ABSTRACT
In some organs, such as the brain, endothelial cells form a robust and highly selective blood-to-tissue barrier. However, in other organs, such as the intestine, endothelial cells provide less stringent permeability, to allow rapid exchange of solutes and nutrients where needed. To maintain the structural and functional integrity of the highly dynamic blood–brain and gut–vascular barriers, endothelial cells form highly specialized cell-cell junctions, known as adherens junctions and tight junctions. Claudins are a family of four-membrane-spanning proteins at tight junctions and they have both barrier-forming and pore-forming properties. Tissue-specific expression of claudins has been linked to different diseases that are characterized by barrier impairment. In this review, we summarize the more recent progress in the field of the claudins, with particular attention to their expression and function in the blood–brain barrier and the recently described gut–vascular barrier, under physiological and pathological conditions.
Abbreviations: 22q11DS 22q11 deletion syndrome; ACKR1 atypical chemokine receptor 1; AD Alzheimer disease; AQP aquaporin; ATP adenosine triphosphate; Aβ amyloid β; BAC bacterial artificial chromosome; BBB blood-brain barrier; C/EBP-α CCAAT/enhancer-binding protein α; cAMP cyclic adenosine monophosphate (or 3ʹ,5ʹ-cyclic adenosine monophosphate); CD cluster of differentiation; CNS central nervous system; DSRED discosoma red; EAE experimental autoimmune encephalomyelitis; ECV304 immortalized endothelial cell line established from the vein of an apparently normal human umbilical cord; EGFP enhanced green fluorescent protein; ESAM endothelial cell-selective adhesion molecule; GLUT-1 glucose transporter 1; GVB gut-vascular barrier; H2B histone H2B; HAPP human amyloid precursor protein; HEK human embryonic kidney; JACOP junction-associated coiled coil protein; JAM junctional adhesion molecules; LYVE1 lymphatic vessel endothelial hyaluronan receptor 1; MADCAM1 mucosal vascular addressin cell adhesion molecule 1; MAPK mitogen-activated protein kinase; MCAO middle cerebral artery occlusion; MMP metalloprotease; MS multiple sclerosis; MUPP multi-PDZ domain protein; PATJ PALS-1-associated tight junction protein; PDGFR-α platelet-derived growth factor receptor α polypeptide; PDGFR-β platelet-derived growth factor receptor β polypeptide; RHO rho-associated protein kinase; ROCK rho-associated, coiled-coil-containing protein kinase; RT-qPCR real time quantitative polymerase chain reactions; PDGFR-β soluble platelet-derived growth factor receptor, β polypeptide; T24 human urinary bladder carcinoma cells; TG2576 transgenic mice expressing the human amyloid precursor protein; TNF-α tumor necrosis factor α; WTwild-type; ZO zonula occludens.
Introduction of the blood-brain barrier and the gut-vascular barrier
The central nervous system (CNS) is a very sensitive structure and requires a homeostatic environment and protection from the constantly changing milieu in the blood stream, as well as from infectious agents and toxins. In the adult, maintenance of the CNS microenvironment and protection of the CNS itself is assigned to three barriers: the brain–cerebrospinal fluid barrier, the blood-cerebrospinal fluid barrier, and the blood–brain barrier (BBB). The BBB has been the most studied, with its discovery arising from the work of Paul Ehrlich, Max Lewandowski and Edwin Goldman. In 1885, Paul Ehrlich showed that when a dye was injected into animals it stained all of the tissues of the body but not the brainCitation1. However, ironically, he attributed his findings to a lack of affinity of the dyes for the brain tissue, and never arrived at the idea of an active barrier function. Later, in 1900, Max Lewandowski observed that toxins did not pass across brain capillaries, and, based also on Ehlrich’s experiments, he concluded with the concept of the BBBCitation1. Finally, Edwin Goldman, a student of Paul Ehrlich, injected dyes into the cerebrospinal fluid of the subarachnoid space of animals and saw that only the brain was stained, and not the body.Citation1 Then, in 1967, the combination of electron microscopy and intravenous injection of horseradish peroxidase into mice offered the opportunity to better describe the localization of the BBB in the capillaries and postcapillary venules of the CNS.Citation2 ()
Figure 1. Structures of blood-brain and intestinal barriers
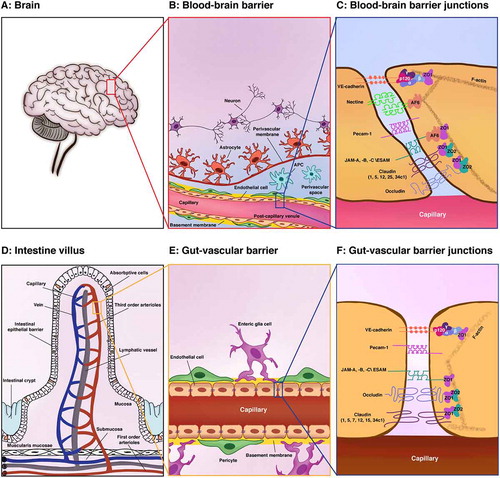
Likewise, in 2015, Spadoni and colleaguesCitation9 injected mice intravenously with fluorescein isothiocyanate–dextran of different molecular sizes, and analyzed the leakage of the dye into the intestine. While molecules of 4 kDa passed through the endothelial barrier into the blood, molecules of 70 kDa did not. This indicated the concept of a gut–vascular barrier (GVB) that allows selective access of the intestinal luminal contents to the liver, which would thus provide tolerance against dietary antigens and commensal microbiotaCitation9 (). Consistent with this, they reported leakage of 70 kDa dextran into the circulation during infection with Salmonella enterica serovar Typhimurium. This alteration of barrier permeability was due to S. enterica-induced down-regulation of endothelial Wnt signaling, which in turn produced an increase in plasmalemma vesicle-associated protein, a molecular component of the fenestrated endothelium that controls vascular permeability.Citation9
The GVB provides a second intestinal barrier, and together with the intestinal–epithelial barrier, these guarantee the physical exclusion of gut microbiota from the systemic circulation ()). The intestinal–epithelial barrier is a semipermeable barrier that is formed by the monolayer of enterocytes that covers the entire mucosa.Citation10 Furthermore, specialized enterocytes also limit bacterial invasion, such as the goblet cells that produce mucus, and the Paneth cells that secrete antimicrobial peptides.Citation11 Just as the intestinal–epithelial barrier selectively regulates what enters the host tissue, the intestinal vasculature must also be highly selective for what enters the circulation.Citation12
The GVB shares many key morphological and functional characteristics with the well-known BBB (). The main components of the BBB are the brain endothelial cells, which are in close contact with the pericytes, with both sheathed in the protective protein cover known as the basement membrane, which is produced by both cell types. Pericytes are recruited to vessels early in development, and they stabilize the vessels by their effects on brain endothelial cells, whereby they acquire more BBB characteristics. Brain endothelial cells and pericytes form the vascular unit of the BBB. The end-feet that project from the astrocytes that are attached on these endothelial cells and pericytes then function as a bridge between the vascular unit and the neurons, as well as promoting the maturation of the BBB. The highly coordinated activity that arises from these multiple cell types, which also include the vascular unit, glia and neurons, is known as the neurovascular unit ()). The perivascular space then lies at the level of the post-capillary vessels, between the vascular unit and the astrocytes, and this is defined by the basement membrane and the parenchymal membrane that is produced by the astrocytes. This space contains antigen-presenting cells (i.e., microglia, macrophages), which have functions in immunosurveillance. Constant communication between the neurovascular unit and the other brain cell types assure the functions of the BBB as a physical, metabolic, transport and immunological barrier (for BBB reviews, seeCitation13–16).
Similarly, in the intestine, where the ‘gut–vascular unit’ consists of blood capillaries that are associated with the pericytes and enteric glial cells ()). It is likely that these pericytes and enteric glial cells are essential for intestinal homeostasis, and that they can influence the endothelial barrier function, thus resembling the astrocytes in the brain. Indeed, it has been reported that transplantation of enteric glia into damaged spinal cord can accelerate repair of the vasculature at a site of injury and induction of the BBB properties.Citation17
The GVB allows the diffusion from lumen to blood of molecules as large 4 kDa, which is eightfold the maximal size reported for the BBB. This will be because these barriers need to fulfill distinct, and diverse, functions. Indeed, the main role of the BBB is to avoid uncontrolled movement of any substances from the blood into the brain parenchyma, to protect the CNS from the constantly changing milieu of the blood stream.Citation18 To accomplish these functions, brain endothelial cells have low pinocytotic activity, lack fenestration, and are interconnected with the complex and continuous tight junctions, thus controlling junction permeability. The tight junctions closest to the apical membrane maintain the BBB integrity by preventing diffusion of proteins between the luminal and abluminal membrane compartments and restricting the paracellular pathway. This effectively prevents the passage of polar, hydrophilic drugs through the endothelial cell layer. Conversely, the intestinal endothelium is located underneath the epithelial layer, and it needs to be permeable to nutrients, due to the absorptive function of the gut ()). Thus, the endothelium in the GVB collaborates with enteroglial cells and pericytes to form tight and adherens junctions according to the needs of the overlying epithelial tissue. On the other hand, the GVB acts as the last and potentially most important line of defense to prevent pathogens from invading the bloodstream, and the subsequent systemic consequences.
Tight junctions include the transmembrane proteins claudins, occludin, junctional adhesion molecules (JAMs) and endothelial cell-selective adhesion molecule (ESAM), and the cytoplasmic zonula occludens proteins (ZO-1/2). Cingulin, 7H6, Afadin/AF6 and junction-associated coiled-coil protein (JACOP) are also cytoplasmic protein adaptors that connect ZO-1 to the cytoskeletonCitation19,Citation20 ()).
The tightness of the cerebral capillary junctions is reflected in their high trans-endothelial electrical resistance. Typically, electrical resistances of ~2000 Ω cm2 are observed in vivo in pial microvessels on the surface of the brain as compared to 1 Ω cm2 to 3 Ω cm2 in mesenteric capillaries.Citation21,Citation22 This difference in the trans-endothelial electrical resistance might be due to the distinctive composition of the tight junctions and adherens junctions in the BBB compared to the GVB.
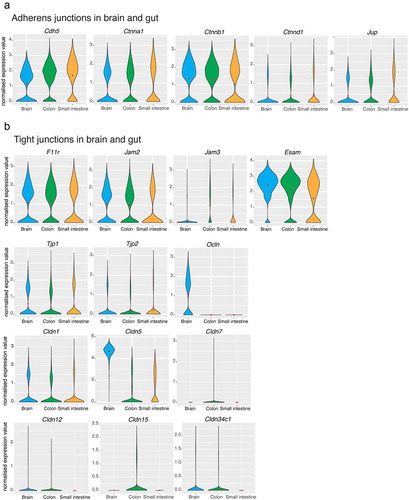
Due to the endothelial cell heterogeneity between and within tissues with different functions, the vasculature of two segments of the intestine (i.e., colon, small intestine) has been compared to the brain vessels.Citation3 Exploiting the single-cell transcriptome dataset from Carmeliet and colleagues, we provide here evidence that in the adherens junctions of the brain, colon and small intestine endothelial cells, the gene expression levels are similar for VE-cadherin (Cdh5), α-catenin (Ctnna1), β-catenin (Ctnnb1), p120 (Ctnnd1) and plakoglobin (Jup). Similarly, in the tight junctions, the expression levels of junctional adhesion molecule-A (F11r), junctional adhesion molecule-B (Jam-2), endothelial cell adhesion molecule (Esam), zonula occludens-1 (Tjp1) and zonula occludens-2 (Tjp2) are comparable ()). Instead, the expression levels for occludin (Ocln) and claudin-5 (Cldn5) are remarkably high in the brain. In addition, a number of the claudins, including claudins-7, −12, −15 and −34c1, show differential expression between the brain and the intestine ()). Interestingly, these data on claudins distributions suggested that the diverse barrier functions of endothelial tissues reflect the use of the different claudins and indicate that claudin expression patterns might indeed be responsible for the known variations in permeability.
Figure 2. Single-cell gene expression profiles of adherens and tight junctions in the brain and gut
For instance, it is well known that the presence of high levels of claudin-5 in the BBB is responsible for the restriction of molecules and ions from blood to brain parenchyma.Citation23,Citation24 However, the relationship between the expression of claudins and the protective role of the GVB to prevent the harmful elements from entering the blood stream from the intestine lumen remains to be defined.
In this review, we summarize the more recent progress on claudins, with particular attention to their expression and function in the BBB and the recently discovered GVB, under both physiological and pathological conditions. We will describe in detail each single barrier separately, because of the scarce availability of information for the GVB compared to the BBB. The reader is referred to several reviews for detailed discussions of the claudins in the other vascular barriers.Citation25–28
Functional and structural properties of claudins
Claudins were originally purified from chicken liver by Mikio Furuse et al. in 1998.Citation29 They identified a protein of 22 kDa inside the tight junction fraction, and its purification and peptide sequencing allowed them to clone two full-length cDNAs that encoded two related proteins of 211 and 230 residues. These proteins were named claudins, which means ‘close’ in Latin.
Since then, the number of proteins recognized as members of the claudin family has been growing continually, with 27 members already characterized in mammals.Citation30 However, more claudins are still being identified, such as claudin-34c1, for which there is experimental evidence at the transcript level for the vascular beds of different organs.Citation3
As members of tetraspan tight junction proteins, claudins serve two main purposes: channel forming and sealing. These functions are not mutually exclusive for each claudin (i.e., claudins-4, −7, −8) as they strongly depend on the cell type in which these proteins are expressed.Citation26 Claudins control the paracellular permeability to ions, larger solutes and water by forming channels across tight junctions. Channel-forming claudins were initially identified through decreased transepithelial electrical resistance of overexpressing cell layers. For instance, claudin-2 forms channels for monovalent inorganic cationsCitation31 and water,Citation32 while claudin-7 forms channels for Na+.Citation33 Moreover, claudin-15 can act as a Na+ channel and a Cl – barrier in vitro, which depends on the cellular context.Citation34 Interestingly, claudin-12 appears to increase cell membrane Ca2+ permeability in vitro, and its expression is up-regulated by vitamin D, which suggests a role for vitamin D in the uptake of Ca2+.Citation35
Sealing is another fundamental function of claudins. This property has been supported by several studies carried out on claudins distributions, which have suggested that the diverse barrier functions of a variety of endothelial tissues depend on the expression of their particular claudins. Claudin expression patterns might be responsible for the variations in permeability across a wide range of tissues, such as the BBB, GVB, blood–testis barrier, retinal barrier and thymic barrier. As knockout models for claudins-1 and −5 have been shown to be lethal, the role of claudins in the maintenance of vascular and epithelial barriers is undoubtedly crucial.Citation36,Citation37 In the brain and in the retina, the presence of a barrier between the vascular lumen, the neuronal layers and the parenchyma maintains a regulated microenvironment and correct neuronal function. Indeed, claudin-5 is the predominant claudin expressed in endothelial tight junctions and has a pivotal role in the barrier properties of distinct tissues. For instance, in the BBB, claudin-5 has an important role in forming a barrier to small molecules, through its strong interactions with the members of the claudin family. Claudin-5 can form homophilic interactions (i.e., claudin-5/claudin-5) or it can bind to claudin-3Citation37Citation38 and claudin-1,Citation39 thus explaining the sealed barrier of this anatomic district. However, several recent reports have postulated numerous mathematical and computational configurations for ionic pores of claudin-5, although supporting experimental data are still not available.Citation40,Citation41
In the intestinal epithelium, claudins show differential expression patterns, and by their barrier function, these proteins prevent the unlimited passage of water and solutes, and the invasion of luminal antigens,Citation42 as was shown through the transepithelial electrical resistance, which has been shown to increase upon overexpression of claudins-1, −4, −5 and −7.Citation43 Interestingly, in 2001, Rahner et al. demonstrated the presence of claudins along the basolateral membrane of gastrointestinal epithelial cells, which suggested that the functions of the basal and lateral membrane compartments depend on the presence of specific claudin molecules.Citation44 It is plausible to assume that claudins have a role in the trafficking of endosomal vesicles from the basolateral membrane to apical tight junctions, which undergo continuous remodeling.Citation45 Furthermore, basolateral claudins might have accessory pore functions, to facilitate discretion of ion transport at tight junctions or to create signaling complexes that transfer information into the cells. Indeed, claudins-1, −2, and −7 are implicated in the regulation of the interfaces between cells and the extracellular matrix through the interactions with integrin molecules in focal adhesions.Citation46 Thus, claudins-1, −2, and −7 regulate normal cellular homeostasis under physiological conditions and facilitate adhesion of metastatic cells under pathological conditions. The diversity of transepithelial electrical resistance and paracellular ion flow is due to the differential expression of claudin members in the gastrointestinal tract. In humans, qRT-PCR analysis revealed the predominant expression of claudins-2 and −15 in the proximal part of the gastrointestinal tract,Citation47 while claudins-3, −4, −7, and −8 show higher expression in the more distal parts. Moreover, in the colon, claudin-1 is expressed and localized at the apex of epithelial cells,Citation48 while claudins-4 and −7 are detected in both the tight junctions and the lateral membranes of the cell surface.Citation49 In the small intestine, claudin-7 is expressed along the basolateral and apical surfaces of epithelial cells, while in the ileum and colon, claudin-8 is distributed on the basolateral membrane of epithelial cells.Citation50,Citation51
Recent reports have demonstrated the unconventional localization of claudins in the nuclear district.Citation52 First, in 2005, Dhawan et al.Citation53 demonstrated the unexpected nuclear presence of claudin-1 in patients with human primary colon cancer and in cancer-related metastasis, while claudin-1 was localized on the basolateral membrane in the normal human colonic mucosa.Citation53 Similarly, numerous studies have shown localization of claudin-1 in the nucleus of melanoma cells and in thyroid carcinoma cells.Citation54,Citation55 Later, the nuclear localization of other claudins was highlighted under pathological conditions: claudin-2 was identified in the nucleus in human lung adenocarcinoma cells. The nuclear distribution of claudin-2 increases cell proliferation in human lung adenocarcinoma cells,Citation56 as for claudin-3 in breast cancer linesCitation57 and claudin-4 in endometrial cancer cells.Citation58
The functions of the claudins are strictly dependent on their structures and post-translational modifications. Claudins constitute a protein family with a molecular mass that ranges from 18 kDa to 27 kDa. They are integral membrane proteins that are characterized by four hydrophobic transmembrane domains, a short N-terminus on the cytoplasmic side (~7 residues), two extracellular loops (ECLs; ECL1, ~52 residues; ECL2, 15–33 residues) and a C-terminus cytoplasmic domain that shows considerable length differences between claudins (21–63 residues)Citation29,Citation59().
Figure 3. Schematic structure of claudins
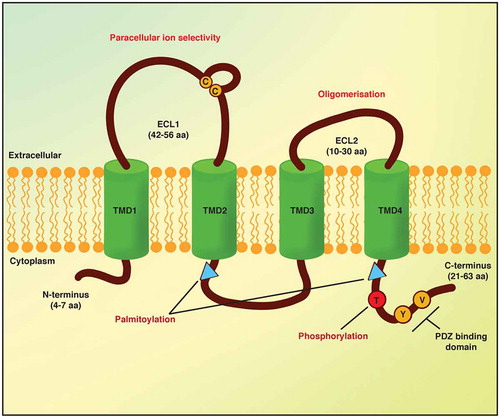
The C-terminus cytoplasmic domain of claudins is predicted to be intracellular and it is fundamental for claudin stability and the intracellular transport of tight junctions.Citation60,Citation61 Based on the length of the C-terminus domain, claudins can be subdivided into two groups: the ‘classic’ and ‘non-classic’ claudins.Citation62 The classic claudins comprise claudins-1–9, −14, −17 and −19, and these show high structural similarity and have short C-terminus cytosolic domains. The non-classic claudins comprise claudins-10–12, −15, −16, −18, −21 and −24, which are characterized by long C-terminus domains, through which they can interact with scaffold proteins in the cytoplasm.Citation63 In claudins-1, −2, −4, −5 and −16, this domain can be phosphorylated, which controls some of their barrier functions (). Furthermore, this phosphorylation is mediated by different kinases and is linked to claudin functional modulation as part of the tight junction assembly, as well as to control the function of the tight junctions. For instance, when claudin-5 is phosphorylated on Thr-207 by cAMP-dependent protein kinase A, barrier permeability in the brain endothelium is decreased,Citation64 while in the lung endothelium, it results in increased permeability of small molecules of the claudin-5–based barrier.Citation65 In addition, the permeability of the brain endothelium is increased by Rho/ROCK signaling, where its activation leads to phosphorylation of claudin-5 Ser and Tyr residues,Citation66 while phosphorylation by MAPK of Thr-203 in claudin-1 in lung endothelial cells decreases the barrier permeability.Citation67
The region spanning from the fourth transmembrane domain to the C-terminus and the region of the intercellular loop after the second transmembrane domain are both characterized by a conserved pair of cysteines, which for claudin-14 can be palmitoylated (). When this palmitoylation is impaired, claudin-14 loses its correct localization at the tight junction, which highlights the importance of this palmitoylation in the protein trafficking.Citation68
Ubiquitination represents another post-translational modification that can regulate functional aspects of claudins. This is an ATP-dependent modification of proteins catalyzed by enzymes that activate, conjugate and favor the binding of ubiquitin to lysine residues of a target protein.Citation69 The fate of a protein depends on its ubiquitination, in terms of its trafficking (endocytosis) and lysosomal degradation.Citation70 For claudins, the C-terminus domain, and more specifically their PDZ domains, show the common requisites for ubiquitination. This process can occur on claudins-1, −2 and −4, and in this case, it also appears to affect their endocytosis and lysosomal degradation.Citation71
Interactions with scaffolding proteins indirectly link claudins to the actin cytoskeleton and regulate claudins functions. It is very common for claudins to have a PDZ binding motif at the C-terminus domain, through which they can interact with scaffolding proteins ZO-1/2/3, PATJ and MUPP1.Citation72–74 Indeed, for most claudins, the end of the C-terminus contains a hydrophobic motif of two peptides that is crucial for binding to PDZ domains on several of the tight junction scaffolding proteins (). However, this motif is not present in claudins-12, −22, −25 and −27.
The first, and longer, claudin extracellular loop (ECL1) is composed of 42–56 residues, and it is located between the first and second transmembrane domains. It contains a highly conserved signature motif across claudins, which suggests that its involvement in the main functions of this protein family. ECL1 defines the charge selectivity and magnitude of the small-ion permeability of the paracellular pathwayCitation75 and shows a wide variation in the numbers and positions of its charged amino acids, which characterizes each of the different claudins. For instance, claudin-4 ECL1 shows the exchange of basic for acidic residues that leads to increased cation permeability, while conversely, claudin-15 ECL1 shows the replacement of acidic residues for basic residues, thus reversing the paracellular charge selectivity from binding to cations over anions.
The amino-acidic sequence of the second extracellular loop (ECL2) is highly conserved. Chimera studies have shown that ECL2 is not involved in the control of paracellular permeability,Citation74 while it is fundamental for interactions between claudins.Citation76 Several types of interactions can occur between claudins, as homophilic and heterophilic, in both cis and trans. This means that claudins can interact with each other along the plasma membrane of the same cell (cis-interaction) or of an opposing cell (trans-interaction). Moreover, any particular claudin can interact with the same family member (homophilic interactions) or with other family members (heterophilic interactions).
For instance, trans-interactions between self-associated molecules of claudin-5 are strongly dependent on amino-acid substitutions in the binding core of ECL2, which supports a substantial contribution of ECL2 in the holding and narrowing function of this claudin.Citation77 Indeed, studies by Piontek and colleagues on claudin-5–transfected HEK cells suggested a role in the formation of tight junction strands via trans-interactions.Citation46 Based on their molecular modeling, they also hypothesized that ECL2 is folded in a helix-turn-helix motif and can form a dimer between different claudins of adjacent cell membranes through hydrophobic interactions of conserved aromatic residues. Moreover, studies carried out on claudin-2/claudin-2 dimers have shown that cis-interactions occur through the ECL2Citation78 domain. In addition, it has been demonstrated that ECL2 of claudins-3 and −4 is a receptor for Clostridium perfringens enterotoxin.Citation79,Citation80 The C-terminus part of C. perfringens enterotoxin can bind claudins,Citation81,Citation82 while the N-terminus part can shape pores in the plasma membrane and promote cell death.
The identification and characterization of the claudins family still leaves the need to explain how they participate in the generation of heterogeneity observed in diverse tissues, which emphasizes their crucial roles in the development and maintenance of vertebrate tissues.
Claudins of the blood–brain barrier
As indicated above, the BBB is formed by a tightly packed monolayer of non-fenestrated endothelial cells that line the brain capillaries and are enveloped by pericytes and perivascular astrocytes ()). Cerebral capillaries account for 85% of the blood vessel length in the brain, which provides an endothelium surface area of ~12 m2 for molecular exchange with the brain, with an approximate 1:1 ratio of capillaries to neurons.Citation83 Specialized endothelial cells along the cerebral vasculature are linked together mechanically by tight junction protein complexes that eliminate the paracellular space between the neighboring endothelial cells ()).
A wide range of studies has suggested that claudins-1, −3, −5 and −12 are expressed in the BBB as integral components of tight junctions, with claudin-5 showing the highest expression in brain endothelial cells ()). This expression of claudin-5 in brain endothelial cells has been shown to occur as early as embryonic day 12.5 in mice,Citation84 and to be maintained to adult life in different experimental models, including mice, rats and chickens.Citation84–86 In-vitro co-culturing of rat astrocytes with pig brain endothelial cells induced increases in claudin-5 expression under conditions of cell contact.Citation87 In the same context, glioma-conditioned medium has been shown to up-regulate claudins-1, −3 and −5 in the human ECV304 cell BBB in-vitro model; of note; however, these are not endothelium-derived cells as they originally came from human T24 urinary bladder carcinoma cells.Citation88 Moreover, brain endothelial cells from rats and chickens show gradual decreases in the expression of claudins-1 and −5, occludin and ZO-1 when cultured in vitro. In addition, in rats, the tight junction morphology in-vitro changes from a ratio of protoplasmic:extracellular faces of 55:45 to 16:84 in vivo. These data suggest a loss of BBB function as protoplasmic-face-associated tight junctions are linked to better sealing of the BBB.Citation85 Claudin-5 was connected to extracellular-face-associated tight junctions when Morita et al. transfected L-fibroblasts with a claudin-5 vector, which resulted in a switch in the tight junction morphology from protoplasmic face associated to extracellular face associated.Citation84 Furthermore, this study speculated that the switch of extracellular-face-associated tight junctions in the embryonic mouse brain (at E12.5) to protoplasmic-face-associated tight junctions in the adult is caused by early expression of claudin-5, with the other claudins expressed during maturation of the brain endothelial cells. Such early and high levels of claudin-5 in the BBB support the controlled permeability of the BBB. For this reason, to examine the involvement of claudin-5 in the development of the BBB, Nitta and colleagues generated claudin-5 deficient (claudin5−/-) mice.Citation37 Although these claudin5−/- mice showed the formation of normal tight junctions, with no bleeding or edema, and normal blood vessel development, their endothelia showed increased permeability to low-molecular-weight dyes. Tracer experiments in claudin5−/- mouse embryos (18.5E) revealed that small molecules ≤800 Da, such as Hoechst (452 Da) and gadolinium-diethylenetriamine penta-acetic acid (742 Da), crossed the BBB, but microperoxidase (1.9 kDa) and tetramethylrhodamine-conjugated dextran (10 kDa) did not.Citation37 Moreover, size-selective, transient BBB opening was seen in siRNA-induced claudin-5 knockdown mice. The permeation of small molecules of up to 742 Da from the brain microvessels, but not of molecules of 4.4 kDa, was seen for up to 48 h after the injection of the claudin-5 siRNA in these mice, with no significant adverse effects seen. Furthermore, the administration of neuropeptide thyrotropin-releasing hormone (360 Da) into the tail vein of the mice after injection of the claudin-5 siRNA inhibited the permeation for up to five times longer than that seen for the non-targeting siRNA control mice.Citation89 Finally, we recently showed that JAM-A promotes C/EBP-α binding to the claudin-5 promoter, which induces claudin-5 transcription and enhances the endothelial barrier function in mice. On the contrary, JAM-A–null mice have decreased claudin-5 expression and increased brain permeability to tracers with molecular weights <500 Da.Citation90
Claudin-12 is another claudin that is expressed in mouse brain. In 2003, while studying the tight junction of their claudin-5−/- mice embryos at E18.5, Nitta et al. also showed that this absence of claudin-5 did not affect the expression of claudin-12, which was concentrated at ZO-1–positive tight junctions.Citation37 However, 5 y later, Ohtsuki et al. reported that claudin-12 is not specific to brain endothelial cells in adult mice.Citation86 They isolated Pecam-1+ and Pecam-1− cells from wild-type (WT) adult mouse brains using magnetic cell sorting. Here, they showed higher claudin-12 mRNA levels in total brain tissue compared to these Pecam-1+ and Pecam-1− cells. In agreement with the study of Ohtsuki et al. (2008), in 2019, Castro Dias et al. used knockout claudin-12LacZ/LacZ reporter mice and demonstrated that according to the β-galactosidase signal in the brain, claudin-12 was expressed at very low levels in the endothelium and pericytes, and significantly more in the astrocytes and neurons.Citation91 They also used these claudin-12LacZ/LacZ mice to show that none of the commercial antibodies can be used for Western blotting as bands of claudin-12 were seen for both WT and claudin-12LacZ/LacZ mice. Furthermore, they did not see any changes in claudin-5, occludin and ZO-1 in freshly isolated brain endothelial cells; they thus concluded that claudin-12 is not needed for the tight junction function of the BBB. In 2008, Shimizu et al. described the barrier properties in terms of transporters and tight junctions using RT-qPCR and Western blotting in cultured pericyte cell lines from different organs, including the brain, lungs and peripheral nerves. Among the several transporters that they also found in the brain endothelial cells, the tight junctions of all three pericyte cell lines expressed claudin-12, ZO-1/2 and JAM-A, but not claudin-5.Citation92
Claudin-1 has been shown to be expressed in rat and chicken brain endothelial cells in vivo and in vitro, and to be up-regulated by a glioma-conditioned medium in ECV304 cells.Citation85,Citation88 However, Ohtsuki and colleagues demonstrated that claudin-1 is 41.5-fold more enriched in endothelial cells from Pecam-1− compared to Pecam-1+ mouse brains. Their data suggested that like claudin-12, claudin-1 is not specific for brain endothelial cells.Citation86
Early studies on claudin-3 have demonstrated that it is expressed in brain endothelial cells. In particular, similar to claudins-1 and −5, claudin-3 is expressed and up-regulated in ECV304 cells treated with glioma-conditioned medium.Citation88 Moreover, it has been shown that claudin-3 is a target of Wnt/β-catenin signaling and is up-regulated in vitro upon β-catenin stabilization or Wnt3a treatment in mouse brain endothelial cells. Inactivation of β-catenin or inhibition of its signaling had the opposite effect. In vivo, stabilization of β-catenin increased claudin-3 expression in brain endothelial cells of embryos (E18.5) and pups at postnatal day 4 (P4). Inactivation of β-catenin in vivo decreased the expression of claudin-3 in the brain endothelial cells of pups at P4, P7 and P14.Citation93 Further experiments in adult mouse brains confirmed the expression and localization of claudin-3 at the tight junctions of brain endothelial cells.Citation94 However, Ohtsuki et al. reported that although claudin-3 mRNA was detected in adult mouse brains, it was not endothelium specific.Citation86 The same study indicated the expression of other claudins as endothelium specific, including for claudins-8, −10, −15, −17, −19, −20, −22 and −23, and that claudins-10 and −22 had comparable mRNA levels to occludin.
Recently, Haseloff and colleagues compared the gene expression profiles of claudins in human and mouse brain endothelia obtained by laser capture microdissection and in primary brain endothelial cells and immortalized brain endothelial cells-3.Citation95 In contrast to in-vitro BBB models, claudin-5 mRNA in vivo is just one of another six high-abundance, tetraspanning tight junction protein transcripts. The mRNAs for claudins-11, −12 and −25 and for occludin (in human, mouse), and for claudins-1 and −27 (in human) are in the same quantitative range within species, which suggests that a complex of complementary proteins (mainly claudins) operates within the paracellular space of the BBB in vivo. This joint operation is lost in vitro, where claudin-5 becomes dominant, and the absence of several highly abundant claudins might also account for the relatively low tightness of in-vitro BBB models.Citation96
Single-cell RNA sequencing has provided the opportunity to investigate the transcriptional profiles of individual cells, and to thus categorize cells more accurately in terms of specific clusters. Two recent studies used single-cell RNA sequencing to better describe the BBB and the endothelium throughout the mouse body,Citation3–5 where with the exception of claudin-5 the various claudins have shown discrepancies with older data. In the first studies,Citation4,Citation5 four vascular and vessel-associated cell types of transgenic reporter mouse models were used. Cldn5(BAC)-GFP was used to define endothelial cells, Pdgfrb (BAC)-EGFP;Cspg4-DsRed to define mural cells, Tagln-Cre;R26-stop-tdTomato to define astrocytes, and Pdgfra-H2BGFP to define perivascular fibroblast-like cells. In total, 3,436 single-cell transcriptomes were analyzed from adult mice, of which around 1,500 were from endothelial cells, 1,000 from pericytes, and the remaining ~1,000 from other cells, such as vascular smooth muscle cells, astrocytes, oligodendrocytes, microglia and perivascular and meningeal fibroblast-like cells. Twenty-one sequences of claudins mRNA were detected, 13 of which were expressed in <10 individual cells, and were thus considered to be background noise. Claudin-3 was detected in four cells, one of which was an endothelial cell. Claudin-1 was detected in only a subpopulation of fibroblasts, claudin-5 was specific to endothelial cells, claudin-10 only to astrocytes, claudin-11 to oligodendrocytes and fibroblasts, claudin-14 only to a few oligodendrocytes, and claudin-15 to a few pericytes and smooth muscle cells, while claudins-12 and −25 were ubiquitously expressed.
The second single-cell RNA sequencing study examined endothelial cell heterogeneity across 11 tissues, including the brain.Citation3 In the CD45−CD31+ brain endothelial cells were identified that were selected by sorting for only four different claudins, among which claudins-1, −12 and −34c1 were low, while claudin-5 was 10-fold to 100-fold more expressed. While claudins-1, −5 and −12 have been reported to be expressed in brain endothelial cells, claudin-34c1 has only been mentioned in this one study to date.
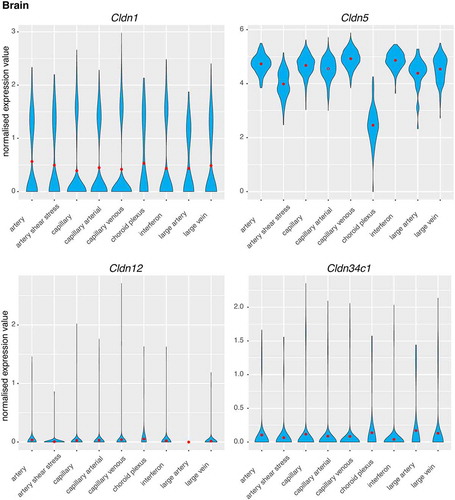
Due to intra-tissue endothelial cell heterogeneity and to differentiate putative functions in the vessels, endothelial cells have been divided into the following nine vessel-related phenotypes, based on marker gene expression analysis: artery; artery sheer stress; capillary; capillary arterial; capillary venous; choroid plexus; interferon; large artery; and large vein. Claudins-1, −12 and −34c1 showed similar expression levels across all of the phenotypes of endothelial cells, with the exception of the absence of claudin-12 in large artery (). Claudin-5 showed similar expression for all of the endothelial cell phenotypes, apart from a small decrease for the artery sheer stress, and a 50% decrease in the choroid plexus ().
Figure 4. Single-cell gene expression profiles of claudins in the brain
For claudin-3 expression in brain endothelial cells, the data of Castro Dias et al. (2019) are in agreement with both of these single-cell sequencing studies. To clarify things for the debate, they generated mice with a total knockout of claudin-3. These mice showed an intact BBB, with no indications of claudin-3 mRNA and protein in the brain endothelial cells as assessed by RT-qPCR and Western blotting. They concluded that in previous claudin-3 immunofluorescence studies, the antibodies used had cross-reacted with other unknown antigen(s) that were also present in the claudin-3 knockout mice.Citation97
Claudins in the gut–vascular barrier
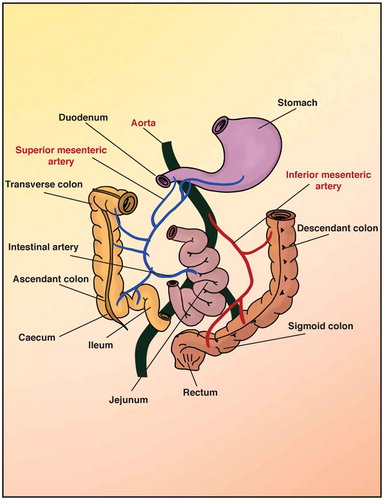
The main function of the gastrointestinal circulation is to deliver the required oxygen for the secretory, absorptive and motor activities of the intestine, with the ‘return delivery’ of CO2 for its removal, and also of the absorbed nutrients and water for all of the organs of the body, using both the blood and the lymphatic vasculature. The intestines are divided into the small and large intestines. The small intestine is subdivided into the duodenum, jejunum and ileum (). The large intestine starts with the cecum and continues with the colon, to end with the rectum and anus.Citation7 The blood supply to the intestines comes from different vessels, but all of these derive from the aorta, which is divided into the celiac trunk, the superior mesenteric artery, and the inferior mesenteric arteryCitation98 (). The arterial blood vessels enter the intestine via the surface of the serosa, which is in contact with the mesentery (the structure that gives support to the vasculature in the peritoneum). Then, these arterial blood vessels enter the villi, to give rise to the branched network made of arterioles and venules, and the capillaries that penetrate the three main tissue layers of the mucosa, muscularis and submucosaCitation99 ()). From the muscular layer, the first-order arterioles are in the submucosa, and the second-order arterioles derive from these, which end up as the third-order arterioles. These last penetrate into the mucosa and along to the tip of the villi, where they become capillaries and form a mesh-like structure.Citation100 In contrast, for the microvasculature of the colon, the branches of the arterioles and capillaries pass along the luminal surface of the mucosa and form a network around the glands, and are much closer to the epithelium than in the small intestineCitation101 ()).
Figure 5. Vascular bed in the gastrointestinal tract
In 2015, Spadoni et al. described a novel anatomical structure in the murine and human intestines that they called the GVB.Citation9 The GVB consists of the blood capillaries located just below the intestinal epithelium (for review, seeCitation102). The GVB is composed of closely interacting intestinal vascular endothelial cells, pericytes and enteroglial cells (). Among the tight junctions here, claudin-5 is expressed primarily in lymphatic endothelial tight junctions. Claudin-12 has been associated with other cell types in the lamina propria.Citation35 Moreover, the transcriptional profile of CD45−CD31+CD105+LYVE1− blood endothelial cells purified from the small intestine of mice confirmed the expression of claudin-12 and revealed the expression of the other claudins, such as claudins-2, −3, −4, −7, −15 and −23Citation8 ()). While the expression of some of these proteins, such as claudin-12, has been validated by immunofluorescence, the expression of these other claudins needs to be further analyzed.Citation9
Recently, Carmeliet and coworkers performed single-cell RNA sequencing of endothelial cells from 11 adult mouse tissues.Citation3 Among these, colon and small intestine endothelial cells showed high expression of gene sets involved in vascular basement membrane/collagen deposition, vascular endothelial growth factor signaling, endothelial cell migration, and vascular barrier integrity and maintenance,Citation103,Citation104 in agreement with their known roles in the maintenance of the gut vascular barrier.Citation9 Based on marker-gene expression, the clustering of the different endothelial cell populations identified in the intestine showed the following phenotypes: arterial endothelial cells, with the large artery and artery endothelial cell subclusters; capillary endothelial cells, with all of the capillary endothelial cell subclusters and capillary–arterial endothelial cells; lymphatic endothelial cells, with all of lymphatic endothelial cell subclusters; and endothelial cells restricted to only some tissues (Madcam1+ veins, Aqp7+ capillaries) ().
Figure 6. Single-cell gene expression profiles of claudins in the intestine
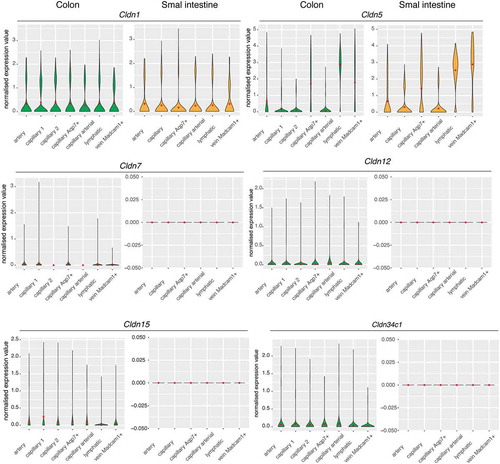
Here, claudin-5 was widely expressed in the distinct vascular beds of both the colon and the small intestine. Specifically, both of these showed high transcript levels of claudin-5 mainly in the lymphatic endothelial cells. Moreover, claudin-5 was expressed in venous endothelial cells that showed enriched expression of endothelial venule markers (Madcam, Lrg1, Ackr1) as defined as Madcam1+ veins.Citation105,Citation106 These represent a specialized subtype of endothelial cells that are adapted for recruiting/trafficking of lymphocytes from the blood to the tissue interstitiumCitation107 (). A remarkable level of claudin-5 transcript was detected in capillary endothelial cells with elevated transcript levels of genes involved in the uptake and metabolism of glycerol and fatty acids (Aqp7, Tcf15, Cd36, Fabp5). These are defined as Aqp7+ capillaries, and they might be involved in the removal of glycerol from enterocytes into the portal system, to bypass the lacteal system (). Arteries, capillaries and arterial capillaries also expressed detectable mRNA levels of claudin-5, which suggested an essential role of claudin-5 in the maintenance of the structural integrity of the gut vasculature and the control of the diffusion of molecules through the intercellular space.
Although claudin-1 was expressed at similar levels in the distinct vascular beds of both the colon and small intestine, claudin-12 was revealed only in the colon, which appears to reflect a function in ion transport rather than in endothelium sealingCitation35 (). Interestingly, the claudins-7, −15 and −34c1 transcripts showed very little, if any, expression in the colon vasculature, but not in the small intestine vascular bed, in agreement with the roles of these claudins as channels that strictly control the paracellular movement of solutesCitation63 ().
Claudin-related diseases of the central nervous system
The main property of the intact BBB is its controlled permeability through the barrier itself for molecules, cells and microorganisms, via the paracellular and transcellular routes. Reactive oxygen species, metalloproteases (MMPs), angiogenic factors, pathogens and immune cell activation and infiltration can cause impairment of the paracellular and transcellular routes, and consequently, CNS damage. This can lead to the development of CNS diseases or their exacerbation, depending on the occurrence of the disease relative to the BBB impairment.Citation108
Here, we summarize the involvement of claudins in several diseases in which claudins dysregulation and mislocalisation increase the permeability of the BBB to peptides, proteins and immune cells, which can lead to, or exacerbate, diseases. These diseases are: Alzheimer’s disease (AD), ischemic stroke, multiple sclerosis (MS) and schizophrenia.
Alzheimer’s disease
Alzheimer’s disease is a chronic neurodegenerative disease that causes severe dementia that is characterized by memory loss, impaired reasoning, and personality alterations. The pathological hallmarks of this disease include the formation of amyloid beta (Αβ) plaques in the brain parenchyma and around blood vessels and neurofibrillary tangles in the neurons, which are composed of hyperphosphorylated tau proteins.Citation109,Citation110 Histological analysis of postmortem human AD brains has shown BBB breakdown as defined by plasma albumin and immunoglobulins around amyloid plaques.Citation111 In 2019, Nation and colleagues presented evidence of BBB impairment by measuring soluble platelet-derived growth factor receptor-β (sPDGFRβ) as a biomarker of damaged capillaries in patients with AD. However, they showed the circulation of sPDGFRβ by cerebrospinal fluid independent of amyloid plaques and tau status by positron emission tomography, which suggested vascular dysfunction as a component of AD pathology.Citation112 Αβ toxicity and neuroinflammation have been associated with down-regulation of tight junction proteins in patients with AD who suffer from cerebral amyloid angiopathy at the capillary level. Two studies by Carrano and colleagues showed that in postmortem brains of patients with cerebral amyloid angiopathy, at the capillary level there was a remarkable reduction, or even complete loss, of claudin-5, among the other tight junction proteins. The capillaries were positive for Αβ plaques or were associated with activated microglia that were positive for nicotinamide adenine dinucleotide phosphate oxidase 2, an enzyme that is responsible for reactive oxygen species production.Citation113,Citation114 Although Viggars et al. correlated increased levels of albumin and fibrinogen to more progressed AD pathology in human postmortem brains, the expression levels of claudin-5, occludin and ZO-1 were not affected.Citation115 Nevertheless, BBB impairment is well accepted as a pathological characteristic of human AD pathology.Citation112
Studies in animal models of AD have also shown contradictory data regarding BBB breakdown and claudins modulation, although this is due to the clinical manifestation of AD in the different animal models. In the Tg2576 AD mouse model, multiple studies have shown increased levels of albumin, low molecular weight tracers, IgG and fibrin in the brain, which were accompanied by decreased levels of tight junction proteins. This model shows overexpression of the human amyloid precursor protein (hAPP) with the Swedish mutation (K670N, M671).Citation116,Citation117 Specifically, Winkler et al. described how deficiency of glucose transporter 1 (Glut-1) affects BBB integrity in humans with a family history of AD. They generated the double transgene AD/Glut-1–deficient mice and showed that at 6 months old, these showed significant down-regulation of tight junction claudin-5 and occludin, and increased plasma proteins in the brain parenchyma, including fibrin and IgG, compared to their control littermates.Citation117 In the same context, Hartz and colleagues described the toxic effects of Αβ on the BBB in vivo and in vitro using mice overexpressing hAPP (i.e., the Tg2576 mouse model). Isolated microvessels from these WT and hAPP mice showed down-regulation of the tight junction proteins claudin-1 (by 84%) and claudin-5 (by 43%), while the MMP-2 and MMP-9 proteins were up-regulated by 90% and 145%, respectively. Cultured brain endothelial cells from these hAPP mice showed a 1.5-fold increase in permeability to Texas red dye (641 Da), compared to WT endothelial cells. Finally, they showed a positive correlation for increasing concentrations of human Αβ isoform 1–40 (Αβ1-40) and greater loss of claudin-1 and claudin-5 in the WT mouse endothelial cells in vitro.Citation118 Counter-intuitively, Keaney et al. suggested targeting claudin-5 and occludin for drug therapies in AD after showing a greater ratio of plasma:brain Αβ1-40 levels in the Tg2576 mice treated with siRNAs against claudin-5 and occludin, which thus revealed Αβ1-40 flux from brain to blood. Moreover, the systematic administration of claudin-5 and occludin siRNAs every 21 d over 9 months in Tg2576 mice improved their cognitive function as measured by T-maze assessment.Citation119 In contrast to studies on Tg2576 mice, experiments with AD mouse models (including SP2-APP, human Tau P301L, P301S) and mice lacking apolipoprotein E or expressing the human protein have shown no claudin-5 decrease or BBB impairment as assessed by tracer leakage (range, 86 Da to 150 kDa).Citation120
Ischemic stroke
Following myocardial infarction, strokes are the second leading cause of death in humans worldwide, with ischemic stroke occurring more often than hemorrhagic stroke. The obstruction of a brain vessel by a forming or circulating clot leads to ischemic stroke.Citation121 Nutrient and oxygen deprivation cause rapid neuronal cell death in the area where the blood is not flowing. Late reperfusion and neuronal cell death can cause a cascade of adverse effects, including activation of glial cells and endothelial cells, tight junction dismantling, and disruption of the BBB due to the production of reactive oxygen species, MMPs, growth factors, cytokines and chemokines. These effects induce initial local inflammation and then immune cell infiltration into the brain parenchyma, which can later lead to further inflammation outspread and brain damage.Citation122 While some studies have proposed the increased BBB permeability in stroke to be an effect of increased vesicular trafficking or nonselective channels,Citation123–125 other studies have attributed this to tight junction protein expression and localization. The effects on expression and localization of tight junction proteins of blood reperfusion or reoxygenation over short and long periods of time have been widely described in animal models. Willis et al. (2010) studied the effects of hypoxia (a major component of stroke) and reoxygenation on tight junctions in a rat model of global hypoxia. When the rats were exposed to 6% oxygen for 30 min to 60 min they showed increased vascular permeability to 4 kDa and 10 kDa dyes and to endogenous IgG, with a more severe phenotype after 60 min, compared to normoxia-exposed rats. Moreover, claudin-5 staining showed a diffuse pattern, with decreased intensity in the microvessels of the hypoxic rats. Reoxygenation for 15 min increased claudin-5 localization to the tight junctions and its expression but not in the control rats. This study also provided evidence of increased expression and activation of the protein kinase Cθ and ζ isoforms, which disrupted the tight junctions.Citation126 In contrast, Bauer and colleagues exposed mice to 8% oxygen for 48 h and reported decreased levels of occludin but not claudin-5 or ZO-1, in total brain lysates. Moreover, immunolabeling of the brains from these mice showed discontinuous and diffuse staining of ZO-1 and occludin, but not of claudin-5. They thus suggested that inhibition of vascular endothelial growth factor and MMP9 might reverse tight junction disassembly.Citation127 These studies by Willis et al. and Bauer et al. demonstrated that expression and localization of hypoxia-mediated tight junction proteins can vary according to the time under hypoxia and the mechanisms that are activated.
Moving to more representative rat stroke models, where middle cerebral artery occlusion (MCAO) has been applied, Jiao and colleagues demonstrated time-dependent changes in tight junctions, which included claudin-5.Citation128 They showed that in isolated brain microvessels, the highest mRNA and protein decreases for claudin-5 were seen in rats following 2 h MCAO and 3 h and 72 h of reperfusion, when the infarct sizes were mostly increased. Similarly, in a permanent MCAO rat model, it was shown that after 8 h and 120 h of reperfusion, the increased Evans blue extravasation was synchronized with decreased expression of claudin-5 and occludin in the ischemic penumbra brain tissues, thus demonstrating the biphasic mode of tight junction expression.Citation129 Although the earlier studies focused on claudin-5, in the recent study of Winkler et al. (2021), they showed that following 60 min of MCAO and 3 h reperfusion, claudin-1 and claudin-12 were decreased in claudin-3 knockout mice. The WT mice treated similarly showed claudin-1, claudin-3 and claudin-12 down-regulation.Citation95 Interestingly, in WT mice, 48 h of reperfusion after MCAO induced enlarged infarct volume and edema, but conserved tight junction formation, whereas in the claudin-3 knockout mice, the junctional localization of claudin-5 and the edema and infarction volume were reduced. They thus suggested a prophylactic role on tight junctions for claudin-3, along with correlation to a worse stroke phenotype.Citation130 A negative effect of claudin-1 in BBB recovery was demonstrated by Sladojevic and coworkers in post-stroke microvessels of human and mouse origin.Citation131 They showed that the rarely present claudin-1 in the brain capillaries was up-regulated after stroke at the mRNA and protein levels and was incorporated into the tight junctions, while claudin-5 was down-regulated. This claudin-1 competed with claudin-5 for the ZO-1 interaction, which led to BBB leakiness and decreased BBB recovery. Inhibition of claudin-1 returned the BBB permeability, and as a consequence, improved neurological recovery.Citation131
Multiple sclerosis
Multiple sclerosis is a chronic autoimmune CNS disorder that is characterized by progressive loss of neuronal myelin, with inflammation, scar tissue and lesions in the brain and the spinal cord.Citation132 As confirmed by gadolinium-diethylenetriamine penta-acetic acid enhancement, BBB impairment can precede MS lesion development and the pathological symptoms of this disease.Citation133 In humans, MS lesions, serum protein leakage, and tight junction abnormalities have been shown in brain endothelial cells.Citation134–136 Although imperfect, the experimental autoimmune encephalomyelitis (EAE) mouse model has been used to study immune cell extravasation and MS pathology in humans. Early immunolabeling studies on EAE mouse brains showed loss of claudin-3, but not claudin-5, in tight junctions of venules surrounded by inflammatory cuffs.Citation94 In contrast, Errede et al. (2012) showed that mice with mild EAE have linear, but discontinuous, claudin-5 localization, while mice with severe EAE lose claudin-5 linearity in the microvessels close to or far from demyelinated neurons.Citation137 In addition, Wang et al. (2016) reported decreased claudin-5 protein levels in whole EAE mouse brain tissue lysates, and increased Evans blue leakage.Citation138 Treatment of these mice with resveratrol, an anti-inflammatory and antioxidative with neuroprotective properties, ameliorated the EAE-induced loss of claudin-5, and decreased Evans blue extravasation. Similar data to these resveratrol effects on claudin-5 and Evans blue were shown for a Kν1.3 (voltage-gated K+ channel) blocker in EAE rats.Citation139 Finally, in 2019, Uchida et al. analyzed the involvement of claudin-11 in the disruption of the BBB in MS using the brains of postmortem human MS patients and EAE mice. They showed significant down-regulation of claudin-11 in the microcapillaries in the brain and spinal cord from both the humans and mice. Moreover, claudin-11 knockdown in vitro in human brain capillary endothelial cells increased the monolayer permeability to fluorescein isothiocyanate–dextran.Citation140 Discrepancies in the expression of claudins or other tight junction proteins in these studies might be due to differences in the EAE models (e.g., disease progression) and brain areas analyzed.
Schizophrenia
Schizophrenia is a chronic and severe mental disorder that affects over 20 million people worldwide. Common symptoms include distortions in thinking, perception, emotions, language and self-awareness. Several studies have shown that BBB-associated tight junction disruption is a hallmark of schizophrenia.Citation141 Indeed, transcriptomic studies of the prefrontal cortex have revealed that 12 out of 21 tight-junction-related genes are down-regulated in schizophrenia patients compared to controls.Citation142 Likewise, genetic studies have shown that individuals with the chromosomal abnormality 22q11 deletion syndrome (22q11DS) have a 30-fold increased lifetime risk of developing schizophrenia, due to the microdeletions in the chromosomal region 22q11.21.Citation143–145
Claudin-5 is located within this deleted region in 22q11DS, which means that these individuals are haplo-insufficient for claudin-5, and therefore they only produce 50% of the claudin-5 compared to the normal population. Campbell and colleagues showed that there is a significant association between the rs10314 allele and a diagnosis of schizophrenia in 22q11DS patients, which suggested that these individuals are producing only about 25% as much claudin-5 compared to the normal population, and therefore they might have greater BBB permeability.Citation146 Loss of claudin-5 in the hippocampus and medial frontal cortex is associated with schizophrenia-related behaviors in rodents, where treatments with antipsychotic drugs directly increase claudin-5 expression.Citation146 Similarly, in postmortem human brain tissue from schizophrenia patients, the levels of claudin-5 are decreased in the vessels of the hippocampus, while the mRNA and protein levels of claudins-5 and −12 and ZO-1 are associated with the age of onset and duration of schizophrenia.Citation141 In addition, in postmortem specimens from individuals with schizophrenia, claudin-5 levels were decreased in the frontal cortex, and this reduction was associated with protein kinase A signaling.Citation147
Finally, deficit schizophrenia is characterized by a breakdown of both the tight junctions and adherens junctions of the paracellular pathways in the gut and BBB. It has been shown that the IgA response to paracellular proteins, E-cadherin, claudin-5 and β-catenin were significantly increased and positively associated with deficit schizophrenia versus non-deficit schizophrenia and controls.Citation148
Diseases related to the gut–vascular barrier
The maintenance of GVB is fundamental to the control of translocation of antigens into the bloodstream and protection from entry of the microbiota. Independent of the intestinal epithelial barrier, endothelial barrier modifications can lead to some pathological conditions, such as inflammatory bowel disease,Citation149 irritable bowel syndrome, deficit schizophrenia,Citation148 and liver disease.Citation150,Citation151 Altered endothelial barrier function has often been linked to pathogens and immune activation, and is closely associated with changes in tight junctions, such as for ZO-1 and occludin. Although the vascular contribution of claudins in these diseases is currently poorly understood, the regulation of claudin expression in the intestinal epithelial barrier also suggests a role for claudins in intestinal diseases (for review, seeCitation42). It has been shown that in patients with chronic intestinal pseudo-obstruction, the gut epithelial and vascular barriers are impaired due to the loss of the tight junction proteins to form oligomers, and the up-regulation of claudin-4, which in turn induces experimental glial cell activation.Citation152 Liu and colleagues established an in-vitro intestinal endothelial barrier model composed of rat intestinal microvascular endothelial cells, and they exposed this model to TNF-α, a cytokine that is involved in many intestinal diseases and that can induce altered barrier function and increased vascular permeability. TNF-αdecreased the expression of the tight junction proteins, including ZO-1, occludin and claudin-1, and increased claudin-2 expression. Claudin-2 is a mediator of the leaky gut barrier during intestinal inflammation,Citation153 and has been reported to be up-regulated in the progression of inflammatory bowel disease.Citation149 This will contribute to the regulation of intestinal inter-endothelial tight junctions and the maintenance of the endothelial barrier during inflammatory stimulation.
Naringin is known for its anti-inflammatory activities in experimental ulcerative colitis and intestinal tumors,Citation154 and it has been shown to increase the expression of claudin-1 and reduce that of claudin-2 in TNF-α–challenged rat intestinal microvascular endothelial cells. On this basis, Liu and colleagues proposed the application of naringin as a potential agent in intestinal disorders. In addition, rat intestinal microvascular endothelial cells exposed to lipopolysaccharide (as a model of sepsis) not only dramatically increased endothelial permeability to fluorescein isothiocyanate–dextran but also decreased trans-endothelial electrical resistance and showed reduced expression of claudin-12, β-catenin and VE-cadherin, compared to control cells. These effects of lipopolysaccharide were antagonized by berberine, which rescued the expression of claudin-12Citation155.
In addition, and regardless of the involvement of claudins, GVB impairment has been detected in patients with celiac disease,Citation9 ankylosing spondylitisCitation156 and colorectal cancer with liver metastasis,Citation157 and in mouse models of cirrhosisCitation150 and nonalcoholic steatohepatitis.Citation151–158 These complex diseases involve the intestine and the liver, and they have been associated with increased vascular permeability, and linked to increased expression of plasmalemma vesicle-associated proteins in intestinal vessels.
Conclusion and perspectives
The composition of the tight junction components closely correlates with barrier function. Claudins are key components of tight junctions that regulate paracellular permeability. Although much effort has been devoted to an understanding of the structural and functional aspects, and the regulation and physiology of the different members of the claudin family, the question as to how claudins regulate the selective permeability has not been answered yet. Some claudins serve as barriers, while others serve as pores in the epithelium, thus generating the heterogeneity observed in the tightness of the various junctions of diverse tissues. Indeed, endothelial barriers can be more or less stringent, depending on the organ that they protect. The well-studied BBB defends the brain from the entry of serum proteins, inflammatory cells, pathogens and other substances, to thus maintain CNS homeostasis. Less stringent barriers, such as the GVB, still help to avoid dissemination of unwanted molecules throughout the organism.
In this review, we have described the claudin composition in the vasculature of the BBB and GVB. While for the BBB the roles of the different claudins and their regulation in homeostatic and pathological scenarios have been widely reported, detailed evidence is missing for the recently identified GVB. The overall information is summarized in .
Table 1. Expression of claudins in the blood-brain and gut-vascular barriers under both physiological and pathological conditions
Claudins-1 and −12 are expressed at similar levels in the brain and gut endothelium. Conversely, claudin-5 is highly enriched in the vascular bed of the brain, compared to the intestine, which thus suggests that claudin-5 might be critical in the regulation of the stringency of both of these barriers. Regardless of the expression levels of the different claudins, their distinctive polymerization through heteromeric and heterotypic claudin–claudin interactions might explain the unique endothelial barrier properties of both brain and gut. Further studies are needed to explore the involvement of claudins in the mechanisms that control vascular barriers.
In conclusion, as claudins have an essential role in vascular homeostasis and disease, targeting claudin-related signaling pathways might provide new therapeutic strategies for the treatment of a broad spectrum of human diseases in the near future.
Conflicts of interest
The authors declare that they have no potential conflicts of interest to disclose.
Acknowledgments
We thank Elisabetta Dejana for the critical reading of the manuscript and Christopher P. Berrie, for editorial assistance. This study was supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC; Investigator Grants 18683 and 21320), Initial Training Networks BtRAIN grant 675619, and the CARIPLO (Cassa di Risparmio delle Provincie Lombarde) Foundation (2016-0461).
Additional information
Funding
Notes on contributors
Monica Giannotta
Monica Giannotta, A.A. Scalise, N. Kakogiannos wrote the article. F. Zanardi and F. Iannelli performed single-cell data analysis. The final form of the article has been approved by all of the authors.
References
- Liddelow SA. Fluids and barriers of the CNS: a historical viewpoint. Fluids Barriers CNS. 2011;8(1):1. doi:https://doi.org/10.1186/2045-8118-8-2.
- Reese TS, Karnovsky MJ. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol. 1967;34(1):207–28. doi:https://doi.org/10.1083/jcb.34.1.207.
- Kalucka J, De Rooij LPMH, Goveia J, Rohlenova K, Dumas SJ, Meta E, Conchinha NV, Taverna F, Teuwen L-A, Veys K, et al. Single-cell transcriptome atlas of murine endothelial cells. Cell. 2020;180(4):764–779 e720. doi:https://doi.org/10.1016/j.cell.2020.01.015.
- He L, Vanlandewijck M, Mäe MA, Andrae J, Ando K, Del Gaudio F, Nahar K, Lebouvier T, Laviña B, Gouveia L, et al. Single-cell RNA sequencing of mouse brain and lung vascular and vessel-associated cell types. Sci Data. 2018;5(1):180160. doi:https://doi.org/10.1038/sdata.2018.160.
- Vanlandewijck M, He L, Mäe MA, Andrae J, Ando K, Del Gaudio F, Nahar K, Lebouvier T, Laviña B, Gouveia L, et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature. 2018;554(7693):475–480. doi:https://doi.org/10.1038/nature25739.
- Castro Dias M, Mapunda JA, Vladymyrov M, Engelhardt B. Structure and junctional complexes of endothelial, epithelial and glial brain barriers. Int J Mol Sci. 2019;20(21):5372. doi:https://doi.org/10.3390/ijms20215372.
- Birbrair A. Pericyte biology: development, homeostasis, and disease. Adv Exp Med Biol. 2018;1109:1–3.
- Spadoni I, Pietrelli A, Pesole G, Rescigno M. Gene expression profile of endothelial cells during perturbation of the gut vascular barrier. Gut Microbes. 2016;7(6):540–548. doi:https://doi.org/10.1080/19490976.2016.1239681.
- Spadoni I, Zagato E, Bertocchi A, Paolinelli R, Hot E, Di Sabatino A, Caprioli F, Bottiglieri L, Oldani A, Viale G, et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science. 2015;350(6262):830–834. doi:https://doi.org/10.1126/science.aad0135.
- Allam-Ndoul B, Castonguay-Paradis S, Veilleux A. Gut microbiota and intestinal trans-epithelial permeability. Int J Mol Sci. 2020;21(17):6402. doi:https://doi.org/10.3390/ijms21176402.
- Farre R, Fiorani M, Abdu Rahiman S, Matteoli G. Intestinal permeability, inflammation and the role of nutrients. Nutrients. 2020;12(4):1185. doi:https://doi.org/10.3390/nu12041185.
- Gentile ME, King IL, Knoll LJ. Blood and guts: the intestinal vasculature during health and helminth infection. PLoS Pathog. 2018;14(7):e1007045. doi:https://doi.org/10.1371/journal.ppat.1007045.
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. doi:https://doi.org/10.1038/nrn1824.
- Bechmann I, Galea I, Perry VH. What is the blood-brain barrier (not)? Trends Immunol. 2007;28(1):5–11. doi:https://doi.org/10.1016/j.it.2006.11.007.
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37(1):13–25. doi:https://doi.org/10.1016/j.nbd.2009.07.030.
- Serlin Y, Shelef I, Knyazer B, Friedman A. Anatomy and physiology of the blood-brain barrier. Semin Cell Dev Biol. 2015;38:2–6. doi:https://doi.org/10.1016/j.semcdb.2015.01.002.
- Jiang S, Khan MI, Lu Y, Werstiuk ES, Rathbone MP. Acceleration of blood-brain barrier formation after transplantation of enteric glia into spinal cords of rats. Exp Brain Res. 2005;162(1):56–62. doi:https://doi.org/10.1007/s00221-004-2119-3.
- Paolinelli R, Corada M, Orsenigo F, Dejana E. The molecular basis of the blood brain barrier differentiation and maintenance. Is it still a mystery? Pharmacol Res. 2011;63(3):165–171. doi:https://doi.org/10.1016/j.phrs.2010.11.012.
- Wolburg H, Lippoldt A. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul Pharmacol. 2002;38(6):323–337. doi:https://doi.org/10.1016/S1537-1891(02)00200-8.
- Wolburg H, Noell S, Mack A, Wolburg-Buchholz K, Fallier-Becker P. Brain endothelial cells and the glio-vascular complex. Cell Tissue Res. 2009;335(1):75–96. doi:https://doi.org/10.1007/s00441-008-0658-9.
- Butt AM, Jones HC, Abbott NJ. Electrical resistance across the blood-brain barrier in anaesthetized rats: a developmental study. J Physiol. 1990;429(1):47–62. doi:https://doi.org/10.1113/jphysiol.1990.sp018243.
- Crone C, Christensen O. Electrical resistance of a capillary endothelium. J Gen Physiol. 1981;77(4):349–371. doi:https://doi.org/10.1085/jgp.77.4.349.
- Lv J, Hu W, Yang Z, Li T, Jiang S, Ma Z, Chen F, Yang Y. Focusing on claudin-5: a promising candidate in the regulation of BBB to treat ischemic stroke. Prog Neurobiol. 2018;161:79–96. doi:https://doi.org/10.1016/j.pneurobio.2017.12.001.
- Wang L, Li Z, Zhang X, Wang S, Zhu C, Miao J, Chen L, Cui L, Qiao H. Protective effect of shikonin in experimental ischemic stroke: attenuated TLR4, p-p38MAPK, NF-κB, TNF-α and MMP-9 expression, up-regulated claudin-5 expression, ameliorated BBB permeability. Neurochem Res. 2014;39(1):97–106. doi:https://doi.org/10.1007/s11064-013-1194-x.
- Reinhold AK, Rittner HL. Barrier function in the peripheral and central nervous system-a review. Pflugers Arch. 2017;469(1):123–134. doi:https://doi.org/10.1007/s00424-016-1920-8.
- Goncalves A, Ambrosio AF, Fernandes R. Regulation of claudins in blood-tissue barriers under physiological and pathological states. Tissue Barriers. 2013;1(3):e24782. doi:https://doi.org/10.4161/tisb.24782.
- Turksen K, Troy TC. Barriers built on claudins. J Cell Sci. 2004;117(12):2435–2447. doi:https://doi.org/10.1242/jcs.01235.
- Nagatake T, Zhao Y-C, Ito T, Itoh M, Kometani K, Furuse M, Saika A, Node E, Kunisawa J, Minato N, et al. Selective expression of claudin-5 in thymic endothelial cells regulates the blood-thymus barrier and T-cell export. Int Immunol. 2021;33(3):171–182. doi:https://doi.org/10.1093/intimm/dxaa069.
- Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and −2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141(7):1539–1550. doi:https://doi.org/10.1083/jcb.141.7.1539.
- Furuse M, Tsukita S. Claudins in occluding junctions of humans and flies. Trends Cell Biol. 2006;16(4):181–188. doi:https://doi.org/10.1016/j.tcb.2006.02.006.
- Amasheh S, Meiri N, Gitter AH, Schöneberg T, Mankertz J, Schulzke JD, Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci. 2002;115(24):4969–4976. doi:https://doi.org/10.1242/jcs.00165.
- Rosenthal R, Milatz S, Krug SM, Oelrich B, Schulzke J-D, Amasheh S, Günzel D, Fromm M. Claudin-2, a component of the tight junction, forms a paracellular water channel. J Cell Sci. 2010;123(11):1913–1921. doi:https://doi.org/10.1242/jcs.060665.
- Alexandre MD, Lu Q, Chen Y-H. Overexpression of claudin-7 decreases the paracellular Cl– conductance and increases the paracellular <sup>Na+ conductance in LLC-PK1 cells. J Cell Sci. 2005;118(12):2683–2693. doi:https://doi.org/10.1242/jcs.02406.
- Colegio OR, Van Itallie CM, McCrea HJ, Rahner C, Anderson JM. Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am J Physiol Cell Physiol. 2002;283(1):C142–147. doi:https://doi.org/10.1152/ajpcell.00038.2002.
- Fujita H, Sugimoto K, Inatomi S, Maeda T, Osanai M, Uchiyama Y, Yamamoto Y, Wada T, Kojima T, Yokozaki H, et al. Tight Junction Proteins Claudin-2 and −12 Are Critical for Vitamin D-dependent Ca2+Absorption between Enterocytes. Mol Biol Cell. 2008;19(5):1912–1921. doi:https://doi.org/10.1091/mbc.e07-09-0973.
- Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, Noda T, Kubo A, Tsukita S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol. 2002;156(6):1099–1111. doi:https://doi.org/10.1083/jcb.200110122.
- Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161(3):653–660. doi:https://doi.org/10.1083/jcb.200302070.
- Piontek J, Fritzsche S, Cording J, Richter S, Hartwig J, Walter M, Yu D, Turner JR, Gehring C, Rahn H-P, et al. Elucidating the principles of the molecular organization of heteropolymeric tight junction strands. Cell Mol Life Sci. 2011;68(23):3903–3918. doi:https://doi.org/10.1007/s00018-011-0680-z.
- Coyne CB, Gambling TM, Boucher RC, Carson JL, Johnson LG. Role of claudin interactions in airway tight junctional permeability. Am J Physiol Lung Cell Mol Physiol. 2003;285(5):L1166–1178. doi:https://doi.org/10.1152/ajplung.00182.2003.
- Rajagopal N, Irudayanathan FJ, Nangia S. Computational Nanoscopy of Tight Junctions at the Blood-Brain Barrier Interface. Int J Mol Sci. 2019;20(22):5583. doi:https://doi.org/10.3390/ijms20225583.
- Fisher D, Mentor S. Are claudin-5 tight junction proteins in the blood-brain barrier porous? Neural Regen Res. 2020;15(10):1838–1839. doi:https://doi.org/10.4103/1673-5374.280308.
- Barmeyer C, Schulzke JD, Fromm M. Claudin-related intestinal diseases. Semin Cell Dev Biol. 2015;42:30–38. doi:https://doi.org/10.1016/j.semcdb.2015.05.006.
- Tsukita S, Yamazaki Y, Katsuno T, Tamura A, Tsukita S. Tight junction-based epithelial microenvironment and cell proliferation. Oncogene. 2008;27(55):6930–6938. doi:https://doi.org/10.1038/onc.2008.344.
- Rahner C, Mitic LL, Anderson JM. Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology. 2001;120(2):411–422. doi:https://doi.org/10.1053/gast.2001.21736.
- Shen L, Weber CR, Turner JR. The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. J Cell Biol. 2008;181(4):683–695. doi:https://doi.org/10.1083/jcb.200711165.
- Iwamoto DV, Calderwood DA. Regulation of integrin-mediated adhesions. Curr Opin Cell Biol. 2015;36:41–47. doi:https://doi.org/10.1016/j.ceb.2015.06.009.
- Lameris AL, Huybers S, Kaukinen K, Mäkelä TH, Bindels RJ, Hoenderop JG, Nevalainen PI. Expression profiling of claudins in the human gastrointestinal tract in health and during inflammatory bowel disease. Scand J Gastroenterol. 2013;48(1):58–69. doi:https://doi.org/10.3109/00365521.2012.741616.
- Bertiaux-Vandaele N, Youmba SB, Belmonte L, Lecleire S, Antonietti M, Gourcerol G, Leroi A-M, Déchelotte P, Ménard J-F, Ducrotté P, et al. The expression and the cellular distribution of the tight junction proteins are altered in irritable bowel syndrome patients with differences according to the disease subtype. Am J Gastroenterol. 2011;106(12):2165–2173. doi:https://doi.org/10.1038/ajg.2011.257.
- Oshima T, Miwa H, Joh T. Changes in the expression of claudins in active ulcerative colitis. J Gastroenterol Hepatol. 2008;23(Suppl 2):S146–150. doi:https://doi.org/10.1111/j.1440-1746.2008.05405.x.
- Ding L, Lu Z, Foreman O, Tatum R, Lu Q, Renegar R, Cao J, Chen Y. Inflammation and disruption of the mucosal architecture in claudin-7-deficient mice. Gastroenterology. 2012;142(2):305–315. doi:https://doi.org/10.1053/j.gastro.2011.10.025.
- Fujita H, Chiba H, Yokozaki H, Sakai N, Sugimoto K, Wada T, Kojima T, Yamashita T, Sawada N. Differential expression and subcellular localization of claudin-7, −8, −12, −13, and −15 along the mouse intestine. J Histochem Cytochem. 2006;54(8):933–944. doi:https://doi.org/10.1369/jhc.6A6944.2006.
- Hagen SJ. Non-canonical functions of claudin proteins: beyond the regulation of cell-cell adhesions. Tissue Barriers. 2017;5(2):e1327839. doi:https://doi.org/10.1080/21688370.2017.1327839.
- Dhawan P, Singh AB, Deane NG, No Y, Shiou S-R, Schmidt C, Neff J, Washington MK, Beauchamp RD. Claudin-1 regulates cellular transformation and metastatic behavior in colon cancer. J Clin Invest. 2005;115(7):1765–1776. doi:https://doi.org/10.1172/JCI24543.
- French AD, Fiori JL, Camilli TC, Leotlela PD, O’Connell MP, Frank BP, Subaran S, Indig FE, Taub DD, Weeraratna AT, et al. PKC and PKA phosphorylation affect the subcellular localization of claudin-1 in melanoma cells. Int J Med Sci. 2009;6:93–101. doi:https://doi.org/10.7150/ijms.6.93.
- Zwanziger D, Badziong J, Ting S, Moeller LC, Schmid KW, Siebolts U, Wickenhauser C, Dralle H, Fuehrer D. The impact of CLAUDIN-1 on follicular thyroid carcinoma aggressiveness. Endocr Relat Cancer. 2015;22(5):819–830. doi:https://doi.org/10.1530/ERC-14-0502.
- Ikari A, Watanabe R, Sato T, Taga S, Shimobaba S, Yamaguchi M, Yamazaki Y, Endo S, Matsunaga T, Sugatani J, et al. Nuclear distribution of claudin-2 increases cell proliferation in human lung adenocarcinoma cells. Biochim Biophys Acta. 2014;1843(9):2079–2088. doi:https://doi.org/10.1016/j.bbamcr.2014.05.017.
- Todd MC, Petty HM, King JM, Piana Marshall BN, Sheller RA, Cuevas ME. Overexpression and delocalization of claudin-3 protein in MCF-7 and MDA-MB-415 breast cancer cell lines. Oncol Lett. 2015;10(1):156–162. doi:https://doi.org/10.3892/ol.2015.3160.
- Cuevas ME, Gaska JM, Gist AC, King JM, Sheller RA, Todd MC. Estrogen-dependent expression and subcellular localization of the tight junction protein claudin-4 in HEC-1A endometrial cancer cells. Int J Oncol. 2015;47(2):650–656. doi:https://doi.org/10.3892/ijo.2015.3030.
- Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2(4):285–293. doi:https://doi.org/10.1038/35067088.
- Ruffer C, Gerke V. The C-terminal cytoplasmic tail of claudins 1 and 5 but not its PDZ-binding motif is required for apical localization at epithelial and endothelial tight junctions. Eur J Cell Biol. 2004;83(4):135–144. doi:https://doi.org/10.1078/0171-9335-00366.
- Arabzadeh A, Troy TC, Turksen K. Role of the Cldn6 cytoplasmic tail domain in membrane targeting and epidermal differentiation in vivo. Mol Cell Biol. 2006;26(15):5876–5887. doi:https://doi.org/10.1128/MCB.02342-05.
- Lal-Nag M, Morin PJ. The claudins. Genome Biol. 2009;10(8):235. doi:https://doi.org/10.1186/gb-2009-10-8-235.
- Gunzel D, Yu AS. Claudins and the modulation of tight junction permeability. Physiol Rev. 2013;93(2):525–569. doi:https://doi.org/10.1152/physrev.00019.2012.
- Ishizaki T, Chiba H, Kojima T, Fujibe M, Soma T, Miyajima H, Nagasawa K, Wada I, Sawada N. Cyclic AMP induces phosphorylation of claudin-5 immunoprecipitates and expression of claudin-5 gene in blood-brain-barrier endothelial cells via protein kinase A-dependent and -independent pathways. Exp Cell Res. 2003;290(2):275–288. doi:https://doi.org/10.1016/S0014-4827(03)00354-9.
- Soma T, Chiba H, Kato-Mori Y, Wada T, Yamashita T, Kojima T, Sawada N. Thr(207) of claudin-5 is involved in size-selective loosening of the endothelial barrier by cyclic AMP. Exp Cell Res. 2004;300(1):202–212. doi:https://doi.org/10.1016/j.yexcr.2004.07.012.
- Persidsky Y, Heilman D, Haorah J, Zelivyanskaya M, Persidsky R, Weber GA, Shimokawa H, Kaibuchi K, Ikezu T. Rho-mediated regulation of tight junctions during monocyte migration across the blood-brain barrier in HIV-1 encephalitis (HIVE). Blood. 2006;107(12):4770–4780. doi:https://doi.org/10.1182/blood-2005-11-4721.
- Fujibe M, Chiba H, Kojima T, Soma T, Wada T, Yamashita T, Sawada N. Thr203 of claudin-1, a putative phosphorylation site for MAP kinase, is required to promote the barrier function of tight junctions. Exp Cell Res. 2004;295(1):36–47. doi:https://doi.org/10.1016/j.yexcr.2003.12.014.
- Van Itallie CM, Gambling TM, Carson JL, Anderson JM. Palmitoylation of claudins is required for efficient tight junction localization. J Cell Sci. 2005;118(7):1427–1436. doi:https://doi.org/10.1242/jcs.01735.
- Chau V, Tobias J, Bachmair A, Marriott D, Ecker D, Gonda D, Varshavsky A. A multiubiquitin chain is confined to specific lysine in a targeted short-lived protein. Science. 1989;243(4898):1576–1583. doi:https://doi.org/10.1126/science.2538923.
- Kerscher O, Felberbaum R, Hochstrasser M. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu Rev Cell Dev Biol. 2006;22(1):159–180. doi:https://doi.org/10.1146/annurev.cellbio.22.010605.093503.
- Takahashi S, Iwamoto N, Sasaki H, Ohashi M, Oda Y, Tsukita S, Furuse M. The E3 ubiquitin ligase LNX1p80 promotes the removal of claudins from tight junctions in MDCK cells. J Cell Sci. 2009;122(7):985–994. doi:https://doi.org/10.1242/jcs.040055.
- Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol. 1999;147(6):1351–1363. doi:https://doi.org/10.1083/jcb.147.6.1351.
- Hamazaki Y, Itoh M, Sasaki H, Furuse M, Tsukita S. Multi-PDZ domain protein 1 (MUPP1) is concentrated at tight junctions through its possible interaction with claudin-1 and junctional adhesion molecule. J Biol Chem. 2002;277(1):455–461. doi:https://doi.org/10.1074/jbc.M109005200.
- Roh MH, Liu CJ, Laurinec S, Margolis B. The carboxyl terminus of zona occludens-3 binds and recruits a mammalian homologue of discs lost to tight junctions. J Biol Chem. 2002;277(30):27501–27509. doi:https://doi.org/10.1074/jbc.M201177200.
- Colegio OR, Van Itallie C, Rahner C, Anderson JM. Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am J Physiol Cell Physiol. 2003;284(6):C1346–1354. doi:https://doi.org/10.1152/ajpcell.00547.2002.
- Blasig IE, Winkler L, Lassowski B, Mueller SL, Zuleger N, Krause E, Krause G, Gast K, Kolbe M, Piontek J, et al. On the self-association potential of transmembrane tight junction proteins. Cell Mol Life Sci. 2006;63(4):505–514. doi:https://doi.org/10.1007/s00018-005-5472-x.
- Piontek J, Winkler L, Wolburg H, Müller SL, Zuleger N, Piehl C, Wiesner B, Krause G, Blasig IE. Formation of tight junction: determinants of homophilic interaction between classic claudins. FASEB J. 2008;22(1):146–158. doi:https://doi.org/10.1096/fj.07-8319com.
- Van Itallie CM, Mitic LL, Anderson JM. Claudin-2 forms homodimers and is a component of a high molecular weight protein complex. J Biol Chem. 2011;286(5):3442–3450. doi:https://doi.org/10.1074/jbc.M110.195578.
- Fujita K, Katahira J, Horiguchi Y, Sonoda N, Furuse M, Tsukita S. Clostridium perfringens enterotoxin binds to the second extracellular loop of claudin-3, a tight junction integral membrane protein. FEBS Lett. 2000;476(3):258–261. doi:https://doi.org/10.1016/S0014-5793(00)01744-0.
- Katahira J, Sugiyama H, Inoue N, Horiguchi Y, Matsuda M, Sugimoto N. Clostridium perfringens enterotoxin utilizes two structurally related membrane proteins as functional receptors in vivo. J Biol Chem. 1997;272(42):26652–26658. doi:https://doi.org/10.1074/jbc.272.42.26652.
- Hanna PC, Mietzner TA, Schoolnik GK, McClane BA. Localization of the receptor-binding region of Clostridium perfringens enterotoxin utilizing cloned toxin fragments and synthetic peptides. The 30 C-terminal amino acids define a functional binding region. J Biol Chem. 1991;266(17):11037–11043. doi:https://doi.org/10.1016/S0021-9258(18)99124-6.
- Sonoda N, Furuse M, Sasaki H, Yonemura S, Katahira J, Horiguchi Y, Tsukita S. Clostridium perfringens enterotoxin fragment removes specific claudins from tight junction strands: evidence for direct involvement of claudins in tight junction barrier. J Cell Biol. 1999;147(1):195–204. doi:https://doi.org/10.1083/jcb.147.1.195.
- Kreczmanski P, Heinsen H, Mantua V, Woltersdorf F, Masson T, Ulfig N, Schmidt-Kastner R, Korr H, Steinbusch HWM, Hof PR, et al. Microvessel length density, total length, and length per neuron in five subcortical regions in schizophrenia. Acta Neuropathol. 2009;117(4):409–421. doi:https://doi.org/10.1007/s00401-009-0482-7.
- Morita K, Sasaki H, Furuse M, Tsukita S. Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol. 1999;147(1):185–194. doi:https://doi.org/10.1083/jcb.147.1.185.
- Liebner S, Kniesel U, Kalbacher H, Wolburg H. Correlation of tight junction morphology with the expression of tight junction proteins in blood-brain barrier endothelial cells. Eur J Cell Biol. 2000;79(10):707–717. doi:https://doi.org/10.1078/0171-9335-00101.
- Ohtsuki S, Yamaguchi H, Katsukura Y, Asashima T, Terasaki T. mRNA expression levels of tight junction protein genes in mouse brain capillary endothelial cells highly purified by magnetic cell sorting. J Neurochem. 2008;104(1):147–154.doi:https://doi.org/10.1111/j.1471-4159.2007.05008.x.
- Cooper I, Cohen-Kashi-Malina K, Teichberg VI. Claudin-5 expression in in-vitro models of the blood-brain barrier. Methods Mol Biol. 2011;762:347–354.
- Neuhaus W, Wirth M, Plattner VE, Germann B, Gabor F, Noe CR. Expression of claudin-1, claudin-3 and claudin-5 in human blood-brain barrier mimicking cell line ECV304 is inducible by glioma-conditioned media. Neurosci Lett. 2008;446(2–3):59–64. doi:https://doi.org/10.1016/j.neulet.2008.09.025.
- Campbell M, Kiang A-S, Kenna PF, Kerskens C, Blau C, O’Dwyer L, Tivnan A, Kelly JA, Brankin B, Farrar G-J, et al. RNAi-mediated reversible opening of the blood-brain barrier. J Gene Med. 2008;10(8):930–947. doi:https://doi.org/10.1002/jgm.1211.
- Kakogiannos N, Ferrari L, Giampietro C, Scalise AA, Maderna C, Ravà M, Taddei A, Lampugnani MG, Pisati F, Malinverno M, et al. JAM-A Acts via C/EBP-α to promote claudin-5 expression and enhance endothelial barrier function. Circ Res. 2020;127(8):1056–1073. doi:https://doi.org/10.1161/CIRCRESAHA.120.316742.
- Castro Dias M, Coisne C, Baden P, Enzmann G, Garrett L, Becker L, Hölter SM, Hrabě De Angelis M, Deutsch U, Engelhardt B, et al. Claudin-12 is not required for blood–brain barrier tight junction function. Fluids and Barriers of the CNS. 2019;16(1):30. doi:https://doi.org/10.1186/s12987-019-0150-9.
- Shimizu F, Sano Y, Maeda T, Abe M-A, Nakayama H, Takahashi R-I, Ueda M, Ohtsuki S, Terasaki T, Obinata M, et al. Peripheral nerve pericytes originating from the blood-nerve barrier expresses tight junctional molecules and transporters as barrier-forming cells. J Cell Physiol. 2008;217(2):388–399. doi:https://doi.org/10.1002/jcp.21508.
- Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, et al. Wnt/β-catenin signaling controls development of the blood-brain barrier. J Cell Biol. 2008;183(3):409–417. doi:https://doi.org/10.1083/jcb.200806024.
- Wolburg H, Wolburg-Buchholz K, Kraus J, Rascher-Eggstein G, Liebner S, Hamm S, Duffner F, Grote E-H, Risau W, Engelhardt B, et al. Localization of claudin-3 in tight junctions of the blood-brain barrier is selectively lost during experimental autoimmune encephalomyelitis and human glioblastoma multiforme. Acta Neuropathol. 2003;105(6):586–592. doi:https://doi.org/10.1007/s00401-003-0688-z.
- Berndt P, Winkler L, Cording J, Breitkreuz-Korff O, Rex A, Dithmer S, Rausch V, Blasig R, Richter M, Sporbert A, et al. Tight junction proteins at the blood-brain barrier: far more than claudin-5. Cell Mol Life Sci. 2019;76(10):1987–2002. doi:https://doi.org/10.1007/s00018-019-03030-7.
- Pardridge WM, Triguero D, Yang J, Cancilla PA. Comparison of in-vitro and in-vivo models of drug transcytosis through the blood-brain barrier. J Pharmacol Exp Ther. 1990;253:884–891.
- Castro Dias M, Coisne C, Lazarevic I, Baden P, Hata M, Iwamoto N, Francisco DMF, Vanlandewijck M, He L, Baier FA, et al. Claudin-3-deficient C57BL/6J mice display intact brain barriers. Sci Rep. 2019;9(1):203. doi:https://doi.org/10.1038/s41598-018-36731-3.
- Kachlik D, Baca V, Stingl J. The spatial arrangement of the human large intestinal wall blood circulation. J Anat. 2010;216(3):335–343. doi:https://doi.org/10.1111/j.1469-7580.2009.01199.x.
- Granger DN, Holm L, Kvietys P. The gastrointestinal circulation: physiology and pathophysiology. Compr Physiol. 2015;5:1541–1583.
- Thorburn T, Aali M, Lehmann C, Aali M, Lehmann C, Lehmann C. Immune response to systemic inflammation in the intestinal microcirculation. Front Biosci (Landmark Ed). 2018;23(2):782–795. doi:https://doi.org/10.2741/4616.
- Kvietys PR. The Gastrointestinal Circulation. Colloquium Series on Integrated Systems Physiology: From Molecule to Function, San Rafael (CA): Morgan & Claypool Life Sciences, 2010, 2(1): 1–127. https://doi.org/10.4199/C00009ED1V01Y201002ISP005
- Spadoni I, Fornasa G, Rescigno M. Organ-specific protection mediated by cooperation between vascular and epithelial barriers. Nat Rev Immunol. 2017;17(12):761–773. doi:https://doi.org/10.1038/nri.2017.100.
- Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML, et al. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130(4):691–703. doi:https://doi.org/10.1016/j.cell.2007.06.054.
- Murakami M, Nguyen LT, Zhang ZW, Moodie KL, Carmeliet P, Stan RV, Simons M. The FGF system has a key role in regulating vascular integrity. J Clin Invest. 2008;118(10):3355–3366. doi:https://doi.org/10.1172/JCI35298.
- Kashiwazaki M, Tanaka T, Kanda H, Ebisuno Y, Izawa D, Fukuma N, Akimitsu N, Sekimizu K, Monden M, Miyasaka M. A high endothelial venule-expressing promiscuous chemokine receptor DARC can bind inflammatory, but not lymphoid, chemokines and is dispensable for lymphocyte homing under physiological conditions. Int Immunol. 2003;15(10):1219–1227. doi:https://doi.org/10.1093/intimm/dxg121.
- Saito K, Tanaka T, Kanda H, Ebisuno Y, Izawa D, Kawamoto S, Okubo K, Miyasaka M. Gene expression profiling of mucosal addressin cell adhesion molecule-1+ high endothelial venule cells (HEV) and Identification of a Leucine-Rich HEV Glycoprotein as a HEV Marker. J Immunol. 2002;168(3):1050–1059. doi:https://doi.org/10.4049/jimmunol.168.3.1050.
- Girard JP, Moussion C, Forster R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol. 2012;12(11):762–773. doi:https://doi.org/10.1038/nri3298.
- Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19(12):1584–1596. doi:https://doi.org/10.1038/nm.3407.
- Alonso AD, Cohen LS, Corbo C, Morozova V, ElIdrissi A, Phillips G, Kleiman FE. Hyperphosphorylation of Tau associates with changes in its function beyond microtubule stability. Front Cell Neurosci. 2018;12:338. doi:https://doi.org/10.3389/fncel.2018.00338.
- Rao CV, Asch AS, Carr DJJ, Yamada HY. “Amyloid-β accumulation cycle” as a prevention and/or therapy target for Alzheimer’s disease. Aging Cell. 2020;19(3):e13109. doi:https://doi.org/10.1111/acel.13109.
- Wisniewski HM, Vorbrodt AW, Wegiel J. Amyloid angiopathy and blood-brain barrier changes in Alzheimer’s disease. Ann N Y Acad Sci. 1997;826:161–172. doi:https://doi.org/10.1111/j.1749-6632.1997.tb48468.x.
- Nation DA, Sweeney MD, Montagne A, Sagare AP, D’Orazio LM, Pachicano M, Sepehrband F, Nelson AR, Buennagel DP, Harrington MG, et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019;25(2):270–276. doi:https://doi.org/10.1038/s41591-018-0297-y.
- Carrano A, Hoozemans JJM, Van Der Vies SM, Rozemuller AJM, Van Horssen J, De Vries HE. Amyloid β induces oxidative stress-mediated blood-brain barrier changes in capillary amyloid angiopathy. Antioxid Redox Signal. 2011;15(5):1167–1178. doi:https://doi.org/10.1089/ars.2011.3895.
- Carrano A, Hoozemans JJM, Van Der Vies SM, Van Horssen J, De Vries HE, Rozemuller AJM. Neuroinflammation and blood-brain barrier changes in capillary amyloid angiopathy. Neurodegener Dis. 2012;10(1–4):329–331. doi:https://doi.org/10.1159/000334916.
- Viggars AP, Wharton SB, Simpson JE, Matthews FE, Brayne C, Savva GM, Garwood C, Drew D, Shaw PJ, Ince PG, et al. Alterations in the blood brain barrier in ageing cerebral cortex in relationship to Alzheimer-type pathology: a study in the MRC-CFAS population neuropathology cohort. Neurosci Lett. 2011;505(1):25–30. doi:https://doi.org/10.1016/j.neulet.2011.09.049.
- Ujiie M, Dickstein DL, Carlow DA, Jefferies WA. Blood-brain barrier permeability precedes senile plaque formation in an Alzheimer disease model. Microcirculation. 2003;10(6):463–470. doi:https://doi.org/10.1038/sj.mn.7800212.
- Winkler EA, Nishida Y, Sagare AP, Rege SV, Bell RD, Perlmutter D, Sengillo JD, Hillman S, Kong P, Nelson AR, et al. GLUT1 reductions exacerbate Alzheimer’s disease vasculo-neuronal dysfunction and degeneration. Nat Neurosci. 2015;18(4):521–530. doi:https://doi.org/10.1038/nn.3966.
- Hartz AM, Bauer B, Soldner ELB, Wolf A, Boy S, Backhaus R, Mihaljevic I, Bogdahn U, Klünemann HH, Schuierer G, et al. Amyloid-β contributes to blood-brain barrier leakage in transgenic human amyloid precursor protein mice and in humans with cerebral amyloid angiopathy. Stroke. 2012;43(2):514–523. doi:https://doi.org/10.1161/STROKEAHA.111.627562.
- Keaney J, Walsh DM, O’Malley T, Hudson N, Crosbie DE, Loftus T, Sheehan F, McDaid J, Humphries MM, Callanan JJ, et al. Autoregulated paracellular clearance of amyloid-β across the blood-brain barrier. Sci Adv. 2015;1(8):e1500472. doi:https://doi.org/10.1126/sciadv.1500472.
- Bien-Ly N, Boswell C, Jeet S, Beach T, Hoyte K, Luk W, Shihadeh V, Ulufatu S, Foreman O, Lu Y, et al. Lack of widespread BBB disruption in Alzheimer’s disease models: focus on therapeutic antibodies. Neuron. 2015;88(2):289–297. doi:https://doi.org/10.1016/j.neuron.2015.09.036.
- Davis SM, Donnan GA. Clinical practice. Secondary prevention after ischemic stroke or transient ischemic attack. N Engl J Med. 2012;366(20):1914–1922. doi:https://doi.org/10.1056/NEJMcp1107281.
- Yang C, Hawkins KE, Dore S, Candelario-Jalil E. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am J Physiol Cell Physiol. 2019;316(2):C135–C153. doi:https://doi.org/10.1152/ajpcell.00136.2018.
- Knowland D, Arac A, Sekiguchi K, Hsu M, Lutz S, Perrino J, Steinberg G, Barres B, Nimmerjahn A, Agalliu D, et al. Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke. Neuron. 2014;82(3):603–617. doi:https://doi.org/10.1016/j.neuron.2014.03.003.
- Krueger M, Bechmann I, Immig K, Reichenbach A, Härtig W, Michalski D. Blood-brain barrier breakdown involves four distinct stages of vascular damage in various models of experimental focal cerebral ischemia. J Cereb Blood Flow Metab. 2015;35(2):292–303. doi:https://doi.org/10.1038/jcbfm.2014.199.
- De Bock M, Van Haver V, Vandenbroucke RE, Decrock E, Wang N, Leybaert L. Into rather unexplored terrain-transcellular transport across the blood-brain barrier. Glia. 2016 Jul;64(7):1097–1123. doi: https://doi.org/10.1002/glia.22960.
- Willis CL, Meske DS, Davis TP. Protein kinase C activation modulates reversible increase in cortical blood-brain barrier permeability and tight junction protein expression during hypoxia and posthypoxic reoxygenation. J Cereb Blood Flow Metab. 2010;30(11):1847–1859. doi:https://doi.org/10.1038/jcbfm.2010.119.
- Bauer AT, Burgers HF, Rabie T, Marti HH. Matrix metalloproteinase-9 mediates hypoxia-induced vascular leakage in the brain via tight junction rearrangement. J Cereb Blood Flow Metab. 2010;30(4):837–848. doi:https://doi.org/10.1038/jcbfm.2009.248.
- Jiao H, Wang Z, Liu Y, Wang P, Xue Y. Specific role of tight junction proteins claudin-5, occludin, and ZO-1 of the blood-brain barrier in a focal cerebral ischemic insult. J Mol Neurosci. 2011;44(2):130–139. doi:https://doi.org/10.1007/s12031-011-9496-4.
- Liu P, Zhang R, Liu D, Wang J, Yuan C, Zhao X, Li Y, Ji X, Chi T, Zou L, et al. Time-course investigation of blood-brain barrier permeability and tight junction protein changes in a rat model of permanent focal ischemia. J Physiol Sci. 2018;68(2):121–127. doi:https://doi.org/10.1007/s12576-016-0516-6.
- Winkler L, Blasig R, Breitkreuz-Korff O, Berndt P, Dithmer S, Helms HC, Puchkov D, Devraj K, Kaya M, Qin Z, et al. Tight junctions in the blood-brain barrier promote edema formation and infarct size in stroke - ambivalent effects of sealing proteins. J Cereb Blood Flow Metab. 2021;41(1):132–145. doi:https://doi.org/10.1177/0271678X20904687.
- Sladojevic N, Stamatovic SM, Johnson AM, Choi J, Hu A, Dithmer S, Blasig IE, Keep RF, Andjelkovic AV. Claudin-1-dependent destabilization of the blood-brain barrier in chronic stroke. J Neurosci. 2019;39:743–757. doi:https://doi.org/10.1523/JNEUROSCI.1432-18.2018.
- Goldenberg MM. Multiple sclerosis review. P T. 2012;37:175–184.
- Kermode AG, Thompson AJ, Tofts P, Macmanus DG, Kendall BE, Kingsley DPE, Moseley IF, Rudge P, Mcdonald WI. Breakdown of the blood-brain barrier precedes symptoms and other MRI signs of new lesions in multiple sclerosis. Pathogenetic and clinical implications. Brain. 1990;113(Pt 5):1477–1489. doi:https://doi.org/10.1093/brain/113.5.1477.
- Plumb J, McQuaid S, Mirakhur M, Kirk J. Abnormal endothelial tight junctions in active lesions and normal-appearing white matter in multiple sclerosis. Brain Pathol. 2002;12(2):154–169. doi:https://doi.org/10.1111/j.1750-3639.2002.tb00430.x.
- Kirk J, Plumb J, Mirakhur M, McQuaid S. Tight junctional abnormality in multiple sclerosis white matter affects all calibres of vessel and is associated with blood-brain barrier leakage and active demyelination. J Pathol. 2003;201(2):319–327. doi:https://doi.org/10.1002/path.1434.
- Van Horssen J, Brink BP, De Vries HE, Van Der Valk P, Bo L. The blood-brain barrier in cortical multiple sclerosis lesions. J Neuropathol Exp Neurol. 2007;66(4):321–328. doi:https://doi.org/10.1097/nen.0b013e318040b2de.
- Errede M, Girolamo F, Ferrara G, Strippoli M, Morando S, Boldrin V, Rizzi M, Uccelli A, Perris R, Bendotti C, et al. Blood-brain barrier alterations in the cerebral cortex in experimental autoimmune encephalomyelitis. J Neuropathol Exp Neurol. 2012;71(10):840–854. doi:https://doi.org/10.1097/NEN.0b013e31826ac110.
- Wang D, Li S-P, Fu J-S, Zhang S, Bai L, Guo L. Resveratrol defends blood-brain barrier integrity in experimental autoimmune encephalomyelitis mice. J Neurophysiol. 2016;116(5):2173–2179. doi:https://doi.org/10.1152/jn.00510.2016.
- Huang J, Han S, Sun Q, Zhao Y, Liu J, Yuan X, Mao W, Peng B, Liu W, Yin J, et al. Kv1.3 channel blocker (ImKTx88) maintains blood-brain barrier in experimental autoimmune encephalomyelitis. Cell Biosci. 2017;7(1):31. doi:https://doi.org/10.1186/s13578-017-0158-2.
- Uchida Y, Sumiya T, Tachikawa M, Yamakawa T, Murata S, Yagi Y, Sato K, Stephan A, Ito K, Ohtsuki S, et al. Involvement of claudin-11 in disruption of blood-brain, -spinal cord, and -arachnoid barriers in multiple sclerosis. Mol Neurobiol. 2019;56(3):2039–2056. doi:https://doi.org/10.1007/s12035-018-1207-5.
- Greene C, Hanley N, Campbell M. Blood-brain barrier associated tight junction disruption is a hallmark feature of major psychiatric disorders. Transl Psychiatry. 2020;10(1):373. doi:https://doi.org/10.1038/s41398-020-01054-3.
- Enwright Iii JF, Huo Z, Arion D, Corradi JP, Tseng G, Lewis DA. Transcriptome alterations of prefrontal cortical parvalbumin neurons in schizophrenia. Mol Psychiatry. 2018;23(7):1606–1613. doi:https://doi.org/10.1038/mp.2017.216.
- Murphy KC. Schizophrenia and velo-cardio-facial syndrome. Lancet. 2002;359(9304):426–430. doi:https://doi.org/10.1016/S0140-6736(02)07604-3.
- Williams NM. Molecular mechanisms in 22q11 deletion syndrome. Schizophr Bull. 2011;37(5):882–889. doi:https://doi.org/10.1093/schbul/sbr095.
- Kao A, Mariani J, McDonald-McGinn DM, Maisenbacher MK, Brooks-Kayal AR, Zackai EH, Lynch DR. Increased prevalence of unprovoked seizures in patients with a 22q11.2 deletion. Am J Med Genet A. 2004;129A(1):29–34. doi:https://doi.org/10.1002/ajmg.a.30133.
- Greene C, Kealy J, Humphries MM, Gong Y, Hou J, Hudson N, Cassidy LM, Martiniano R, Shashi V, Hooper SR, et al. Dose-dependent expression of claudin-5 is a modifying factor in schizophrenia. Mol Psychiatry. 2018;23(11):2156–2166. doi:https://doi.org/10.1038/mp.2017.156.
- Nishiura K, Ichikawa-Tomikawa N, Sugimoto K, Kunii Y, Kashiwagi K, Tanaka M, Yokoyama Y, Hino M, Sugino T, Yabe H, et al. PKA activation and endothelial claudin-5 breakdown in the schizophrenic prefrontal cortex. Oncotarget. 2017;8(55):93382–93391. doi:https://doi.org/10.18632/oncotarget.21850.
- Maes M, Sirivichayakul S, Kanchanatawan B, Vodjani A. Breakdown of the paracellular tight and adherens junctions in the gut and blood brain barrier and damage to the vascular barrier in patients with deficit schizophrenia. Neurotox Res. 2019;36(2):306–322. doi:https://doi.org/10.1007/s12640-019-00054-6.
- Groschwitz KR, Hogan SP. Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol. 2009 quiz 21-22;124(1):3–20. doi:https://doi.org/10.1016/j.jaci.2009.05.038.
- Sorribas M, Jakob MO, Yilmaz B, Li H, Stutz D, Noser Y, De Gottardi A, Moghadamrad S, Hassan M, Albillos A, et al. FXR modulates the gut-vascular barrier by regulating the entry sites for bacterial translocation in experimental cirrhosis. J Hepatol. 2019;71(6):1126–1140. doi:https://doi.org/10.1016/j.jhep.2019.06.017.
- Cheng C, Tan J, Qian W, Zhang L, Hou X. Gut inflammation exacerbates hepatic injury in the high-fat diet induced NAFLD mouse: attention to the gut-vascular barrier dysfunction. Life Sci. 2018;209:157–166. doi:https://doi.org/10.1016/j.lfs.2018.08.017.
- Boschetti E, Accarino A, Malagelada C, Malagelada JR, Cogliandro RF, Gori A, Tugnoli V, Giancola F, Bianco F, Bonora E, et al. Gut epithelial and vascular barrier abnormalities in patients with chronic intestinal pseudo-obstruction. Neurogastroenterol Motil. 2019;31(8):e13652. doi:https://doi.org/10.1111/nmo.13652.
- Luettig J, Rosenthal R, Barmeyer C, Schulzke JD. Claudin-2 as a mediator of leaky gut barrier during intestinal inflammation. Tissue Barriers. 2015;3(1–2):e977176. doi:https://doi.org/10.4161/21688370.2014.977176.
- Liu P, Bian Y, Fan Y, Zhong J, Liu Z. Protective effect of naringin on in-vitro gut-vascular barrier disruption of intestinal microvascular endothelial cells induced by TNF-α. J Agric Food Chem. 2020;68(1):168–175. doi:https://doi.org/10.1021/acs.jafc.9b06347.
- He Y, Yuan X, Zuo H, Sun Y, Feng A. Berberine exerts a protective effect on gut-vascular barrier via the modulation of the Wnt/β-catenin signaling pathway during sepsis. Cell Physiol Biochem. 2018;49(4):1342–1351. doi:https://doi.org/10.1159/000493412.
- Ciccia F, Guggino G, Rizzo A, Alessandro R, Luchetti MM, Milling S, Saieva L, Cypers H, Stampone T, Di Benedetto P, et al. Dysbiosis and zonulin up-regulation alter gut epithelial and vascular barriers in patients with ankylosing spondylitis. Ann Rheum Dis. 2017;76(6):1123–1132. doi:https://doi.org/10.1136/annrheumdis-2016-210000.
- Bertocchi A, Carloni S, Ravenda PS, Bertalot G, Spadoni I, Lo Cascio A, Gandini S, Lizier M, Braga D, Asnicar F, et al. Gut vascular barrier impairment leads to intestinal bacteria dissemination and colorectal cancer metastasis to liver. Cancer Cell. 2021. doi:https://doi.org/10.1016/j.ccell.2021.03.004.
- Mouries J, Brescia P, Silvestri A, Spadoni I, Sorribas M, Wiest R, Mileti E, Galbiati M, Invernizzi P, Adorini L, et al. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J Hepatol. 2019;71(6):1216–1228. doi:https://doi.org/10.1016/j.jhep.2019.08.005.
