ABSTRACT
Oropharyngeal, airway, intestinal, and genital mucosal epithelia are the main portals of entry for the majority of human pathogenic viruses. To initiate systemic infection, viruses must first be transmitted across the mucosal epithelium and then spread across the body. However, mucosal epithelia have well-developed tight junctions, which have a strong barrier function that plays a critical role in preventing the spread and dissemination of viral pathogens. Viruses can overcome these barriers by disrupting the tight junctions of mucosal epithelia, which facilitate paracellular viral penetration and initiate systemic disease. Disruption of tight and adherens junctions may also release the sequestered viral receptors within the junctional areas, and liberation of hidden receptors may facilitate viral infection of mucosal epithelia. This review focuses on possible molecular mechanisms of virus-associated disruption of mucosal epithelial junctions and its role in transmucosal viral transmission and spread.
Introduction
Accumulating evidence indicates that pathogenic viruses can overcome the epithelial mucosal barrier function by disrupting tight junctions, thereby opening up paracellular space and facilitating viral penetration and spread. Furthermore, virus-induced disruption of epithelial tight junctions may lead to the release of hidden viral receptors in the junctional areas. This may facilitate viral infection of mucosal epithelial cells, thus initiating transmission and spread of pathogenic viruses. Virus-induced disruption of tight junctions could take place by direct interaction of viral proteins with epithelial cell receptors and junctional proteins or by an indirect effect through activating multiple proinflammatory cytokines and other proteins in the mucosal epithelial environment.Citation1–3 This review describes the possible molecular mechanisms of virus-associated disruption of epithelial junctions and their role in the transmucosal transmission of viral infection.
Intercellular tight and adherens junctions of mucosal epithelia are critical to mucosal barrier function
The oropharyngeal, ectocervical, vaginal, and foreskin epithelia consist of a multilayered, stratified squamous epithelium supported by an underlying layer of fibrous connective tissue, the lamina propria ().Citation4–7 The endocervical and intestinal mucosa is covered with monostratified simple epithelium. All mucosal epithelia form multiple intercellular junctions, including tight and adherens junctions,Citation4,Citation5,Citation8–15 which are critical for maintaining the morphologic and physiologic features of mucosal epithelia and their barrier function.
Figure 1. Detection of tight junction proteins in oral (buccal, tonsil), cervical and intestinal (jejunal region) epithelia. Representative immunofluorescence images of tight junction proteins occludin (red) in multistratified buccal, tonsil, and ectocervical mucosal epithelia and ZO-1 (red) in a single-cell layer of intestinal epithelium are shown. Oral (buccal and tonsil) squamous mucosal epithelia have well-developed tight junctions in the strata granulosum, spinosum, and parabasal layers. Cervical epithelia form tight junctions in the strata spinosum and parabasal layers. A single-cell layer of intestinal epithelium also has well-developed tight junctions. The tight junctions are localized at the upper lateral membranes of epithelial cells and seal intercellular spaces, preventing paracellular penetration by viruses. Nuclei are stained blue. Dashed white lines separate epithelium from the lamina propria. GR, granulosum; SP, spinosum; BL, basal; LP, lamina propria; EP, epithelium. Confocal microscopy. Original magnification: for buccal, tonsil and cervical x400, for intestinal x630
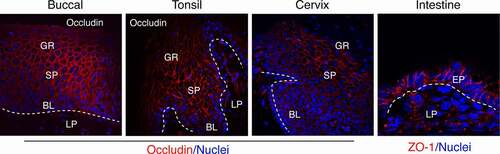
Tight junctions of mucosal epithelium form the physical tissue barrier between epithelial cells that seals the paracellular space and protects the body from the environmentCitation9,Citation10,Citation12,Citation15–17 (). Tight junctions comprise the integral membrane proteins of the claudin family, which has 27 members and is responsible for the formation of tight junction strands, i.e., heteropolymers, which are embedded within the plasma membrane, to delineate the border between the apical and basolateral membrane domains.Citation18–20 Tight junctions also have other proteins, including occludin, tricellulin, and MarvelD3, which are a staple of tight junctions, responsible for their maintenance and regulation of function.Citation21–23 The transmembrane proteins occludin and the claudins are associated with the PDZ domain, which contains the cytoplasmic proteins zonula occludens-1 (ZO-1), −2, and −3Citation24,Citation25 (). The zonula occludens mediate linkage of occludin and claudins to the actin cytoskeleton.Citation26 Other cytoplasmic tight junction-associated proteins, including cingulin, PALS1 (the protein associated with Lin-seven 1), MUPP1 (multi-PDZ domain protein 1), MAGI1 and −2 (membrane-associated guanylate kinase), PATJ (the protein associated with tight junctions), ASIP/PARS3, and Par6, play a role in the interaction of transmembrane tight junction proteins with the actin cytoskeleton and in the regulation of tight junction signaling.Citation27–30 Interaction of the transmembrane tight junction proteins facilitates the formation of tight junctions between adjacent epithelial cells near the apical surface, sealing the paracellular space between epithelial cells.Citation31 Junctional adhesion molecules 1 (JAM-1), −2, and −3 and coxsackievirus and adenovirus receptor (CAR) are immunoglobulin family members with single transmembrane domains. These domains are specifically localized at the tight junctions of epithelial cells and are involved in the regulation of junctional integrity and paracellular permeabilityCitation32 (). JAM-1, which is directly bound and sequestered in the epithelial tight junction areas, regulates paracellular permeability and tight junction resealing.Citation32–38 An adhesion protein, nectin-1, is also localized within the epithelial junctions and serves as a receptor for herpes simplex virus (HSV).Citation39–41 Intercellular adherens junctions are formed by homotypic interaction of the transmembrane protein E-cadherin, which is connected to intracellular proteins p120 and β and α catenins and the actin cytoskeleton.Citation42 Both tight and adherens junctions are connected through the actin cytoskeleton.Citation42
Figure 2. Model of organization of epithelial tight junctions and their disruption. Tight junctions are formed between epithelial cells of oral, genital, and intestinal mucosa by lateral interaction of integral membrane proteins occludin and claudins, which are associated with the cytoplasmic proteins ZO-1, −2, and −3 (left). These proteins link occludin and claudins to the actin cytoskeleton. Adherens junctions are formed in the lateral membranes of epithelial cells by homophilic interactions of E-cadherins. Junctional adhesion molecules (JAMs), coxsackievirus and adenovirus receptor (CAR), and nectin-1 are also localized at the tight and adherens junctions of mucosal epithelia. They may serve as receptors for reovirus, coxsackievirus/adenovirus, and HSV-1, respectively, and are sequestered within the tight and adherens junctions. Interactions of viral pathogens with mucosal epithelial cells may directly or indirectly cause disruption of epithelial junctions by downregulation of junctional protein expression and/or their mislocalization from assembled junctions, leading to dissociation of tight junction proteins from each other and from actin cytoskeleton (right). Disruption of epithelial tight junctions facilitates (i) opening of the paracellular space between epithelial cells and (ii) paracellular penetration by viruses. Disruption of tight and adherens junctions may release sequestered JAMs, CAR, and nectin-1, which may increase viral accessibility
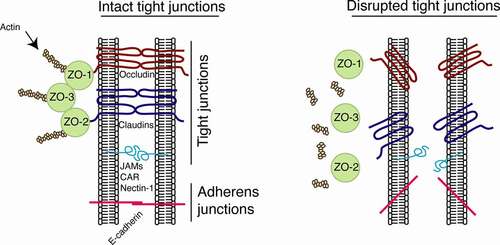
Virus-associated disruption of epithelial tight junctions and its role in viral transmission and disease progression
Coronavirus SARS-COV-2
The newly emerged coronavirus SARS-COV-2, currently causing a global pandemic, leads to severe respiratory disease (COVID-19). SARS-COV-2 is a positive-sense single-stranded RNA virus that belongs to the family Coronaviridae.Citation43–45 SARS-COV-2 shares many features of previously identified SARS-COV virus.Citation43,Citation46 It infects nasopharyngeal, bronchial, and alveolar epithelium,Citation46 which have well-developed tight junctions and polarized organization.Citation47–50 SARS-COV and SARS-COV-2’s entry into the body is mediated by the binding of viral membrane glycoprotein S with its cell surface receptor angiotensin-converting enzyme II (ACE2), which is expressed on the apical membranes of nasopharyngeal and airway epithelium.Citation51–55 Both SARS-COV and SARS-COV-2 binding to ACE2, via the receptor-binding domain of S glycoprotein, induce their conformational changes by exposing the fusion domain. This triggers the fusion of viral and cellular membranes, facilitating viral entry.Citation56 After viral replication, progeny virions are also released from apical membranes; however, the molecular mechanism of apical release of virus is not clear.Citation57,Citation58 At the initial stage of SARS-COV-2 infection, the virus will downregulate the expression of innate immune proteins, including type I interferons in the nasopharyngeal and airway epithelia,Citation59,Citation60 which could delay the development of innate immunity. Thus, at the initial stage of SARS-COV-2 infection, release of progeny virions from apical membranes of upper airway tract and lung epithelial cells may lead to the shedding of progeny virions outside the body through the airway tract and nasopharyngeal/oral cavity, increasing the risk of virus spread.Citation61,Citation62
More intensive spread and replication of SARS-COV-2 within the airway epithelium causes a cytopathic effect that disrupts tight junctions and depolarizes the epithelial cells,Citation63 leading to the spread of virus in lung tissues and cells.Citation64 The multifunctional SARS-COV-2 envelope (E) protein has a DLLV (aspartic acid, leucine, leucine, and valine) hydrophobic motif located at the C-terminal domain, which binds to PALS1 and interrupts its interaction with tight junction proteins. This leads to the disruption of tight junctions and depolarization of airway epithelial cells.Citation65 SARS-COV-2 E protein binding to PALS1 also changes E-cadherin intracellular traffic, reducing its functions and causing depolarization of epithelial cells and disruption of tight junctions.Citation66 Moreover, the extreme C-terminal domain of E protein binds to the second PDZ domain of ZO-1, interfering with its functions and leading to the disruption of tight junctions.Citation67
SARS-COV-2 infection triggers the activation of multiple proinflammatory cytokines, including interferon gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), interleukin 1 (IL-1), IL-6, and IL-18,Citation63,Citation68,Citation69 which may disrupt tight junctions. IFN-γ and TNF-α may cause the disruption of mucosal epithelia by actin cytoskeletal contraction and internalization of tight junction proteins.Citation70–73 IFN-γ and TNF-α induce the activation of myosin light-chain kinase (MLCK), which activates the phosphorylation of myosin light chain (MLC), leading to the reorganization of the membrane-associated actin/myosin cytoskeleton and tight junction remodeling.Citation74 The activation of MLC induces internalization of junctional proteins, including JAM-1, occludin, and claudin-1 and −4, leading to the disappearance of junctional proteins from cell border and junctional areas.Citation72,Citation73,Citation75 In vivo studies showed that TNF-α-induced MLCK activation triggers caveolin-1-dependent internalization of occludin and disassembly of ZO-1 from the junctions of mouse intestinal cells.Citation76 IL-1, −6, and −18 also may play a role in the disruption of tight junctions in epithelial cells.Citation77–80
SARS-COV-2-induced disruption of tight junctions of airway epithelium may facilitate the paracellular spread of virus into other tissues and cells, including endothelial cells,Citation81–83 contributing to the development of pneumonia and vasculitis. Moreover, the major cause of respiratory failure by SARS-COV-2 infection is damage to the epithelial–endothelial barrier of the alveolus, which facilitates flooding of the alveolar lumen with a proinflammatory cytokine containing fluid and inflammatory cells.
Influenza virus. Influenza A virus (IAV) is a well-known respirator viral pathogen that belongs to the Orthomyxoviridae family and causes infection of upper and lower airway epithelium.Citation84 IAV binds to sialic acid on the apical surface of airway epithelial cells; after viral replication, most releases of virus also occur from the apical membranes of epithelial cells, leading to human-to-human spread of virus.Citation85,Citation86 IAV infection of the upper and lower airway epithelial cell in vitro reduces the expression of occludin, claudin-4, and JAM-1, which cause disruption of epithelial junctions and impair the barrier function of airway epithelium.Citation87 IAV induces the disruption of epithelial junctions in the lung, which contributes to the development of edema and pneumonia.Citation88
IAV infection of polarized alveolar epithelial cells results in the loss of claudin-4 expression and the disruption of tight junctions.Citation87 This effect is independent of activation of proinflammatory cytokines, indicating the direct effect of IAV in the downregulation of claudin-4 expression. IAV nonstructural protein 1 (NS1) has consensus motif ESEV in its N terminus, which binds to the PDZ domain of discs large homolog 1 (DLG1) protein,Citation89,Citation90 which may play a critical role in the formation of tight junctions. Claudins bind to PDZ proteins, including DLG1,Citation91 suggesting that IAV-associated downregulation of claudin-4 and disruption of tight junctions in airway epithelial cells could be induced by the interaction between viral NS1 and DLG1, which may subsequently downregulate claudin-4 expression.
IAV infection of lung cells activates mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K) signaling, which initiates the activation of Gli1, a transcription factor in the sonic hedgehog signaling pathway.Citation92 Activation of Gli1 induces Snail expression, which downregulates the expression of adherens junction protein E-cadherin and tight junction proteins ZO-1 and occludin, and increases paracellular permeability.Citation92
Rhinovirus
Rhinoviruses are RNA viruses responsible for the majority of upper respiratory tract infections, which cause otitis, sinusitis, and bronchitis.Citation93 Most rhinovirus serotypes bind to intercellular adhesion molecule (ICAM)-1, and some minor group serotypes bind to low-density lipoprotein receptor.Citation94–97
Rhinovirus infection of polarized airway epithelial cells causes disruption of the epithelial barrier by dissociation of ZO-1 from tight junctions and reduction of paracellular permeability, which is independent of proinflammatory cytokines, including TNF-α, IFN-γ, and IL-1β.Citation98 Intranasal inoculation in mice with rhinovirus causes the loss of ZO-1 from bronchial epithelium tight junctions, indicating that rhinovirus-induced disruption of tight junctions may play a critical role in the development of virus-associated respiratory disease. The molecular mechanisms of rhinovirus-induced ZO-1 dissociation from tight junctions and downregulation of ZO-1 expression are not yet clear.
Human respiratory syncytial virus
Human respiratory syncytial virus (RSV) is a single-stranded virus belonging to the Paramyxoviridae family. RSV infects epithelial cells in the upper airways of adults and in the upper and lower respiratory tracts in infants.Citation99,Citation100 Infections in young children result in severe lower respiratory tract inflammation, leading to the development of bronchiolitis and pneumonia, recurrent wheeze, and childhood asthma.Citation101,Citation102 RSV infection in mice lung cells causes the downregulation of claudin-1 and occludin mRNA expression;Citation103,Citation104 confocal microscopy shows a significant reduction of occludin, claudin-1, claudin-2, claudin-18, ZO-1, and E-cadherin expression in the lung epithelium. RSV infection in mice lung cells induces the activation of proinflammatory chemokines, including IL-1β, −6, and −12, monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α and −1β), granulocyte colony-stimulating factor (G-CSF), KC, and RANTES, which is well correlated with an increase in viral load, lung inflammation, and reduced airway epithelial barrier function.Citation103 These data indicate that RSV of lung epithelium activates proinflammatory cytokines that disrupt tight junctions via MAPK upregulation,Citation105 facilitating viral spread and the progression of lung disease.
Rotavirus
Rotaviruses are double-stranded RNA viruses belonging to the Reoviridae family. Rotaviruses infect the differentiated columnar intestinal epithelial cells and cause severe diarrhea in infants and young children.Citation106,Citation107 Rotavirus infection of polarized intestinal epithelial cells leads to the disruption of tight junctions.Citation108 The viral nonstructural protein NSP4 enterotoxin causes a decrease in transepithelial resistance (TER) of epithelial cells and an increase in their paracellular permeability.Citation109,Citation110 NSP4 prevents the targeting of ZO-1 in tight junctions and induces the disruption and/or reorganization of F-actin.Citation110 Expression of tight junction proteins claudin-1 and occludin is also drastically altered and becomes cytoplasmic during infection. Claudin-1 redistribution is well correlated with the decline in TER of polarized intestinal epithelial cells.Citation109
Rotavirus structural VP4 capsid protein is essential for virus–cell interactions and is cleaved by trypsin into peptides VP5 and VP8.Citation111 In rotavirus-infected cells, VP8 is responsible for the reduction of TER and inhibits the development of newly formed tight junctions.Citation111 The treatment of cells with VP8 causes redistribution of ZO-1, occludin, and claudin-3 from assembled tight junctions into the cytoplasm. VP8-induced disruption of tight junctions releases the hidden α2β1, αvβ3, and αxβ2 integrins in the tight junction areas.Citation111,Citation112 Integrins α2β1, αvβ3, and αxβ2 serve as receptors for rotavirus.Citation113–116 Thus, VP8-induced disruption of tight junctions increases the accessibility of virions to integrins released from the junctional areas and promotes viral spread.
Coxsackievirus group B (CVB) and adenovirus
Coxsackievirus belongs to the Picornaviridae family and infects skin or intestinal mucosal epithelium.Citation117 Disease manifestation may include painful blisters on the mouth and small tender lesions on the palms of the hands and the bottoms of the feet. Adenovirus belongs to the Adenoviridae family and infects upper and lower airway epithelium; it causes respiratory illness, including the common cold, pneumonia, croup, and bronchitis.Citation118,Citation119 Adenovirus also could be involved in gastroenteritis, conjunctivitis, and cystitis.Citation120
CAR is sequestered by the tight junction and therefore is not accessible to virus from the apical surface of mucosal epithelium.Citation121–123 Initially, CVB interacts with the glycophosphatidylinositol (GPI)-anchored protein decay-accelerating factor on the apical cell surface of epithelial cells. This activates ABL kinase, triggering Rac-dependent actin rearrangements that allow virus to move to the tight junction. This causes a transient disruption of the tight junction by occludin internalization via caveolin-associated endocytosis.Citation121,Citation122,Citation124,Citation125 Disruption of tight junctions leads to the release of sequestered receptor CAR, which facilitates viral entry and spread.
Adenovirus infection is initiated by high-affinity binding of adenovirus fiber protein to the CAR, which subsequently initiates viral binding to entry receptors αvβ3, αvβ5, α5β1, and αvβ1 integrins.Citation126–130 Mouse adenovirus disrupts tight junctions in endothelial cells, reducing the expression of claudin-5 and occludin,Citation131 suggesting that the interaction of human adenovirus with airway epithelium may also disrupt junctions, releasing sequestered CAR for viral binding. Airway epithelium may express the isoform of CAR, CAR,ExCitation8 on the apical surface of epithelial cells, which bind to the virus. IL-8 may increase the expression of CAR,ExCitation8 facilitating adenovirus infection from the apical surface of airway epithelial cells.Citation132,Citation133
Reovirus
Reoviruses are nonenveloped RNA genomic viruses that belong to the Reoviridae family, and they infect epithelia of the respiratory and intestinal tracts of children and adults; infection causes mild respiratory or gastrointestinal illness.Citation134 Reovirus infection in neonates may spread to other organs, including the central nervous system, and cause serious neurologic disorders.Citation135,Citation136 Reovirus attachment to the mucosal airway and the intestinal epithelium is facilitated by binding of a filamentous viral protein, σ1, to JAM-A,Citation137–141 which is sequestered in the epithelial tight junctions.Citation32–38 Binding of reovirus σ1 to sequestered JAM-A is not clear; it is possible that the interaction of reovirus with epithelial cells may activate unidentified signaling, which may transiently disrupt tight junctions, releasing JAM-1 for σ1 binding.
Herpes simplex virus 1
HSV-1 belongs to the Herpesviridae family, which infects oral mucosal epithelia, initiating systemic viral spread in the body.Citation142 After primary infection with mucosal epithelia, HSV-1 establishes a lifelong latency in sensory neurons and, upon reactivation, infects mucocutaneous sites.Citation142 HSV-1 may cause mild illness or sporadic, severe, and life-threatening skin disease, including atopic dermatitis, which may lead to disseminated skin infection (eczema herpeticum).Citation143
Formation of polarization and tight junctions of Madin-Darby canine kidney (MDCK) cells substantially reduces HSV-1 infection and viral spread compared with nonpolarized cells without tight junctions.Citation144 The disruption of tight junctions (ZO-1) and adherens junctions (E-cadherin) by calcium depletion also results in increasing virus infectivity.Citation39,Citation144 Ex vivo infection of murine skin tissue with HSV-1 showed that viral infection may occur if tight junctions are disrupted, indicating that the paracellular spread of the virus is critical for HSV-1 infection and spread.Citation145,Citation146
HSV-1 is a common opportunistic viral pathogen that causes multiple oral and genital disorders, such as ulcers and necrotic lesions in human immunodeficiency virus (HIV)-infected individuals. Although the increased risk of HSV infection and reactivation may be mediated in part by HIV-induced immune dysfunction, prolonged interaction of the HIV proteins tat and gp120 and cell-free HIV virions with oral epithelial cells induces the disruption of tight and adherens junctions and thereby facilitates the rapid spread of HSV in the epithelium.Citation41 This is due to the liberation of HSV-1 glycoprotein D receptor nectin-1, which is hidden in the epithelial junctions.Citation39–41
HIV-associated activation of MAPK leads to the upregulation of transcription factor NF-κB and matrix metalloproteinase-9 (MMP-9).Citation147 This induces the disruption of tight and adherens junctions, increasing HSV-1 cell-to-cell spread. Inhibition of HIV-associated MAPK activation abolishes NF-κB and MMP-9 upregulation and reduces HSV-1 spread. Inactivation of MMP-9 also reduces HIV-promoted HSV-1 spread. Thus, HIV-1-activated MAPK/NF-κB and MMP-9 play a critical role in the disruption of oral epithelial junctions and HSV-1 cell-to-cell spread.Citation147
Human cytomegalovirus
Human cytomegalovirus (HCMV) is an important human pathogen of the Herpesviridae family that initially infects salivary gland epithelial cells and subsequently spreads in the body, establishing latency in the myeloid progenitor cells.Citation148–150 HCMV is associated with opportunistic infection during HIV/AIDS disease.Citation151 It manifests as oral mucosal lesions, esophagitis, retinitis, pneumonia, hepatitis, gastrointestinal inflammation, or encephalopathy.Citation152–156
HCMV often reactivates asymptomatically in antiretroviral therapy-suppressed HIV-infected individuals.Citation157 In HIV-infected individuals, HCMV may replicate in the gastrointestinal tract, infecting the endothelial, stromal, and intestinal epithelial cells, and can cause erosive and ulcerative disorders.Citation158–160 HCMV disrupts tight junctions of polarized intestinal cells, significantly downregulating ZO-1 expression, reducing TER, and increasing epithelial barrier permeability.Citation161 Furthermore, HCMV-associated disruption of intestinal epithelium integrity could be attributed to activation of IL-1β, IL-6, and TNF-α by productive viral infection.Citation161 HCMV-induced disruption of tight junctions is critical for viral infection and viral spread. Inhibition of HCMV replication preserves epithelial polarity and tight junction integrity.
Human papillomavirus
Human papillomavirus (HPV) is an oncogenic virus of the Papillomaviridae family that infects oropharyngeal and genital mucosal epithelial cells. Multiple high-risk oncogenic HPVs, including HPV16 and HVP18, have been identified as the etiological agents of many oropharyngeal and almost all anogenital carcinomas.Citation162,Citation163 Two oncoproteins, E6 and E7, play a critical role in the induction and development of HPV-associated neoplasia. Accumulated evidence indicates that E6 and E7 oncoproteins may disrupt epithelial junctions via multiple mechanisms.
MAPK is upregulated in HPV-associated neoplastic tissues;Citation164 this is correlated with the overexpression of the oncogenic HPV E6 protein.Citation165 HPV16 E6 activates MAPK signaling through the Ras-related protein 1 pathway.Citation165,Citation166 HPV E6-activated MAPK may lead to the disruption of epithelial junctions by downregulating the expression of tight junction proteins ZO-1 and occludin through NF-κB signaling and/or by aberrant internalization via caveolin-mediated endocytosis.Citation167–169
E6 may also disrupt tight junctions through the direct interaction of tight junction-associated proteins. E6 proteins from all oncogenic HPV types, including HPV16 and −18, contain a C-terminal PDZ-binding motif (X-S/T-X-V/L) that binds to the PDZ domain of the tight junction-associated proteins PATJ, MAGI1 and −2, and MUPP1.Citation30,Citation170–172 This interaction results in the degradation of the E6-bound PDZ domain proteins,Citation30,Citation170–172 causing disconnection of occludin and claudins from cortical actin and tight junctions. HPV-induced disruption of tight junctions may reduce the barrier function of oral and genital mucosal epithelia, facilitating paracellular penetration of multiple viral pathogens, including HIV, HCMV, and HSV.
HPV oncoproteins E6/E7 in epithelial cells may also reduce the expression of adherens junction protein E-cadherin: the epithelial cells acquire a spindle-like morphology.Citation173,Citation174 E6 and E7 induce the expression of MMP-2 and −9,Citation175,Citation176 which cleave E-cadherin,Citation177,Citation178 leading to the loss of adherens junctions. Thus, HPV E6/E7 may disrupt both tight and adherens junctions in epithelial cells, which may initiate the development of epithelial–mesenchymal transition (EMT).Citation179,Citation180
The role of EMT has been shown in most epithelial cancers, including HPV-associated neoplastic processes.Citation181,Citation182 Reduction in E-cadherin expression with the acquisition of the mesenchymal phenotype has been observed in HPV16-infected cervical cancers.Citation183 EMT is a normal epigenetic process in embryonic development that coordinates and regulates the differentiation of cell lineage identity.Citation184 However, the EMT phenotype also plays an important role in neoplastic processes, facilitating growth and metastasis of tumor cells.Citation185,Citation186 During cancer-associated EMT, epithelial cells lose cell–cell junctions and become proliferative and invasive.Citation187 Thus, HPV-induced disruption of epithelial tight and adherens junctions may contribute to the development of neoplastic progression through EMT mechanisms.
Human immunodeficiency virus
HIV-1 is a retrovirus that infects CD4+ T lymphocytes, monocytes/macrophages, and dendritic cells. It causes AIDS, which leads to progressive immune dysfunction, allowing the development of opportunistic infections, cancer, and other disorders. HIV-susceptible CD4+ T lymphocytes, monocytes/macrophages, and dendritic cells are localized under genital, intestinal, and oropharyngeal mucosal epithelium. To gain access to these target cells, HIV must first translocate through the genital, intestinal, and oropharyngeal mucosal epithelium, all of which have well-developed tight junctions preventing virus penetration. Disruption of the tight junction of mucosal epithelium opens up paracellular pathways that allow HIV paracellular penetration toward intramucosal/submucosal virus-susceptible immune cells.Citation5,Citation188
In vitro studies using polarized endocervical, ectocervical, intestinal, and oral epithelia have shown that the direct interaction of HIV-1 with the apical (mucosal) surface of epithelial cells disrupts tight junction proteins (claudin-1 and −4, occludin, and ZO-1) and results in increased paracellular permeability.Citation41,Citation147,Citation189 Treatment of polarized epithelial cells with purified HIV-1 envelope protein gp120 disrupts tight junctions,Citation4,Citation41,Citation147,Citation189 indicating that the interaction of viral envelope with the mucosal surface is critical for the disruption of epithelial junctions and paracellular penetration by HIV.
HIV-1-induced disruption of intestinal and endocervical junctions is associated with the upregulation of proinflammatory cytokines.Citation1,Citation189 Exposure of oral and intestinal epithelial cells to HIV-1 induces the expression of TNF-α and IL-6 and −8.Citation1 HIV interaction with endometrial cells also increases the expression of TNF-α, IL-1β, and IL-6. HIV gp120 interacts with Toll-like receptors (TLRs) TLR2 and TLR4 on endometrial and endocervical epithelial cells, leading to NF-κB activation, which upregulates the expression of cytokines, including TNF-α.Citation190 HIV-1 gp120 binding to heparan sulfate proteoglycan (HSPG) is important in the interaction between gp120 and TLRs and in the activation of NF-κB. Interestingly, activated TNF-α-stimulated NF-κB signaling inhibits the expression of ZO-1.Citation71 Thus, it is possible that HIV-associated induction of expression of TNF-α and activation of NF-κB may maintain each other’s active status, leading to substantial disruption of epithelial junctions.
HIV gp120-induced disruption of oral epithelial junctions is associated with the activation of MAPK.Citation41,Citation147 HIV gp120 interacts with galactosylceramide (GalCer), CCR5, and/or CXCR4 on the oral, genital, and intestinal epithelial surface,Citation14,Citation191–196 which induces intracellular calcium elevation and activation of MAPK signaling.Citation197,Citation198 This causes the upregulation of transcription factor NF-κB and MMP-9, which digest junctional proteins, thereby disrupting tight and adherens junctions.Citation147
Prolonged interaction of cell-free HIV-1 virions and viral envelope protein gp120 with oral, cervical, and foreskin epithelial cells induces the disruption of tight and adherens junctions, which leads to an EMT.Citation199 HIV-induced disruption of tight junctions and EMT may reduce the barrier function of mucosal epithelia, promoting the paracellular spread of HSV-1 and HPV16.Citation4,Citation147 Furthermore, HIV-1-induced EMT may contribute to the development of HPV malignancy.
As described above, interaction of HIV-1 with genital, intestinal, and oropharyngeal mucosal epithelium may induce the disruption of tight junctions through multiple mechanisms. Disruption of tight junctions facilitates the paracellular penetration of HIV-1 into mucosal epithelia, where the virus infects CD4+ T lymphocytes, monocytes/macrophages, and dendritic cells, thereby initiating systemic HIV/AIDS disease.
Conclusions and future perspectives
The oral, airway, intestinal, and genital mucosal epithelia with well-developed tight junctions have highly efficient biological barrier function that prevent paracellular penetration by viral pathogens. However, the interaction of viruses with mucosal epithelia disrupts tight junctions, reducing their barrier function and allowing paracellular penetration of viruses into the body (). Viruses could induce the disruption of epithelial tight junctions through the interaction of viral proteins with tight junction proteins and/or virus-activated signaling pathways. Virus-induced activation of signaling pathways, including MAPK, PI3K, and NF-κB, may lead to the induction of expression of proinflammatory cytokines, which disrupt epithelial junctions by reducing the expression of tight junction proteins ZO-1, occludin, and claudins and/or by their mislocalization from assembled tight junctions. MAPK- and NF-κB-activated MMPs may cause the degradation of junctional proteins. Virus-induced disruption of epithelial junctions may release sequestered viral receptors from the disrupted junctional areas, allowing better access of virions to their receptors.
Table 1. Interaction of human pathogenic viruses with mucosal epithelial junctional proteins
Prevention of disruption and repair of tight junctions may significantly reduce transmucosal viral transmission. Therefore, it is critical to identify the important viral and cellular target proteins and signaling pathways that play a key role in the disruption of epithelial junctions. Establishment of efficient prophylactic and therapeutic approaches for preventing the disruption of tight junctions and repairing damaged junctions may protect mucosal epithelia from virus-associated disruption and spread of pathogenic viruses.
Abbreviations
ZO-1, −2, and −3, zonula occludens; PALS1, the protein associated with Lin-seven 1; MUPP1, multi-PDZ domain protein 1; MAGI1 and −2, membrane-associated guanylate kinase; PATJ, the protein associated with tight junctions; JAM-1, −2, −3, and -A, junctional adhesion molecules; CAR, coxsackievirus and adenovirus receptor; HSV-1, herpes simplex virus 1; ACE2, angiotensin-converting enzyme II; E, SARS-COV-2 envelope protein; IFN-γ, interferon gamma; TNF-α, tumor necrosis factor alpha; IL-1, −1β, −6, −8, −12, and −18, interleukins; MLCK, myosin light-chain kinase; MLC, myosin light chain; IAV, influenza A virus; TLRs, Toll-like receptors; NS-1, IAV nonstructural protein 1; DLG1, discs large homolog 1 protein; MAPK, mitogen-activated protein kinase; PI3K, phosphoinositide 3-kinase; ICAM-1, intercellular adhesion molecule; RSV, human respiratory syncytial virus; MCP-1, monocyte chemoattractant protein-1; MIP-1α and −1β, macrophage inflammatory protein-1α and −1β; G-CSF, granulocyte colony-stimulating factor; TER, transitional endoplasmic reticulum; CVB, coxsackievirus group B; GPI, glycophosphatidylinositol; MDCK, Madin-Darby canine kidney; HIV, human immunodeficiency virus; NF-κB, transcription factor; MMP-9, matrix metalloproteinase-9; HCMV, human cytomegalovirus; HPV, human papillomavirus; EMT, epithelial–mesenchymal transition; HSPG, heparan sulfate proteoglycan; GalCer, galactosylceramide; PDZ (Postsynaptic density 95, PSD-85; Discs large, Dlg; Zonula occludens-1, ZO-1).
Disclosure conflicts of interest statement
No potential conflict of interest was reported by the author(s).
Acknowledgments
I thank Drs. Deborah Greenspan, Piri Veluppillai, Karen Smith-McCuine, and Kristina Rosbe for providing biopsy samples, and Rossana Herrera for excellent technical assistance. This project was supported by the NIDCR R01DE028129 and NCI R01CA232887 grants.
Additional information
Funding
References
- Tugizov S. Human immunodeficiency virus-associated disruption of mucosal barriers and its role in HIV transmission and pathogenesis of HIV/AIDS disease. Tissue Barriers. 2016;4(e1159276):1. doi:https://doi.org/10.1080/21688370.2016.1159276.
- Torres-Flores JM, Arias CF. Tight Junctions Go Viral! Viruses. 2015;7(9):5145–18. doi:https://doi.org/10.3390/v7092865.
- Guttman JA, Finlay BB. Tight junctions as targets of infectious agents. Biochim Biophys Acta. 2009;1788(832–841). doi:https://doi.org/10.1016/j.bbamem.2008.10.028.
- Tugizov SM, Herrera R, Chin-Hong P, Veluppillai P, Greenspan D, Michael Berry J, Pilcher CD, Shiboski CH, Jay N, Rubin M, et al. HIV-associated disruption of mucosal epithelium facilitates paracellular penetration by human papillomavirus. Virology. 2013;446(378–388):378–388. doi:https://doi.org/10.1016/j.virol.2013.08.018.
- Tugizov SM, Herrera R, Veluppillai P, Greenspan D, Soros V, Greene WC, Levy JA, Palefsky JM. Differential transmission of HIV traversing fetal oral/intestinal epithelia and adult oral epithelia. J Virol. 2012;86(2556–2570):2556–2570. doi:https://doi.org/10.1128/JVI.06578-11.
- Anderson DJ, Marathe J, Pudney J. The Structure of the Human Vaginal Stratum Corneum and its Role in Immune Defense. Am J Reprod Immunol. 2014;71(618–623):618–623. doi:https://doi.org/10.1111/aji.12230.
- Dinh MH, McRaven MD. Keratinization of the adult male foreskin and implications for male circumcision. AIDS. 2010;24(899–906):1381–1382. doi:https://doi.org/10.1097/QAD.0b013e3283367779.
- Schluter H, Wepf R, Moll I, Franke WW. Sealing the live part of the skin: the integrated meshwork of desmosomes, tight junctions and curvilinear ridge structures in the cells of the uppermost granular layer of the human epidermis. Eur J Cell Biol. 2004;83(11–12):655–665. doi:https://doi.org/10.1078/0171-9335-00434.
- Langbein L, Pape U-F, Grund C, Kuhn C, Praetzel S, Moll I, Moll R, Franke WW. Tight junction-related structures in the absence of a lumen: occludin, claudins and tight junction plaque proteins in densely packed cell formations of stratified epithelia and squamous cell carcinomas. Eur J Cell Biol. 2003;82(8):385–400. doi:https://doi.org/10.1078/0171-9335-00330.
- Langbein L, Grund C, Kuhn C, Praetzel S, Kartenbeck J, Brandner JM, Moll I, Franke WW. Tight junctions and compositionally related junctional structures in mammalian stratified epithelia and cell cultures derived therefrom. Eur J Cell Biol. 2002;81(419–435):419–435. doi:https://doi.org/10.1078/0171-9335-00270.
- Brandner JM, Kief S, Grund C, Rendl M, Houdek P, Kuhn C, Tschachler E, Franke WW, Moll I. Organization and formation of the tight junction system in human epidermis and cultured keratinocytes. Eur J Cell Biol. 2002;81(253–263):253–263. doi:https://doi.org/10.1078/0171-9335-00244.
- Takano K, Kojima T, Go M, Murata M, Ichimiya S, Himi T, Sawada N. HLA-DR- and CD11c-positive dendritic cells penetrate beyond well-developed epithelial tight junctions in human nasal mucosa of allergic rhinitis. J Histochem Cytochem. 2005;53(611–619):611–619. doi:https://doi.org/10.1369/jhc.4A6539.2005.
- Blaskewicz CD, Pudney J, Anderson DJ. Structure and function of intercellular junctions in human cervical and vaginal mucosal epithelia. Biol Reprod. 2011;85(97–104):97–104. doi:https://doi.org/10.1095/biolreprod.110.090423.
- Tugizov SM, Herrera R, Veluppillai P, Greenspan D, Soros V, Greene WC, Levy JA, Palefsky JM. HIV is inactivated after transepithelial migration via adult oral epithelial cells but not fetal epithelial cells. Virology. 2011;409(211–222):211–222. doi:https://doi.org/10.1016/j.virol.2010.10.004.
- Go M, Kojima T, Takano K-I, Murata M, Ichimiya S, Tsubota H, Himi T, Sawada N. Expression and function of tight junctions in the crypt epithelium of human palatine tonsils. J Histochem Cytochem. 2004;52(1627–1638):1627–1638. doi:https://doi.org/10.1369/jhc.4A6339.2004.
- Sawada N, Murata M, Kikuchi K, Osanai M, Tobioka H, Kojima T, Chiba H. Tight junctions and human diseases. Med Electron Microsc. 2003;36(147–156):147–156. doi:https://doi.org/10.1007/s00795-003-0219-y.
- Bouschbacher M, Bomsel M, Verronèse E, Gofflo S, Ganor Y, Dezutter-Dambuyant C, Valladeau J. Early events in HIV transmission through a human reconstructed vaginal mucosa. AIDS. 2008;22(1257–1266):1257–1266. doi:https://doi.org/10.1097/QAD.0b013e3282f736f400002030-200807110-00002. [pii]
- Tsukita S, Tanaka H, The Claudins: TA. From Tight Junctions to Biological Systems. Trends Biochem Sci. 2019;44(141–152):141–152. doi:https://doi.org/10.1016/j.tibs.2018.09.008.
- Tsukita S, Furuse M. Occludin and claudins in tight-junction strands: leading or supporting players? Trends Cell Biol. 1999;9(7):268–273. doi:https://doi.org/10.1016/s0962-8924(99)01578-0.
- Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2(285–293):285–293. doi:https://doi.org/10.1038/35067088.
- Cording J, Berg J, Käding N, Bellmann C, Tscheik C, Westphal JK, Milatz S, Günzel D, Wolburg H, Piontek J, et al. In tight junctions, claudins regulate the interactions between occludin, tricellulin and marvelD3, which, inversely, modulate claudin oligomerization. J Cell Sci. 2013;126(554–564):554–564. doi:https://doi.org/10.1242/jcs.114306.
- Westphal JK, Dörfel MJ, Krug SM, Cording JD, Piontek J, Blasig IE, Tauber R, Fromm M, Huber O. Tricellulin forms homomeric and heteromeric tight junctional complexes. Cell Mol Life Sci. 2010;67(2057–2068):2057–2068. doi:https://doi.org/10.1007/s00018-010-0313-y.
- Raleigh DR, Marchiando AM, Zhang Y, Shen L, Sasaki H, Wang Y, Long M, Turner JR. Tight junction-associated MARVEL proteins marveld3, tricellulin, and occludin have distinct but overlapping functions. Mol Biol Cell. 2010;21(1200–1213):1200–1213. doi:https://doi.org/10.1091/mbc.E09-08-0734.
- Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol. 1999;147(1351–1363):1351–1363. doi:https://doi.org/10.1083/jcb.147.6.1351.
- Bauer H, Zweimueller-Mayer J, Steinbacher P, Lametschwandtner A, Bauer HC. The dual role of zonula occludens (ZO) proteins. J Biomed Biotechnol. 2010;2010(402593):1–11. doi:https://doi.org/10.1155/2010/402593.
- Niessen CM. Tight junctions/adherens junctions: basic structure and function. J Invest Dermatol. 2525-2532;127.
- Zhuravleva K, Goertz O, Wölkart G, Guillemot L, Petzelbauer P, Lehnhardt M, Schmidt K, Citi S, Schossleitner K. The tight junction protein cingulin regulates the vascular response to burn injury in a mouse model. Microvasc Res. 2020;132(104067):104067. doi:https://doi.org/10.1016/j.mvr.2020.104067.
- Guillemot L, Paschoud S, Pulimeno P, Foglia A, Citi S. The cytoplasmic plaque of tight junctions: a scaffolding and signalling center. Biochim Biophys Acta. 2008;1778(601–613):601–613. doi:https://doi.org/10.1016/j.bbamem.2007.09.032.
- Sugihara-Mizuno Y, Adachi M, Kobayashi Y, Hamazaki Y, Nishimura M, Imai T, Furuse M, Tsukita S. Molecular characterization of angiomotin/JEAP family proteins: interaction with MUPP1/Patj and their endogenous properties. Genes Cells. 2007;12(473–486):473–486. doi:https://doi.org/10.1111/j.1365-2443.2007.01066.x.
- Storrs CH, Silverstein SJ. PATJ, a tight junction-associated PDZ protein, is a novel degradation target of high-risk human papillomavirus E6 and the alternatively spliced isoform 18 E6. J Virol. 4080-4090:81.
- Zihni C, Mills C, Matter K, Balda MS. Tight junctions: from simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol. 2016;17(564–580). doi:https://doi.org/10.1038/nrm.2016.80.
- Naik UP, Eckfeld K. Junctional adhesion molecule 1 (JAM-1). J Biol Regul Homeost Agents. 2003;17(341–347).
- The BG. JAM family of junctional adhesion molecules. Curr Opin Cell Biol. 2003;15(525–530). doi:https://doi.org/10.1016/s0955-0674(03)00104-2.
- Bazzoni G, Martı́nez-Estrada OM, Orsenigo F, Cordenonsi M, Citi S, Dejana E. Interaction of junctional adhesion molecule with the tight junction components ZO-1, cingulin, and occludin. J Biol Chem. 2000;275(20520–20526):20520–20526. doi:https://doi.org/10.1074/jbc.M905251199.
- Martinez-Estrada OM, Villa A, Breviario F, Orsenigo F, Dejana E, Bazzoni G. Association of junctional adhesion molecule with calcium/calmodulin-dependent serine protein kinase (CASK/LIN-2) in human epithelial caco-2 cells. J Biol Chem. 2001;276(9291–9296):9291–9296. doi:https://doi.org/10.1074/jbc.M006991200.
- Mandell KJ, Babbin BA, Nusrat A, Parkos CA. Junctional adhesion molecule 1 regulates epithelial cell morphology through effects on beta1 integrins and Rap1 activity. J Biol Chem. 2005;280(11665–11674):11665–11674. doi:https://doi.org/10.1074/jbc.M412650200.
- Mandell KJ, McCall IC, Parkos CA. Involvement of the junctional adhesion molecule-1 (JAM1) homodimer interface in regulation of epithelial barrier function. J Biol Chem. 2004;279(16254–16262):16254–16262. doi:https://doi.org/10.1074/jbc.M309483200.
- Mandell KJ, Parkos CA. The JAM family of proteins. Adv Drug Deliv Rev. 2005;57(857–867):857–867. doi:https://doi.org/10.1016/j.addr.2005.01.005.
- Yoon M, Spear PG. Disruption of adherens junctions liberates nectin-1 to serve as receptor for herpes simplex virus and pseudorabies virus entry. J Virol. 2002;76(7203–7208):7203–7208. doi:https://doi.org/10.1128/JVI.76.14.7203-7208.2002.
- Krummenacher C, Nicola AV, Whitbeck JC, Lou H, Hou W, Lambris JD, Geraghty RJ, Spear PG, Cohen GH, Eisenberg RJ, et al. Herpes simplex virus glycoprotein D can bind to poliovirus receptor-related protein 1 or herpesvirus entry mediator, two structurally unrelated mediators of virus entry. J Virol. 1998;72(9):7064–7074. doi:https://doi.org/10.1128/JVI.72.9.7064-7074.1998.
- Sufiawati I, Tugizov SM. HIV-Associated Disruption of Tight and Adherens Junctions of Oral Epithelial Cells Facilitates HSV-1 Infection and Spread. PloS One. 2014;9(e88803):e88803. doi:https://doi.org/10.1371/journal.pone.0088803.
- Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778(660–669):660–669. doi:https://doi.org/10.1016/j.bbamem.2007.07.012.
- V’Kovski P, Kratzel A, Steiner S, Stalder H, Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol. 2021;19(3):155–170. doi:https://doi.org/10.1038/s41579-020-00468-6.
- Woo PC, Huang Y, Lau SK, Yuen KY. Coronavirus genomics and bioinformatics analysis. Viruses. 2010;2(1804–1820):1804–1820. doi:https://doi.org/10.3390/v2081803.
- Woo PC, Lau SK, Huang Y, Yuen KY. Coronavirus diversity, phylogeny and interspecies jumping. Exp Biol Med (Maywood). 2009;234(1117–1127):1117–1127. doi:https://doi.org/10.3181/0903-MR-94.
- Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): a Review. JAMA. 2020;324(782–793):782. doi:https://doi.org/10.1001/jama.2020.12839.
- Tugizov SM, Berline JW, Palefsky JM. Epstein-Barr virus infection of polarized tongue and nasopharyngeal epithelial cells. Nat Med. 2003;9(307–314):477. doi:https://doi.org/10.1038/nm0403-477d.
- Tucker SP, Compans RW. Virus infection of polarized epithelial cells. Adv Virus Res. 1993;42(187–247).
- Hao S, Ning K, Kuz CA, Vorhies K, Yan Z, Qiu J. Long-Term Modeling of SARS-CoV-2 Infection of In Vitro Cultured Polarized Human Airway Epithelium. MBio. 2020;11(6). doi:https://doi.org/10.1128/mBio.02852-20.
- Harcourt JL, Haynes LM. Establishing a Liquid-covered Culture of Polarized Human Airway Epithelial Calu-3 Cells to Study Host Cell Response to Respiratory Pathogens In vitro. J Vis Exp. 2013;(72). doi:https://doi.org/10.3791/50157.
- Daniel Wrapp, Nianshuang Wang, Kizzmekia S Corbett, Jory A Goldsmith, Ching-Lin Hsieh, Olubukola Abiona, Barney S Graham, Jason S McLellan, et al. Cryo-EM Structure of the 2019-nCoV Spike in the. Conformation P. bioRxiv. doi:https://doi.org/10.1101/2020.02.11.944462. 2020.
- Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(450–454):450–454. doi:https://doi.org/10.1038/nature02145.
- Wenhui Li, Chengsheng Zhang, Jianhua Sui, Jens H Kuhn, Michael J Moore, Shiwen Luo, Swee-Kee Wong, I-Chueh Huang, Keming Xu, Natalya Vasilieva, et al. Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J. 2005;24(1634–1643):1634–1643. doi:https://doi.org/10.1038/sj.emboj.7600640.
- Wan Y, Shang J, Graham R, Baric RS, Li F, Gallagher T. Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J Virol. 2020;94(7). doi:https://doi.org/10.1128/JVI.00127-20.
- Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, Talavera-López C, Maatz H, Reichart D, Sampaziotis F, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26(681–687):681–687. doi:https://doi.org/10.1038/s41591-020-0868-6.
- Inoue Y, Tanaka N, Tanaka Y, Inoue S, Morita K, Zhuang M, Hattori T, Sugamura K. Clathrin-dependent entry of severe acute respiratory syndrome coronavirus into target cells expressing ACE2 with the cytoplasmic tail deleted. J Virol. 2007;81(8722–8729):8722–8729. doi:https://doi.org/10.1128/JVI.00253-07.
- Sims AC, Burkett SE, Yount B, Pickles RJ. SARS-CoV replication and pathogenesis in an in vitro model of the human conducting airway epithelium. Virus Res. 2008;133(33–44):33–44. doi:https://doi.org/10.1016/j.virusres.2007.03.013.
- Tseng CT, Tseng J, Perrone L, Worthy M, Popov V, Peters CJ. Apical entry and release of severe acute respiratory syndrome-associated coronavirus in polarized Calu-3 lung epithelial cells. J Virol. 2005;79(9470–9479):9470–9479. doi:https://doi.org/10.1128/JVI.79.15.9470-9479.2005.
- Lin L, Lu L, Cao W, Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. 2020;9(727–732):727–732. doi:https://doi.org/10.1080/22221751.2020.1746199.
- Sa Ribero M, Jouvenet N, Dreux M, Nisole S, Stapleford K. Interplay between SARS-CoV-2 and the type I interferon response. PLoS Pathog. 2020;16(e1008737):e1008737. doi:https://doi.org/10.1371/journal.ppat.1008737.
- Liu P, Cai J, Jia R, Xia S, Wang X, Cao L, Zeng M, Xu J. Dynamic surveillance of SARS-CoV-2 shedding and neutralizing antibody in children with COVID-19. Emerg Microbes Infect. 2020;9(1254–1258):1254–1258. doi:https://doi.org/10.1080/22221751.2020.1772677.
- Wang H, Liu Q, Hu J, Zhou M, Yu M-Q, Li K-Y, Xu D, Xiao Y, Yang J-Y, Lu Y-J, et al. Nasopharyngeal Swabs Are More Sensitive Than Oropharyngeal Swabs for COVID-19 Diagnosis and Monitoring the SARS-CoV-2 Load. Front Med (Lausanne). 2020;7(334). doi:https://doi.org/10.3389/fmed.2020.00334
- Deinhardt-Emmer S, Böttcher S, Häring C, Giebeler L, Henke A, Zell R, Jungwirth J, Jordan PM, Werz O, Hornung F, et al. SARS-CoV-2 causes severe epithelial inflammation and barrier dysfunction. J Virol. 2021;95(10). doi:https://doi.org/10.1128/JVI.00110-21
- Zhu N, Wang W, Liu Z, Liang C, Wang W, Ye F, Huang B, Zhao L, Wang H, Zhou W, et al. Morphogenesis and cytopathic effect of SARS-CoV-2 infection in human airway epithelial cells. Nat Commun. 2020;11(1). doi:https://doi.org/10.1038/s41467-020-17796-z
- De Maio F, Lo Cascio E, Babini G, Sali M, Della Longa S, Tilocca B, Roncada P, Arcovito A, Sanguinetti M, Scambia G, et al. Improved binding of SARS-CoV-2 Envelope protein to tight junction-associated PALS1 could play a key role in COVID-19 pathogenesis. Microbes Infect. 2020;22(592–597):592–597. doi:https://doi.org/10.1016/j.micinf.2020.08.006.
- Teoh KT, Siu Y-L, Chan W-L, Schlüter MA, Liu C-J, Peiris JSM, Bruzzone R, Margolis B, Nal B. The SARS coronavirus E protein interacts with PALS1 and alters tight junction formation and epithelial morphogenesis. Mol Biol Cell. 2010;21(3838–3852):3838–3852. doi:https://doi.org/10.1091/mbc.E10-04-0338.
- Shepley-McTaggart A, Sagum CA, Oliva I, Rybakovsky E, DiGuilio K, Liang J, Bedford MT, Cassel J, Sudol M, Mullin JM, et al. SARS-CoV-2 Envelope (E) Protein Interacts with PDZ-Domain-2 of Host Tight Junction Protein ZO1. bioRxiv. 2020. doi:https://doi.org/10.1101/2020.12.22.422708.
- Huang KJ, Su I-J, Theron M, Wu Y-C, Lai S-K, Liu -C-C, Lei H-Y. An interferon-gamma-related cytokine storm in SARS patients. J Med Virol. 2005;75(185–194):185–194. doi:https://doi.org/10.1002/jmv.20255.
- Song P, Li W, Xie J, Hou Y, You C. Cytokine storm induced by SARS-CoV-2. Clin Chim Acta. 2020;509(280–287):280–287. doi:https://doi.org/10.1016/j.cca.2020.06.017.
- Bruewer M, Utech M, Ivanov AI, Hopkins AM, Parkos CA, Nusrat A. Interferon-gamma induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. FASEB J. 2005;19(8):923–933.
- Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, Said HM. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol. 2004;286(G367–376):G367–G376. doi:https://doi.org/10.1152/ajpgi.00173.2003.
- Ma TY, Boivin MA, Ye D, Pedram A, Said HM. Mechanism of TNF-{alpha} modulation of Caco-2 intestinal epithelial tight junction barrier: role of myosin light-chain kinase protein expression. Am J Physiol Gastrointest Liver Physiol. 2005;288(G422–430):G422–G430. doi:https://doi.org/10.1152/ajpgi.00412.2004.
- Ye D, Ma I, Ma TY. Molecular mechanism of tumor necrosis factor-alpha modulation of intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol. 2006;290(G496–504):G496–G504. doi:https://doi.org/10.1152/ajpgi.00318.2005.
- Utech M, Ivanov AI, Samarin SN, Bruewer M, Turner JR, Mrsny RJ, Parkos CA, Nusrat A. Mechanism of IFN-gamma-induced endocytosis of tight junction proteins: myosin II-dependent vacuolarization of the apical plasma membrane. Mol Biol Cell. 2005;16(5040–5052):5040–5052. doi:https://doi.org/10.1091/mbc.E05-03-0193.
- Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, Nusrat A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171(6164–6172):6164–6172. doi:https://doi.org/10.4049/jimmunol.171.11.6164.
- Marchiando AM, Shen L, Graham WV, Weber CR, Schwarz BT, Austin JR, Raleigh DR, Guan Y, Watson AJM, Montrose MH, et al. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J Cell Biol. 2010;189(111–126):111–126. doi:https://doi.org/10.1083/jcb.200902153.
- Yang F, Zhao K, Zhang X, Zhang J, Xu B. ATP Induces Disruption of Tight Junction Proteins via IL-1 Beta-Dependent MMP-9 Activation of Human Blood-Brain Barrier In Vitro. Neural Plast. 2016;2016(8928530):1–12. doi:https://doi.org/10.1155/2016/8928530.
- Wardill HR, Logan RM, Bowen JM, Van Sebille YZ, Gibson RJ. Tight junction defects are seen in the buccal mucosa of patients receiving standard dose chemotherapy for cancer. Support Care Cancer. 2016;24(1779–1788):1779–1788. doi:https://doi.org/10.1007/s00520-015-2964-6.
- Lapointe TK, Buret AG. Interleukin-18 facilitates neutrophil transmigration via myosin light chain kinase-dependent disruption of occludin, without altering epithelial permeability. Am J Physiol Gastrointest Liver Physiol. 2012;302(G343–351):G343–G351. doi:https://doi.org/10.1152/ajpgi.00202.2011.
- Al-Sadi R, Guo S, Dokladny K, Smith MA, Ye D, Kaza A, Watterson DM, Ma TY. Mechanism of Interleukin-1β Induced-Increase in Mouse Intestinal Permeability In Vivo. J Interferon Cytokine Res. 2012;32(474–484):474–484. doi:https://doi.org/10.1089/jir.2012.0031.
- Maccio U, Zinkernagel AS, Shambat SM, Zeng X, Cathomas G, Ruschitzka F, Schuepbach RA, Moch H, Varga Z. SARS-CoV-2 leads to a small vessel endotheliitis in the heart. EBioMedicine. 2021;63(103182):103182. doi:https://doi.org/10.1016/j.ebiom.2020.103182.
- Varga Z. [Endotheliitis in COVID-19]. Pathologe. 2020;41(99–102):99–102. doi:https://doi.org/10.1007/s00292-020-00875-9.
- Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(1417–1418):1417–1418. doi:https://doi.org/10.1016/S0140-6736(20)30937-5.
- Dou D, Revol R, Ostbye H, Wang H, Daniels DR. Influenza A Virus Cell Entry, Replication, Virion Assembly and Movement. Front Immunol. 2018;9(1581). doi:https://doi.org/10.3389/fimmu.2018.01581.
- de Graaf M, Fouchier RA. Role of receptor binding specificity in influenza A virus transmission and pathogenesis. EMBO J. 2014;33(823–841):1614. doi:https://doi.org/10.1002/embj.201387442.
- Chan MC, Chan RW, Yu WC, Ho CC, Chui WH, Lo CK, Yuen KM, Guan Y, Nicholls JM, Peiris JM, et al. Influenza H5N1 virus infection of polarized human alveolar epithelial cells and lung microvascular endothelial cells. Respir Res. 2009;10(102). doi:https://doi.org/10.1186/1465-9921-10-102
- Short KR, Kasper J, Van Der Aa S, Andeweg AC, Zaaraoui-Boutahar F, Goeijenbier M, Richard M, Herold S, Becker C, Scott DP, et al. Influenza virus damages the alveolar barrier by disrupting epithelial cell tight junctions. Eur Respir J. 2016;47(954–966):954–966. doi:https://doi.org/10.1183/13993003.01282-2015.
- LeMessurier KS, Tiwary M, Morin NP, Samarasinghe AE. Respiratory Barrier as a Safeguard and Regulator of Defense Against Influenza A Virus and Streptococcus pneumoniae. Front Immunol. 2020;11(3). doi:https://doi.org/10.3389/fimmu.2020.00003.
- Golebiewski L, Liu H, Javier RT, Rice AP. The avian influenza virus NS1 ESEV PDZ binding motif associates with Dlg1 and Scribble to disrupt cellular tight junctions. J Virol. 2011;85(10639–10648):10639–10648. doi:https://doi.org/10.1128/JVI.05070-11.
- Liu H, Golebiewski L, Dow EC, Krug RM, Javier RT, Rice AP. The ESEV PDZ-binding motif of the avian influenza A virus NS1 protein protects infected cells from apoptosis by directly targeting Scribble. J Virol. 2010;84(11164–11174):11164–11174. doi:https://doi.org/10.1128/JVI.01278-10.
- Brennan K, Offiah G, McSherry EA, Hopkins AM. Tight junctions: a barrier to the initiation and progression of breast cancer? J Biomed Biotechnol. 2010;2010:460607. doi:https://doi.org/10.1155/2010/460607.
- Ruan T, Sun J, Liu W, Prinz RA, Peng D, Liu X, Xu X. H1N1 Influenza Virus Cross-Activates Gli1 to Disrupt the Intercellular Junctions of Alveolar Epithelial Cells. Cell Rep. 2020;31(107801):107801. doi:https://doi.org/10.1016/j.celrep.2020.107801.
- Jennings LC, Dick EC. Transmission and control of rhinovirus colds. Eur J Epidemiol. 1987;3(327–335):327–335. doi:https://doi.org/10.1007/BF00145641.
- Bella J, Rossmann MG. Review: rhinoviruses and their ICAM receptors. J Struct Biol. 1999;128(69–74):69–74. doi:https://doi.org/10.1006/jsbi.1999.4143.
- Bella J, Rossmann MGICAM-1. receptors and cold viruses. Pharm Acta Helv. 2000;74(291–297):291–297. doi:https://doi.org/10.1016/s0031-6865(99)00056-4.
- Kolatkar PR, Bella J, Olson NH, Bator CM, Baker TS, Rossmann MG. Structural studies of two rhinovirus serotypes complexed with fragments of their cellular receptor. EMBO J. 1999;18(6249–6259):6249–6259. doi:https://doi.org/10.1093/emboj/18.22.6249.
- Rossmann MG, Bella J, Kolatkar PR, He Y, Wimmer E, Kuhn RJ, Baker TS. Cell recognition and entry by rhino- and enteroviruses. Virology. 2000;269(239–247):239–247. doi:https://doi.org/10.1006/viro.2000.0258.
- Sajjan U, Wang Q, Zhao Y, Gruenert DC, Hershenson MB. Rhinovirus disrupts the barrier function of polarized airway epithelial cells. Am J Respir Crit Care Med. 2008;178(1271–1281):1271–1281. doi:https://doi.org/10.1164/rccm.200801-136OC.
- Johnson JE, Gonzales RA, Olson SJ, Wright PF, Graham BS. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod Pathol. 2007;20(108–119):108–119. doi:https://doi.org/10.1038/modpathol.3800725.
- Pickles RJ, DeVincenzo JP. Respiratory syncytial virus (RSV) and its propensity for causing bronchiolitis. J Pathol. 2015;235(266–276):266–276. doi:https://doi.org/10.1002/path.4462.
- Tourdot S, Mathie S, Hussell T, Edwards L, Wang H, Openshaw PJM, Schwarze J, Lloyd CM. Respiratory syncytial virus infection provokes airway remodelling in allergen-exposed mice in absence of prior allergen sensitization. Clin Exp Allergy. 2008;38(1016–1024):1016–1024. doi:https://doi.org/10.1111/j.1365-2222.2008.02974.x.
- Lambert L, Sagfors AM, Openshaw PJ, Culley FJ. Immunity to RSV in Early-Life. Front Immunol. 2014;5(466). doi:https://doi.org/10.3389/fimmu.2014.00466.
- Kast JI, McFarlane AJ, Głobińska A, Sokolowska M, Wawrzyniak P, Sanak M, Schwarze J, Akdis CA, Wanke K. Respiratory syncytial virus infection influences tight junction integrity. Clin Exp Immunol. 2017;190(351–359):351–359. doi:https://doi.org/10.1111/cei.13042.
- Smallcombe CC, Linfield DT, Harford TJ, Bokun V, Ivanov AI, Piedimonte G, Rezaee F. Disruption of the airway epithelial barrier in a murine model of respiratory syncytial virus infection. Am J Physiol Lung Cell Mol Physiol. 2019;316(2):L358–L368. doi:https://doi.org/10.1152/ajplung.00345.2018.
- Petecchia L, Sabatini F, Usai C, Caci E, Varesio L, Rossi GA. Cytokines induce tight junction disassembly in airway cells via an EGFR-dependent MAPK/ERK1/2-pathway. Lab Invest. 2012;92(1140–1148):1140–1148. doi:https://doi.org/10.1038/labinvest.2012.67.
- Estes MK, Kang G, Zeng CQ, Crawford SE, Ciarlet M. Pathogenesis of rotavirus gastroenteritis. Novartis Found Symp. 2001;238:82–96. discussion 96-100. doi:https://doi.org/10.1002/0470846534.ch6.
- Crawford SE, Ramani S, Tate JE, Parashar UD, Svensson L, Hagbom M, Franco MA, Greenberg HB, O’Ryan M, Kang G, et al. Rotavirus infection. Nat Rev Dis Primers. 2017;3(17083). doi:https://doi.org/10.1038/nrdp.2017.83
- Obert G, Peiffer I, Servin AL. Rotavirus-induced structural and functional alterations in tight junctions of polarized intestinal Caco-2 cell monolayers. J Virol. 2000;74(4645–4651):4645–4651. doi:https://doi.org/10.1128/jvi.74.10.4645-4651.2000.
- Dickman KG, Hempson SJ, Anderson J, Lippe S, Zhao L, Burakoff R, Shaw RD. Rotavirus alters paracellular permeability and energy metabolism in Caco-2 cells. Am J Physiol Gastrointest Liver Physiol. 2000;279(G757–766):G757–G766. doi:https://doi.org/10.1152/ajpgi.2000.279.4.G757.
- Tafazoli F, Zeng CQ, Estes MK, Magnusson KE, Svensson L. NSP4 enterotoxin of rotavirus induces paracellular leakage in polarized epithelial cells. J Virol. 2001;75(1540–1546):1540–1546. doi:https://doi.org/10.1128/JVI.75.3.1540-1546.2001.
- Nava P, Lopez S, Arias CF, Islas S, Gonzalez-Mariscal L. The rotavirus surface protein VP8 modulates the gate and fence function of tight junctions in epithelial cells. J Cell Sci. 2004;117(5509–5519):5509–5519. doi:https://doi.org/10.1242/jcs.01425.
- Tafazoli F, Holmstrom A, Forsberg A, Magnusson KE, O’Brien AD. Apically Exposed, Tight Junction-Associated β1-Integrins Allow Binding and YopE-Mediated Perturbation of Epithelial Barriers by Wild-Type Yersinia Bacteria. Infect Immun. 2000;68(5335–5343):5335–5343. doi:https://doi.org/10.1128/iai.68.9.5335-5343.2000.
- Arias CF, Guerrero CA, Méndez E, Zárate S, Isa P, Espinosa R, Romero P, López S. Early events of rotavirus infection: the search for the receptor(s). Novartis Found Symp. 2001;238:47–60. discussion 60-43. doi:https://doi.org/10.1002/0470846534.ch4.
- Guerrero CA, Mendez E, Zarate S, Isa P, Lopez S, Arias CF. Integrin alpha(v)beta(3) mediates rotavirus cell entry. Proc Natl Acad Sci U S A. 2000;97(14644–14649):14644–14649. doi:https://doi.org/10.1073/pnas.250299897.
- Zarate S, Espinosa R, Romero P, Guerrero CA, Arias CF, López S. Integrin alpha2beta1 mediates the cell attachment of the rotavirus neuraminidase-resistant variant nar3. Virology. 2000;278(50–54):50–54. doi:https://doi.org/10.1006/viro.2000.0660.
- Coulson BS, Londrigan SL, Lee DJ. Rotavirus contains integrin ligand sequences and a disintegrin-like domain that are implicated in virus entry into cells. Proc Natl Acad Sci U S A. 1997;94(5389–5394):5389–5394. doi:https://doi.org/10.1073/pnas.94.10.5389.
- Abban CY, Meneses PI. Usage of heparan sulfate, integrins, and FAK in HPV16 infection. Virology. 2010;403(1–16):1–16. doi:https://doi.org/10.1016/j.virol.2010.04.007.
- Lynch JP 3rd, Fishbein M, Echavarria M. Adenovirus. Semin Respir Crit Care Med. 2011;32(4):494–511. doi:https://doi.org/10.1055/s-0031-1283287.
- Lynch JP 3rd, Kajon AE. Adenovirus: epidemiology, Global Spread of Novel Serotypes, and Advances in Treatment and Prevention. Semin Respir Crit Care Med. 2016;37(586–602). doi:https://doi.org/10.1055/s-0036-1584923.
- Tebruegge M, Curtis N. Adenovirus: an overview for pediatric infectious diseases specialists. Pediatr Infect Dis J. 2012;31(626–627):626–627. doi:https://doi.org/10.1097/INF.0b013e318250b066.
- Coyne CB, Bergelson JM. CAR: a virus receptor within the tight junction. Adv Drug Deliv Rev. 2005;57(6):869–882.
- Coyne CB, Bergelson JM. Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell. 2006;124(1):119–131. doi:https://doi.org/10.1016/j.cell.2005.10.035. [pii]10.1016/j.cell.2005.10.035
- Cohen CJ, Shieh JTC, Pickles RJ, Okegawa T, Hsieh J-T, Bergelson JM. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc Natl Acad Sci U S A. 2001;98(15191–15196):15191–15196. doi:https://doi.org/10.1073/pnas.261452898. [pii]
- Coyne CB, Shen L, Turner JR, Bergelson JM. Coxsackievirus entry across epithelial tight junctions requires occludin and the small GTPases Rab34 and Rab5. Cell Host Microbe. 2007;2(3):181–192. doi:https://doi.org/10.1016/j.chom.2007.07.003.
- Coyne CB, Voelker T, Pichla SL, Bergelson JM. The coxsackievirus and adenovirus receptor interacts with the multi-PDZ domain protein-1 (MUPP-1) within the tight junction. J Biol Chem. 2004;279(46):48079–48084. doi:https://doi.org/10.1074/jbc.M409061200. [pii]
- Nemerow GR, Cheresh DA, Wickham TJ. Adenovirus entry into host cells: a role for alpha(v) integrins. Trends Cell Biol. 1994;4(52–55):52–55. doi:https://doi.org/10.1016/0962-8924(94)90010-8.
- Wickham TJ, Filardo EJ, Cheresh DA, Nemerow GR. Integrin alpha v beta 5 selectively promotes adenovirus mediated cell membrane permeabilization. J Cell Biol. 1994;127(257–264):257–264. doi:https://doi.org/10.1083/jcb.127.1.257.
- Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73(309–319):309–319. doi:https://doi.org/10.1016/0092-8674(93)90231-e.
- Davison E, Diaz RM, Hart IR, Santis G, Marshall JF. Integrin alpha5beta1-mediated adenovirus infection is enhanced by the integrin-activating antibody TS2/16. J Virol. 1997;71(6204–6207):6204–6207. doi:https://doi.org/10.1128/JVI.71.8.6204-6207.1997.
- Li E, Brown SL, Stupack DG, Puente XS, Cheresh DA, Nemerow GR. Integrin alpha(v)beta1 is an adenovirus coreceptor. J Virol. 2001;75(5405–5409):5405–5409. doi:https://doi.org/10.1128/JVI.75.11.5405-5409.2001.
- Gralinski LE, Ashley SL, Dixon SD, Spindler KR. Mouse adenovirus type 1-induced breakdown of the blood-brain barrier. J Virol. 2009;83(9398–9410):9398–9410. doi:https://doi.org/10.1128/JVI.00954-09.
- Kotha PL, Sharma P, Kolawole AO, Yan R, Alghamri MS, Brockman TL, Gomez-Cambronero J, Excoffon KJDA. Adenovirus entry from the apical surface of polarized epithelia is facilitated by the host innate immune response. PLoS Pathog. 2015;11(e1004696):e1004696. doi:https://doi.org/10.1371/journal.ppat.1004696.
- Excoffon KJ, Bowers JR, Sharma P1. Alternative splicing of viral receptors: a review of the diverse morphologies and physiologies of adenoviral receptors. Recent Res Dev Virol. 2014;9:1–24.
- Forrest JC, Dermody TS. Reovirus receptors and pathogenesis. J Virol. 2003;77(9109–9115):9109–9115. doi:https://doi.org/10.1128/jvi.77.17.9109-9115.2003.
- Weiner HL, Drayna D, Averill DR Jr., Fields BN. Molecular basis of reovirus virulence: role of the S1 gene. Proc Natl Acad Sci U S A. 1977;74(5744–5748):5744–5748. doi:https://doi.org/10.1073/pnas.74.12.5744.
- Weiner HL, Powers ML, Fields BN. Absolute linkage of virulence and central nervous system cell tropism of reoviruses to viral hemagglutinin. J Infect Dis. 1980;141(609–616):609–616. doi:https://doi.org/10.1093/infdis/141.5.609.
- Barton ES, Forrest JC, Connolly JL, Chappell JD, Liu Y, Schnell FJ, Nusrat A, Parkos CA, Dermody TS. Junction adhesion molecule is a receptor for reovirus. Cell. 2001;104(441–451):441–451. doi:https://doi.org/10.1016/s0092-8674(01)00231-8.
- Campbell JA, Schelling P, Wetzel JD, Johnson EM, Forrest JC, Wilson GAR, Aurrand-Lions M, Imhof BA, Stehle T, Dermody TS, et al. Junctional adhesion molecule a serves as a receptor for prototype and field-isolate strains of mammalian reovirus. J Virol. 2005;79(7967–7978):7967–7978. doi:https://doi.org/10.1128/JVI.79.13.7967-7978.2005.
- Forrest JC, Campbell JA, Schelling P, Stehle T, Dermody TS. Structure-function analysis of reovirus binding to junctional adhesion molecule 1. Implications for the mechanism of reovirus attachment. J Biol Chem. 2003;278(48):48434–48444. doi:https://doi.org/10.1074/jbc.M305649200.
- Prota AE, Campbell JA, Schelling P, Forrest JC, Watson MJ, Peters TR, Aurrand-Lions M, Imhof BA, Dermody TS, Stehle T, et al. Crystal structure of human junctional adhesion molecule 1: implications for reovirus binding. Proc Natl Acad Sci U S A. 2003;100(5366–5371):5366–5371. doi:https://doi.org/10.1073/pnas.0937718100.
- Schelling P, Guglielmi KM, Kirchner E, Paetzold B, Dermody TS, Stehle T. The reovirus sigma1 aspartic acid sandwich: a trimerization motif poised for conformational change. J Biol Chem. 2007;282(11582–11589):11582–11589. doi:https://doi.org/10.1074/jbc.M610805200.
- Widener RW, Whitley RJ. Herpes simplex virus. Handb Clin Neurol. 2014;123(251–263). doi:https://doi.org/10.1016/B978-0-444-53488-0.00011-0.
- Bussmann C, Peng WM, Bieber T, Novak N. Molecular pathogenesis and clinical implications of eczema herpeticum. Expert Rev Mol Med. 2008;10(e21). doi:https://doi.org/10.1017/S1462399408000756.
- Hayashi K. Role of tight junctions of polarized epithelial MDCK cells in the replication of herpes simplex virus type 1. J Med Virol. 1995;47(323–329):323–329. doi:https://doi.org/10.1002/jmv.1890470406.
- Rahn E, Petermann P, Thier K, Bloch W, Morgner J, Wickström SA, Knebel-Mörsdorf D. Invasion of Herpes Simplex Virus Type 1 into Murine Epidermis: an Ex Vivo Infection Study. J Invest Dermatol. 2015;135(3009–3016):3009–3016. doi:https://doi.org/10.1038/jid.2015.290.
- Rahn E, Thier K, Petermann P, Rübsam M, Staeheli P, Iden S, Niessen CM, Knebel-Mörsdorf D. Epithelial Barriers in Murine Skin during Herpes Simplex Virus 1 Infection: the Role of Tight Junction Formation. J Invest Dermatol. 2017;137(884–893):884–893. doi:https://doi.org/10.1016/j.jid.2016.11.027.
- Sufiawati I, Tugizov SM. HIV-induced matrix metalloproteinase-9 activation through mitogen-activated protein kinase signalling promotes HSV-1 cell-to-cell spread in oral epithelial cells. J Gen Virol. 2018;99(937–947):937–947. doi:https://doi.org/10.1099/jgv.0.001075.
- Gabrielli L, Bonasoni MP, Chiereghin A, Piccirilli G, Santini D, Pavia C, Turello G, Squarzoni D, Lazzarotto T. Salivary glands and human congenital cytomegalovirus infection: what happens in early fetal life? J Med Virol. 2017;89(2):318–323. doi:https://doi.org/10.1002/jmv.24628.
- Manning WC, Stoddart CA, Lagenaur LA, Abenes GB, Mocarski ES. Cytomegalovirus determinant of replication in salivary glands. J Virol. 1992;66(3794–3802):3794–3802. doi:https://doi.org/10.1128/JVI.66.6.3794-3802.1992.
- Forte E, Zhang Z, Thorp EB, Cytomegalovirus Latency HM. Reactivation: an Intricate Interplay With the Host Immune Response. Front Cell Infect Microbiol. 2020;10(130). doi:https://doi.org/10.3389/fcimb.2020.00130.
- Griffiths P, Baraniak I, Reeves M. The pathogenesis of human cytomegalovirus. J Pathol. 2015;235(288–297):288–297. doi:https://doi.org/10.1002/path.4437.
- Drew WL. Cytomegalovirus infection in patients with AIDS. Clin Infect Dis. 1992;14(608–615):608–615. doi:https://doi.org/10.1093/clinids/14.2.608-a.
- Kovacs A, Schluchter M, Easley K, Demmler G, Shearer W, Russa PL, Pitt J, Cooper E, Goldfarb J, Hodes D, et al. Cytomegalovirus infection and HIV-1 disease progression in infants born to HIV-1-infected women. Pediatric Pulmonary and Cardiovascular Complications of Vertically Transmitted HIV Infection Study Group. N Engl J Med. 1999;341(2):77–84. doi:https://doi.org/10.1056/NEJM199907083410203.
- Nigro G, Krzysztofiak A, Gattinara GC, Mango T, Mazzocco M, Porcaro MA, Provvedi S, Booth JC. Rapid progression of HIV disease in children with cytomegalovirus DNAemia. AIDS. 1996;10:1127–1133.
- Chandwani S, KAUL A, Bebenroth D, Kim M, John DD, FIDELIA A, HASSEL A, BORKOWSKY W, KRASINSKI K. Cytomegalovirus infection in human immunodeficiency virus type 1-infected children. Pediatr Infect Dis J. 1996;15(310–314):310–314. doi:https://doi.org/10.1097/00006454-199604000-00006.
- Gie RP, Goussard P. CMV pneumonia in HIV-infected and HIV-uninfected infants: a neglected disease? Int J Tuberc Lung Dis. 2017;21(12):1209–1210. doi:https://doi.org/10.5588/ijtld.17.0714.
- Freeman ML, Lederman MM, Gianella S. Partners in Crime: the Role of CMV in Immune Dysregulation and Clinical Outcome During HIV Infection. Curr HIV/AIDS Rep. 2016;13(10–19):10–19. doi:https://doi.org/10.1007/s11904-016-0297-9.
- Goodgame RW. Gastrointestinal cytomegalovirus disease. Ann Intern Med. 1993;119(924–935):924. doi:https://doi.org/10.7326/0003-4819-119-9-199311010-00010.
- Goodgame RW. Viral infections of the gastrointestinal tract. Curr Gastroenterol Rep. 1999;1(292–300):292–300. doi:https://doi.org/10.1007/s11894-999-0112-5.
- Francis ND, Boylston AW, Roberts AH, Parkin JM, Pinching AJ. Cytomegalovirus infection in gastrointestinal tracts of patients infected with HIV-1 or AIDS. J Clin Pathol. 1989;42(1055–1064):1055–1064. doi:https://doi.org/10.1136/jcp.42.10.1055.
- Maidji E, Somsouk M, Rivera JM, Hunt PW, Stoddart CA. Replication of CMV in the gut of HIV-infected individuals and epithelial barrier dysfunction. PLoS Pathog. 2017;13(e1006202):e1006202. doi:https://doi.org/10.1371/journal.ppat.1006202.
- Vidal L, Gillison ML. Human papillomavirus in HNSCC: recognition of a distinct disease type. Hematol Oncol Clin North Am. 2008;22(6):1125–1142. doi:https://doi.org/10.1016/j.hoc.2008.08.006. vii
- Palefsky JM. Antiretroviral therapy and anal cancer: the good, the bad, and the unknown. Sex Transm Dis. 2012;39(501–503):501–503. doi:https://doi.org/10.1097/OLQ.0b013e31825f7921.
- Engelbrecht AM, Gebhardt S, Louw L. Ex vivo study of MAPK profiles correlated with parameters of apoptosis during cervical carcinogenesis. Cancer Lett. 2006;235(93–99):93–99. doi:https://doi.org/10.1016/j.canlet.2005.04.005.
- Tang X, Zhang Q, Nishitani J, Brown J, Shi S, Le AD. Overexpression of human papillomavirus type 16 oncoproteins enhances hypoxia-inducible factor 1 alpha protein accumulation and vascular endothelial growth factor expression in human cervical carcinoma cells. Clin Cancer Res. 2007;13(2568–2576):2568–2576. doi:https://doi.org/10.1158/1078-0432.CCR-06-2704.
- Chakrabarti O, Veeraraghavalu K, Tergaonkar V, Liu Y, Androphy EJ, Stanley MA, Krishna S. Human papillomavirus type 16 E6 amino acid 83 variants enhance E6-mediated MAPK signaling and differentially regulate tumorigenesis by notch signaling and oncogenic Ras. J Virol. 2004;78(5934–5945):5934–5945. doi:https://doi.org/10.1128/JVI.78.11.5934-5945.2004.
- Zhong Y, Smart EJ, Weksler B, Couraud P-O, Hennig B, Toborek M. Caveolin-1 regulates human immunodeficiency virus-1 Tat-induced alterations of tight junction protein expression via modulation of the Ras signaling. J Neurosci. 2008;28(7788–7796):7788–7796. doi:https://doi.org/10.1523/JNEUROSCI.0061-08.2008.
- Song L, Ge S, Pachter JS. Caveolin-1 regulates expression of junction-associated proteins in brain microvascular endothelial cells. Blood. 2007;109(1515–1523):1515–1523. doi:https://doi.org/10.1182/blood-2006-07-034009.
- Gonzalez-Mariscal L, Tapia R, Chamorro D. Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta. 2008;1778:729–756. doi:https://doi.org/10.1016/j.bbamem.2007.08.018.
- Latorre IJ, Roh MH, Frese KK, Weiss RS, Margolis B, Javier RT. Viral oncoprotein-induced mislocalization of select PDZ proteins disrupts tight junctions and causes polarity defects in epithelial cells. J Cell Sci. 2005;118(18):4283–4293.
- Thomas M, Laura R, Hepner K, Guccione E, Sawyers C, Lasky L, Banks L. Oncogenic human papillomavirus E6 proteins target the MAGI-2 and MAGI-3 proteins for degradation. Oncogene. 2002;21(5088–5096):5088–5096. doi:https://doi.org/10.1038/sj.onc.1205668.
- Grm HS, Banks L. Degradation of hDlg and MAGIs by human papillomavirus E6 is E6-AP-independent. J Gen Virol. 2004;85(2815–2819):2815–2819. doi:https://doi.org/10.1099/vir.0.80035-085/10/2815. [pii]
- Hellner K, Mar J, Fang F, Quackenbush J, Munger K. HPV16 E7 oncogene expression in normal human epithelial cells causes molecular changes indicative of an epithelial to mesenchymal transition. Virology. 2009;391(57–63):57–63. doi:https://doi.org/10.1016/j.virol.2009.05.036.
- Laurson J, Khan S, Chung R, Cross K, Raj K. Epigenetic repression of E-cadherin by human papillomavirus 16 E7 protein. Carcinogenesis. 2010;31(918–926):918–926. doi:https://doi.org/10.1093/carcin/bgq027.
- Shiau MY, Fan L-C, Yang S-C, Tsao C-H, Lee H, Cheng Y-W, Lai L-C, Chang Y-H. Human Papillomavirus Up-Regulates MMP-2 and MMP-9 Expression and Activity by Inducing Interleukin-8 in Lung Adenocarcinomas. PloS One. 2013;8(e54423). doi:https://doi.org/10.1371/journal.pone.0054423.
- Cardeal LB, Boccardo E, Termini L, Rabachini T, Andreoli MA, di Loreto C, Filho AL, Villa LL, Maria-Engler SS. HPV16 oncoproteins induce MMPs/RECK-TIMP-2 imbalance in primary keratinocytes: possible implications in cervical carcinogenesis. PloS One. 2012;7(e33585):e33585. doi:https://doi.org/10.1371/journal.pone.0033585.
- Symowicz J, Adley BP, Gleason KJ, Johnson JJ, Ghosh S, Fishman DA, Hudson LG, Stack MS. Engagement of collagen-binding integrins promotes matrix metalloproteinase-9-dependent E-cadherin ectodomain shedding in ovarian carcinoma cells. Cancer Res. 2007;67(2030–2039):2030–2039. doi:https://doi.org/10.1158/0008-5472.CAN-06-2808.
- You TK, Kim KM, Noh SJ, Bae JS, Jang KY, Chung MJ, Moon WS, Kang MJ, Lee DG, Park HS, et al. Expressions of E-cadherin, Cortactin and MMP-9 in Pseudoepitheliomatous Hyperplasia and Squamous Cell Carcinoma of the Head and Neck: their Relationships with Clinicopathologic Factors and Prognostic Implication. Korean J Pathol. 2012;46(331–340):331. doi:https://doi.org/10.4132/KoreanJPathol.2012.46.4.331.
- David JM, Rajasekaran AK. Dishonorable discharge: the oncogenic roles of cleaved E-cadherin fragments. Cancer Res. 2012;72(2917–2923):2917–2923. doi:https://doi.org/10.1158/0008-5472.CAN-11-3498.
- Ikenouchi J, Matsuda M, Furuse M, Tsukita S. Regulation of tight junctions during the epithelium-mesenchyme transition: direct repression of the gene expression of claudins/occludin by Snail. J Cell Sci. 2003;116(1959–1967):1959–1967. doi:https://doi.org/10.1242/jcs.00389.
- Lee MY, Shen MR. Epithelial-mesenchymal transition in cervical carcinoma. Am J Transl Res. 2012;4(1–13).
- Hsu YM, Chen Y-F, Chou C-Y, Tang M-J, Chen JH, Wilkins RJ, Ellory JC, Shen M-R. KCl cotransporter-3 down-regulates E-cadherin/beta-catenin complex to promote epithelial-mesenchymal transition. Cancer Res. 2007;67(11064–11073):11064–11073. doi:https://doi.org/10.1158/0008-5472.CAN-07-2443.
- Lee MY, Chou CY, Tang MJ, Shen MR. Epithelial-mesenchymal transition in cervical cancer: correlation with tumor progression, epidermal growth factor receptor overexpression, and snail up-regulation. Clin Cancer Res. 2008;14(4743–4750):4743–4750. doi:https://doi.org/10.1158/1078-0432.CCR-08-0234.
- Ocana OH, Nieto MA. Epithelial plasticity, stemness and pluripotency. Cell Res. 2010;20(1086–1088):1086–1088. doi:https://doi.org/10.1038/cr.2010.127.
- Nieto MA, Cano A. The epithelial-mesenchymal transition under control: global programs to regulate epithelial plasticity. Semin Cancer Biol. 2012;22(361–368):361–368. doi:https://doi.org/10.1016/j.semcancer.2012.05.003.
- Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(178–196). doi:https://doi.org/10.1038/nrm3758.
- Talbot LJ, Bhattacharya SD, Kuo PC. Epithelial-mesenchymal transition, the tumor microenvironment, and metastatic behavior of epithelial malignancies. Int J Biochem Mol Biol. 2012;3:117–136.
- Carias AM, McCoombe S, McRaven M, Anderson M, Galloway N, Vandergrift N, Fought AJ, Lurain J, Duplantis M, Veazey RS, et al. Defining the interaction of HIV-1 with the mucosal barriers of the female reproductive tract. J Virol. 2013;87(11388–11400):11388–11400. doi:https://doi.org/10.1128/JVI.01377-13.
- Aisha N, Olivia C, Wendy N. Dobson-Belaire, Michel Ouellet, Michel J. T, Scott D. Gray-Owen, A. Larry Arsenault, and Charu K. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog. 2010;6(e1000852):e1000852. doi:https://doi.org/10.1371/journal.ppat.1000852.
- Nazli A, Kafka JK, Ferreira VH, Anipindi V, Mueller K, Osborne BJ, Dizzell S, Chauvin S, Mian MF, Ouellet M, et al. HIV-1 gp120 induces TLR2- and TLR4-mediated innate immune activation in human female genital epithelium. Journal of Immunology. 2013;191(4246–4258):4246–4258. doi:https://doi.org/10.4049/jimmunol.1301482.
- Gupta S, Gach JS, Becerra JC, Phan TB, Pudney J, Moldoveanu Z, Joseph SB, Landucci G, Supnet MJ, Ping L-H, et al. The Neonatal Fc receptor (FcRn) enhances human immunodeficiency virus type 1 (HIV-1) transcytosis across epithelial cells. PLoS Pathog. 2013;9(e1003776). doi:https://doi.org/10.1371/journal.ppat.1003776
- Bomsel M. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat Med. 1997;3(42–47):42–47. doi:https://doi.org/10.1038/nm0197-42.
- Bobardt MD. Primary intestinal epithelial cells selectively transfer R5 HIV-1 to CCR5+ cells. Nat Med. 2002;8:150–156.
- Gang M, Xiping W, Xiaoyun W, Marty T. Sellers, Julie M. Decker, Zina M, Jan M. Orenstein, Martin F. Graham, John C. Kappes, Jiri Mestecky, et al. Primary intestinal epithelial cells selectively transfer R5 HIV-1 to CCR5+ cells. Nat Med. 2002;8:150–156.
- Kohli A, Islam A, Moyes DL, Murciano C, Shen C, Challacombe SJ, Naglik JR. Oral and vaginal epithelial cell lines bind and transfer cell-free infectious HIV-1 to permissive cells but are not productively infected. PloS One. 2014;9(e98077):e98077. doi:https://doi.org/10.1371/journal.pone.0098077.
- Rossana H, Michael M, Kristina R, Zhimin F, Aaron W, and Sharof T. Human beta-defensins 2 and −3 cointernalize with human immunodeficiency virus via heparan sulfate proteoglycans and reduce infectivity of intracellular virions in tonsil epithelial cells. Virology. 2016;487(172–187):172–187. doi:https://doi.org/10.1016/j.virol.2015.09.025.
- Maresca M, Mahfoud R, Garmy N, Kotler DP, Fantini J, Clayton F. The virotoxin model of HIV-1 enteropathy: involvement of GPR15/Bob and galactosylceramide in the cytopathic effects induced by HIV-1 gp120 in the HT-29-D4 intestinal cell line. J Biomed Sci. 2003;10(156–166):156–166.
- Del Corno M, Liu Q-H, Schols D, de Clercq E, Gessani S, Freedman BD, Collman RG. HIV-1 gp120 and chemokine activation of Pyk2 and mitogen-activated protein kinases in primary macrophages mediated by calcium-dependent, pertussis toxin-insensitive chemokine receptor signaling. Blood. 2001;98(10):2909–2916. doi:https://doi.org/10.1182/blood.V98.10.2909.
- Lien K, Mayer W, Herrera R, Rosbe K, Tugizov SM. HIV-1proteins gp120 and tat induce the epithelial-mesenchymal transition in oral and genital mucosal epithelial cells. PLoS One. 2019;14(e0226343):e0226343. doi:https://doi.org/10.1371/journal.pone.0226343.
