is mandatory for CD8(+) T cell-mediated contact hypersensitivity. J Exp Med. 1999;189:779–786. doi:10.1084/jem.189.5.779.
- Modi BG, Neustadter J, Binda E, Lewis J, Filler RB, Roberts SJ, Kwong BY, Reddy S, Overton JD, Galan A, et al. Langerhans cells facilitate epithelial DNA damage and squamous cell carcinoma. Science. 2012;335:104–108. doi:10.1126/science.1211600.
- Kaplan DH, Igyarto BZ, Gaspari AA. Early immune events in the induction of allergic contact dermatitis. Nat Rev Immunol. 2012;12:114–124. doi:10.1038/nri3150.
- Engelbrecht TN, Schroeter A, Hauss T, Neubert RH. Lipophilic penetration enhancers and their impact to the bilayer structure of stratum corneum lipid model membranes: neutron diffraction studies based on the example oleic acid. Biochim Biophys Acta. 2011;1808:2798–2806. doi:10.1016/j.bbamem.2011.08.012.
- Steinbrink K, Sorg C, Macher E. Low zone tolerance to contact allergens in mice: a functional role for CD8+ T helper type 2 cells. J Exp Med. 1996;183:759–768. doi:10.1084/jem.183.3.759.
- Matzinger P, Kamala T. Tissue-based class control: the other side of tolerance. Nat Rev Immunol. 2011;11:221–230. doi:10.1038/nri2940.
- Sarantopoulos S, Cardones AR, Sullivan KM. How I treat refractory chronic graft-versus-host disease. Blood. 2019;133:1191–1200. doi:10.1182/blood-2018-04-785899.
- Migden MR, Khushalani NI, Chang ALS, Lewis KD, Schmults CD, Hernandez-Aya L, Meier F, Schadendorf D, Guminski A, Hauschild A, Wong DJ, et al. Cemiplimab in locally advanced cutaneous squamous cell carcinoma: results from an open-label, phase 2, single-arm trial. Lancet Oncol. 2020;21:294–305.
- Kojima T, Hara H, Yamaguchi K, Hironaka S, Iwasa S, Kato K, Tsushima T, Yasui H, Ura T, Muro K, et al. Phase II study of nivolumab (ONO-4538/BMS-936558) in patients with esophageal cancer: preliminary report of overall survival. J Clin Oncol. 2016;34:TPS175–TPS. doi:10.1200/jco.2016.34.4_suppl.tps175.
- Santin A, Deng W, Frumovitz MM, Huh WK, Khleif S, Lankes HA, Ratner E, O’Cearbhaill R, Jazaeri AA, Birrer M, et al. A phase II evaluation of nivolumab, a fully human antibody against PD-1, in the treatment of persistent or recurrent cervical cancer. J Clin Oncol. 2018;36:5536. doi:10.1200/JCO.2018.36.15_suppl.5536.
- Amouzegar A, Chauhan SK, Dana R. Alloimmunity and tolerance in corneal transplantation. J Immunol. 2016;196:3983–3991. doi:10.4049/jimmunol.1600251.
- Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–1192.
- Worbs T, Hammerschmidt SI, Forster R. Dendritic cell migration in health and disease. Nat Rev Immunol. 2017;17:30–48.
- Ohnmacht C, Pullner A, King SB, Drexler I, Meier S, Brocker T, Voehringer D. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J Exp Med. 2009;206:549–559.
- Birnberg T, Bar-On L, Sapoznikov A, Caton ML, Cervantes-Barragan L, Makia D, Krauthgamer R, Brenner O, Ludewig B, Brockschnieder D, et al. Lack of conventional dendritic cells is compatible with normal development and T cell homeostasis, but causes myeloid proliferative syndrome. Immunity. 2008;29:986–997. doi:10.1016/j.immuni.2008.10.012.
- Doebel T, Voisin B, Nagao K. Langerhans cells - the macrophage in dendritic cell clothing. Trends Immunol. 2017;38:817–828. doi:10.1016/j.it.2017.06.008.
- Hovav AH. Mucosal and skin langerhans cells - nurture calls. Trends Immunol. 2018;39:788–800. doi:10.1016/j.it.2018.08.007.
- Streilein JW, Toews GB, Bergstresser PR. Corneal allografts fail to express Ia antigens. Nature. 1979;282:326–327.
- Hattori T, Chauhan SK, Lee H, Ueno H, Dana R, Kaplan DH, Saban DR. Characterization of Langerin-expressing dendritic cell subsets in the normal cornea. Invest Ophthalmol Vis Sci. 2011;52:4598–4604. doi:10.1167/iovs.10-6741.
- Chang SY, Cha HR, Chang JH, Ko HJ, Yang H, Malissen B, Iwata M, Kweon M. Lack of retinoic acid leads to increased langerin-expressing dendritic cells in gut-associated lymphoid tissues. Gastroenterology. 2010;138:1468–78,78 e1-6. doi:10.1053/j.gastro.2009.11.006.
- de Witte L, Nabatov A, Pion M, Fluitsma D, de Jong MA, de Gruijl T, Piguet V, Kooyk YV, Geijtenbeek TBH. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat Med. 2007;13:367–371. doi:10.1038/nm1541.
- Dupont C, Kalach N, Soulaines P, Legoue-Morillon S, Piloquet H, Benhamou PH. Cow’s milk epicutaneous immunotherapy in children: a pilot trial of safety, acceptability, and impact on allergic reactivity. J Allergy Clin Immunol. 2010;125:1165–1167.
- Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol. 2015;16:343–353. doi:10.1038/ni.3123.
- Sigmundsdottir H, Butcher EC. Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. Nat Immunol. 2008;9:981–987.
- Imakado S, Bickenbach JR, Bundman DS, Rothnagel JA, Attar PS, Wang XJ, Walczak VR, Wisniewski S, Pote J, Gordon JS, et al. Targeting expression of a dominant-negative retinoic acid receptor mutant in the epidermis of transgenic mice results in loss of barrier function. Genes Dev. 1995;9:317–329. doi:10.1101/gad.9.3.317.
- Attar PS, Wertz PW, McArthur M, Imakado S, Bickenbach JR, Roop DR. Inhibition of retinoid signaling in transgenic mice alters lipid processing and disrupts epidermal barrier function. Mol Endocrinol. 1997;11:792–800. doi:10.1210/mend.11.6.0010.
- Xie Z, Komuves L, Yu QC, Elalieh H, Ng DC, Leary C, Chang S, Crumrine D, Bikle DD, Yoshizawa T, et al. Lack of the vitamin D receptor is associated with reduced epidermal differentiation and hair follicle growth. J Invest Dermatol. 2002;118:11–16. doi:10.1046/j.1523-1747.2002.01644.x.
- Li M, Hener P, Zhang Z, Kato S, Metzger D, Chambon P. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc Natl Acad Sci U S A. 2006;103:11736–11741. doi:10.1073/pnas.0604575103.
- Li M, Messaddeq N, Teletin M, Pasquali JL, Metzger D, Chambon P. Retinoid X receptor ablation in adult mouse keratinocytes generates an atopic dermatitis triggered by thymic stromal lymphopoietin. Proc Natl Acad Sci U S A. 2005;102:14795–14800. doi:10.1073/pnas.0507385102.
- Ruzicka T, Larsen FG, Galewicz D, Horvath A, Coenraads PJ, Thestrup-Pedersen K, Ortonne JP, Zouboulis CC, Harsch M, Brown TC, et al. Oral alitretinoin (9-cis-retinoic acid) therapy for chronic hand dermatitis in patients refractory to standard therapy: results of a randomized, double-blind, placebo-controlled, multicenter trial. Arch Dermatol. 2004;140:1453–1459.
- Digiovanna JJ, Mauro T, Milstone LM, Schmuth M, Toro JR. Systemic retinoids in the management of ichthyoses and related skin types. Dermatol Ther. 2013;26:26–38.
- Agace WW, Persson EK. How vitamin A metabolizing dendritic cells are generated in the gut mucosa. Trends Immunol. 2012;33:42–48. doi:10.1016/j.it.2011.10.001.
- Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi:10.1016/j.immuni.2004.08.011.
- Kumar S, Sandell LL, Trainor PA, Koentgen F, Duester G. Alcohol and aldehyde dehydrogenases: retinoid metabolic effects in mouse knockout models. Biochim Biophys Acta. 2012;1821:198–205.
- Clugston RD, Blaner WS. The adverse effects of alcohol on vitamin A metabolism. Nutrients. 2012;4:356–371. doi:10.3390/nu4050356.
- Jackson SF, Fell HB. Epidermal fine structure in embryonic chicken skin during atypical differentiation induced by vitamin A in culture. Dev Biol. 1963;7:394–419. doi:10.1016/0012-1606(63)90132-5.
- Hosokawa S, Shinzato M, Kaneko C, Shamoto M. Migration and maturation of Langerhans cells in squamous metaplasia of the rat trachea induced by vitamin A deficiency. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;63:159–166. doi:10.1007/BF02899256.
- Chorro L, Sarde A, Li M, Woollard KJ, Chambon P, Malissen B, Kissenpfennig A, Barbaroux J-B, Groves R, Geissmann F. Langerhans cell (LC) proliferation mediates neonatal development, homeostasis, and inflammation-associated expansion of the epidermal LC network. J Exp Med. 2009;206:3089–3100.
- Koenig U, Amatschek S, Mildner M, Eckhart L, Tschachler E. Aldehyde dehydrogenase 1A3 is transcriptionally activated by all-trans-retinoic acid in human epidermal keratinocytes. Biochem Biophys Res Commun. 2010;400:207–211. doi:10.1016/j.bbrc.2010.08.035.
- Madison KC. Barrier function of the skin: “la raison d’etre” of the epidermis. J Invest Dermatol. 2003;121:231–241. doi:10.1046/j.1523-1747.2003.12359.x.
