ABSTRACT
The epithelium forms a smart barrier to the external environment that can remodel whilst maintaining tissue integrity, a feature important for development, homeostasis, and function. Its dysregulation can lead to diseases ranging from cancer to vision loss. Epithelial remodeling requires reorganization of a thin sheet of actomyosin cortex under the plasma membrane of polarized cells that form basolateral contacts with neighboring cells and the extracellular matrix (ECM). Rho GTPases act as spatiotemporal molecular switches in this process, controlling localized actomyosin dynamics. However, the molecular mechanisms that control actomyosin dynamics at the apical cortex are poorly understood. This review focusses on a growing body of evidence that suggest myotonic dystrophy kinase-related Cdc42-binding kinase (MRCK) plays a conserved role in morphogenetic signaling at the apical cortex in diverse cell and tissue remodeling processes. The possible molecular and mechanistic basis for the diverse functions of MRCK at the apical pole will also be discussed.
Introduction
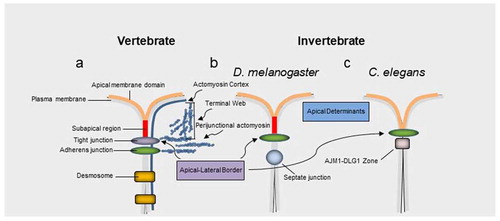
Epithelial cells polarize by developing distinct cell surface domains with different biochemical compositions and functionsCitation1. The apical membrane domain in vertebrate epithelia is defined by the position of the upmost apical portion of tight junctions. These multiprotein complexes have been implicated in a variety of processes in health and disease, beyond their tradition barrier function.Citation2,Citation3 Tight junctions form an apical junctional complex with adherens junctions () and are associated with the apicobasal polarity machinery.Citation7 Invertebrates such as D. melanogaster and C. elegans contain adherens junctions as the most apical junctional structureCitation2 (). The apical junctional complex, contributes to the cytoskeleton via an F-actin perijunctional belt and, in highly apically differentiated epithelia, an F-actin rich structural network known as the terminal web ().Citation2,Citation3,Citation8 The terminal web is normally linked to specialized organ specific brush border membranes. Evidence also suggests that similar to invertebrate D. melanogaster, vertebrate epithelia also contain a subapical domain above the highest positioned junctional structureCitation4,Citation5 ().
Rho GTPases are critical components, of signaling pathways that regulate the cytoskeleton, to guide diverse cellular functions including proliferation, migration, adhesion, polarization, and specialization of the apical plasma membrane.Citation3 This is due to their role as localized molecular switches that cycle between active GTP bound and inactive GDP bound states to spatiotemporally control cell and tissue morphology. The active GTP-bound state allows association with an effector protein that regulates cytoskeletal reorganization to drive cell and tissue morphogenesis.Citation9,Citation10 Effector proteins include Neural Wiskott-Aldrich syndrome protein (N-WASP), the p21-activated kinase (PAK), Rho-associated kinase (ROCK) and closely related MRCK. The diverse functions of Rho GTPases, despite their relatively low number is due to the vast number of their regulators that include guanine nucleotide exchange factors (GEFs) that promote the exchange of GDP for GTP, and GTPase-activating proteins (GAPs) that stimulate GTP hydrolysis. These regulators possess distinct localization profiles that largely define the molecular switch properties of Rho GTPases.
The functions of various Rho GTPase signaling pathways at cell-cell and cell-extracellular matrix (ECM) adhesions have been extensively studied.Citation2,Citation11–13 However, the role(s) and significance of Rho GTPase signaling at the apical membrane domain, during cell and tissue morphogenesis, is poorly understood. Over the past few years, a growing body of evidence support a conserved role for the Cdc42 effector MRCK as an apical driver of morphogenesis in different developmental and homeostatic contexts.
Figure 1. Apical junctions across vertebrates and invertebrates. a, In vertebrates tight junctions are positioned apical to adherens junctions. b, c, Invertebrate adherens junctions are often the most apically positioned junctional structure. The equivalent structure to tight junctions in many invertebrate epithelia, such as D. melanogaster, the septate junction, is positioned basal to the adherens junction. Aside from regulating barrier properties apical junctions also play an important role in apicobasal polarity via at least two protein complexes formed by the partitioning defective 3 (PAR3)-PAR6-atypical protein kinase (aPKC)-Cdc42 complex and the protein crumbs homologue 3 (CRB3)-protein associated with Lin-7 1 (PALS1)-PALS1-associated tight junction (PATJ) complex. In vertebrates these complexes have traditionally been associated with tight junctions and their homologues in D. melanogaster associate with a subapical region (SAR) apical to adherens junctions. However, an equivalent signaling zone has been proposed in vertebrates.Citation4,Citation5 Note, the actin cortex, filamentous actin of the perijunctional belt and terminal web are highlighted in blue. The apical junction molecule 1 (AJM1)–discs large homologue 1 (DLG1) complexCitation6 in C. elegans is involved in barrier function, similar to tight junctions in vertebrates and septate junctions in many invertebrates
Structure of MRCK
There are 3 related MRCK proteins MRCKα, β and γ that are part of a PKA, G, C (AGC) superfamilyCitation14 (). MRCKα and β, both ubiquitously expressedCitation15 (), display the most homology with 85% amino acid similarity across their kinase domains and 61% total amino acid identity ().Citation16 MRCKγ, which may be restricted to fewer tissuesCitation16,Citation17 (), is closest to MRCKβ with 72% amino acid identity over its kinase domain and 44% identity over the total amino acid sequence ().Citation18 Although MRCK proteins were largely identified on the basis of their binding properties to Cdc42-GTP, subsequent studies have demonstrated that MRCKs can act as a Cdc42 and/or Rac effector via their Cdc42/Rac interactive binding (CRIB) domain.Citation19–21 The Rho effector ROCK1 and ROCK2 kinases are related to the MRCKs (), especially the three-dimensional spatial organization of their kinase domains.Citation22,Citation23 Whilst MRCKs carry out their effector functions via their CRIB domains, ROCK kinases possess a Rho-binding domain (RBD) that act downstream of Rho GTPase. In MRCKs the protein kinase C conserved region 1 (C1), at least in the case of MRCKα and MRCKβ, can bind phorbol esters at high affinitiesCitation24,Citation25 that may promote kinase activationCitation24 and/or membrane translocation.Citation25 The pleckstrin homology (PH)-like domains of MRCKs contain a similar three-dimensional structure and are thought to be important for subcellular localization via binding to other proteins or lipids. MRCKα,β,γ all contain a citron homology (CH) domain that lies adjacent to the PH-like domain.Citation26 The spatial arrangement of CH-PH domains is conserved and suggest a cooperative action in possible functions including protein-protein interactions that may contribute to subcellular distribution. It has also been proposed using in vitro assays that the function of Cdc42-GTP may not be to increase kinase activity toward myosin light chain (MLC) but membrane recruitment, bringing MRCK in close proximity to cortical MLC to activate it.Citation19
Figure 2. Domain organization of MRCKs and ROCK1/2. a, Homology within the MRCKs is observed over N-terminal kinase domains (KD), C-terminal C1, citron homology domain (CH), pleckstrin homology domain (PH) and Cdc42- and Rac-interactive binding domain (CRIB). b, The N-terminal kinase domain of ROCK1/2 displays significant homology with the MRCKs and has several common substrates (refer to ). The C-terminal domain of ROCK1/2, that is far less homologous to the MRCKs, includes a Rho-binding domain (RBD), cysteine-rich domain (CRD), different spatially organized PH domain, and lacks C1, CH and CRIB domains
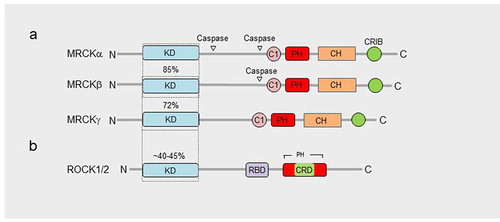
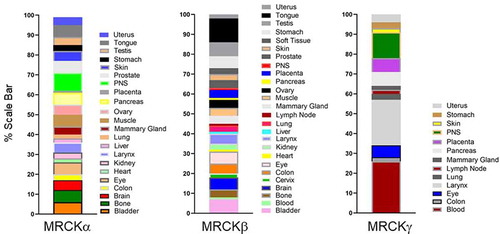
Figure 3. Tissue distribution of MRCKs. MRCKα and β are ubiquitously expressed, whereas MRCKγ is restricted to fewer tissues. The blood, larynx, and peripheral nervous system are indicated to have the highest levels of MRCKγ expression. Determination of relative tissue distributions of MRCKs has been previously described.Citation16
MRCK as a driver of apical domain organization
A. Apical expansion and brush border induction
Polarization requires the segregation of polarity determinants, including partitioning defective (PAR) proteins, into distinct domains.Citation29,Citation30 In response to Cdc42 activation the Par6-aPKC complex transiently binds to Par3, in epithelial cells, dissociating from it to demarcate the apical/lateral border, and segregates into the developing apical domain.Citation4,Citation31,Citation32 The apical domain of epithelia often undergoes a morphogenic transformation leading to functional actin-rich structures such as the brush border membrane of absorptive epithelia.
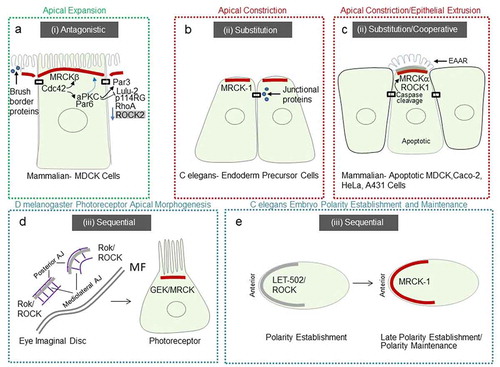
In mammalian MDCK kidney epithelial cells, full specialization of the apical membrane occurs via apical polarity determinants driven by Cdc42 and its apical GEF Dbl3Citation4. A hypothetical model of antagonism between Cdc42-driven apical polarity determinants, activated by Dbl3, and RhoA-ROCK2-driven perijunctional actomyosin contractility as a defining step in apical domain organization was previously described.Citation3 Recent work substantiates this hypothesis and suggests a role for MRCKβ as a determinant of apical domain organization. Following activation of Cdc42 by Dbl3, recruitment of MRCKβ to the apical cortex promotes polarized activation of myosinII.Citation27 MRCKβ recruitment is coupled and coordinated with Par6-aPKCζ apical recruitment to drive a dual effector mechanism (). aPKC is recruited to TJ to deactivate the RhoA/ROCK2 activator p114RhoGEF via inhibition of LULU-2, and phosphorylate Par3 to apically exclude it and define the apical-basolateral border.Citation4,Citation27,Citation31–33 Phosphorylation of Par3 by aPKC destabilizes the interaction and results in distribution of Par6-aPKC along an expanding apical membrane domainCitation4 a process that is dependent on MRCKβ-activated myosinII.Citation27 Apical domain development is concomitant with an apical enrichment of cytosolic components that promote brush border induction.Citation27,Citation34
Figure 4. Schematic representation of conserved MRCK activity, at the apical pole
B. Apical constriction
Apical constriction, an opposing process to apical expansion, describes a shrinking of the apical cell surface and is an important morphogenic event during developmental processes such as neural tube formation in vertebrates and gastrulation in many systems.Citation35 Apical constriction involves medio-apical actomyosin networks, positioned at the apical cortex, under tension that generate forceCitation36–38 and perijunctional actomyosin belts that contract via a purse-string mechanism.Citation39,Citation40 Since medio-apical networks are connected to junctions a fundamental question has persisted, as to how these two structures are maintained, coupled, and coordinated spatially and temporally.
The gastrulation movements in the early C. elegans embryo have provided a useful model to address this question. Contraction of apical actomyosin networks is required for the internalization of endoderm precursor cells (EPCs).Citation41,Citation42 For actomyosin-based contractile force to drive tissue morphogenesis, the force must be mechanically transmitted to adjacent cells. The force-bearing bridge between the actomyosin cortices of neighboring cells is the cadherin-catenin complex (CCC).Citation43,Citation44 MRCK-1 is required for the activation of myosin at the apical cortex of gastrulating cells and apical constriction.Citation45 MRCK-1 localizes at the apical pole of apically constricting endoderm precursor cells, activating actomyosin contractility, that enriches junctional proteins α-catenin, β-catenin, and cadherinCitation45 (). This study indicates that MRCK-1 links developmental patterning mechanisms to cytoskeletal force generation to drive apical constriction.
C. Epithelial cell extrusion
Epithelial cells need to maintain a closed sheet during homeostasis. Such a state requires synchronization of the junctional actomyosin belt between neighboring cells that control precise removal of aged or damaged cells by a process called epithelial cell extrusion. Loss of epithelial homeostasis is associated with different diseases, for example, cancer.Citation3,Citation46
Actomyosin reorganization occurs in both the apoptotic cell and neighboring cells that coordinate with each other to facilitate removal of the apoptotic cell whilst quickly replacing a potential space.Citation47–49 Apoptotic cells use the sphingosine-1-phosphate (S1P) receptor 2 pathway to mediate their status to neighboring cells, resulting in the assembly of a basal actomyosin constriction ring.Citation50 However, the physical role of apoptotic cells during epithelial extrusion was less clear until recently.
Apoptosis is triggered by several pathways that converge into a cascade of caspases that drive hallmark features including activation of cytoplasmic endonucleases, release of immunomodulation proteins, nuclear fragmentation, and the formation of blebs and apoptotic bodies.Citation51–54 Although caspases were initially proposed to degrade the actin cytoskeletonCitation55,Citation56 consequent work indicated that the actin cytoskeleton is the main driving force in apoptotic cells,Citation57 being responsible for most of the associated morphological processes.Citation47,Citation58–61 Evidence suggests that ROCK1 kinase activity on myosin light chain 2 (MLC2), is increased by caspase 3 driven proteolytic cleavage causing contraction of the cortical actomyosin network that drives blebbing.Citation59,Citation62 A subsequent study demonstrated that epithelial extrusion is a biphasic process, involving basal ring constriction that is generally preceded by the formation of a dense apical actin structure.Citation48 More recently MRCKα has been identified as a key downstream kinase of cell morphogenesis in the apoptotic pathway, that initiates epithelial extrusionCitation63 (). During this process MRCKα is constitutively activated by proteolytic cleavage at aspartate 478 residue. MRCKα activity increases MLC2 phosphorylation at the apical pole which drives the formation of an extrusion apical actin ring (EAAR) in an apoptotic cell. The EAAR structure pulls actin bundles, resulting in cell body compaction and removal, by producing cell-autonomous forces as an early event of epithelial extrusion. In addition to MRCKα, caspase-mediated cleavage irreversibly activates ROCK1Citation59,Citation62 and MLCK,Citation64 resulting in constitutive phosphorylation of MLC2. Such a ubiquitous activation of myosin is thought to underly a requirement for a rapid and substantial burst of myosin activity.Citation63
MRCKs role in cancer
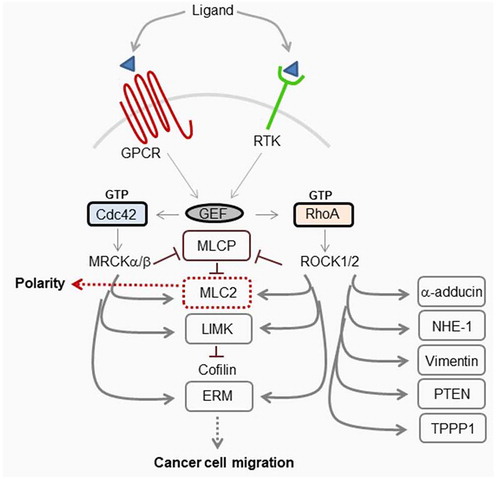
The importance of Cdc42-MRCK and RhoA-ROCK1/2 in both polarity and cell motility () place them as important factors in both normal cellular processes and cancer. MRCK may possess tumorigenic properties due to increased kinase activity,Citation65 that may be independent of Cdc42Citation19, and/or its overexpression in certain types of tumor.Citation28 Additionally, the dysregulation of Cdc42 due to overexpression,Citation66,Citation67 or by GEFs, may contribute to the tumorigenic properties of MRCK. Since Cdc42 GEFs are activated by a diverse spectrum of cell surface receptors including G-protein coupled receptors, growth factor receptors, integrins and cytokines it is not surprising that several GEFS have been identified to be dysregulated in cancer.Citation68 The ability of Cdc42-signaling to contribute to cell migration would mean that under oncogenic conditions, due to other signaling factors, it could potentially behave as a tumorigenic factor in the absence of its own direct dysregulation. RhoA and its GEFs Vav and Trio are also overexpressed in particular cancers although, as with Cdc42, its inherent ability to function in both migration and polarity means it may act as either a tumorigenic factor or tumor suppressor.Citation69,Citation70 Rho-ROCK and Cdc42-MRCK-signaling have been demonstrated to converge during actomyosin-dependent cell motility.Citation71 Rho-ROCK is important for tumor cell migration through a three-dimensional matrix with a rounded morphology, whereas Cdc42-MRCK co-operates with Rho-ROCK to generate and maintain elongated morphology and invasion. It is thought that proteins that function in tumor cell invasion and metastasis also contribute to the growth of primary tumors.Citation72 Therefore, the development of specific MRCK inhibitors is expected to have beneficial effects on reducing tumor growth and progressionCitation65
Figure 5. Schematic representation of MRCK and ROCK1/2 signaling
Conclusions and future perspectives
The apical membrane of polarized epithelial cells undergo tissue and developmental process-specific modifications that rely on actomyosin based forces to drive shape changes. In recent years studies from a variety of laboratories have identified a conserved role for MRCK in yet diverse apical domain organization processes. The precise role MRCK plays at the apical domain may be partly due to structural characteristics of the protein variants.
It has been proposed that the carboxyl-terminus of MRCK comprised of C1, PH, CH, and CRIB domains may specify its membrane localization in response to extracellular signals. For instance, vasopressin treatment of kidney cells from the collecting duct promotes increased apical plasma membrane localization of MRCKβ.Citation73 The bringing of MRCK in close proximity to MLC2, by Cdc42-GTP binding to its CRIB domain,Citation19 may therefore bridge localization with functional activity. Since the spatial organization of the carboxyl-terminus CH-PH domains is conserved and flanked by a CRIB domain in all 3 MRCKs, this may suggest that under certain stimuli MRCKs may possess an inherent capacity to function as a regulator of the apical cortex.Citation16,Citation27,Citation45,Citation63,Citation73 Subtle differences between MRCKs may determine functional specificity. For instance, during apoptosis both MRCKα and MRCKβ are cleaved by caspases at their coil-coiled regions.Citation63 However, MRCKα contains additional cleavage sequences that may increase its susceptibility to caspase cleavage (), defining its distinct role in apoptosis (). Intriguingly, apical localization of MRCKα in mammalian epithelial cells was functionally enhanced toward MLC2 by caspase cleavage and removal of its carboxyl-terminus. Thus, additional regions of MRCK may contribute to its localization.
Another factor that is likely to define the precise role of MRCK at the apical pole is its spatiotemporal relationship to ROCK1/2. For instance, both MRCKα and C. elegans MRCK-1 regulate apical constrictionCitation45,Citation63 (). MRCKα is important for apical constriction during apoptosis,Citation63 whereas MRCK-1 regulates apical constriction during gastrulation.Citation45 In C. elegans MRCK-1 seems to function by substituting the role of ROCK, to drive actomyosin contractility at the apical pole and the enrichment of junctional proteins that may modulate force transmission between neighboring cells (). During D. melanogaster gastrulation polarized myosin activation is due to Rok/ROCK.Citation74,Citation75 In mammalian epithelial cells p114RhoGEF was previously reported to drive junction assembly, and apical constriction, although ROCK was reported to function directly at junctions in these cells.Citation33,Citation76 Apical constriction in vertebrates during invagination processes is also dependent on ROCK.Citation39 During apoptosis of mammalian epithelial cells, the precise relationship between MRCKα and ROCK1, i.e., cooperative and/or one of substitution, needs further investigation and may depend on the cellular model.Citation63 The EAAR structure reported to be mediated by MRCKα activation of myosinII, during apoptosis (), seems to be a distinct structure from apical constriction structures previously described.Citation63 The shared localization of the EAAR with MRCK and ROCK may suggest that its mechanism is derived from the base mechanism of apical constriction.
In vertebrate mammalian epithelial cells MRCKβ plays an important role in apical morphogenesis during apical expansion and brush border induction through Cdc42-dependent activation of myosinII.Citation4,Citation27 The apical distribution of MRCKβ is conserved with MRCK-1 in C. elegans and MRCKα in mammalian epithelial cells and would be expected to drive a similar contractile force-generating process that controls apical constriction. The key difference in the fate of the apical domain seems to be the spatiotemporal relationship between MRCK and ROCK. The cooperative or substitution role MRCKα and MRCK-1 play with ROCK in apoptosis and gastrulation, is contrasted during apical expansion and brush border induction with an antagonistic one. Such an antagonistic behavior may be facilitated by differences between the carboxyl-terminus regions of MRCK and ROCKCitation77 (), which may be important for orchestrating spatially distinct actomyosin regulatory mechanisms via controlling distinct subcellular recruitment. Indeed, as MDCK epithelial cells undergo polarization and apical specification, MRCKβ is coupled to another Cdc42 effector, complexed Par6-aPKCζ, that downregulates the pro-apical constriction LULU-2-p114RhoGEF-RhoA-ROCK2 pathway localized at the apical junctional complexCitation27,Citation33,Citation78 (). Thus, MRCKβ plays a more complex role in apical expansion, both facilitating and defining an expanding apical membrane domain through a similar contractile base mechanism at the apical pole displayed by MRCKα and MRCK-1, yet antagonizing perijunctional contractility. It is notable that both MRCKβ and MRCK-1 promote concentration of cytosolic brush border and junctional proteins, respectively, during their function at the apical domainCitation27,Citation45 (). Although polarization of subcellular domains may facilitate cytosolic segregation of proteins,Citation79 future work is required to precisely understand how apical contractility generated by MRCK drives process specific localization of these factors.
In D. melanogaster Genghis Khan (GEK), the orthologue of MRCK, was demonstrated to localize at the apical pole of photoreceptor cells and control actomyosin contractility dependent apical morphogenesis.Citation27 In early eye discs Rho-1-Rok/ROCK signaling drives junctional remodelingCitation80–82 before Cdc42-GEK activation leads to a reorientation of the actomyosin contractility gradient to control apical morphogenesisCitation27 (). Future work to understand the precise mode by which Rok/ROCK activity is superseded by GEK/MRCK activity may provide further insight into the underlying mechanisms of apical domain organization during photoreceptor development in D. melanogaster. A similar conservation of a sequential function between RhoA and Cdc42 signaling is observed during polarization of the C. elegans embryo. RhoA orthologue RHO-1 is required for the establishment of polarity, whereas CDC-42 is required for polarity maintenance (). Interestingly, CGEF-1, the orthologue of mammalian Dbl3, is the robust activator of CDC-42 that together with a GAP at the posterior pole, CHIN-1, restricts CDC-42 activity to the anterior pole.Citation83 Furthermore, polarized distribution of non-muscle myosinII at the anterior pole during the maintenance phase, is achieved by restricting CDC-42 activity and thereby the activity of its effector MRCK-1 at this position. A more recent study indicates that CDC-42 activity at the anterior pole increases during the late establishment phase of polarity, suggesting that MRCK-1 function may be activated during this phase.Citation84 Recent work in D. melanogaster hair follicle epithelia has implicated GEK/MRCK as a Cdc42 effector at the apical pole. RhoGAP19D, was found to suppress Cdc42 activity at the lateral domain and exclude it to the apical pole.Citation85 The orthologue of RhoGAP19D in C elegans, PAC-1, carries out a similar function in the early embryoCitation86 and was found to be required for polarized activity of MRCK-1 in endodermal precursor cells.Citation45 In hair follicle epithelia GEK/MRCK localized laterally in rho 19d inactive mutant clones resulting in increased lateral contractility, apical expansion, and epithelial buckling leading to invasion into the adjacent tissue.Citation85 Previous work suggested that myosin phosphorylation at the apical cortex is important for follicle cell shape which is only partly dependent on Rok.Citation87 Future work is required to determine whether GEK/MRCK and Rok function cooperatively, for example, in these cells.
In summary, an increasing body of evidence suggests that MRCK plays a major role in apical domain organization and morphogenesis, in different developmental and homeostatic contexts. The spatiotemporal functional relationship between MRCK and ROCK may be a major defining factor in the fate of the apical domain. Future work to understand the interplay between MRCK and ROCK signaling at the cell cortex, is therefore likely to provide insight into control mechanisms of cell and tissue morphogenesis. For example, it would be of interest to determine whether an antagonistic relationship affects tension gradients at the cortex to control apical domain development during epithelial polarity establishment and morphogenesis. Another important question is whether MRCK plays a role in controlling the apical cortex during tissue-specific functions by the mature epithelium. Since, MRCK and ROCK also possess the ability to function in both polarity and tumorigenesis, understanding their complex relationship is likely to provide important insight into cancer progression.
Acknowledgments
This work was partly supported by grants from the BBSRC (BB/N014855/1), Moorfields Eye Charity, Rosetrees Trust, and University College London Technology Fund.
Additional information
Funding
References
- Mellman I, Nelson WJ. Coordinated protein sorting, targeting and distribution in polarized cells. Nat Rev Mol Cell Biol. 2008;9(833–845):1–12. doi:https://doi.org/10.1038/nrm2525.
- Zihni C, Mills C, Matter K, Balda MS. Tight junctions: from simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol. 2016;17(564–580):564–580. doi:https://doi.org/10.1038/nrm.2016.80.
- Zihni C, Terry SJ. RhoGTPase signalling at epithelial tight junctions: bridging the GAP between polarity and cancer. Int J Biochem Cell Biol. 2015;64(120–125):120–125. doi:https://doi.org/10.1016/j.biocel.2015.02.020.
- Zihni C, Munro PMG, Elbediwy A, Keep NH, Terry SJ, Harris J, Balda MS, Matter K. Dbl3 drives Cdc42 signaling at the apical margin to regulate junction position and apical differentiation. J Cell Biol. 2014;204(111–127):111–127. doi:https://doi.org/10.1083/jcb.201304064.
- Tan B, Yatim SMJM, Peng S, Gunaratne J, Hunziker W, Ludwig A. The mammalian crumbs complex defines a distinct polarity domain apical of epithelial tight junctions. Curr Biol. 2020;30:2791–2804. e2796. doi:https://doi.org/10.1016/j.cub.2020.05.032.
- Asano A, Asano K, Sasaki H, Furuse M, Tsukita S. Claudins in Caenorhabditis elegans: their distribution and barrier function in the epithelium. Curr Biol. 2003;13(1042–1046):1042–1046. doi:https://doi.org/10.1016/s0960-9822(03)00395-6.
- Diaz-Diaz C, Baonza G, Martin-Belmonte F. The vertebrate epithelial apical junctional complex: dynamic interplay between Rho GTPase activity and cell polarization processes. Biochim Biophys Acta Biomembr. 2020;1862(183398):183398. doi:https://doi.org/10.1016/j.bbamem.2020.183398.
- Hull BE, Staehelin LA. The terminal web. A reevaluation of its structure and function. J Cell Biol. 1979;81:67–82. doi:https://doi.org/10.1083/jcb.81.1.67.
- Hall A. Rho family GTPases. Biochem Soc Trans. 2012;40(1378–1382):1378–1382. doi:https://doi.org/10.1042/BST20120103.
- Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9(690–701):690–701. doi:https://doi.org/10.1038/nrm2476.
- McCormack J, Welsh NJ, Braga VM. Cycling around cell-cell adhesion with Rho GTPase regulators. J Cell Sci. 2013;126(379–391):379–391. doi:https://doi.org/10.1242/jcs.097923.
- Samarin S, Nusrat A. Regulation of epithelial apical junctional complex by Rho family GTPases. Front Biosci (Landmark Ed). 2009;14(1129–1142):1129–1142. doi:https://doi.org/10.2741/3298.
- Terry S, Nie M, Matter K, Balda MS. Rho signaling and tight junction functions. Physiology (Bethesda). 2010;25(16–26):16–26. doi:https://doi.org/10.1152/physiol.00034.2009.
- Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol. 2010;11(9–22):9–22. doi:https://doi.org/10.1038/nrm2822.
- Liu X, Yu X, Zack DJ, Zhu H, Qian J. TiGER: a database for tissue-specific gene expression and regulation. BMC Bioinform. 2008;9(271):271. doi:https://doi.org/10.1186/1471-2105-9-271.
- Unbekandt M, Olson MF. The actin-myosin regulatory MRCK kinases: regulation, biological functions and associations with human cancer. J Mol Med (Berl). 2014;92(217–225):217–225. doi:https://doi.org/10.1007/s00109-014-1133-6.
- Ng Y, Tan I, Lim L, Leung T. Expression of the human myotonic dystrophy kinase-related Cdc42-binding kinase gamma is regulated by promoter DNA methylation and Sp1 binding. J Biol Chem. 2004;279(34156–34164):34156–34164. doi:https://doi.org/10.1074/jbc.M405252200.
- Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988;16(10881–10890):10881–10890. doi:https://doi.org/10.1093/nar/16.22.10881.
- Leung T, Chen XQ, Tan I, Manser E, Lim L. Myotonic dystrophy kinase-related Cdc42-binding kinase acts as a Cdc42 effector in promoting cytoskeletal reorganization. Mol Cell Biol. 1998;18(130–140):130–140. doi:https://doi.org/10.1128/mcb.18.1.130.
- Luo L, Lee T, Tsai L, Tang G, Jan LY, Jan YN. Genghis Khan (Gek) as a putative effector for Drosophila Cdc42 and regulator of actin polymerization. Proc Natl Acad Sci U S A. 1997;94(12963–12968). doi:https://doi.org/10.1073/pnas.94.24.12963.
- Schwarz J, Proff J, Hävemeier A, Ladwein M, Rottner K, Barlag B, Pich A, Tatge H, Just I, Gerhard R, et al. Serine-71 phosphorylation of Rac1 modulates downstream signaling. PLoS One. 2012;7(e44358):e44358. doi:https://doi.org/10.1371/journal.pone.0044358.
- Heikkila T, Wheatley E, Crighton D, Schroder E, Boakes A, Kaye SJ, Mezna M, Pang L, Rushbrooke M, Turnbull A, et al. Co-crystal structures of inhibitors with MRCKbeta, a key regulator of tumor cell invasion. PLoS One. 2011;6(e24825):e24825. doi:https://doi.org/10.1371/journal.pone.0024825.
- Clayton NS, Ridley AJ. Targeting Rho GTPase signaling networks in cancer. Front Cell Dev Biol. 2020;8(222). doi:https://doi.org/10.3389/fcell.2020.00222.
- Tan I, Seow KT, Lim L, Leung T. Intermolecular and intramolecular interactions regulate catalytic activity of myotonic dystrophy kinase-related Cdc42-binding kinase alpha. Mol Cell Biol. 2001;21(2767–2778):2767–2778. doi:https://doi.org/10.1128/MCB.21.8.2767-2778.2001.
- Choi SH, Czifra G, Kedei N, Lewin NE, Lazar J, Pu Y, Marquez VE, Blumberg PM. Characterization of the interaction of phorbol esters with the C1 domain of MRCK (myotonic dystrophy kinase-related Cdc42 binding kinase) alpha/beta. J Biol Chem. 2008;283(10543–10549):10543–10549. doi:https://doi.org/10.1074/jbc.M707463200.
- Madaule P, Eda M, Watanabe N, Fujisawa K, Matsuoka T, Bito H, Ishizaki T, Narumiya S. Role of citron kinase as a target of the small GTPase Rho in cytokinesis. Nature. 1998;394(491–494):491–494. doi:https://doi.org/10.1038/28873.
- Zihni C, Vlassaks E, Terry S, Carlton J, Leung TKC, Olson M, Pichaud F, Balda MS, Matter K. An apical MRCK-driven morphogenetic pathway controls epithelial polarity. Nat Cell Biol. 2017;19(1049–1060):1049–1060. doi:https://doi.org/10.1038/ncb3592.
- Kale VP, Hengst JA, Desai DH, Amin SG, Yun JK. The regulatory roles of ROCK and MRCK kinases in the plasticity of cancer cell migration. Cancer Lett. 2015;361(185–196):185–196. doi:https://doi.org/10.1016/j.canlet.2015.03.017.
- Cowan CR, Hyman AA. Acto-myosin reorganization and PAR polarity in C. elegans. Development. 2007;134(6):1035–1043. doi:https://doi.org/10.1242/dev.000513.
- St Johnston D, Ahringer J. Cell polarity in eggs and epithelia: parallels and diversity. Cell. 2010;141(757–774):757–774. doi:https://doi.org/10.1016/j.cell.2010.05.011.
- Morais-de-sa E, Mirouse V, St Johnston D. aPKC phosphorylation of Bazooka defines the apical/lateral border in Drosophila epithelial cells. Cell. 2010;141(509–523):509–523. doi:https://doi.org/10.1016/j.cell.2010.02.040.
- Walther RF, Pichaud F. Crumbs/DaPKC-dependent apical exclusion of Bazooka promotes photoreceptor polarity remodeling. Curr Biol. 2010;20(1065–1074):1065–1074. doi:https://doi.org/10.1016/j.cub.2010.04.049.
- Nakajima H, Tanoue T. The circumferential actomyosin belt in epithelial cells is regulated by the Lulu2-p114RhoGEF system. Small GTPases. 2012;3(91–96):91–96. doi:https://doi.org/10.4161/sgtp.19112.
- Sauvanet C, Wayt J, Pelaseyed T, Bretscher A. Structure, regulation, and functional diversity of microvilli on the apical domain of epithelial cells. Annu Rev Cell Dev Biol. 2015;31(593–621):593–621. doi:https://doi.org/10.1146/annurev-cellbio-100814-125234.
- Sawyer JM, Harrell JR, Shemer G, Sullivan-Brown J, Roh-Johnson M, Goldstein B. Apical constriction: a cell shape change that can drive morphogenesis. Dev Biol. 2010;341(5–19):5–19. doi:https://doi.org/10.1016/j.ydbio.2009.09.009.
- Ma X, Lynch HE, Scully PC, Hutson MS. Probing embryonic tissue mechanics with laser hole drilling. Phys Biol. 2009;6(36004):036004. doi:https://doi.org/10.1088/1478-3975/6/3/036004.
- Roh-Johnson M, Shemer G, Higgins CD, McClellan JH, Werts AD, Tulu US, Gao L, Betzig E, Kiehart DP, Goldstein B, et al. Triggering a cell shape change by exploiting preexisting actomyosin contractions. Science. 2012;335(1232–1235):1232–1235. doi:https://doi.org/10.1126/science.1217869.
- Samarage CR, White M, Álvarez Y, Fierro-González J, Henon Y, Jesudason E, Bissiere S, Fouras A, Plachta N. Cortical Tension Allocates the First Inner Cells of the Mammalian Embryo. Dev Cell. 2015;34(435–447):435–447. doi:https://doi.org/10.1016/j.devcel.2015.07.004.
- Martin AC, Goldstein B. Apical constriction: themes and variations on a cellular mechanism driving morphogenesis. Development. 2014;141(1987–1998):1987–1998. doi:https://doi.org/10.1242/dev.102228.
- Rodriguez-Diaz A, Toyama Y, Abravanel DL, Wiemann JM, Wells AR, Tulu US, Edwards GS, Kiehart DP. Actomyosin purse strings: renewable resources that make morphogenesis robust and resilient. Hfsp J. 2008;2(220–237):220–237. doi:https://doi.org/10.2976/1.2955565.
- Nance J, Priess JR. Cell polarity and gastrulation in C. elegans. Development. 2002;129:387–397.
- Lee JY, Goldstein B. Mechanisms of cell positioning during C. elegans gastrulation. Development. 2003;130:307–320. doi:https://doi.org/10.1242/dev.00211.
- Martin AC, Gelbart M, Fernandez-Gonzalez R, Kaschube M, Wieschaus EF. Integration of contractile forces during tissue invagination. J Cell Biol. 2010;188(735–749):735–749. doi:https://doi.org/10.1083/jcb.200910099.
- Borghi N, Sorokina M, Shcherbakova OG, Weis WI, Pruitt BL, Nelson WJ, Dunn AR. E-cadherin is under constitutive actomyosin-generated tension that is increased at cell-cell contacts upon externally applied stretch. Proc Natl Acad Sci U S A. 2012;109(12568–12573):12568–12573. doi:https://doi.org/10.1073/pnas.1204390109.
- Marston DJ, Higgins C, Peters K, Cupp T, Dickinson D, Pani A, Moore R, Cox A, Kiehart D, Goldstein B, et al. MRCK-1 Drives Apical Constriction in C. elegans by Linking Developmental Patterning to Force Generation. Curr Biol. 2016;26:2079–2089. doi:https://doi.org/10.1016/j.cub.2016.06.010.
- Macara IG, Guyer R, Richardson G, Huo Y, Ahmed SM. Epithelial homeostasis. Curr Biol. 2014;24(R815–825):R815–R825. doi:https://doi.org/10.1016/j.cub.2014.06.068.
- Rosenblatt J, Raff MC, Cramer LP. An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Curr Biol. 2001;11(1847–1857):1847–1857. doi:https://doi.org/10.1016/s0960-9822(01)00587-5.
- Kuipers D, Mehonic A, Kajita M, Peter L, Fujita Y, Duke T, Charras G, Gale JE. Epithelial repair is a two-stage process driven first by dying cells and then by their neighbours. J Cell Sci. 2014;127(1229–1241):1229–1241. doi:https://doi.org/10.1242/jcs.138289.
- Wu SK, Lagendijk AK, Hogan BM, Gomez GA, Yap AS. Active contractility at E-cadherin junctions and its implications for cell extrusion in cancer. Cell Cycle. 2015;14(315–322):315–322. doi:https://doi.org/10.4161/15384101.2014.989127.
- Gu Y, Forostyan T, Sabbadini R, Rosenblatt J. Epithelial cell extrusion requires the sphingosine-1-phosphate receptor 2 pathway. J Cell Biol. 2011;193(667–676):667–676. doi:https://doi.org/10.1083/jcb.201010075.
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(495–516):495–516. doi:https://doi.org/10.1080/01926230701320337.
- Cullen SP, Martin SJ. Caspase activation pathways: some recent progress. Cell Death Differ. 2009;16(935–938):935–938. doi:https://doi.org/10.1038/cdd.2009.59.
- Black RA, Kronheim SR, Merriam JE, March CJ, Hopp TP. A pre-aspartate-specific protease from human leukocytes that cleaves pro-interleukin-1 beta. J Biol Chem. 1989;264(5323–5326):5323–5326. doi:https://doi.org/10.1016/S0021-9258(18)83546-3.
- Crawford ED, Wells JA. Caspase substrates and cellular remodeling. Annu Rev Biochem. 2011;80(1055–1087):1055–1087. doi:https://doi.org/10.1146/annurev-biochem-061809-121639.
- Brown SB, Bailey K, Savill J. Actin is cleaved during constitutive apoptosis. Biochem J. 1997;323(Pt 1):233–237. doi:https://doi.org/10.1042/bj3230233.
- Mashima T, Naito M, Tsuruo T. Caspase-mediated cleavage of cytoskeletal actin plays a positive role in the process of morphological apoptosis. Oncogene. 1999;18(2423–2430):2423–2430. doi:https://doi.org/10.1038/sj.onc.1202558.
- Desouza M, Gunning PW, Stehn JR. The actin cytoskeleton as a sensor and mediator of apoptosis. Bioarchitecture. 2012;2(75–87):75–87. doi:https://doi.org/10.4161/bioa.20975.
- Croft DR, Coleman ML, Li S, Robertson D, Sullivan T, Stewart CL, Olson MF. Actin-myosin-based contraction is responsible for apoptotic nuclear disintegration. J Cell Biol. 2005;168(245–255):245–255. doi:https://doi.org/10.1083/jcb.200409049.
- Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol. 2001;3(339–345):339–345. doi:https://doi.org/10.1038/35070009.
- Wickman GR, Julian L, Mardilovich K, Schumacher S, Munro J, Rath N, Zander SA, Mleczak A, Sumpton D, Morrice N, et al. Blebs produced by actin-myosin contraction during apoptosis release damage-associated molecular pattern proteins before secondary necrosis occurs. Cell Death Differ. 2013;20(1293–1305):1293–1305. doi:https://doi.org/10.1038/cdd.2013.69.
- Nunez R, Sancho-Martinez SM, Novoa JM, Lopez-Hernandez FJ. Apoptotic volume decrease as a geometric determinant for cell dismantling into apoptotic bodies. Cell Death Differ. 2010;17(1665–1671):1665–1671. doi:https://doi.org/10.1038/cdd.2010.96.
- Sebbagh M, Renvoizé C, Hamelin J, Riché N, Bertoglio J, Bréard J. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol. 2001;3(346–352):346–352. doi:https://doi.org/10.1038/35070019.
- Gagliardi PA, Somale D, Puliafito A, Chiaverina G, Di Blasio L, Oneto M, Bianchini P, Bussolino F, Primo L. MRCKalpha is activated by caspase cleavage to assemble an apical actin ring for epithelial cell extrusion. J Cell Biol. 2018;217(231–249):231–249. doi:https://doi.org/10.1083/jcb.201703044.
- Petrache I, Crow MT, Neuss M, Garcia JG. Central involvement of Rho family GTPases in TNF-alpha-mediated bovine pulmonary endothelial cell apoptosis. Biochem Biophys Res Commun. 2003;306(244–249):244–249. doi:https://doi.org/10.1016/s0006-291x(03)00945-8.
- Unbekandt M, Belshaw S, Bower J, Clarke M, Cordes J, Crighton D, Croft DR, Drysdale MJ, Garnett MJ, Gill K, et al. Discovery of potent and selective MRCK inhibitors with therapeutic effect on skin cancer. Cancer Res. 2018;78(2096–2114):2096–2114. doi:https://doi.org/10.1158/0008-5472.CAN-17-2870.
- Fritz G, Just I, Kaina B. Rho GTPases are over-expressed in human tumors. Int J Cancer. 1999;81(682–687):682–687. doi:https://doi.org/10.1002/(sici)1097-0215(19990531)81:5<682::aid-ijc2>3.0.co;2-b.
- Kamai T, Yamanishi T, Shirataki H, Takagi K, Asami H, Ito Y, Yoshida K-I. Overexpression of RhoA, Rac1, and Cdc42 GTPases is associated with progression in testicular cancer. Clin Cancer Res. 2004;10(4799–4805):4799–4805. doi:https://doi.org/10.1158/1078-0432.CCR-0436-03.
- Maldonado MDM, Medina JI, Velazquez L, Dharmawardhane S. Targeting Rac and Cdc42 GEFs in metastatic cancer. Front Cell Dev Biol. 2020;8(201). doi:https://doi.org/10.3389/fcell.2020.00201.
- Takami Y, Higashi M, Kumagai S, Kuo PC, Kawana H, Koda K, Miyazaki M, Harigaya K. The activity of RhoA is correlated with lymph node metastasis in human colorectal cancer. Dig Dis Sci. 2008;53(467–473):467–473. doi:https://doi.org/10.1007/s10620-007-9887-0.
- Rodrigues P, Macaya I, Bazzocco S, Mazzolini R, Andretta E, Dopeso H, Mateo-Lozano S, Bilić J, Cartón-García F, Nieto R, et al. RHOA inactivation enhances Wnt signalling and promotes colorectal cancer. Nat Commun. 2014;5(5458). doi:https://doi.org/10.1038/ncomms6458
- Wilkinson S, Paterson HF, Marshall CJ. Cdc42-MRCK and Rho-ROCK signalling cooperate in myosin phosphorylation and cell invasion. Nat Cell Biol. 2005;7(255–261):255–261. doi:https://doi.org/10.1038/ncb1230.
- Waclaw B, Bozic I, Pittman ME, Hruban RH, Vogelstein B, Nowak MA. A spatial model predicts that dispersal and cell turnover limit intratumour heterogeneity. Nature. 2015;525(261–264):261–264. doi:https://doi.org/10.1038/nature14971.
- Loo CS, Chen C-W, Wang P-J, Chen P-Y, Lin S-Y, Khoo K-H, Fenton RA, Knepper MA, Yu M-J. Quantitative apical membrane proteomics reveals vasopressin-induced actin dynamics in collecting duct cells. Proc Natl Acad Sci U S A. 2013;110(17119–17124):17119–17124. doi:https://doi.org/10.1073/pnas.1309219110.
- Barrett K, Leptin M, Settleman J. The Rho GTPase and a putative RhoGEF mediate a signaling pathway for the cell shape changes in Drosophila gastrulation. Cell. 1997;91(905–915):905–915. doi:https://doi.org/10.1016/s0092-8674(00)80482-1.
- Hacker U, Perrimon N. DRhoGEF2 encodes a member of the Dbl family of oncogenes and controls cell shape changes during gastrulation in Drosophila. Genes Dev. 1998;12(274–284):274–284. doi:https://doi.org/10.1101/gad.12.2.274.
- Terry SJ, Zihni C, Elbediwy A, Vitiello E, Leefa Chong San IV, Balda MS, Matter K. Spatially restricted activation of RhoA signalling at epithelial junctions by p114RhoGEF drives junction formation and morphogenesis. Nat Cell Biol. 2011;13(159–166):159–166. doi:https://doi.org/10.1038/ncb2156.
- Zhao Z, Manser E. Myotonic dystrophy kinase-related Cdc42-binding kinases (MRCK), the ROCK-like effectors of Cdc42 and Rac1. Small GTPases. 2015;6(81–88):81–88. doi:https://doi.org/10.1080/21541248.2014.1000699.
- Nakajima H, Tanoue T. Lulu2 regulates the circumferential actomyosin tensile system in epithelial cells through p114RhoGEF. J Cell Biol. 2011;195(245–261):245–261. doi:https://doi.org/10.1083/jcb.201104118.
- Hoege C, Hyman AA. Principles of PAR polarity in Caenorhabditis elegans embryos. Nat Rev Mol Cell Biol. 2013;14(315–322):315–322. doi:https://doi.org/10.1038/nrm3558.
- Robertson F, Pinal N, Fichelson P, Pichaud F. Atonal and EGFR signalling orchestrate rok- and Drak-dependent adherens junction remodelling during ommatidia morphogenesis. Development. 2012;139(3432–3441):3432–3441. doi:https://doi.org/10.1242/dev.080762.
- Corrigall D, Walther RF, Rodriguez L, Fichelson P, Pichaud F. Hedgehog signaling is a principal inducer of Myosin-II-driven cell ingression in Drosophila epithelia. Dev Cell. 2007;13(730–742):730–742. doi:https://doi.org/10.1016/j.devcel.2007.09.015.
- Escudero LM, Bischoff M, Freeman M. Myosin II regulates complex cellular arrangement and epithelial architecture in Drosophila. Dev Cell. 2007;13(717–729):717–729. doi:https://doi.org/10.1016/j.devcel.2007.09.002.
- Kumfer KT, Cook SJ, Squirrell JM, Eliceiri KW, Peel N, O’Connell KF, White JG. CGEF-1 and CHIN-1 Regulate CDC-42 activity during asymmetric division in the Caenorhabditis elegans Embryo. Mol Biol Cell. 2010;21(266–277):266–277. doi:https://doi.org/10.1091/mbc.E09-01-0060.
- Wang SC, Low TYF, Nishimura Y, Gole L, Yu W, Motegi F. Cortical forces and CDC-42 control clustering of PAR proteins for Caenorhabditis elegans embryonic polarization. Nat Cell Biol. 2017;19(988–995):988–995. doi:https://doi.org/10.1038/ncb3577.
- Fic W, Bastock R, Raimondi F, Los E, Inoue Y, Gallop JL, Russell RB, St Johnston D. RhoGAP19D inhibits Cdc42 laterally to control epithelial cell shape and prevent invasion. J Cell Biol. 2021;220(4). doi:https://doi.org/10.1083/jcb.202009116.
- Anderson DC, Gill JS, Cinalli RM, Nance J. Polarization of the C. elegans embryo by RhoGAP-mediated exclusion of PAR-6 from cell contacts. Science. 2008;320(5884):1771–1774. doi:https://doi.org/10.1126/science.1156063.
- Wang Y, Riechmann V. The role of the actomyosin cytoskeleton in coordination of tissue growth during Drosophila oogenesis. Curr Biol. 2007;17(1349–1355):1349–1355. doi:https://doi.org/10.1016/j.cub.2007.06.067.
