ABSTRACT
Background: Obesity is a complex disease involving the accumulation of body fat that can inflict a substantial risk to health due to the potent role it plays in the development of a series of chronic diseases including cardiovascular diseases (CVD), nonalcoholic fatty liver diseases (NAFLD), kidney diseases, diabetes, and some cancers. Despite all efforts made, no therapy has succeeded in reversing the obesity pandemic and its associated diseases. Herein, the aim was to study the effect of adipose-derived mesenchymal stem cells on obesity-induced multi-organ injuries in a diet-induced obese mouse model. Male C57BL/6 mice were fed with regular chow diet or high fat diet (HFD) to induce obesity for 15 weeks after which the mice were administered two doses of adipose-derived mesenchymal stem cells (ASC-treated groups) or media as control (media-treated groups). Animals were sacrificed and adipose, hepatic, renal, and cardiac tissues were obtained for histopathological evaluation. Mice on HFD showed excessive pathological alterations such as epididymal adipose tissue expansion, hepatic fat accumulation, glomerular swelling, and cardiomyocyte hypertrophy. However, treatment with ASCs significantly reversed the significant histopathological abnormalities induced by obesity. In conclusion, this study demonstrated the therapeutic effects of adipose-derived mesenchymal stem cells on obesity-associated complications such as NAFLD, CVD, and kidney disorders in a diet-induced obese animal model, which were partly due to the attenuation of inflammatory cytokines such as TNF-α and IL-6.
Background
According to the World Health Organization (WHO), obesity is defined as “abnormal or excessive fat accumulation that presents a risk to health”.Citation1 Obesity is a complex disease, whose worldwide prevalence has risen at an unprecedented pace in the last 40 years, thus reaching pandemic levels.Citation2 It also poses a serious public health challenge due to the potent role obesity plays in the development of a vast number of chronic diseases such as nonalcoholic fatty liver disease (NAFLD), cardiovascular disease (CVD), kidney disease, diabetes, and some cancers.Citation3–6 The mechanisms by which obesity leads to the pathogenesis of these diseases have still not been fully elucidated, although it is believed that inflammation is a contributing factor.Citation7 Increased lipid accumulation and adipose tissue expansion in response to obesity are often associated with reduction in adipose tissue blood flow and thereby leading to hypoxia.Citation8,Citation9 Adipose tissue hypoxia results in the activation of many signaling pathways that stimulate the release of pro-inflammatory molecules.Citation10 Those inflammatory signals ultimately induce adipocyte death, which in turn triggers macrophage activation and infiltration into adipose tissue to scavenge the dead adipocytes, which subsequently exaggerate the inflammatory response by producing pro-inflammatory cytokines IL-6 and TNF-α; hence, leading to tissue injury.Citation10,Citation11
Despite all efforts made, no therapy has succeeded in reversing the obesity pandemic and its associated diseases including NAFLD and other extra-hepatic chronic diseases, such as CVD and renal disorders. The use of stem cells for clinical applications has bloomed recently and mesenchymal stem cells (MSCs) in particular have been largely utilized in current years for the treatment of several diseases owing to their self-renewal features, multidifferentiation potential as well as their anti-inflammatory, immunomodulatory, and homing capacities that promote tissue repair.Citation12–16 Besides, the relative ease by which MSCs can be isolated from adult tissues, such as adipose tissue, has made these remarkable cells an attractive option for therapeutic applications.Citation17 Herein, we investigated whether intraperitoneal delivery of human adipose-derived mesenchymal stem cells can promote therapeutic effects on obesity-related complications such as NAFLD, CVD, and kidney disorders in a diet-induced obese animal model.
Methods
Animals
Male C57BL/6 mice were housed in a controlled environment with free access to water and food and a 12:12 hour light/dark cycle with a temperature of 22 ± 2°C and 60% relative humidity. All animal procedures used were approved by the Institutional Animal Care and Use Committee (IACUC) at the American University of Beirut with an approval number 18-12-510. A period of 7 days was used as an adaptation period before the experimentation began.
Experimental design
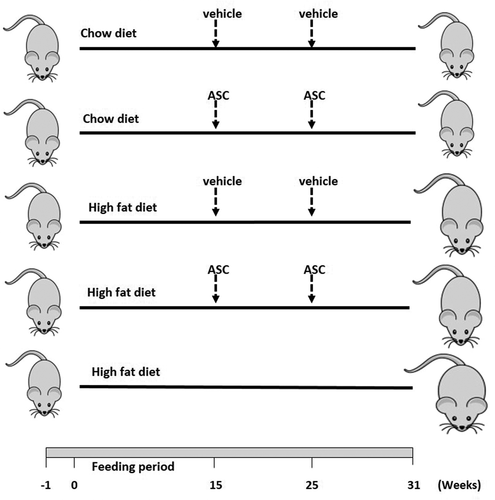
The 9-week-old mice were chronically fed with either the regular chow diet or the high fat diet (HFD) (Research Diet, Inc, D12492, NJ, USA) for 15 weeks. Chow diet contains 10 cal% fat, 20 cal% proteins, and 70 cal% carbohydrates, while the HFD has 60 cal% fat, 20 cal% proteins, and 20 cal% carbohydrates. These diets were supplemented for 15 weeks after which the mice were fed with regular chow diet and administered intraperitoneally with either the DMEM/F12 medium as control (media-treated groups) or two doses of 4.2 × 107 cells/kg body weight of adipose-derived mesenchymal stem cells (ASC-treated groups) with an elapse of 10 weeks. Obese untreated mice (HFD group) were maintained on the HFD until the end of the experiment (31 weeks). All animals were sacrificed 6 weeks after the second injection. The experimental design is summarized in .
Figure 1. Schematic representation of animal design. Male C57BL/6 mice (n = 25) were fed with chow or high fat diet for 15 weeks. Obese and lean mice were then administered either adipose-derived mesenchymal stem cells (ASCs) or vehicle (media) on week 15 and week 25. A group of mice was left on HFD for 31 weeks. n = 5 for each group
Cell culture
Human adipose-derived mesenchymal stem cells (ASCs), a kind gift from Reviva Regenerative Medicine Center, Bsalim, Lebanon, were characterized and cultured in DMEM/F12 medium supplemented with 10% FBS and 1% antibiotic-antimycotic at 37°C and 5% CO2 atmosphere.Citation18 The medium was changed twice a week and cells were passaged when reaching 80–90% confluency. Cells were used up to passage 5 for animal treatments.
Histopathological analysis
At the end of the study, all mice groups were sacrificed using chloroform and the liver, kidney, heart, and epididymal adipose tissues were harvested, washed with saline, blotted dry with a paper towel and weighed. Tissues were then fixed overnight in 4% paraformaldehyde in phosphate buffer saline (PBS) at 4°C ready to be embedded in paraffin for sectioning using microtome (Leica, Germany). For histopathological assessment, sections of 5-μm thickness were prepared and stained with hematoxylin and eosin (H&E, Sigma-Aldrich). Kidney sections were also stained with periodic acid-Schiff (PAS, Sigma-Aldrich) to better examine the glomerular structure and quantify the glomerular tuft area. Besides, Masson’s trichrome staining was conducted on hepatic, renal, and cardiac sections according to a standard protocol to assess fibrosis. All sections were examined using a light microscope with images captured by Olympus digital camera (Optiphot 2; Nikon, Tokyo, Japan). It is worth mentioning that all the imaging was blinded to avoid bias. Areas of individual fat cells in adipose tissues and cardiomyocytes in cardiac tissues as well as number of lipid droplets in liver sections of each group were quantified using the Image analysis software (ImageJ, NIH). Glomerular fibrosis was also assessed by measuring the blue stained area in the glomerulus to total glomerular area as previously described.Citation19 For each mouse, four random sections were selected with five microscopic fields per section.
Inflammatory cytokine analysis
At the end of the experiment, blood was collected and serum isolated and stored at −20°C until further analysis. Enzyme-linked immunosorbent assay (ELISA) using specific SimpleStep ELISA kits (Abcam) was used to determine serum Interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) concentrations according to the manufacturer’s instructions. The optical density was measured at 450 nm.
Statistical analysis
Data were analyzed using statistical package for social sciences, SPSS 24 (SPSS, Inc., Chicago, IL, USA) and expressed as mean ± standard error of mean (SEM). Comparisons between groups were performed using one-way analysis of variance (ANOVA) followed by Tukey post hoc test. P value <.05 was considered to be statistically significant.
Results
Effect of ASC administration on adipose tissue
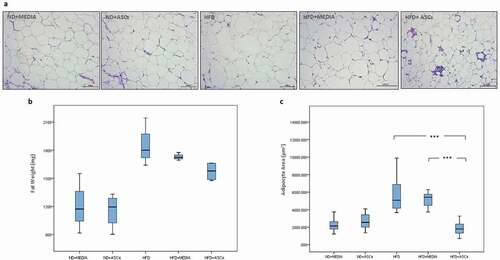
To assess the effects of ASC infusion on the adipose tissue, epididymal fat weight and adipocyte area were determined. HFD intake in mice resulted in a significant increase in the weight of epididymal fat tissue; however, there was no significant difference in fat weight between HFD-fed group and HFD mice receiving ASCs (). Moreover, feeding with a HFD was accompanied by a significant increase in adipocyte area (6477 μm2) as compared to chow-fed controls (2328 and 2668 μm2). However, the average area of adipocytes was significantly reduced in HFD mice injected intraperitoneally with ASCs reaching 1890 μm2 (), suggesting that infusion with ASCs was sufficient to shrink the size of adipose cells augmented by the HFD feeding.
Figure 2. Adipose-derived mesenchymal stem cells attenuate epididymal adipose tissue expansion in HFD-induced obese mice. Representative images of hematoxylin and eosin (H&E)-stained sections (a) and weight (b) of epididymal adipose tissue. Adipocyte area was also quantified (c). n = 5. *** represents statistical significance between groups at P < .001
ASC administration reverses liver damage
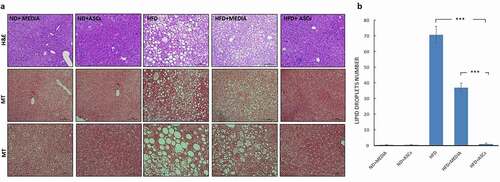
Visual assessment of the gross morphology of the livers from mice of different groups showed massive changes in the HFD mice compared to the controls (ND+media and ND+ASCs). While control groups exhibited ordinary reddish-brown, soft bright surfaces and normal size livers, the HFD groups (HFD and HFD+media) were paler with no luster and much larger in size. However, livers of mice treated with adipose-derived stem cells presented a morphology similar to the normal groups (). Furthermore, liver weights were significantly increased in HFD groups compared to those of normal groups. Interestingly, treatment with stem cells was sufficient to reverse this liver weight gain seen in obese mice (). Histologically, H&E staining of liver sections showed normal structure and intact architecture in control groups (ND+media and ND+ASCs) with healthy hepatocytes. On the contrary, HFD and HFD+media groups demonstrated increased accumulation of lipid droplets (steatosis) and hepatocyte ballooning that were remarkably inhibited when mice were treated with ASCs (), with almost no visible changes in the ASC-treated hepatic sections as compared to the control ones. Masson’s trichrome staining was also performed on liver sections to assess liver fibrosis. No evidence of blue stained fibrous tissue was detected in all mice groups ().
Figure 3. Adipose-derived mesenchymal stem cells reverse liver enlargement induced by HFD feeding in C57BL/6 mice. (a) Gross morphology and (b) weight of the liver in treated mice groups. Data are presented as mean ± SEM; n = 5. *** represents statistical significance between groups at P < .001
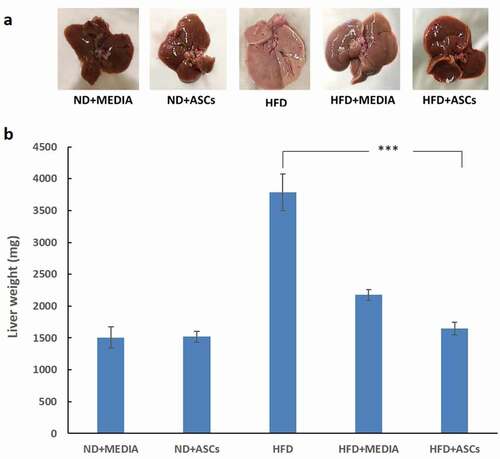
Figure 4. Adipose-derived mesenchymal stem cells suppress liver steatosis and hepatocyte ballooning induced by HFD in mice. (a) Representative images of Hematoxylin/eosin (H&E) and Masson’s Trichrome (MT)-stained liver sections obtained from treated groups and (b) quantification of lipid droplet numbers. Data are presented as mean ± SEM. *** represents statistical significance between groups at P < .001
Histopathological changes in the renal tissue
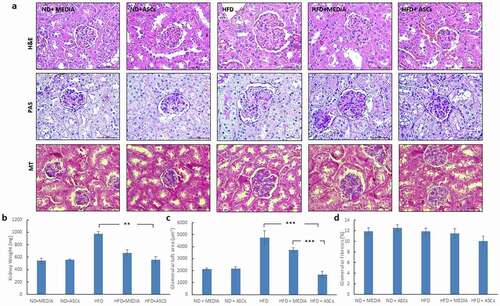
We then investigated any renal alterations in obese mice, untreated or treated with ASCs. The kidney weight was the heaviest in obese mice fed with the HFD for 31 weeks, followed by mice fed with the HFD for 15 weeks (HFD+media). However, HFD mice treated with two doses of ASCs on the 15th and 25th weeks, showed kidney weights similar to those of normal chow-fed controls (ND+media and ND+ASCs) (). To evaluate renal histopathological changes, H&E and PAS staining were performed (). Glomerular tuft area tended to be larger in mice on HFD (HFD and HFD+media groups) as compared to mice on chow diet. Interestingly, treatment with ASCs significantly decreased glomerular tuft area (). No alterations in glomerular fibrosis was detected in all groups as demonstrated by Masson’s trichrome staining ().
Figure 5. Adipose-derived mesenchymal stem cells decrease glomerular swelling observed in the kidneys of HFD-fed mice. (a) Representative images of Hematoxylin/eosin (H&E), periodic acid-Schiff (PAS) and Masson’s Trichrome (MT)-stained kidney sections obtained from different animal groups. (b) Kidney weight, (c) glomerular tuft area and d) % of glomerular fibrosis were also quantified. Data are presented as mean ± SEM. ** and *** represent statistical difference between groups at P < .01 and P < .001 significance, respectively
Effect of ASC treatment on the heart
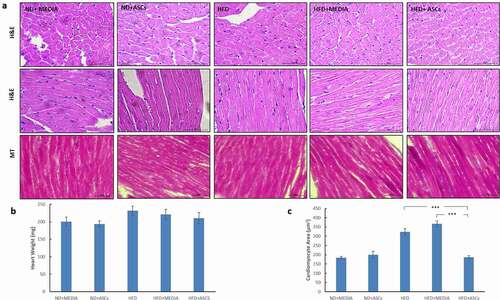
The heart weights were heavier in the HFD groups (HFD and HFD+media) than in the chow-fed groups (ND+media and ND+ASCs). Treatment of HFD-fed mice with ASCs reduced heart weight, but this decrease was not significant (). Moreover, histopathological H&E staining results demonstrated increased cardiomyocyte cross-sectional area in untreated or vehicle-treated HFD mice (). The mean cardiomyocyte area was 60–70% higher in mice fed with the HFD (HFD and HFD+media) versus control mice. Nevertheless, treatment with stem cells significantly reduced cardiomyocyte area to values similar to that of control groups (). Adjacent sections were also stained with Masson’s trichrome to assess myocardial fibrosis. Feeding with a HFD for 15 and 31 weeks had no effect on fibrosis as illustrated by lack of blue staining ().
Figure 6. Adipose-derived mesenchymal stem cells attenuate cardiomyocyte hypertrophy induced by HFD in mice. (a) Representative images of transverse (upper panel) and longitudinal (middle and lower panels) cardiac muscle tissue sections from different animal groups stained with Hematoxylin/eosin (H&E) and Masson’s Trichrome (MT) stains. (b) Heart weight and (c) cardiomyocyte area were also quantified. Data are presented as mean ± SEM. *** represents statistical significance between groups at P < .001
Inflammatory cytokines
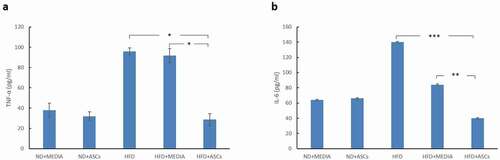
The concentrations of serum IL-6 were significantly higher (P < .001) in the HFD group as compared to the chow-fed groups (ND+media and ND+ASCs). Replacement of the HFD with a normal chow diet after 15 weeks led to decrease in the IL-6 levels in the HFD+media group, but was still markedly higher than the control groups. However, treatment with ASCs caused significant reduction in the systemic IL-6 levels (). In addition, chronic HFD feeding (HFD group) caused a similar increase in serum TNF-α levels as compared to lean mice. Treating with ASCs notably lowered the concentrations of TNF-α to about the same levels as that of the control groups ().
Figure 7. Adipose-derived mesenchymal stem cells treatment suppresses TNF-α and IL-6 expression in the serum of HFD-fed mice. ELISA analyses of TNF-α (a) and IL-6 (b) expression in various animal groups. *, **, and *** represent statistical difference between groups at P < .05, P < .01 and P < .001 significance, respectively
Discussion
Obesity has become a public health concern worldwide due to its poor control and its association with multiple organ injuries.Citation20 In recent years, many studies have demonstrated a role of obesity in the development of several diseases involving the liver, heart, kidneys as well as many other organs.Citation21–23 Despite advances in obesity treatment, therapeutic approaches are not yet optimal, especially in the long run. Therefore, increasing efforts have been made to find and develop new treatments that can reduce the accumulation of fat in the adipose tissue and reverse obesity-associated organ injuries. In this study, adipose-derived mesenchymal stem cells were used in diet-induced obese mice to explore their effect on obesity-induced multi-organ damage. Male C57BL/6 mice were utilized because they lack hormonal changes related to estrous stages, which could affect certain parameters.Citation24 It is worth mentioning that there are various animal models that have been used as a preclinical research tool to study obesity and its associated diseases. Such available models are either genetic such as transgenic mice or dietary which involves manipulation in the food environment such as the diet-induced obesity (DIO) model.Citation25 It is believed that DIO animals are better than most of the genetically modified models (leptin receptor-deficient db/db mice, MC4 receptor-deficient mice or leptin-deficient ob/ob mice) at imitating the state of common obesity in humans and may possibly be the best choice for testing potential therapeutics.Citation25,Citation26 In this study, we explored the therapeutic effects of adipose-derived mesenchymal stem cells on the epididymal fat, liver, kidneys, and hearts of DIO mice. Mice were fed a HFD to induce obesity. In our previous study, we have demonstrated substantial increase in body weights and blood glucose levels in HFD-fed mice.Citation27
Herein, histological evaluation demonstrated increased adipocyte areas and fat accumulation in the liver of HFD-fed mice, which were both markedly attenuated when mice were treated with ASCs. This excessive buildup of fat in the liver is referred to as nonalcoholic fatty liver disease (NAFLD). NAFLD has become one of the most common causes of chronic liver disease with a prevalence of 25% worldwide and it is expected to become the leading cause of liver transplant in the next decade.Citation28,Citation29 Therefore, due to the high prevalence rates of NAFLD globally, close association with cardiometabolic disorders and absence of approved pharmacological treatment, there is a surge for developing a treatment for NAFLD. Our results demonstrated that treatment with ASCs markedly suppressed fat vacuoles and lipid accumulation in the liver of obese mice and subsequently the liver returned to normal red brownish color. Also, consistent with our results, a previous study showed that compact bone-derived MSCs reversed the expansion of subcutaneous adipose tissue and attenuated NAFLD when HFD-fed mice were intravenously injected twice with 1 × 106 MSCs.Citation30
Obesity was also pointed out as a potent risk factor for the development of kidney disease. Therefore, changes in the kidney structure were examined histologically. Normal glomerular architecture was observed in ND groups; however, there was definite increase in glomerular size when mice were fed a HFD with no other significant changes found. Previously, Altunkaynak et al. showed that feeding a high-fat diet to female Sprague Dawley rats for a period of 3 months induced many kidney alterations such as mononuclear cell infiltration, glomerular necrosis and atrophy, and increase in the connective tissue in the kidneys.Citation31 The fact that these major kidney alterations were absent in our study is that in spite the C57BL/6 strain being obesity prone, these mice are relatively resistant to renal injury.Citation32 Interestingly, treatment with ASCs effectively reduced the glomerular swelling observed in the kidneys of HFD-fed mice. This finding is similar to that in a recent study in which multiple adipose-derived MSCs attenuated the glomerular hypertrophy identified in HFD/streptozotocin rat model.Citation33
The chronic HFD feeding in C57BL/6 mice clearly induced cardiac hypertrophy as indicated by the heavier heart weights and especially the increased cardiomyocyte cross-sectional areas in the HFD groups. Increase in cardiomyocyte size is considered as a kind of gold standard in determining cardiac hypertrophy.Citation34 A similar study demonstrated that chronic HFD feeding for 11 months induced both myocardial hypertrophy and fibrosis in CD1 male mice.Citation35 However, our results did not show any cardiac fibrosis as illustrated by the Masson’s trichrome staining. This could be due to the fact that the feeding period in our study did not exceed 31 weeks (7.2 months), which might not be sufficient to induce the buildup of fibrosis suggesting that only a mild degree of cardiac hypertrophy occurred. Interestingly, when the HFD was replaced by chow diet after 15 weeks in the HFD+media group, this was not sufficient to ameliorate the cardiac alterations; however, treatment with ASCs was able to significantly mitigate the enlargement of cardiomyocytes, a hallmark of cardiac hypertrophy. In agreement with our findings, Daltro et al. (2017) have shown that treatment with mouse bone marrow-derived MSCs improved the histopathological cardiac abnormalities induced by obesity in mice fed with a HFD.Citation36
Moreover, this study showed that serum levels of pro-inflammatory cytokines, TNF-α and IL-6 were significantly higher in the HFD group compared to control groups. Similar to previous reports in the literature, obesity generates excess of fat that in turn secretes pro-inflammatory proteins into the circulation.Citation37,Citation38 Nevertheless, ASC infusion downregulated TNF-α and IL-6 levels, suggesting that the stem cell treatment was sufficient to suppress HFD-induced inflammation, and thereby contribute to the protective effect on the obesity-associated multiple organ injuries.
Conclusions
Our results suggest that adipose-derived mesenchymal stem cells exerted a therapeutic effect on adipose, cardiac, renal, and hepatic alterations in a HFD-induced obese animal model. Our findings provided strong evidence that these cells can significantly reverse epididymal adipose tissue expansion, inhibit fat accumulation in NAFLD and antagonize glomerular swelling and cardiomyocyte hypertrophy associated with obesity, at least in part, via ameliorating inflammation. However, despite the promising beneficial effects of adipose-derived mesenchymal stem cells in this experimental model, the question remains as to whether these therapeutic findings can be extrapolated to clinical reality in humans.
Author contributions
F.S. was involved in planning the work, the histopathological processing, staining, and imaging as well as Image J analysis. F.S. also drafted the manuscript and designed the figures. H.J. handled the animals and performed the ELISA experiments. A.E. was involved in preparing the IACUC forms, ordering C57BL/6 mice and contributed to the final version of the manuscript.
Acknowledgments
Open Access funding provided by the Qatar National Library.
The authors thank Mrs Nivine Masri and Mrs Lama Hanbali for help in microscopic imaging. The authors also extend their acknowledgments to Dr Khodr Issa for his assistance in animal dissection and Dr Charbel Khalil from Reviva Regenerative Medicine Center, Lebanon, for the kind gift of ASCs.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Obesity and overweight [Internet]. [accessed 2020 Nov 3]. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
- Blüher M. Obesity: global epidemiology and pathogenesis [Internet]. Nat Rev Endocrinol. Nature Publishing Group. 2019 [accessed 2020 Oct 30];15:1–11. doi:https://doi.org/10.1038/s41574-019-0176-8.
- Fan R, Wang J, Du J. Association between body mass index and fatty liver risk: a dose-response analysis. Sci Rep [Internet]]. 2018 Dec 1 [accessed 2020 Nov 3];8(1):1–7. www.nature.com/scientificreports/.
- Cercato C, Fonseca FA. Cardiovascular risk and obesity [Internet]. Diabetol Metab Syndr. BioMed Central Ltd. 2019 [accessed 2020 Nov 3];11:74. doi:https://doi.org/10.1186/s13098-019-0468-0.
- Ejerblad E, Fored CM, Lindblad P, Fryzek J, McLaughlin JK, Nyrén O. Obesity and risk for chronic renal failure. J Am Soc Nephrol [Internet]. 2006 Jun 1 [accessed 2020 Nov 3];17(6):1695–1702. www.jasn.org.
- Lega IC, Lipscombe LL. Review: diabetes, obesity, and cancer-pathophysiology and clinical implications [Internet]. . Endocr Rev; 2020 [accessed 2020 Nov 3];41(1); 33–52. https://academic.oup.com/edrv/article/41/1/33/5625127.
- Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y. Obesity & inflammation: the linking mechanism & the complications. Arch Med Sci [Internet]. 2017 [accessed 2020 Nov 3];13(4):851–863. /pmc/articles/PMC5507106/?report=abstract.
- Ye J. Emerging role of adipose tissue hypoxia in obesity and insulin resistance [Internet]. Int J Obes. NIH Public Access. 2009 [accessed 2020 Nov 3];33:54–66. doi:https://doi.org/10.1038/ijo.2008.229.
- Engin A The pathogenesis of obesity-associated adipose tissue inflammation. In: Advances in Experimental Medicine and Biology [Internet]. New York LLC: Springer; 2017 [accessed 2020 Nov 3]. 221–245. https://link.springer.com/chapter/10.1007/978-3-319-48382-5_9.
- Zhang C, Luo X, Zhang D, Deng B, Tong J, Zhang M, Chen L, Duan H, Niu W. Hypoxic adipocytes induce macrophages to release inflammatory cytokines that render skeletal muscle cells insulin resistant. Biochem Biophys Res Commun. 2020 Jan 15;521(3):625–631. doi:https://doi.org/10.1016/j.bbrc.2019.10.162.
- Marseglia L, Manti S, D’Angelo G, Nicotera A, Parisi E, Di Rosa G, Gitto E, Arrigo T. Oxidative stress in obesity: a critical component in human diseases [Internet]. Int J Molecular Sci MDPI AG. 2015 [accessed 2020 Nov 3];16:378–400. /pmc/articles/PMC4307252/?report=abstract.
- Matsushita K, Dzau VJ. Mesenchymal stem cells in obesity: insights for translational applications [Internet]. Laboratory Investigation. Nature Publishing Group. 2017 [accessed 2020 Oct 7];97:1158–1166. doi:https://doi.org/10.1038/labinvest.2017.42.
- Saleh F, Itani L, Calugi S, Grave RD, El Ghoch M. Adipose-derived mesenchymal stem cells in the treatment of obesity: a systematic review of longitudinal studies on preclinical evidence. Curr Stem Cell Res Ther [Internet]. 2018 May 15 [accessed 2018 Jun 8];13. http://www.ncbi.nlm.nih.gov/pubmed/29766824.
- Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells [Internet]. Blood. 2007 [accessed 2020 Nov 3];110:3499–3506. https://pubmed.ncbi.nlm.nih.gov/17664353/.
- Iyer SS, Rojas M. Anti-inflammatory effects of mesenchymal stem cells: novel concept for future therapies. Expert Opin Biol Ther. 2008;8:569–581. doi:https://doi.org/10.1517/14712598.8.5.569.
- Rifai L, Saleh FA. Conventional and alternative mesenchymal stem cell therapies for the treatment of diabetes. In: advances in experimental medicine and biology [Internet]. Adv Exp Med Biol. 2020; accessed 2021 Mar 18. https://pubmed.ncbi.nlm.nih.gov/33385177/.
- Gir P, Oni G, Brown SA, Mojallal A, Rohrich RJ. Human adipose stem cells: current clinical applications [Internet]. Plast Reconstr Surg. 2012 [accessed 2020 Nov 3];129:1277–1290. https://pubmed.ncbi.nlm.nih.gov/22634645/.
- Alió JL, Alió Del Barrio JL, El Zarif M, Azaar A, Makdissy N, Khalil C, Harb W, El Achkar I, Jawad ZA, De Miguel MP, et al. Regenerative surgery of the corneal stroma for advanced keratoconus: 1-year outcomes. Am J Ophthalmol [Internet]. 2019 Jul 1 [accessed 2021 Jul 1];203:53–68. https://europepmc.org/article/med/30772348.
- Lucía Gutiérrez M, Guevara J, Alejandro Barrera L. Semi-automatic grading system in histologic and immunohistochemistry analysis to evaluate in vitro chondrogenesis [Internet]. Universitas Scientiarum. 2012 [accessed 2020 Dec 3]; 17. www.javeriana.edu.co/universitas_scientiarum.
- Khaodhiar L, McCowen KC, Blackburn GL. Obesity and its comorbid conditions. Clin Cornerstone. 1999 Jan 1;2(3):17–31. doi:https://doi.org/10.1016/S1098-3597(99)90002-9.
- Rong Y, Chun-Yan N, Hong-Xin Z, Lu Y, Wen W, Yu T. Association of adolescent obesity with nonalcoholic fatty liver disease and related risk factors in Xi’an, China. Ann Hepatol [Internet]. 2018 Jan 1 [accessed 2020 Nov 3];17(1):85–91. http://www.elsevier.es/en-revista-annals-hepatology-16-articulo-association-adolescent-obesity-with-nonalcoholic-S1665268119301103.
- Carbone S, Canada JM, Billingsley HE, Siddiqui MS, Elagizi A, Lavie CJ. Obesity paradox in cardiovascular disease: where do we stand? [Internet]. Vasc Health Risk Manag. Dove Medical Press Ltd. 2019 [accessed 2020 Nov 3];15:89–100. doi:https://doi.org/10.2147/VHRM.S168946.
- Kovesdy CP, Furth SL, Zoccali C. Obesity and kidney disease: hidden consequences of the epidemic [Internet]. Can J Kidney Health Dis. SAGE Publications Ltd. 2017 [cited 2020 Nov 3]; 4. Available from: /pmc/articles/PMC5433675/?report=abstract
- Mauvais-Jarvis F, Arnold AP, Reue KA. Guide for the design of pre-clinical studies on sex differences in metabolism [Internet]. Cell Metab. Cell Press. 2017 [accessed 2020 Oct 7];25:1216–1230. doi:https://doi.org/10.1016/j.cmet.2017.04.033.
- Lutz TA, Woods SC. Overview of animal models of obesity. Curr Protoc Pharmacol [Internet]. 2012 Sep [accessed 2020 Oct 7];58(Chapter 5\(SUPPL.58)). https://pubmed.ncbi.nlm.nih.gov/22948848/
- Barrett P, Mercer JG, Morgan PJ. Preclinical models for obesity research [Internet]. DMM Dis Models Mechanisms. Company of Biologists Ltd. 2016 [accessed 2020 Oct 7];9:1245–1255. doi:https://doi.org/10.1242/dmm.026443.
- Jaber H, Issa K, Eid A, Saleh FA. The therapeutic effects of adipose-derived mesenchymal stem cells on obesity and its associated diseases in diet-induced obese mice. Sci Rep [Internet]. 2021;11(1):6291. https://doi.org/10.1038/s41598-021-85917-9.
- Pais R, Barritt AS, Calmus Y, Scatton O, Runge T, Lebray P, Poynard T, Ratziu V, Conti F. NAFLD and liver transplantation: current burden and expected challenges. J Hepatol. 2016;65(6):1245–1257. doi:https://doi.org/10.1016/j.jhep.2016.07.033.
- Mitra S, De A, Chowdhury A. Epidemiology of non-alcoholic and alcoholic fatty liver diseases. Trans Gastroenterol Hepatol. 2020;5:16. doi:https://doi.org/10.21037/tgh.2019.09.08.
- Wang H, Zhang H, Huang B, Miao G, Yan X, Gao G, Luo Y, Chen H, Chen W, Yang L. Mesenchymal stem cells reverse high-fat diet-induced non-alcoholic fatty liver disease through suppression of CD4+ T lymphocytes in mice. Mol Med Rep [Internet]. 2018 Mar 1 [accessed 2020 Nov 3];17(3).3769–3774. http://www.spandidos-publications.com/10.3892/mmr.2017.8326/abstract.
- Altunkaynak ME, Özbek E, Altunkaynak BZ, Can İ, Unal D, Unal B. The effects of high-fat diet on the renal structure and morphometric parametric of kidneys in rats. J Anat [Internet]]. 2008 Jun 1 [accessed 2021 Mar 18];212(6):845–852. doi:https://doi.org/10.1111/j.1469-7580.2008.00902.x.
- Wicks SE, Nguyen TT, Breaux C, Kruger C, Stadler K. Diet-induced obesity and kidney disease - In search of a susceptible mouse model. Biochimie [Internet]. 2016 May 1 [accessed 2020 Nov 3];124:65–73. https://pubmed.ncbi.nlm.nih.gov/26248309/.
- Yu S, Cheng Y, Zhang L, Yin Y, Xue J, Li B, Gong Z, Gao J, Mu Y. Treatment with adipose tissue-derived mesenchymal stem cells exerts anti-diabetic effects, improves long-term complications, and attenuates inflammation in type 2 diabetic rats. Stem Cell Res Ther [Internet]. 2019 Nov 20 [accessed 2020 Nov 4];10(1).1–18. https://link.springer.com/articles/10.1186/s13287-019-1474–8.
- Coelho-Filho OR, Shah RV, Mitchell R, Neilan TG, Moreno H, Simonson B, Kwong R, Rosenzweig A, Das S, Jerosch-Herold M, et al. Quantification of cardiomyocyte hypertrophy by cardiac magnetic resonance: implications for early cardiac remodeling. Circulation [Internet]. 2013 Sep 10 [accessed 2020 Nov 4];128(11):1225–1233. https://pubmed.ncbi.nlm.nih.gov/23912910/.
- Wang Z, Li L, Zhao H, Peng S, Zuo Z. Chronic high fat diet induces cardiac hypertrophy and fibrosis in mice. Metabolism [Internet]. 2015 [accessed 2020 Nov 4];64(8):917–925. https://pubmed.ncbi.nlm.nih.gov/25982698/.
- Daltro PS, Barreto BC, Silva PG, Neto PC, PHF SF, Santana Neta D, Carvalho GB, Silva DN, Paredes BD, de Alcantara AC et al. Therapy with mesenchymal stromal cells or conditioned medium reverse cardiac alterations in a high-fat diet–induced obesity model. Cytotherapy. 2017 Oct 1;19(10):1176–1188. doi:https://doi.org/10.1016/j.jcyt.2017.07.002.
- Kern L, Mittenbühler MJ, Vesting AJ, Ostermann AL, Wunderlich CM, Wunderlich FT. Obesity-induced TNFα and IL-6 signaling: the missing link between obesity and inflammation- driven liver and colorectal cancers [Internet]. Cancers. MDPI AG. 2019 [accessed 2020 Dec 4];11. /pmc/articles/PMC6356226/?report=abstract.
- Goyal R, Faizy AF, Siddiqui SS, Singhai M. Evaluation of TNF-α and IL-6 levels in obese and non-obese diabetics: pre- and postinsulin effects. N Am J Med Sci [Internet]. 2012 Apr 11 [accessed 2020 Dec 4];4(4):180–184. /pmc/articles/PMC3334258/?report=abstract.
