ABSTRACT
In spite of clinical advancements and improved diagnostic techniques, breast cancers are the leading cause of cancer-associated deaths in women worldwide. Although 70% of early breast cancers can be cured, there are no efficient therapies against metastatic breast cancers. Several factors including connexins and gap junctions play roles in breast tumorigenesis. Connexins are critical for cellular processes as a linkage between connexin mutations and hereditary disorders demonstrated their importance for tissue homeostasis. Further, alterations in their expression, localization and channel activities were observed in many cancers including breast cancer. Both channel-dependent and independent functions of connexins were reported in initiation and progression of cancers. Unlike initial reports suggesting tumor suppressor functions, connexins and gap junctions have stage, context and isoform dependent effects in breast cancers similar to other cancers. In this review, we tried to describe the current understanding of connexins in tumorigenesis specifically in breast cancers.
Introduction
Breast cancer is the most frequently observed and the second most lethal cancer type in women worldwide.Citation1 Although 70–80% of the breast cancer cases are treatable, current therapies are still insufficient for metastatic breast cancers, which remain as one of the most incurable diseases.Citation2 Breast cancer treatments can be either loco-regional therapy, where assigned drugs are directly administered to the tumor site or systemic therapy, where drugs spread through the whole body.Citation2 In addition, one of the crucial pieces of information required in the decision of therapeutic strategy is the determination of the breast cancer subtype, as breast cancers are categorized into different subgroups based on their molecular and histological differences due to intertumoral cellular heterogeneity.Citation2,Citation3 As seen in other cancer types such as liver and lung cancers, both geneticCitation4 and non-genetic factors play roles in breast cancer initiation and progression.Citation5,Citation6 Although the exact mechanisms in the initiation of breast cancers are not known, several genes are mutated, amplified or lost in addition to epigenetic alterations.
Both intrinsic and extrinsic factors have critical roles in breast cancer initiation and progression. Breast cancer grows into a complex microenvironment and crosstalk among cancer cells and between cancer cells and their microenvironment can alter cellular processes. Direct cell-to-cell contacts and communication in addition to signals delivered from the extracellular milieu greatly contribute to the management of cellular programs.Citation7,Citation8 Cell-to-cell interactions and communications can be mediated by cell adhesion proteins that form adherent junctions and tight junctions and by intercellular channels formed by gap junctions (GJ), respectively. Gap junction channels are composed of connexin subunits and facilitate the direct linkage between the cytoplasm of adjacent cells.Citation9 Significant proportion of cellular communication in tissues is mediated by gap junctions and their aberrant expressions and/or functions are associated with hereditary genetic diseases and cancers.Citation10 Alterations of gap junction channel activities, changes in localization and expression of connexins have been reported in various cancers including breast cancer,Citation11–13 and this review will mainly focus on the roles of connexins and their channels in cancers with specific emphasis on breast cancers.
Structure and function of connexins
Gap junctions permit the exchange of ions, secondary messengers and metabolites with a molecular size of 1200–1800 Da such as noncoding RNAs, ATP, cyclic nucleotides, glucose, small peptides and inositol triphosphate (IP3) between neighboring cells.Citation14,Citation15 Besides, GJIC (Gap Junctional Intercellular Communication) is critical in proliferation, apoptosis, differentiation, and maintenance of tissue homeostasis.Citation16 Connexins are the basic subunit of the gap junction channels,Citation17 and they have four α-helical transmembrane domains with an intracellular amino and a carboxyl terminals connected by two extracellular and one cytoplasmic loops.Citation16,Citation18 21 different connexin members with conserved sequence, organization and function have been reported in the human genome with molecular weights ranging from 26 kDa to 62 kDa.Citation19,Citation20 The amino-terminal and transmembrane domains have the highest sequence conservation among the family members while the cytoplasmic loop and the carboxyl-terminal tail are the most diverse domains among different subtypes in terms of their length and amino acid sequences.Citation21,Citation22 In addition, the C-terminal tail is one of the main determinants of the size and interacting partners of the connexins.Citation23,Citation24
Currently, two different connexin nomenclatures are in use.Citation25 The first and the most widely used one is based on Cx abbreviation for connexin together with their molecular mass in kDa, such as connexin with a molecular weight of 32 is known as Cx32.Citation26 Alternatively, connexins are named according to their subgroups (α, β, γ, δ, ε) which are determined based on their sequence similarity and cytoplasmic tail length.Citation27,Citation28 This nomenclature uses the abbreviation of gap junction (Gj) and their serial number determined based on their discovery order within the subgroups, for instance, Gjb1 is used for Cx32, which was the first discovered connexin in beta group.Citation28
Even though the basis of gap junctions was established in the 60s, cloning and sequencing of Cx43 from the heart tissue in 1987 laid the foundations of the characterization of connexins and gap junctionsCitation29 and the next 30 years have focused on the identification of other members and their biosynthetic pathways in addition to their roles in cellular processes and tissue homeostasis.Citation21 Briefly, gap junction channel biosynthesis starts with the oligomerization of six connexin subunits into connexons (hemichannels) at the ER-Golgi network. Then, the hemichannels are transported to the plasma membrane with vesicles where they can dock with other connexons from adjacent cells to complete the formation of gap junctions with a pore size of 8–16 Å.Citation17 Additionally, connexons can function as non-junctional hemichannels on the plasma membrane, leading to communication between the cytoplasm and the extracellular environment ().Citation30,Citation31
Figure 1. Connexins and gap junction organizations. Six connexins oligomerize to form connexons (hemichannels) that can be either homomeric or heteromeric if they are formed from one type or more than one type of connexins, respectively. After the transport of connexons to the plasma membrane, they can function as non-junctional hemichannels or align with another connexon to complete the formation of either homotypic gap junction channels composed of the same type of connexon or heterotypic gap junctions consisting of different hemichannels.
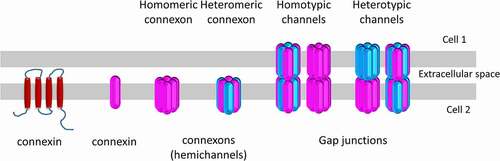
While connexin expression has been reported in almost all tissues, three main principles were proposed about their expression in cells: i) many cells can express more than one isoform, suggesting a compensatory mechanism among connexins;Citation32 ii) compensation among different connexin isoforms cannot always happen;Citation33 and iii) based on expression patterns, Cx43 is the most commonly expressed connexin isoform throughout the human body.Citation32 Since multiple connexin isoforms can be expressed in the same cell, different connexins can oligomerize to form heteromeric hemichannels besides homomeric ones that are formed from only one type of connexin (). For instance, Cx26/Cx32, Cx46/Cx50 and Cx26/Cx30 heteromeric connexons were reported to function in the liver, the lens, and the cochlea, respectively.Citation34–36 Similar organizational combinations can also be observed during the formation of gap junctions as homotypic channels are formed from the same type of connexons while heterotypic ones are formed from different hemichannels (). However, not all co-expressed connexin isoforms can engage into heteromeric or heterotypic oligomerizations. Even though the exact compatibility mechanisms at hemichannel or gap junction channel level are not known, the contributions of amino acid motifs in the cytosolic domains, transmembrane domains and/or extracellular loops were indicated during the oligomerization of connexins in the cytosol and docking of connexons on the plasma membrane which can also be regulated by different pathways in a cell-dependent manner.Citation37–39 The connexin types determine the permeability of channels to different molecules: For instance, homotypic Cx32 gap junctions had a higher permeability to IP3 than Cx43 or Cx26 ones and they also showed a higher permeability to adenosine while Cx43 channels had higher permeability to ATP.Citation40 Moreover, heteromeric combinations can also alter the selectivity of channels compared to homomeric arrangements; while homomeric Cx26 or Cx32 hemichannels were permeable to myoinositol and all inositol phosphates, Cx26/Cx32 heteromeric hemichannels had selective permeability to inositol phosphates.Citation41 Therefore, different subunit and connexon combinations can lead to diverse hemichannel and gap junction channel functions and contribute to the complexity of communication mediated by connexins. During development and adult life, connexin oligomerization can be altered based on the cells/tissues’ need. For example, Cx43 do not form heterotypic channels with Cx26, Cx30 or Cx32 in the mammary gland.Citation42 Meanwhile, Cx26/Cx32 heterotypic channels were observed in later stages of the pregnancy in a greater amount than the homomeric Cx26 channels.Citation43–45 These alterations in the oligomerization of the channels were correlated with the requirement of the transmission of different secondary messengers to maximize the electrical and molecular coupling needed for the milk secretion.Citation46
Gap junction activities are regulated by different factors including voltage, pH, divalent cations and chemical modificationsCitation47–50 and each channel has a different response to these factors: for instance, Cx32, Cx43, and Cx46 open actively at pH 7.2,Citation51,Citation52 whereas Cx57 closes at the same pH value.Citation53 In addition, gap junction channels are also sensitive to intracellular and extracellular Ca2+ changes, as upregulation of Ca2+ leads to the uncoupling of the hemichannels and gap junctions.Citation50,Citation54 Moreover, the functions, trafficking and stability of connexins and their channels were shown to be regulated by post-translational modifications such as phosphorylation, SUMOylation and S-nitrosylation especially at the C-terminal tail and the intercellular loop.Citation55
Phosphorylation of the proteins is one of the key modifications for their proper functioning and abnormalities in its states can interfere with cellular processes.Citation16,Citation55,Citation56 Cx43, which is the most widely distributed connexin isoform in the human body, is shown to have around 30 putative phosphorylation sites.Citation55 Phosphorylation modulates the metabolic and electrical coupling by altering the gap junction gating to regulate various cell processes and maintain tissue homeostasis.Citation57 Cx43 was shown to be dephosphorylated at Ser-325, -328, and -330 during heart ischemia, which led to the loss of Cx43 gap junctions at intercalated discs and its redistribution to the lateral regions of the myocardiocytes.Citation58 Furthermore, the restoration of Cx43-Ser-368 phosphorylation with a Cx43 C-terminal peptide mimetic was shown to improve cardiac function by inhibiting Cx43-ZO-1 interaction, which normally controls Cx43 membrane localization and gap junction plaque size.Citation59 Connexin phosphorylation can be mediated by several oncogenes and proto-oncogenes such as HRAS, c-Src or v-Src, implicating their involvement in tumorigenesis.Citation55 Moreover, phosphorylation of different sites on connexins has diverse effects on the channel activities where phosphorylation of Cx43 at Ser-279 and Ser-282 by kinases including MAPKs led to its clathrin-mediated endocytosis prior to gap junction plaque formation in pancreatic cancer cells.Citation60 In contrast, phosphorylation can have an opposite effect where for example, cAMP-mediated phosphorylation of Cx40 resulted in increased conductance between Cx40 transfected SkHep1-cells.Citation61 The importance of connexin phosphorylation on the GJIC was also demonstrated by the effect of EGFR tyrosine kinase inhibitor gefitinib on oxaliplatin-induced apoptosis where gefitinib increased Cx43-dependent GJIC as a result of reduced Src and PKC expression which normally phosphorylated Cx43 and decreased GJIC between I-10 testicular cancer cells.Citation62 In addition to gap junctions, post-translational modifications are also important for the hemichannel functions. John et al. demonstrated the reduction of Cx43 hemichannel activity upon phosphorylation by MAPKs in the presence of extracellular Ca2+ in the heart tissue. Further, Cx43 dephosphorylation by phosphatases resulted in the opening of hemichannels when the cells were subjected to metabolic inhibiton.Citation63 Similarly, phosphorylation of rodent lens Cx46 decreased hemichannel activity that was mediated by PKC activity in Xenopus oocytes.Citation64 In addition to phosphorylation, other post-translational modifications were also shown to interfere with channel activities. For example, Cx43-nitrosylation increased dye uptake by hemichannelsCitation65 and Cx43 SUMOylation increased Cx43 GJIC in liver cancer stem cells, sensitizing them to the chemotherapy.Citation66 Overall, GJ assembly and function are affected from different intrinsic and extrinsic factors such as kinases, pH, Ca2+. In addition, their interaction with other junctional complexes and cytoskeletal elements play role in their proper localization and functions.
Gap junctions and their association with other junctions and cytoskeletal proteins
Adherens and tight junction proteins which mainly take role in cell-cell adhesion and cellular polarity, respectively, are functionally associated with each other and gap junctional proteins.Citation67,Citation68 Interaction and/or association among junctional complexes can regulate each other’s localization and function under normal physiology or disease state.Citation67,Citation69
The relation between adhesion proteins and GJ assembly was initially demonstrated by an increase in Cx43 mediated GJIC upon the overexpression of adhesion protein E-cadherin in a Ca2+ dependent manner in P3/22 cells.Citation70 Then, a close association between Cx43, desmoplakin and cadherin proteins on the plasma membrane was demonstrated with electron microscopy in rat cardiomyocytes after myocardial infarction which also suggested their physical interaction.Citation71 The association between connexins and cadherins is cell- and isoform-specific and cadherins have differentially affected gap junction assembly. E-cadherin enhanced the assembly of Cx43 gap junction channels whereas N-cadherin led to the endocytosis of Cx43 gap junction plaques in rat liver epithelial, which suggested connexins as the downstream targets for E-cadherin and N-cadherin mediated signaling pathways.Citation72 In contrast, the membranous N-cadherin was required for Cx43 membrane localization in NIH3T3 cellsCitation73 and cellular motility reduced with concomitant decrease in N-cadherin expression upon Cx43 knockdown.Citation73 These observations pointed out the interplay between connexins and adherence junctions in a cell/tissue type-dependent manner.
Cadherins can influence the function, localization and expression of connexins in different cancer types. Cytoplasmic Cx26 expression was correlated with methylation in E-cadherin promoter in samples taken from endometrial cancer patients and down-regulating E-cadherin at post-transcriptional level using anti-sense oligonucleotides altered Cx26 localization from the plasma membrane to the cytoplasm.Citation74 Similarly, Cx43 was positively associated with E-cadherin expression in samples taken from endometrial cancer patients.Citation75 Even though E-cadherin based cell adhesion was not sufficient for the initiation of de novo GJ assembly in squamous carcinoma cells, E-cadherin differentially affected gap junction assembly in an isoform specific manner.Citation76 While Cx32 gap junction assembly was observed only in the presence of E-cadherin, Cx43 gap junction channel formation was not dependent on E-cadherin expression, suggesting the presence of diverse regulatory mechanisms in gap junction assembly.Citation76 Moreover, observation of co-localization of Cx43 GJ with ZO-1 and actin filaments suggested a role for cytoskeletal elements in the initiation and/or stabilization of gap junction assembly.Citation76 Moreover, the reconstitution of Cx43 gap junctions upon Cx43 re-expression in glioma stem cells inhibited proliferation, self-renewal capacity and invasiveness but increased E-cadherin expression which then inhibited the Wnt/β-catenin pathway by recruiting β-catenin to the plasma membrane from the nucleus.Citation77 The association between connexins and adherence junctions at protein level was predominantly shown at the plasma membrane. However, connexins was recently shown to regulate cadherin gene expression as shown in amphibian neural crest cells where N-cadherin expression was directly regulated by Cx43 C-terminal isoform which forms a complex with Polymerase III.Citation78 This observation has brought novel considerations on connexin-cadherin associations that need to be explored further.
Desmosomes also regulate the gap junction assembly and function. A reduced dye transfer between cells and the relocalization of connexins from the plasma membrane to the cytoplasm was observed in cardiac cells upon downregulation of PKP2, plakophilin 2 desmosomal protein.Citation79 Further studies confirmed the mislocalized and decreased Cx43 with desmoplakin loss in the heart.Citation80,Citation81 Moreover, desmoplakin loss resulted in Cx43 phosphorylation by Ras-MAPK pathway which eventually led to the degradation of Cx43 in cardiac cells. In addition, desmoplakin loss caused increased ubiquitination in Cx43 that consequently caused Cx43 degradation in lysosomes.Citation82
Tight junctions are another important intercellular junction complexes and ZO protein family is one of the crucial tight junction component contributing to the gap junction assembly. Gourdie et al. demonstrated an increase in gap junction aggregates in the plasma membrane compared to undocked connexons when the Cx43/ZO-1 interaction was inhibited.Citation83 Moreover, heterocellular interaction between mouse mammary epithelial cell line SCp2 and mouse mammary myoepithelial SCg6 cells resulted in the sequestration of α-catenin, β-catenin and ZO-2 into Cx30, Cx32, and Cx43 gap junction complexes. This heterocellular interaction also regulated the differentiation of mammary epithelial cells in a gap junction-dependent manner.Citation84 A similar association between catenin/ZO-2 and connexins was observed in breast cancer cells. Cx43 overexpression caused reduced nuclear β-catenin level in 2D and 3D cultured human ER(+) breast cancer cell line MCF-7 and in 3D cultured triple-negative breast cancer cell line MDA-MB-231. Increased Cx43 GJs then decreased proliferation and extravasation capability in 3D cultured MCF-7 and MDA-MB-231 cells.Citation85 In addition to ZO family, connexins are also associated with claudins in tight junctions where IL-1B treatment caused loss of Cx43 with a concomitant increase in claudin-1 expression in astrocytes that have high Cx43 expression but no tight junctions, suggesting dynamic changes among junctional complexes under insult.Citation86 Other connexins also interact and/or associate with tight junction components. For example, Cx32 co-localization with ZO-1, ZO-2 and claudin-1 proteins was reported in rat hepatocytes and a positive correlation between expression of connexins and tight junction proteins might implicate the contribution of Cx32 to cellular polarity.Citation87
In mammary gland, the adherent/tight junctions and connexin interaction during puberty, pregnancy, lactation, and involution suggested Cx isoform- and stage-specific association and regulation among junctional complexes. Cx43 interacted with β-catenin in all stages however, its interaction with E-cadherin was dynamic and was observed especially in lactation and post-weaning stages. While Cx43 did not interact with P-cadherin during mammary gland development, it was associated with claudin-3 in late pregnancy and lactation stages. In addition, Cx32 interacted with E-cadherin, β-catenin and Cx26 in lactation stage while no association between Cx30 and adherent or tight junction proteins was observed, demonstrating isoform-specific interaction/regulation between connexins and other junctional complexes in the mammary gland.Citation68
Junctional complexes extensively interact with cytoskeletal elements, regulate their organization in cells and association between cytoskeletal elements and gap junction assembly was also demonstrated by several groups. Cx43 delivery to the cell–cell contact area on the plasma membrane was mediated through interaction with microtubule plus end binding protein TIP EB1. Thus, silencing of TIP EB1 decreased the intercellular communication between Hela cells by interfering with Cx43 GJ assembly.Citation88 Interaction between C-tail of Cx43 and α/β-tubulin also suggested the contribution of Cx43 on microtubule stability.Citation89 In addition to tubulin, the relation between Cx43 and actin filaments was also shown by reduced Cx43-mediated GJIC upon an actin-binding protein drebrin downregulation.Citation90 Cx43 increased the migration of glioma cells by either C-tail dependent or independent mechanisms and actin cytoskeleton reorganization induced by Cx43 carboxyl tail led to the lamellipodia-like migration.Citation91 Moreover, in MCF-7 breast cancer cells, cofilin-coated actin filaments-based protrusions mediated their adherence to the osteogenic cells by Cx43-dependent gap junctions and cadherins. In addition, when Cx43 GJ was inhibited using a Cx43-specific inhibitory peptide or cofilin, migration by tethering was reduced in breast cancer cells,Citation92 demonstrating the interplay between gap junctions and cytoskeletal filaments.
Connexins in cancer
GJIC and connexins are indispensable for tissue homeostasis and human physiology as demonstrated both by the association of connexin mutations with several hereditary diseases and by the link between cancer and abnormal channel activities and/or connexin expression.Citation21 So far, at least nine human hereditary disorders resulting from connexin gene mutations have been reported.Citation32,Citation93 The most common hereditary disease caused by connexin mutations is nonsyndromic sensorineural hearing loss due to mostly Cx26 mutations in addition to Cx30 and Cx31 mutations.Citation94–96 Another tissue affected by the connexin mutations is the lens, where Cx46 and Cx50 mutations resulted in congenital cataracts.Citation97,Citation98 Similarly, Cx32 mutations were reported in X-linked dominant form of Charcot–Marie–Tooth diseaseCitation99 and Cx26 mutations were further linked to syndromic hearing loss with epidermal phenotypes including keratitis–ichthyosis–deafness syndrome.Citation15,Citation100,Citation101 In addition to hereditary diseases, there is a growing evidence demonstrating the involvement of connexins and their channels in many cancers.
Cancer is the second leading cause of death in the world and people have been suffering from the lack of efficient therapies. Ample amounts of evidence suggested the multistep processes comprised of series of genetic alterations behind tumorigenesis. However, as Hanahan and Weinberg suggested cancer is more than a tumor mass and it is a highly complex tissue composed of many different cell types.Citation102 In addition, tumor cells recruit normal cells and lead to the formation of tumor-associated stromal cells, which have also active roles in the development of the tumor.Citation102,Citation103 Thus, it is not surprising that the interaction among cells and between cells and their microenvironment greatly contribute to tumorigenesis. As one of the mediators of cell-to-cell and cell-to-extracellular environment communication, the involvement of gap junctions and connexins was demonstrated in cancer initiation and progression. Specifically, the association of connexins with cancer was initially shown in the 1960s, accelerating the studies to decipher their functions in tumorigenesis.Citation104,Citation105 Initial studies provided evidence for the involvement of GJIC in cancer while later studies demonstrated GJIC-independent functions of connexins in tumorigenesis.
Channel-dependent roles of connexins in cancer
The concept that GJIC modulates tumor growth was suggested in 1966 by detecting the lack of electric coupling between cells from rat liver tumor, implicating the tumor suppressor effect of gap junctions for the first time.Citation14,Citation106 Then, following studies supported the tumor suppressor functions of gap junctions with additional evidences including the downregulation of connexin expression in primary cells derived from different tumors;Citation107,Citation108 decreased tumor growth upon ectopic expression of connexins and higher susceptibility of connexin knockout mice to tumor formation compared to their controls.Citation109–112
Consistent with the initial observations, connexin expression is reduced,Citation13,Citation113 proteins are mis-localized or lostCitation16 in early stages of many tumors. In primary melanoma tumors, the loss of Cx43 gap junction activity was reported within the epithelium and most Cx43 was observed in the cytoplasm.Citation114 In addition, loss of Cx43 dependent GJIC was reported in primary gastric and lung cancers with re-localization of Cx43 to the cytoplasm.Citation115,Citation116 Similar to Cx43, Cx26 expression was predominantly reported in the cytoplasm of primary breast and colorectal cancer cells, supporting the loss of GJIC in these cancers as well.Citation12,Citation117 Furthermore, decreased Cx32mediated GJIC was reported for primary hepatocellular carcinoma with mis-localization of Cx32 to the cytoplasm.Citation118 In agreement with these observations, their ectopic re-expression resulted in the restoration of growth control and decreased the migration potential of cells.Citation112 For instance, Cx32 overexpression decreased growth rate in SKHepI highly aggressive hepatocellular cancer cellsCitation119 and Cx26 overexpression reduced the proliferation of MCF-7 breast cancer cells in addition to their invasion and migration capacity.Citation120 The suppressive effect of GJIC on migration and invasion of HeLa cells upon the re-introduction of Cx32 was demonstrated to be due to increased intercellular adhesion, suggesting both channel and adhesive activity of connexins in cancer.Citation121 GJIC can also play a role in tumorigenesis by regulating other cellular processes including apoptosis.Citation122 When HeLa cells were transfected with Cx43, Cx40 or Cx37 isoforms, they individually induced cell death with the highest apoptosis rate being induced in Cx43-expressing cells and the lowest one being observed in Cx37-containing cells. Further, the induction of apoptosis by Cx43 and Cx40 gap junction channels was shown to be mediated by the transmission of potential death mediators such as Ca2+, cAMP, and IP3 between cells.Citation122 Moreover, promotion of apoptosis by Cx43 GJIC was reported in BC31 rat bladder carcinoma cell line, where Cx43-coupled GJIC promoted the transmission of cell death signals (most likely Ca2+ ions) to the adjacent healthy cells,Citation123 indicating diverse functions of connexins in various cancers.
While the initial studies suggested the anti-tumorigenic property of gap junctions, later studies indicated that the effects of connexins and/or gap junctions in cancer were not so straightforward, and their functions were isoform-, cell type- and cancer-stage dependent. For instance, while increased GJIC was reported as a cause of a decrease in migration potential of different cancer cells, increased GJIC due to Cx26 expression elevated the migration potential in HeLa cells by reducing the cellular adhesion and altering the migration pattern from sheet migration seen in wild-type (WT) cells to single cell or cell cluster migration.Citation124 In addition to their isoform- and cell type-specific functions, connexins/gap junctions can also demonstrate cancer stage dependent effects. Hong and colleagues reported the pro-tumorigenic roles of Cx43 gap junctions in U87MG glioma cells where Cx43-dependent cellular communication between glioma cells and astrocytes increased the invasion of glioma cells via transmission of miRNAs (miR-5096) through gap junction channels.Citation125 Furthermore, Cx43 localized to caveolin-1 containing lipid rafts modulated the invasion capacity of U251 glioblastoma cells where heterocellular gap junctional communication between astrocytes and glioblastoma cells enhanced the invasion characteristics of U251 cells.Citation126
Several studies demonstrated the high expression of connexins in metastatic sites, which might suggest their roles in the adaptation and survival of the tumor cells.Citation127,Citation128 Increased levels of Cx26 and Cx43 were observed in breast cancer metastasis to lymph nodes and a correlation between high Cx26 expression and poor prognosis was reported in several tumor types including renal, pancreatic, glioma and lung.Citation14 Moreover, the restoration of Cx26 GJIC was demonstrated to increase the intravasation and extravasation of both Cx26-transfected F10 melanoma cells and Cx26-high BL6 metastatic melanoma cells by enhancing their interaction with the endothelial cells.Citation128 In contrast, ectopic expression of Cx26 forming proper gap junctions decreased the invasiveness and growth of MCF-7 breast cancer cells, supporting the tumor- or tissue-specific effects of Cx26 in cancer.Citation120
In addition to GJIC-dependent roles, connexins also regulate cancer progression in hemichannel form by releasing autocrine and/or paracrine signals into the extracellular environment.Citation14 Zhou et al. showed the inhibitory effects of Cx43 hemichannels on the bone metastasis of breast cancer cells as a result of ATP release from hemichannels, which was previously shown to suppress the metastasis of the breast cancers through activation of purinergic receptors.Citation129 In addition, increased chemotherapy sensitivity was observed in glioma cells upon Cx43 C-terminal peptide mimetic, αCT1, application, which was suggested to inhibit the Cx43 hemichannels activity,Citation130 suggesting tumor-specific effects of hemichannels similar to gap junction channels.
Channel-independent roles of connexins in cancer
Investigations based on the blocking of gap junctions and/or hemichannels or connexin mutants, leading to non-functional channels, implicated the gap junction channel independent functions of connexins in cancers.Citation131 Connexins can function without forming channels in the cytosol and act as signaling hubs, regulating the activities of other molecules. Cx43, which has one of the longest C-terminal tail among the family members is exposed to several post-translational modifications and can interact with several signaling mediators such as Bax in pancreatic cancer,Citation132 β-catenin in breast cancer,Citation133 hDlg in cervical cancer and regulate their intracellular localization.Citation134 Through these interactions, connexins interfere with cellular processes such as apoptosis and proliferation. For instance, cytoplasmic Cx43 expression was correlated with a significant and cytosolic E-cadherin expression in LH7 lung cancer cells, which was consequently suggested to reduce cell proliferation.Citation135 Similarly, Cx43 induction in Jeg3 malignant trophoblast cells decreased cellular growth through its interaction with NOV protein where co-localization and interaction between Cx43 C-terminal tail and NOV was promoted due to the re-localization of NOV from the nucleus to the plasma membrane.Citation136 Moreover, the effect of connexins on cell proliferation was demonstrated by an increase in p27 protein level upon Cx43 overexpression that then decreased U2OS osteosarcoma cell proliferation by inhibiting the transition from G1 to S phase.Citation137 Similarly, the interaction between Cx32 and tumor suppressor Dlgh1 was associated with altered cell cycle phases and cellular proliferation in hepatocytes where Dlgh1 was co-localized with Cx32 in the plasma membrane and altered the cellular growth by arresting the cells in G0/G1 phase. Further, when Cx32 was downregulated, Dlgh1 localization shifted from the plasma membrane to the nucleus, leading to elevated proliferation of SKHep human hepatoma cells.Citation138 In addition to proliferation, connexins and their interacting partners regulate other processes such as migration and apoptosis. Yang et al. showed a decrease in Cx32 mediated GJIC in hepatocellular carcinoma and a positive correlation between decreased Cx32 expression and decreased E-cadherin expression accompanied by an increase in snail level. As a result, the authors suggested that Cx32 mediated the migration and invasion of HCCs through a snail dependent pathway.Citation139 For apoptosis, co-localization of cytoplasmic Cx26 and Cx43 with pro-apoptotic Bak but not with anti-apoptotic Bcl-2 was reported in breast cancer cells which may propose the participation of connexins in pro-apoptotic signaling pathways,Citation140 demonstrating the diverse functions of connexins and their interacting partners in different cellular mechanisms.
Apart from their direct or indirect interactions with signaling molecules on the plasma membrane or in the cytosol, connexins can also regulate the gene expression. For example, Cx43 carboxyl tail was shown to translocate to the nucleus by interacting with the basic transcription factor-3 and form a complex with Pol III, regulating N-cadherin expression in neural crest cells of Xenopus laevis and in HeLa cells.Citation78 Gene regulations by connexins were also demonstrated for Cx26, which mediated the self-renewability of MDA-MB-231 triple-negative breast cancer cells by forming a complex with NANOG stem cell transcription factor and FAK in the cytoplasm close to the nucleus,Citation141 further supporting the context/cell type-, cancer stage- and isoform-dependent roles of connexins in cancer.
Connexins in mammary gland development
The human mammary gland is a complex organ composed of different tissue types including glandular, fatty and fibrous tissues.Citation142 The mature mammary gland is composed of numerous alveoli developed into lobules, consisting of milk-producing glands. Ducts facilitate milk transportation through the nipples from the lobules and terminal duct lobular units are the places where the breast cancer arises.Citation2,Citation44 While at birth mammary gland is only composed of improperly developed ducts; it undergoes a wide range of remodeling during pregnancy and involution (post-pregnancy). In puberty, improperly developed, rudimentary ducts start to fill the mammary fat tissue by elongating and branching. During pregnancy, ducts branching extends and alveoli develop to eventually produce milk. After weaning, involution occurs and glands are remodeled back to the structure before pregnancy by widespread apoptosis.Citation44 The cyclic changes are repeated in each pregnancy and involution, and are managed by several signaling pathways controlled by the hormones and local growth factors. Mammary gland development and remodeling require precise intercellular communication where the gap junctions have major roles.Citation44,Citation46 Moreover, connexin expression, localization and function are strictly regulated from birth to post-pregnancy to accompany the dynamic features behind the mammary gland differentiation and remodeling. Studies on the rodent mammary glands showed the expression and function of connexins during homeostasis, pregnancy, lactation and involutionCitation44 and while Cx26 and Cx43 were the major connexin isoforms in the mammary gland, the presence of Cx32 and Cx30 was also reported.Citation44,Citation143
The mammary gland is composed of two main cell types: basal and luminal. The luminal epithelial cells form the ducts and alveoli while the basal epithelia composed of myoepithelial cells create the outer layer of glands.Citation142 Cx26 and Cx32 were observed at basolateral regions of the luminal cells of rodent mammary glands and they had the highest expression during lactation followed by a decline at involution. Cx26 was shown to play roles in the proliferation of luminal cells and together with increased Cx32 expression, they were important in milk production.Citation44,Citation144 Further, Cx26/Cx32 heteromeric channels were replaced by homomeric Cx32 channels during the lactating stage, depending on cellular needs. Since homomeric Cx32 channels have wider pores compared to heteromeric Cx26/Cx32 channels, they would allow the transmission of bigger signaling molecules such as cGMP. On the other hand, Cx26 hemichannels are impermeable to cGMP but they can transmit cAMP. The switch between different channel types can accommodate the cells’ need as stoichiometry/balance between cAMP and cGMP is important during mammary gland remodeling from pregnancy to the lactation in addition to milk production.Citation44,Citation46,Citation145 Similar to Cx26 and Cx32, co-localization of Cx30 with Cx26 was also observed in the mammary gland. Cx30 expression increased in late pregnancy and early lactating stage and declined in involution.Citation42 Lastly, Cx43 was found in myoepithelial cells, and its expression was downregulated in mid-pregnancy and almost lost in the lactating stage. Then, it started to re-express in early involution.Citation146 The role of Cx43 in milk ejection was demonstrated by impaired milk ejection in Cx43 knock-in transgenic mice in which one Cx43 allele was replaced with Cx32. In conclusion, connexins are important in mammary gland development and their expression is tightly regulated during remodeling processes in adults as well. While Cx26 and Cx43 were reported to be the main isoforms in the human mammary gland, recent studies demonstrated the expression of Cx32 and Cx30 as well.Citation147 Most studies about the roles of connexins in humans are based on findings in rodents but the involvement of connexins in breast cancers has also suggested their importance for human mammary gland homeostasis.Citation148
Connexins in primary breast cancers
Up to date, even though ample evidence suggested the loss of GJIC in primary breast cancers, contradicting observations were also reported.Citation44,Citation144 In 1991, Laird and colleagues observed that regardless of the histological type, Cx43 protein was undetectable in primary tumor samples taken from 32 breast cancer patients who had not taken any chemotherapy or radiotherapy and no correlation between the estrogen-progesterone level and Cx43 expression was observed.Citation11 Similarly, Cx43 was primarily observed in the cytoplasm without any membranous Cx43 expression.Citation149,Citation150 The weak expression of Cx26 and Cx43 mRNA was also shown in primary breast cancer cell lines, 21MT2, MCF-7, BT549, and SKBR3.Citation151 Furthermore, Cx43 and Cx26 were mostly observed to localize to the cytoplasm rather than the plasma membrane of the breast cancers at the primary tumor sites.Citation12 Another connexin isoform reported in early breast tumors was Cx46, even though its expression has not been shown in normal breast tissues.Citation49 The expression of Cx46 was also demonstrated in MCF-7 cells, which had increased susceptibility to death under hypoxic conditions when Cx46 was downregulated. Later on, the involvement of Cx32 in breast cancer was demonstrated where Cx32 was mostly detected in the cytoplasm of cells in primary tumors,Citation152 before its expression was shown in normal breast tissues,Citation147 suggesting the loss of GJIC in primary tumors due to low connexin expression and/or cytosolic localization.
The correlation between connexin expression in primary breast cancers and patient’s prognosis was also suggested to be isoform- and cancer stage-specific. Chasampalioti et al. showed that high Cx43 levels in the membrane and the cytoplasm led to better patient survival but low levels of Cx43 were associated with high proliferation and poor patient outcome.Citation150 Similarly, Cx43 protein level was correlated with the improved breast cancer outcome in samples taken from 2000 patients and Cx43 was suggested to be an independent prognostic marker.Citation147 In contrast to Cx43, Cx30 protein level was correlated with poorer prognosis whereas Cx26 and Cx32 showed inconsistent associations with prognosis. Cx26 mRNA expression was associated with poorer prognosis in luminal B patients while it was correlated with increased relapse-free survival in ER(+) breast cancer patients. Interestingly, Cx32 mRNA level was linked to increased relapse-free survival while Cx32 protein level was correlated with poorer patient outcome independent from the histological type. As a result, the tumor stage and/or subtype was proposed to be taken into consideration during the determination of the relevance of connexins with prognosis.Citation147
Connexins in breast cancer metastasis
As presented above gap junctional intercellular communication was mostly reduced in primary tumor sites due to either decreased connexin expression or mis-localization of connexins to the cytoplasm. Nicolson and colleagues revealed the first in vitro and in vivo study of the relation between intercellular communication and metastatic malignancy in 1987, where high metastatic potential was negatively correlated with GJIC in mammary adenocarcinoma.Citation153 In the same year, another study examined the intercellular communication between fibroblast and metastatic clones of breast cancer taken from the lungs and a negative correlation between GJIC and metastatic potential of breast cancer in rats was observed.Citation154 Authors suggested that intercellular communication between normal cells and tumor cells would inhibit the metastasis of the breast cancer cells to the lungs.
While mostly cytoplasmic Cx26 and Cx43 with low amount of membranous localization was observed in primary breast cancer tumor samples, membranous Cx26 and Cx43 level was increased in metastatic lymph node.Citation12 Furthermore, more than 50% of Cx26 and Cx43 negative primary tumors had Cx26 and Cx43 expression in lymph node metastasis, suggesting their involvement in metastasis processes.Citation12 Similarly, a positive correlation was also observed between Cx32 overexpression and lymph node metastasis. Even though Cx32 expression was detected in primary tumor cells, no Cx32 expression was shown in non-tumorigenic mammary tissue.Citation152 These studies were breakthroughs showing the dynamic regulations of connexin expression at different stages of the breast cancer. The increased connexin expression was shown in other metastatic tissues, where Stoletov and colleagues showed that Cx43 expression was significantly increased in metastatic brain lesions of breast cancers compared to the healthy brain tissue. Here, Cx43 expression was restricted to a small group of breast tumor population and was observed mostly in tumor cells which were in contact with the blood vessels.Citation155 Silencing of Cx43 decreased the number of extravasated cells and thus reduced the colonization of breast cancer cells to the brain, which was regulated by transcription factor twist as shown by the re-localization of Cx43 from the plasma membrane to the perinuclear region in the absence of twist.Citation155 Similar to Stoletovs’ Cx43 study, Elzarrad and colleagues demonstrated high Cx43 expression in the contact areas between 4T1 breast cancer cell lines and pulmonary microvascular endothelial cells (PMVEC) that was shown to increase the intravasation of Cx43 overexpressing 4T1 cells to the lung sections. Additionally, impaired Cx43 GJ formation decreased the number of intravasated breast cancer cells and furthermore high expression of Cx43 in intratumor vessels suggested the importance of Cx43 in vasculature formation in tumors.Citation156 On the other hand, protective roles of connexins in metastasis of breast cancers was also demonstrated. Defects in Cx43 expression and/or trafficking revealed the association between Cx43 and increased incidence of lung metastasis and mammary gland hyperplasia, which was explained by the escape of the tumor cells through the disrupted myoepithelial cell barrier provided by Cx43 gap junctions.Citation113
Different regulatory mechanisms for Cx43 expression during breast tumorigenesis were reported. Ming et al. showed a negative correlation between mir-200a and Cx43 expression in primary breast cancer tissues where they observed an increase in the migration of MCF-7 non-aggressive breast cancer cell line upon Cx43 overexpression which was attenuated by mir-200a treatment.Citation157 Likewise, migration potential of MDA-MB-231 triple-negative breast cancer cells was decreased by mir-200a treatment with a concomitant decrease in Cx43 level.Citation157 Cx43 expression was further shown to be reduced by mir-206 and mir-381 in MDA-MB-231 cells that consequently decreased migration potential.Citation158,Citation159
Epithelial to Mesenchymal Transition (EMT)
As Brabletz and colleagues suggested in cancer biology, EMT is a much-debated issue and is a highly dynamic process. It is also highly complicated where cancer cells require to immediately adapt to changes that require the alteration of the expression and localization of many molecules during the process.Citation160 One of the contributors of EMT is remodeling intercellular communication and gap junction proteins.
The involvement of connexins in EMT was reported in different cancer types. Cx26 overexpression promoted EMT as a result of decreased E-cadherin and increased vimentin and slug expression through PI3K/AKT pathway in a GJIC-independent manner which also increased chemoresistance of non-small lung cancer HCC-827 and PC9 cell lines.Citation161 Another connexin isoform indicated in EMT was Cx32 that is highly expressed in liver cells. Cx32 expression (mainly localized in the cytoplasm) was reported to have a strong correlation with E-cadherin and vimentin expression in HepG2 doxorubicin resistant cells where the transition of EMT to MET (mesenchymal to epithelial transition) with Cx32 overexpression was demonstrated with increased E-cadherin and decreased vimentin expression.Citation162 The same group also reported a strong positive correlation between Cx32 and E-cadherin expression but a negative correlation between snail expression and Cx32 expression in SMMC-7721 and HepG2 HCC cells.Citation139 They also suggested a negative correlation between Cx32 expression and migration, metastasis and invasion capacity of HCC cells.Citation139 The direct effect of connexins on EMT was shown by the regulation of N-cadherin expression by Cx43. Cx43 carboxyl tail was translocated to the nucleus by interacting with basic transcription factor-3 and enhanced N-cadherin expression during the migration of neural crest cells in Xenopus laevis.Citation78
Although the effects of connexins on EMT have been shown in different tissues and cancer types, there are not many studies in breast cancers. Cx32 was recently shown to have a context-dependent effect on EMT. Cx32 was positively correlated with E-cadherin expression in non-tumorigenic MCF10A cells with increased migration potential but it had a negative correlation with E-cadherin expression in MDA-MB-231 breast cancer cells without altering their migration capacity,Citation163 suggesting the context-dependent roles of connexins in EMT.
Conclusions
Connexins are essentials in human physiology as reported by the linkage of connexin gene mutations with human diseases. In the 1960s, the first evidence on the connexins in cancer progression was reported and their anti- and pro-tumorigenic roles have been documented both in vitro and in vivo. Developments in the field demonstrated that connexin isoforms, tumor types and tumor stages are important determinants for the function of connexins in tumorigenesis.
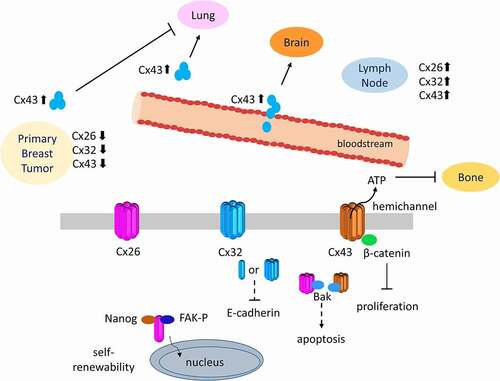
Connexin expression and localization is strictly controlled throughout the mammary gland development and their dynamic regulation is crucial in differentiation and remodeling during pregnancy and involution. Moreover, both anti- and pro-tumorigenic effects of connexins in breast cancers have been shown (). In primary breast cancer tissues, GJIC was lost due to either aberrant connexin localization or decreased connexin expression. On the other hand, increased GJIC or connexin expression was observed during the metastasis of breast cancer (). Cx26, Cx32 and Cx43 overexpression was observed in lymph node metastasis and Cx43 level increased in cells during metastasis to the lungs and the brain. Further, the roles of connexins become more complicated due to their non-channel functions in self-renewability, EMT and cellular proliferation through the interaction of connexins with different proteins and transcription factors. Cx43 interacting with β-catenin or Bak can modulate cell survival while Cx26 together with NANOG and FAK enhances self-renewability of breast cancer cells. Finally, Cx32 overexpression caused reduction in E-cadherin levels in breast cancer cells.
Figure 2. Diverse roles of connexins in the primary and metastatic breast cancers. Connexins and GJIC were downregulated in primary breast cancers while elevation of Cx26, Cx32 and Cx43 expression was observed at metastatic sites of breast cancers. These effects can be regulated either through gap junction/hemichannel activities by modulating the exchange of molecules or channel-independent roles of connexins by interacting with several molecules in the cytosol or on the plasma membrane.
In conclusion, connexins are multifaceted proteins and their expressions, localization, channels and non-channel activities are critical in breast cancers. There are still many questions about their regulation and interacting partners in breast tumorigenesis to understand their diverse roles at different stages of cancer for the development of effective prognostic and therapeutic tools.
Acknowledgments
Financial supports by The Scientific and Technological Research Council of Turkey (114Z874 and 119Z294 to GM) and The Young Investigator Award by the Turkish Academy of Sciences (GM) are highly appreciated. We would like to apologize to the researchers whose studies could not be included due to space constraints.
Disclosure statement
The authors declare no competing or financial interests.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):1–19. doi:https://doi.org/10.3322/caac.21492.
- Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P, Ruddy K, Tsang J, Cardoso F. Breast cancer. Nat Rev Dis Primers. 2019;5:66.
- Perou CMS, Elsen T, Rijn MB, Jeffreyl M, Rees SS, C. A. Pollack JR, Johnsen RDT, Akslen H, Fluge LA, Pergamenschikov Q, et al. Molecular portraits of human breast tumours. nature. 2000;406(6797):6. doi:https://doi.org/10.1038/35021093.
- Shiovitz S, Korde LA. Genetics of breast cancer: a topic in evolution. Annals of Oncology. 2015;26(7):1291–1299. doi:https://doi.org/10.1093/annonc/mdv022.
- Chen WY, Rosner B, Hankinson SE, Colditz GA, Willett WC. Moderate Alcohol Consumption During Adult Life, Drinking Patterns, and Breast Cancer Risk. Jama-J Am Med Assoc. 2011;306(17):1884–1890. doi:https://doi.org/10.1001/jama.2011.1590.
- Danaei G, Vander Hoorn S, Lopez AD, Murray CJL, Ezzati M, Collab CRA. Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet. 2005;366(9499):1784–1793. doi:https://doi.org/10.1016/S0140-6736(05)67725-2.
- Ruffell B, Au A, Rugo HS, Esserman LJ, Hwang ES, Coussens LM. Leukocyte composition of human breast cancer. P Natl Acad Sci USA. 2012;109(8):2796–2801. doi:https://doi.org/10.1073/pnas.1104303108.
- Soysal SD, Tzankov A, Muenst SE. Role of the Tumor Microenvironment in Breast Cancer. Pathobiology. 2015;82:142–152.
- Wei CJ, Xu X, Lo CW. Connexins and cell signaling in development and disease. Annu Rev Cell Dev Biol. 2004;20(1):811–838. doi:https://doi.org/10.1146/annurev.cellbio.19.111301.144309.
- Bonacquisti EE, Nguyen J. Connexin 43 (Cx43) in cancer: implications for therapeutic approaches via gap junctions. Cancer Letters. 2019;442:439–444. doi:https://doi.org/10.1016/j.canlet.2018.10.043.
- Laird DW, Fistouris P, Batist G, Alpert L, Huynh HT, Carystinos GD, Alaoui-Jamali MA. Deficiency of connexin43 gap junctions is an independent marker for breast tumors. Cancer Research. 1999;59:4104–4110.
- Kanczuga-Koda L, Sulkowski S, Lenczewski A, Koda M, Wincewicz A, Baltaziak M, Sulkowska M. Increased expression of connexins 26 and 43 in lymph node metastases of breast cancer. J Clin Pathol. 2006;59(4):429–433. doi:https://doi.org/10.1136/jcp.2005.029272.
- Wilgenbus KK, Kirkpatrick CJ, Knuechel R, Willecke K, Traub O. Expression of Cx26, Cx32 and Cx43 Gap Junction Proteins in Normal and Neoplastic Human Tissues. International Journal of Cancer. 1992;51(4):522–529. doi:https://doi.org/10.1002/ijc.2910510404.
- Aasen T, Leithe E, Graham SV, Kameritsch P, Mayan MD, Mesnil M, Pogoda K, Tabernero A. Connexins in cancer: bridging the gap to the clinic. Oncogene. 2019;38(23):4429–4451. doi:https://doi.org/10.1038/s41388-019-0741-6.
- Mese G, Richard G, White TW. Gap junctions: basic structure and function. J Invest Dermatol. 2007;127(11):2516–2524. doi:https://doi.org/10.1038/sj.jid.5700770.
- Aasen T, Mesnil M, Naus CC, Lampe PD, Laird DW. Gap junctions and cancer: communicating for 50 years (vol 16, pg 775, 2016). Nature Reviews Cancer. 2017;17(1):74. doi:https://doi.org/10.1038/nrc.2016.142.
- Sinha G, Ferrer AI, Moore CA, Naaldijk Y, Rameshwar P. Gap Junctions and Breast Cancer Dormancy. Trends in Cancer. 2020;6(4):348–357. doi:https://doi.org/10.1016/j.trecan.2020.01.013.
- Laird DW, Revel JP. Biochemical and Immunochemical Analysis of the Arrangement of Connexin43 in Rat-Heart Gap Junction Membranes. Journal of Cell Science. 1990;97(1):109–117. doi:https://doi.org/10.1242/jcs.97.1.109.
- Goodenough DA, Paul DL. Gap Junctions. Csh Perspect Biol. 2009;1:1.
- Buratto D, Donati V, Zonta F, Mammano F. Harnessing the therapeutic potential of antibodies targeting connexin hemichannels. Bba-Mol Basis Dis. 2021;1867(4):4. doi:https://doi.org/10.1016/j.bbadis.2020.166047.
- Laird DW, Lampe PD. Therapeutic strategies targeting connexins. Nat Rev Drug Discov. 2018;17:905–921.
- Solan JL, Lampe PD. Connexin43 phosphorylation: structural changes and biological effects. Biochemical Journal. 2009;419(2):261–272. doi:https://doi.org/10.1042/BJ20082319.
- Leithe E, Mesnil M, Aasen T. The connexin 43 C-terminus: a tail of many tales. Biochimica Et Biophysica Acta (Bba)-biomembranes. 2018;1860:48–64.
- Evans WH, De Vuyst E, Leybaert L. The gap junction cellular internet: connexin hemichannels enter the signalling limelight. Biochemical Journal. 2006;397(1):1–14. doi:https://doi.org/10.1042/BJ20060175.
- Sohl G, Willecke K. An update on connexin genes and their nomenclature in mouse and man. Cell Communication and Adhesion. 2003;10(4–6):173–180. doi:https://doi.org/10.1080/cac.10.4-6.173.180.
- Beyer EC, Paul DL, Goodenough DA. Connexin Family of Gap Junction Proteins. J Membrane Biol. 1990;116(3):187–194. doi:https://doi.org/10.1007/BF01868459.
- Kumar NM, Gilula NB. The gap junction communication channel. Cell. 1996;84(3):381–388. doi:https://doi.org/10.1016/S0092-8674(00)81282-9.
- Kumar NM. Molecular biology of the interactions between connexins. Novart Fdn Symp. 1999;219:6–21.
- Beyer EC, Paul DL, Goodenough DA. Connexin43 - a Protein from Rat-Heart Homologous to a Gap Junction Protein from Liver. Journal of Cell Biology. 1987;105(6):2621–2629. doi:https://doi.org/10.1083/jcb.105.6.2621.
- Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Bio. 2003;4(4):285–294. doi:https://doi.org/10.1038/nrm1072.
- Spray DC, Ye ZC, Ransom BR. “Functional connexin hemichannels”: a critical appraisal. Glia. 2006;54(7):758–773. doi:https://doi.org/10.1002/glia.20429.
- Laird DW. Life cycle of connexins in health and disease. Biochemical Journal. 2006;394:527–543.
- White TW. Nonredundant gap junction functions. News Physiol Sci. 2003;18:95–99.
- Sun JJ, Ahmad S, Chen SP, Tang WX, Zhang YP, Chen P, Lin X. Cochlear gap junctions coassembled from Cx26 and 30 show faster intercellular Ca2+ signaling than homomeric counterparts. Am J Physiol-Cell Ph. 2005;288(3):C613–C623. doi:https://doi.org/10.1152/ajpcell.00341.2004.
- Jiang JX, Goodenough DA. Heteromeric connexons in lens gap junction channels. P Natl Acad Sci USA. 1996;93(3):1287–1291. doi:https://doi.org/10.1073/pnas.93.3.1287.
- Hopperstad MG, Srinivas M, Spray DC. Properties of gap junction channels formed by Cx46 alone and in combination with Cx50. Biophysical Journal. 2000;79(4):1954–1966. doi:https://doi.org/10.1016/S0006-3495(00)76444-7.
- Koval M. Pathways and control of connexin oligomerization. Trends Cell Biol. 2006;16(3):159–166. doi:https://doi.org/10.1016/j.tcb.2006.01.006.
- Koval M, Molina SA, Burt JM. Mix and match: investigating heteromeric and heterotypic gap junction channels in model systems and native tissues. Febs Letters. 2014;588(8):1193–1204. doi:https://doi.org/10.1016/j.febslet.2014.02.025.
- Karademir LB, Aoyama H, Yue B, Chen HH, Bai DL. Engineered Cx26 variants established functional heterotypic Cx26/Cx43 and Cx26/Cx40 gap junction channels. Biochemical Journal. 2016;473(10):1391–1403. doi:https://doi.org/10.1042/BCJ20160200.
- Niessen H, Harz H, Bedner P, Kramer K, Willecke K. Selective permeability of different connexin channels to the second messenger inositol 1,4,5-trisphosphate. Journal of Cell Science. 2000;113(8):1365–1372. doi:https://doi.org/10.1242/jcs.113.8.1365.
- Ayad WA, Locke D, Koreen IV, Harris AL. Heteromeric, but not homomeric, connexin channels are selectively permeable to inositol phosphates. Journal of Biological Chemistry. 2006;281(24):16727–16739. doi:https://doi.org/10.1074/jbc.M600136200.
- Talhouk RS, Elble RC, Bassam R, Daher M, Sfeir A, Mosleh LA, El-Khoury H, Hamoui S, Pauli BU, El-Sabban ME. Developmental expression patterns and regulation of connexins in the mouse mammary gland: expression of connexin30 in lactogenesis. Cell Tissue Res. 2005;319(1):49–59. doi:https://doi.org/10.1007/s00441-004-0915-5.
- Locke D, Jamieson S, Stein T, Liu J, Hodgins MB, Harris AL, Gusterson B. Nature of Cx30-containing channels in the adult mouse mammary gland. Cell Tissue Res. 2007;328(1):97–107. doi:https://doi.org/10.1007/s00441-006-0301-6.
- McLachlan E, Shao Q, Laird DW. Connexins and gap junctions in mammary gland development and breast cancer progression. J Membr Biol. 2007;218(1–3):107–121. doi:https://doi.org/10.1007/s00232-007-9052-x.
- Beyer EC, Gemel J, Martinez A, Berthoud VM, Valiunas V, Moreno AP, Brink PR. Heteromeric mixing of connexins: compatibility of partners and functional consequences. Cell Commun Adhes. 2001;8(4–6):199–204. doi:https://doi.org/10.3109/15419060109080723.
- Locke D, Stein T, Davies C, Morris J, Harris AL, Evans WH, Monaghan P, Gusterson B. Altered permeability and modulatory character of connexin channels during mammary gland development. Experimental Cell Research. 2004;298(2):643–660. doi:https://doi.org/10.1016/j.yexcr.2004.05.003.
- Trexler EB, Bennett MVL, Bargiello TA, Verselis VK. Voltage gating and permeation in a gap junction hemichannel. P Natl Acad Sci USA. 1996;93(12):5836–5841. doi:https://doi.org/10.1073/pnas.93.12.5836.
- Oh S, Bargiello TA. Voltage Regulation of Connexin Channel Conductance. Yonsei Med J. 2015;56(1):1–15. doi:https://doi.org/10.3349/ymj.2015.56.1.1.
- Banerjee D. Connexin’s connection in breast cancer growth and progression. International Journal of Cell Biology. 2016;2016:9025905. doi: https://doi.org/10.1155/2016/9025905.
- Peracchia C. Chemical gating of gap junction channels Roles of calcium, pH and, calmodulin. Bba-Biomembranes. 2004;1662(1–2):61–80. doi:https://doi.org/10.1016/j.bbamem.2003.10.020.
- Liu SG, Taffet S, Stoner L, Delmar M, Vallano ML, Jalife J, Structural A. Basis for the Unequal Sensitivity of the Major Cardiac and Liver Gap-Junctions to Intracellular Acidification - the Carboxyl Tail Length. Biophysical Journal. 1993;64(5):1422–1433. doi:https://doi.org/10.1016/S0006-3495(93)81508-X.
- Eckert R. pH gating of lens fibre connexins. Pflug Arch Eur J Phy. 2002;443(5–6):843–851. doi:https://doi.org/10.1007/s00424-001-0760-2.
- Palacios-Prado N, Sonntag S, Skeberdis VA, Willecke K, Bukauskas FF. Gating, permselectivity and pH-dependent modulation of channels formed by connexin57, a major connexin of horizontal cells in the mouse retina. J Physiol-London. 2009;587(13):3251–3269. doi:https://doi.org/10.1113/jphysiol.2009.171496.
- Pfahnl A, Dahl G. Gating of cx46 gap junction hemichannels by calcium and voltage. Pflug Arch Eur J Phy. 1999;437(3):345–353. doi:https://doi.org/10.1007/s004240050788.
- Aasen T, Johnstone S, Vidal-Brime L, Lynn KS, Koval M. Connexins: synthesis, Post-Translational Modifications, and Trafficking in Health and Disease. Int J Mol Sci. 2018;19(5):5. doi:https://doi.org/10.3390/ijms19051296.
- Johnstone SR, Billaud M, Lohman AW, Taddeo EP, Isakson BE. Posttranslational Modifications in Connexins and Pannexins. J Membrane Biol. 2012;245(5–6):319–332. doi:https://doi.org/10.1007/s00232-012-9453-3.
- Pogoda K, Kameritsch P, Retamal MA, Vega JL. Regulation of gap junction channels and hemichannels by phosphorylation and redox changes: a revision. Bmc Cell Biol. 2016;17(1):137–150. doi:https://doi.org/10.1186/s12860-016-0099-3.
- Lampe PD, Cooper CD, King TJ, Burt JM. Analysis of Connexin43 phosphorylated at S325, S328 and S330 in normoxic and ischemic heart. Journal of Cell Science. 2006;119(16):3435–3442. doi:https://doi.org/10.1242/jcs.03089.
- O’Quinn MP, Palatinus JA, Harris BS, Hewett KW, Gourdie RG, Peptide A. Mimetic of the Connexin43 Carboxyl Terminus Reduces Gap Junction Remodeling and Induced Arrhythmia Following Ventricular Injury. Circ Res. 2011;108(6):704–715. doi:https://doi.org/10.1161/CIRCRESAHA.110.235747.
- Johnson KE, Mitra S, Katoch P, Kelsey LS, Johnson KR, Mehta PP. Phosphorylation on Ser-279 and Ser-282 of connexin43 regulates endocytosis and gap junction assembly in pancreatic cancer cells. Molecular Biology of the Cell. 2013;24(6):715–733. doi:https://doi.org/10.1091/mbc.e12-07-0537.
- Van Rijen HV, van Veen TA, Hermans MM, Jongsma HJ. Human connexin40 gap junction channels are modulated by cAMP. Cardiovascular Research. 2000;45(4):941–951. doi:https://doi.org/10.1016/S0008-6363(99)00373-9.
- Wu JF, Ji J, Dong SY, Li BB, Yu ML, Wu DD, Tao L, Tong XH. Gefitinib enhances oxaliplatin-induced apoptosis mediated by Src and PKC-modulated gap junction function. Oncology Reports. 2016;36(6):3251–3258. doi:https://doi.org/10.3892/or.2016.5156.
- John S, Cesario D, Weiss JN. Gap junctional hemichannels in the heart. Acta Physiol Scand. 2003;179(1):23–31. doi:https://doi.org/10.1046/j.1365-201X.2003.01197.x.
- Jedamzik B, Marten I, Ngezahayo A, Ernst A, Kolb HA. Regulation of lens rCx46-formed hemichannels by activation of protein kinase C, external Ca2+ and protons. J Membrane Biol. 2000;173(1):39–46. doi:https://doi.org/10.1007/s002320001005.
- Retamal MA, Cortes CJ, Reuss L, Bennett MVL, Saez JC. S-nitrosylation and permeation through connexin 43 hemichannels in astrocytes: induction by oxidant stress and reversal by reducing agents. P Natl Acad Sci USA. 2006;103(12):4475–4480. doi:https://doi.org/10.1073/pnas.0511118103.
- Shen YM, Li YX, Ma XF, Wan QH, Jiang ZM, Liu YX, Zhang DY, Liu XZ, Wu WH. Connexin 43 SUMOylation improves gap junction functions between liver cancer stem cells and enhances their sensitivity to HSVtk/GCV. International Journal of Oncology. 2018;52:872–880.
- Campbell HK, Maiers JL, DeMali KA. Interplay between tight junctions & adherens junctions. Experimental Cell Research. 2017;358(1):39–44. doi:https://doi.org/10.1016/j.yexcr.2017.03.061.
- Dianati E, Poiraud J, Weber-Ouellette A, Plante I. Connexins, E-cadherin, Claudin-7 and beta-catenin transiently form junctional nexuses during the post-natal mammary gland development. Dev Biol. 2016;416(1):52–68. doi:https://doi.org/10.1016/j.ydbio.2016.06.011.
- Angst BD, Khan LUR, Severs NJ, Whitely K, Rothery S, Thompson RP, Magee AI, Gourdie RG. Dissociated spatial patterning of gap junctions and cell adhesion junctions during postnatal differentiation of ventricular myocardium. Circ Res. 1997;80(1):88–94. doi:https://doi.org/10.1161/01.RES.80.1.88.
- Jongen WMF, Fitzgerald DJ, Asamoto M, Piccoli C, Slaga TJ, Gros D, Takeichi M, Yamasaki H. Regulation of Connexin 43-Mediated Gap Junctional Intercellular Communication by Ca2+ in Mouse Epidermal-Cells Is Controlled by E-Cadherin. Journal of Cell Biology. 1991;114(3):545–555. doi:https://doi.org/10.1083/jcb.114.3.545.
- Matsushita T, Oyamada M, Fujimoto K, Yasuda Y, Masuda S, Wada Y, Oka T, Takamatsu T. Remodeling of cell-cell and cell-extracellular matrix interactions at the border zone of rat myocardial infarcts. Circ Res. 1999;85(11):1046–1055. doi:https://doi.org/10.1161/01.RES.85.11.1046.
- Govindarajan R, Chakraborty S, Johnson KE, Falk MM, Wheelock MJ, Johnson KR, Mehta PP. Assembly of connexin43 into gap junctions is regulated differentially by E-cadherin and N-cadherin in rat liver epithelial cells. Mol Biol Cell. 2010;21:4089–4107.
- Wei CJ, Francis R, Xu X, Lo CW. Connexin43 associated with an N-cadherin-containing multiprotein complex is required for gap junction formation in NIH3T3 cells. Journal of Biological Chemistry. 2005;280(20):19925–19936. doi:https://doi.org/10.1074/jbc.M412921200.
- Nishimura M, Saito T, Yamasaki H, Kudo R. Suppression of gap junctional intercellular communication via 5 ‘ CpG island methylation in promoter region of E-cadherin gene in endometrial cancer cells. Carcinogenesis. 2003;24(10):1615–1623. doi:https://doi.org/10.1093/carcin/bgg121.
- Wincewicz A, Baltaziak M, Kanczuga-Koda L, Lesniewicz T, Rutkowski R, Sobaniec-Lotowska M, Sulkowski S, Koda M, Sulkowska M. Aberrant Distributions and Relationships Among E-cadherin, beta-catenin, and Connexin 26 and 43 in Endometrioid Adenocarcinomas. Int J Gynecol Pathol. 2010;29(4):358–365. doi:https://doi.org/10.1097/PGP.0b013e3181c3c57f.
- Chakraborty S, Mitra S, Falk MM, Caplan SH, Wheelock MJ, Johnson KR, Mehta PP. E-cadherin Differentially Regulates the Assembly of Connexin43 and Connexin32 into Gap Junctions in Human Squamous Carcinoma Cells. Journal of Biological Chemistry. 2010;285(14):10761–10776. doi:https://doi.org/10.1074/jbc.M109.053348.
- Yu SC, Xiao HL, Jiang XF, Wang QL, Li Y, Yang XJ, Ping YF, Duan JJ, Jiang JY, Ye XZ, et al. Connexin 43 Reverses Malignant Phenotypes of Glioma Stem Cells by Modulating E-Cadherin. Stem Cells. 2012;30(2):108–120. doi:https://doi.org/10.1002/stem.1685.
- Kotini M, Barriga EH, Leslie J, Gentzel M, Rauschenberger V, Schambony A, Mayor R. Gap junction protein Connexin-43 is a direct transcriptional regulator of N-cadherin in vivo. Nat Commun. 2018;9(1):3846. doi:https://doi.org/10.1038/s41467-018-06368-x.
- Oxford EM, Musa H, Maass K, Coombs W, Taffet SM, Delmar M. Connexin43 remodeling caused by inhibition of plakophilin-2 expression in cardiac cells. Circ Res. 2007;101(7):703–711. doi:https://doi.org/10.1161/CIRCRESAHA.107.154252.
- Gomes J, Finlay M, Ahmed AK, Ciaccio EJ, Asimaki A, Saffitz JE, Quarta G, Nobles M, Syrris P, Chaubey S, et al. Electrophysiological abnormalities precede overt structural changes in arrhythmogenic right ventricular cardiomyopathy due to mutations in desmoplakin-A combined murine and human study. Eur Heart J. 2012;33(15):1942–1953. doi:https://doi.org/10.1093/eurheartj/ehr472.
- Lyon RC, Mezzano V, Wright AT, Pfeiffer E, Chuang J, Banares K, Castaneda A, Ouyang KF, Cui L, Contu R, et al. Connexin defects underlie arrhythmogenic right ventricular cardiomyopathy in a novel mouse model. Hum Mol Genet. 2014;23(5):1134–1150. doi:https://doi.org/10.1093/hmg/ddt508.
- Kam CY, Dubash AD, Magistrati E, Polo S, Satchell KJF, Sheikh F, Lampe PD, Green KJ. Desmoplakin maintains gap junctions by inhibiting Ras/MAPK and lysosomal degradation of connexin-43. Journal of Cell Biology. 2018;217(9):3219–3235. doi:https://doi.org/10.1083/jcb.201710161.
- Rhett JM, Jourdan J, Gourdie RG. Connexin 43 connexon to gap junction transition is regulated by zonula occludens-1. Molecular Biology of the Cell. 2011;22(9):1516–1528. doi:https://doi.org/10.1091/mbc.e10-06-0548.
- Talhouk RS, Mroue R, Mokalled M, Abi-Mosleh L, Nehme R, Ismail A, Khalil A, Zaatari M, El-Sabban ME. Heterocellular interaction enhances recruitment of alpha and beta-catenins and ZO-2 into functional gap-junction complexes and induces gap junction-dependant differentiation of mammary epithelial cells. Experimental Cell Research. 2008;314(18):3275–3291. doi:https://doi.org/10.1016/j.yexcr.2008.07.030.
- Talhouk RS, Fares MB, Rahme GJ, Hariri HH, Rayess T, Dbouk HA, Bazzoun D, Al-Labban D, El-Sabban ME. Context dependent reversion of tumor phenotype by connexin-43 expression in MDA-MB231 cells and MCF-7 cells: role of beta-catenin/connexin43 association. Exp Cell Res. 2013;319(20):3065–3080. doi:https://doi.org/10.1016/j.yexcr.2013.10.002.
- Duffy HS, John GR, Lee SC, Brosnan CF, Spray DC. Reciprocal regulation of the junctional proteins claudin-1 and connexin43 by interleukin-1 beta in primary human fetal astrocytes. J Neurosci. 2000;20(23):23. doi:https://doi.org/10.1523/JNEUROSCI.20-23-j0004.2000.
- Kojima T, Kokai Y, Chiba H, Yamamoto M, Spray D, Mochizuki Y, Sawada N. Cx32 formation and/or Cx32 mediated intercellular communication induce tight junctions in hepatocytes. Molecular Biology of the Cell. 2000;11:330a–330a.
- Shaw RM, Fay AJ, Puthenveedu MA, von Zastrow M, Jan YN, Jan LY. Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell. 2007;128(3):547–560. doi:https://doi.org/10.1016/j.cell.2006.12.037.
- Giepmans BNG, Verlaan I, Moolenaar WH. Connexin-43 interactions with ZO-1 and alpha- and beta-tubulin. Cell Communication and Adhesion. 2001;8(4–6):219–223. doi:https://doi.org/10.3109/15419060109080727.
- Butkevich E, Hulsmann S, Wenzel D, Shirao T, Duden R, Majoul I. Drebrin is a novel connexin-43 binding partner that links gap junctions to the submembrane cytoskeleton. Curr Biol. 2004;14(8):650–658. doi:https://doi.org/10.1016/j.cub.2004.03.063.
- Crespin S, Bechberger J, Mesnil M, Naus CC, Sin WC. The Carboxy-Terminal Tail of Connexin43 Gap Junction Protein Is Sufficient to Mediate Cytoskeleton Changes in Human Glioma Cells. J Cell Biochem. 2010;110(3):589–597. doi:https://doi.org/10.1002/jcb.22554.
- Muscarella AM, Dai W, Mitchell PG, Zhang WJ, Wang H, Jia LY, Stossi F, Mancini MA, Chiu W, Zhang XHF. Unique cellular protrusions mediate breast cancer cell migration by tethering to osteogenic cells. Npj Breast Cancer. 2020;6(1):1. doi:https://doi.org/10.1038/s41523-020-00183-8.
- Herve JC. Gap junction channels: from protein genes to diseases. Prog Biophys Mol Bio. 2007;94(1–2):1–4. doi:https://doi.org/10.1016/j.pbiomolbio.2007.03.012.
- Petit C, Levilliers J, Hardelin JP. Molecular genetics of hearing loss. Annu Rev Genet. 2001;35(1):589–646. doi:https://doi.org/10.1146/annurev.genet.35.102401.091224.
- Mese G, Londin E, Mui R, Brink PR, White TW. Altered gating properties of functional Cx26 mutants associated with recessive non-syndromic hearing loss. Hum Genet. 2004;115(3):191–199. doi:https://doi.org/10.1007/s00439-004-1142-6.
- Mese G, Valiunas V, Brink PR, White TW. Connexin26 deafness associated mutations show altered permeability to large cationic molecules. Am J Physiol-Cell Ph. 2008;295(4):C966–C974. doi:https://doi.org/10.1152/ajpcell.00008.2008.
- Hall JE. A junction of transparency. Focus on “Functional effects of Cx50 mutations associated with congenital cataracts”. Am J Physiol-Cell Ph. 2014;306(3):C200–C201. doi:https://doi.org/10.1152/ajpcell.00323.2013.
- Pal JD, Liu XQ, Mackay D, Shiels A, Berthoud VM, Beyer EC, Ebihara L. Connexin46 mutations linked to congenital cataract show loss of gap junction channel function. Am J Physiol-Cell Ph. 2000;279(3):C596–C602. doi:https://doi.org/10.1152/ajpcell.2000.279.3.C596.
- Ionasescu V, Searby C, Ionasescu R. Point Mutations of the Connexin32 (Gjb1) Gene in X-Linked Dominant Charcot-Marie-Tooth Neuropathy. Hum Mol Genet. 1994;3(2):355–358. doi:https://doi.org/10.1093/hmg/3.2.355.
- Mese G, Sellitto C, Li LP, Wang HZ, Valiunas V, Richard G, Brink PR, White TW. The Cx26-G45E mutation displays increased hemichannel activity in a mouse model of the lethal form of keratitis-ichthyosis-deafness syndrome. Molecular Biology of the Cell. 2011;22(24):4776–4786. doi:https://doi.org/10.1091/mbc.e11-09-0778.
- Aypek H, Bay V, Mese G. Altered cellular localization and hemichannel activities of KID syndrome associated connexin26 I30N and D50Y mutations. Bmc Cell Biol. 2016;17:5. doi: https://doi.org/10.1186/s12860-016-0081-0.
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi:https://doi.org/10.1016/S0092-8674(00)81683-9.
- Hanahan D, Weinberg RA. Hallmarks of Cancer: the Next Generation. Cell. 2011;144(5):646–674. doi:https://doi.org/10.1016/j.cell.2011.02.013.
- Mehta PP, Bertram JS, Loewenstein WR. Growth-Inhibition of Transformed-Cells Correlates with Their Junctional Communication with Normal-Cells. Cell. 1986;44(1):187–196. doi:https://doi.org/10.1016/0092-8674(86)90497-6.
- Loewenstein WR, Kanno Y. Intercellular communication and the control of tissue growth: lack of communication between cancer cells. Nature. 1966;209(5029):1248–1249. doi:https://doi.org/10.1038/2091248a0.
- Azarnia R, Loewenstein WR. Intercellular Communication and the Control of Growth .10. Alteration of Junctional Permeability by the Src Gene - a Study with Temperature-Sensitive Mutant Rous-Sarcoma Virus. J Membrane Biol. 1984;82(3):191–205. doi:https://doi.org/10.1007/BF01871629.
- Mesnil M, Crespin S, Avanzo JL, Zaidan-Dagli ML. Defective gap junctional intercellular communication in the carcinogenic process. Bba-Biomembranes. 2005;1719(1–2):125–145. doi:https://doi.org/10.1016/j.bbamem.2005.11.004.
- Cronier L, Crespin S, Strale PO, Defamie N, Mesnil M, Junctions G. Cancer: new Functions for an Old Story. Antioxid Redox Sign. 2009;11(2):323–338. doi:https://doi.org/10.1089/ars.2008.2153.
- Hellmann P, Grummer R, Schirrmacher K, Rook M, Traub O, Winterhager E. Transfection with different connexin genes alters growth and differentiation of human choriocarcinoma cells. Experimental Cell Research. 1999;246(2):480–490. doi:https://doi.org/10.1006/excr.1998.4332.
- Zhu D, Caveney S, Kidder GM, Naus CCG. Transfection of C6 Glioma-Cells with Connexin-43 Cdna - Analysis of Expression, Intercellular Coupling, and Cell-Proliferation. P Natl Acad Sci USA. 1991;88(5):1883–1887. doi:https://doi.org/10.1073/pnas.88.5.1883.
- Hirschi KK, Xu CE, Tsukamoto T, Sager R. Gap junction genes Cx26 and Cx43 individually suppress the cancer phenotype of human mammary carcinoma cells and restore differentiation potential. Cell Growth Differ. 1996;7:861–870.
- McLachlan E, Shao Q, Wang HL, Langlois S, Laird DW. Connexins act as tumor suppressors in three-dimensional mammary cell organoids by regulating differentiation and angiogenesis. Cancer Res. 2006;66(20):9886–9894. doi:https://doi.org/10.1158/0008-5472.CAN-05-4302.
- Plante I, Stewart MKG, Barr K, Allan AL, Laird DW. Cx43 suppresses mammary tumor metastasis to the lung in a Cx43 mutant mouse model of human disease. Oncogene. 2011;30(14):1681–1692. doi:https://doi.org/10.1038/onc.2010.551.
- Alaga KC, Crawford M, Dagnino L, Laird DW. Aberrant Cx43 Expression and Mislocalization in Metastatic Human Melanomas. J Cancer. 2017;8(7):1123–1128. doi:https://doi.org/10.7150/jca.18569.
- Tang B, Peng ZH, Yu PW, Yu G, Qian F. Expression and significance of Cx43 and E-cadherin in gastric cancer and metastatic lymph nodes. Medical Oncology. 2011;28(2):502–508. doi:https://doi.org/10.1007/s12032-010-9492-5.
- Zhao JQ, Sun FJ, Liu SS, Yang J, Wu YQ, Li GS, Chen QY, Wang JX. Expression of Connexin 43 and E-cadherin Protein and mRNA in Non-small Cell Lung Cancers in Chinese Patients. Asian Pac J Cancer P. 2013;14(2):639–643. doi:https://doi.org/10.7314/APJCP.2013.14.2.639.
- Ezumi K, Yamamoto H, Murata K, Higashiyama M, Damdinsuren B, Nakamura Y, Kyo N, Okami J, Ngan CY, Takemasa I, et al. Aberrant expression of connexin 26 is associated with lung metastasis of colorectal cancer. Clin Cancer Res. 2008;14(3):677–684. doi:https://doi.org/10.1158/1078-0432.CCR-07-1184.
- Krutovskikh V, Mazzoleni G, Mironov N, Omori Y, Aguelon AM, Mesnil M, Berger F, Partensky C, Yamasaki H, Homologous A. Heterologous Gap-Junctional Intercellular Communication in Primary Human Liver-Tumors Associated with Aberrant Protein Localization but Not Gene Mutation of Connexin-32. International Journal of Cancer. 1994;56(1):87–94. doi:https://doi.org/10.1002/ijc.2910560116.
- Eghbali B, Kessler JA, Reid LM, Roy C, Spray DC. Involvement of Gap-Junctions in Tumorigenesis - Transfection of Tumor-Cells with Connexin-32 Cdna Retards Growth-Invivo. P Natl Acad Sci USA. 1991;88(23):10701–10705. doi:https://doi.org/10.1073/pnas.88.23.10701.
- Momiyama M, Omori Y, Ishizaki Y, Nishikawa Y, Tokairin T, Ogawa J, Enomoto K. Connexin26-mediated gap junctional communication reverses the malignant phenotype of MCF-7 breast cancer cells. Cancer Sci. 2003;94(6):501–507. doi:https://doi.org/10.1111/j.1349-7006.2003.tb01473.x.
- Yang J, Liu B, Wang Q, Yuan DD, Hong XT, Yang Y, Tao L. Connexin 32 and its derived homotypic gap junctional intercellular communication inhibit the migration and invasion of transfected HeLa cells via enhancement of intercellular adhesion. Molecular Medicine Reports. 2011;4:971–979.
- Kameritsch P, Khandoga N, Pohl U, Pogoda K. Gap junctional communication promotes apoptosis in a connexin-type-dependent manner. Cell Death & Disease. 2013;4(4):e584. doi: https://doi.org/10.1038/cddis.2013.105.
- Krutovskikh VA, Piccoli C, Yamasaki H. Gap junction intercellular communication propagates cell death in cancerous cells (vol 21, pg 1989, 2002). Oncogene. 2002;21(28):4471. doi:https://doi.org/10.1038/sj.onc.1205567.
- Polusani SR, Kalmykov EA, Chandrasekhar A, Zucker SN, Nicholson BJ. Cell coupling mediated by connexin 26 selectively contributes to reduced adhesivity and increased migration. Journal of Cell Science. 2016;129:4399–4410.
- Hong XT, Sin WC, Harris AL, Naus CC. Gap junctions modulate glioma invasion by direct transfer of microRNA. Oncotarget. 2015;6(17):15566–15577. doi:https://doi.org/10.18632/oncotarget.3904.
- Strale PO, Clarhaut J, Lamiche C, Cronier L, Mesnil M, Defamie N. Down-Regulation of Connexin43 Expression Reveals the Involvement of Caveolin-1 Containing Lipid Rafts in Human U251 Glioblastoma Cell Invasion. Mol Carcinogen. 2012;51(11):845–860. doi:https://doi.org/10.1002/mc.20853.
- Chen Q, Boire A, Jin X, Valiente M, Er EE, Lopez-Soto A, Jacob LS, Patwa R, Shah H, Xu K, et al. Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature. 2016;533(7604):493-+. doi:https://doi.org/10.1038/nature18268.
- Ito A, Katoh F, Kataoka TR, Okada M, Tsubota N, Asada H, Yoshikawa K, Maeda S, Kitamura Y, Yamasaki H, et al. A role for heterologous gap junctions between melanoma and endothelial cells in metastasis. Journal of Clinical Investigation. 2000;105(9):1189–1197. doi:https://doi.org/10.1172/JCI8257.
- Zhou JZ, Riquelme MA, Gu S, Kar R, Gao X, Sun L, Jiang JX. Osteocytic connexin hemichannels suppress breast cancer growth and bone metastasis. Oncogene. 2016;35(43):5597–5607. doi:https://doi.org/10.1038/onc.2016.101.
- Murphy SF, Varghese RT, Lamouille S, Guo SJ, Pridham KJ, Kanabur P, Osimani AM, Sharma S, Jourdan J, Rodgers CM, et al. Connexin 43 Inhibition Sensitizes Chemoresistant Glioblastoma Cells to Temozolomide. Cancer Research. 2016;76(1):139–149. doi:https://doi.org/10.1158/0008-5472.CAN-15-1286.
- Krutovskikh VA, Troyanovsky SM, Piccoli C, Tsuda H, Asamoto M, Yamasaki H. Differential effect of subcellular localization of communication impairing gap junction protein connexin43 on tumor cell growth in vivo. Oncogene. 2000;19(4):505–513. doi:https://doi.org/10.1038/sj.onc.1203340.
- Sun Y, Zhao XP, Yao YT, Qi XY, Yuan YZ, Hu Y. Connexin 43 interacts with Bax to regulate apoptosis of pancreatic cancer through a gap junction-independent pathway. International Journal of Oncology. 2012;41(3):941–948. doi:https://doi.org/10.3892/ijo.2012.1524.
- Talhouk RS, Fares MB, Rahme GJ, Hariri HH, Rayess T, Dbouk HA, Bazzoun D, Al-Labban D, El-Sabban ME. Context dependent reversion of tumor phenotype by connexin-43 expression in MDA-MB231 cells and MCF-7 cells: role of beta-catenin/connexin43 association. Experimental Cell Research. 2013;319:3065–3080.
- MacDonald AI, Sun P, Hernandez-Lopez H, Aasen T, Hodgins MB, Edward M, Roberts S, Massimi P, Thomas M, Banks L, et al. A functional interaction between the MAGUK protein hDlg and the gap junction protein connexin 43 in cervical tumour cells. Biochemical Journal. 2012;446(1):9–21. doi:https://doi.org/10.1042/BJ20111144.
- Xu HT, Li QC, Zhang YX, Zhao Y, Liu Y, Yang ZQ, Wang EH. Connexin 43 recruits E-cadherin expression and inhibits the malignant behaviour of lung cancer cells. Folia Histochem Cytobiol. 2008;46(3):315–321. doi:https://doi.org/10.2478/v10042-008-0057-9.
- Gellhaus A, Dong XS, Propson S, Maass K, Klein-Hitpass L, Kibschull M, Traub O, Willecke K, Perbal B, Lye SJ, et al. Connexin43 interacts with NOV - A possible mechanism for negative regulation of cell growth in choriocarcinoma cells. Journal of Biological Chemistry. 2004;279(35):36931–36942. doi:https://doi.org/10.1074/jbc.M404073200.
- Zhang YW, Morita I, Ikeda M, Ma KW, Murota S. Connexin43 suppresses proliferation of osteosarcoma U2OS cells through post-transcriptional regulation of p27. Oncogene. 2001;20(31):4138–4149. doi:https://doi.org/10.1038/sj.onc.1204563.
- Duffy HS, Iacobas I, Hotchkiss K, Hirst-Jensen BJ, Bosco A, Dandachi N, Dermietzel R, Sorgen PL, Spray DC. The gap junction protein connexin32 interacts with the Src homology 3/hook domain of discs large homolog 1. J Biol Chem. 2007;282(13):9789–9796. doi:https://doi.org/10.1074/jbc.M605261200.
- Yang Y, Zhang N, Zhu J, Hong XT, Liu H, Ou YR, Su F, Wang R, Li YM, Wu Q. Downregulated connexin32 promotes EMT through the Wnt/beta-catenin pathway by targeting Snail expression in hepatocellular carcinoma. Int J Oncol. 2017;50(6):1977–1988. doi:https://doi.org/10.3892/ijo.2017.3985.
- Kanczuga-Koda L, Sulkowski S, Tomaszewski J, Koda M, Sulkowska M, Przystupa W, Golaszewska J, Baltaziak M. Connexins 26 and 43 correlate with Bak, but not with Bcl-2 protein in breast cancer. Oncology Reports. 2005;14:325–329.
- Thiagarajan PS, Sinyuk M, Turaga SM, Mulkearns-Hubert EE, Hale JS, Rao V, Demelash A, Saygin C, China A, Alban TJ, et al. Cx26 drives self-renewal in triple-negative breast cancer via interaction with NANOG and focal adhesion kinase. Nat Commun. 2018;9(1):578. doi:https://doi.org/10.1038/s41467-018-02938-1.
- Martin F, Stein T, Howlin J. Springer Science+Business Media, Mammary gland development: methods and protocols. New York/USA: Humana Press; 2017. Vol. xi. p. 358.
- Pozzi A, Risek B, Kiang DT, Gilula NB, Kumar NM. Analysis of Multiple Gap Junction Gene-Products in the Rodent and Human Mammary-Gland. Experimental Cell Research. 1995;220:212–219.
- El-Saghir JA, El-Habre ET, El-Sabban ME, Talhouk RS. Connexins: a junctional crossroad to breast cancer. International Journal of Developmental Biology. 2011;55(7–9):773–780. doi:https://doi.org/10.1387/ijdb.113372je.
- Weber PA, Chang HC, Spaeth KE, Nitsche JM, Nicholson BJ. The permeability of gap junction channels to probes of different size is dependent on connexin composition and permeant-pore affinities. Biophys J. 2004;87(2):958–973. doi:https://doi.org/10.1529/biophysj.103.036350.
- Lambe T, Finlay D, Murphy M, Martin F. Differential expression of connexin 43 in mouse mammary cells. Cell Biol Int. 2006;30(5):472–479. doi:https://doi.org/10.1016/j.cellbi.2006.02.008.
- Teleki I, Szasz AM, Maros ME, Gyorffy B, Kulka J, Meggyeshazi N, Kiszner G, Balla P, Samu A, Krenacs T. Correlations of differentially expressed gap junction connexins Cx26, Cx30, Cx32, Cx43 and Cx46 with breast cancer progression and prognosis. PLoS One. 2014;9:e112541.
- Lee SW, Tomasetto C, Sager R. Positive Selection of Candidate Tumor-Suppressor Genes by Subtractive Hybridization. P Natl Acad Sci USA. 1991;88(7):2825–2829. doi:https://doi.org/10.1073/pnas.88.7.2825.
- Conklin C, Huntsman D, Yorida E, Makretsov N, Turbin D, Bechberger JF, Sin WC, Naus CC. Tissue microarray analysis of connexin expression and its prognostic significance in human breast cancer. Cancer Letters. 2007;255(2):284–294. doi:https://doi.org/10.1016/j.canlet.2007.05.001.
- Chasampalioti M, Green AR, Ellis IO, Rakha EA, Jackson AM, Spendlove I, Ramage JM. Connexin 43 is an independent predictor of patient outcome in breast cancer patients. Breast Cancer Res Tr. 2019;174(1):93–102. doi:https://doi.org/10.1007/s10549-018-5063-9.
- Lee SW, Tomasetto C, Paul D, Keyomarsi K, Sager R. Transcriptional downregulation of gap-junction proteins blocks junctional communication in human mammary tumor cell lines. The Journal of Cell Biology. 1992;118(5):1213–1221. doi:https://doi.org/10.1083/jcb.118.5.1213.
- Kanczuga-Koda L, Sulkowska M, Koda M, Rutkowski R, Sulkowski S. Increased expression of gap junction protein–connexin 32 in lymph node metastases of human ductal breast cancer. Folia Histochem Cyto. 2007;45:175–180.
- Nicolson GL, Dulski KM, Trosko JE. Loss of Intercellular Junctional Communication Correlates with Metastatic Potential in Mammary Adenocarcinoma Cells. P Natl Acad Sci USA. 1988;85(2):473–476. doi:https://doi.org/10.1073/pnas.85.2.473.
- Hamada J, Takeichi N, Kobayashi H. Inverse Correlation between the Metastatic Capacity of Cell Clones Derived from a Rat Mammary-Carcinoma and Their Intercellular Communication with Normal Fibroblasts. Jpn J Cancer Res. 1987;78:1175–1178.
- Stoletov K, Strnadel J, Zardouzian E, Momiyama M, Park FD, Kelber JA, Pizzo DP, Hoffman R, VandenBerg SR, Klemke RL. Role of connexins in metastatic breast cancer and melanoma brain colonization. Journal of Cell Science. 2013;126:904–913.
- Elzarrad MK, Haroon A, Willecke K, Dobrowolski R, Gillespie MN, Al-Mehdi AB. Connexin-43 upregulation in micrometastases and tumor vasculature and its role in tumor cell attachment to pulmonary endothelium. BMC Med. 2008;6:20. doi:https://doi.org/10.1186/1741-7015-6-20.
- Ming J, Zhou Y, Du JZ, Fan SH, Pan BB, Wang YH, Fan LJ, Jiang J. Identification of miR-200a as a novel suppressor of connexin 43 in breast cancer cells. Bioscience Rep. 2015;35(5):e00251. doi: https://doi.org/10.1042/BSR20150153.
- Anderson C, Catoe H, Werner R. MIR-206 regulates connexin43 expression during skeletal muscle development. Nucleic Acids Res. 2006;34(20):5863–5871. doi:https://doi.org/10.1093/nar/gkl743.
- Ming J, Zhou Y, Du JZ, Fan SG, Pan BB, Wang YH, Fan LJ, Jiang J. miR-381 suppresses C/EBP alpha-dependent Cx43 expression in breast cancer cells. Bioscience Rep. 2015;35(6):e00266. doi: https://doi.org/10.1042/BSR20150167.
- Brabletz T, Kalluri R, Nieto MA, Weinberg RA. EMT in cancer. Nature Reviews Cancer. 2018;18(2):128-+. doi:https://doi.org/10.1038/nrc.2017.118.
- Yang J, Qin G, Luo M, Chen J, Zhang Q, Li L, Pan L, Qin S. Reciprocal positive regulation between Cx26 and PI3K/Akt pathway confers acquired gefitinib resistance in NSCLC cells via GJIC-independent induction of EMT. Cell Death & Disease. 2015;6(7):e1829. doi: https://doi.org/10.1038/cddis.2015.197.
- Yu M, Han G, Qi B, Wu X. Cx32 reverses epithelial-mesenchymal transition in doxorubicin-resistant hepatocellular carcinoma. Oncol Rep. 2017;37(4):2121–2128. doi:https://doi.org/10.3892/or.2017.5462.
- Adak A, Unal YC, Yucel S, Vural Z, Turan FB, Yalcin-Ozuysal O, Ozcivici E, Mese G. Connexin 32 induces pro-tumorigenic features in MCF10A normal breast cells and MDA-MB-231 metastatic breast cancer cells. Bba-Mol Cell Res. 2020;1867:12.
