ABSTRACT
Claudins are major components of tight junctions that maintain cell polarity and intercellular adhesion. The dynamics of claudins in cancer cells have attracted attention as a therapeutic target. During carcinogenesis, claudin expression is generally downregulated; however, overexpression of claudin-18.2 has been observed in several types of cancers. Upregulated and mislocalized claudin-18.2 expression in cancer cells has been suggested as a therapeutic target. Research on claudin-18.2 has revealed its involvement in carcinogenesis. Clinical trials using zolbetuximab, a monoclonal antibody targeting claudin-18.2, for patients with advanced cancer yielded positive results with few high-grade adverse events; thus, it is expected to be a novel and effective therapeutic. Here, we review current insights into the role that claudin-18.2 plays in basic cancer research and clinical applications. A better understanding of these roles will facilitate the development of new treatment strategies for cancer patients with poor prognoses.
Introduction
Tight junction molecules have intercellular adhesion functions in all cell types. Recently, the dynamics of these proteins in cancer cells have attracted attention. Epithelial cells are held together by several major classes of intracellular junctions, and adherens and tight junctions play essential roles in the development and maintenance of epithelial cells. Tight junctions are typically located on the apical side of epithelial cells, and their components interact with those of neighboring cells. Tight junction proteins are often dysfunctional or altered in various types of cancer cells, and their controlled paracellular permeation and polarity are lost in cancer cellsCitation1. Altered tight junction proteins also modulate cytoskeletal elements and signaling molecules bound to these proteins, resulting in the loss of regulated cell migration and proliferation.Citation2
The two major components of tight junctions are occludin and claudin proteins, which bind to the PDZ domains of zonula occludens (ZO) proteins to anchor the actin cytoskeleton.Citation3,Citation4 Claudins are 20–27-kDa transmembrane proteins that form extremely tight associations with their counterparts on adjacent cells.Citation4 These proteins have four transmembrane domains and two extracellular loops to maintain cell-cell integrity and regulate paracellular ion transport.Citation3 Currently, there are 27 known members of the claudin family and cancer cells have a specific claudin expression pattern according to the tumor cell origin.Citation5 In 2008, Sahin et al. demonstrated that the claudin-18 splice variant 2, claudin-18.2, is highly expressed in several tumors.Citation6 Research on claudin-18.2 has gradually elucidated its expression patterns and functions in cancer cells. Moreover, clinical trials using anti-claudin-18.2 antibodies as a cancer-specific molecular targeted therapy for advanced gastric adenocarcinoma are ongoing.Citation7–9 This accumulating body of evidence indicates that claudin-18.2 is one of the most clinically relevant tight junction proteins.
For successful antibody therapy targeting claudin-18.2, it is important to understand the dynamics, localization, and function of claudin-18.2 in cancer cells. In addition, the efficacy, eligible patients, and adverse events of monoclonal antibodies to claudin-18.2 in cancer patients need to be analyzed together with in vitro and in vivo results. While most reviews describe either the results of basic research or the results of clinical trials, we have summarized both findings to provide a better understanding of the dynamics of claudin-18.2 and cancer therapy targeting claudin-18.2. Here, we review current insights into the role that claudin-18.2 plays in basic cancer research and clinical applications. A better understanding of these roles will facilitate the development of new treatment strategies for cancer.
Claudins in cancer, and therapeutic approaches targeting claudins
Claudins have three main functions: barrier, fence, and intracellular signaling.1,Citation10 The barrier function is the ability to selectively regulate paracellular permeation of water, ions, macromolecules, and immune cells, whereas the fence function separates the apical and basolateral domains and regulates the movement of substances within the plasma membrane.Citation10,Citation11 Tight junctions, including claudins, also act as signaling hubs by binding to multiple signaling molecules. Claudins in cancer cells are known to activate signaling pathways associated with tumor progression and metastasis. For example, claudin-1 activates the c-Abl-Ras-Raf-1-ERK1/2 signaling pathway in hepatocellular carcinomaCitation12 and the Notch/phosphoinositide-3-kinase (PI3K)/Akt signaling pathway in colitis-associated cancer.Citation13 Claudin-2 in colon cancer is substantially upregulated via the epidermal growth factor receptor-ERK1/2 kinase axis and overexpressed claudin-2 increases cell proliferation and tumor growth in vivo.Citation14 Claudin-3 has a suppressive role in epithelial-mesenchymal transition via activation of the Wnt/beta-catenin signaling pathway in lung squamous cell carcinomaCitation15 and hepatocellular carcinoma.Citation16 Thus, the impact of claudins on carcinogenesis has been investigated in various types of cancer cells.
In normal epithelial cells, claudins are mostly present in the apical tight junction fraction, which forms intercellular strands.Citation17 Claudin expression is generally downregulated and mislocalized during carcinogenesis and epithelial-mesenchymal transition of cancer cells, and loss of claudins causes dysfunction of their epithelial polarity and barrier function.Citation2,Citation18 However, overexpression of claudins has also been observed in several types of cancer. The expression of each cancer cell tight junction molecule is abnormally regulated.Citation19 Claudin-18.2 expression of cancer cells was transcriptionally upregulated with the binding of cyclic AMP–responsive element binding protein to the methylated CLDN18.2 promoter region.Citation6 The upregulation of claudins in cancer cells in comparison to normal epithelial cells could be a diagnostic tool and a therapeutic target.Citation20 A recent study has shown that a human IgG1 monoclonal antibody against the second extracellular loop of claudin-3 (h4G3) recognizes claudin-3-expressing tumors rather than normal organs in mouse xenograft models.Citation21 Claudin-3 is overexpressed in various carcinomas, including breast, colorectal, gastric, pancreatic, prostate, and ovarian cancer,Citation22 while it is also expressed in normal tissues such as the colon, rectum, thyroid, salivary glands, pancreas, prostate, liver, and kidney.Citation23 The antibody h4G3 had a lower affinity to normal tissues than to tumor sites in mice bearing xenograft tumors despite its ability to recognize mouse claudin-3, and it had no antitumor efficacy in vivo.Citation21 Thus, although claudin-3, which is present in various organs of the body, has potential value for cancer diagnosis, its specific antibody can cause severe adverse events involving cell death. As claudins are generally expressed in normal epithelial and endothelial cells at various locations,Citation24 the application of anti-claudin antibodies to humans includes the potential for off-target effects and fatal adverse events.
Antibodies against claudins specific to cancer cells are being investigated as novel therapeutic tools for cancer patients. An anti-claudin-6 monoclonal antibody (ASP1650, also called IMAB027) was generated and used in a phase I clinical trial (OVAR; NCT02054351) for patients with advanced and recurrent ovarian cancerCitation25 and in a phase II clinical trial (NCT03760081) for patients with advanced germ cell tumors of testicular cancer.Citation26 Claudin-6 expression is activated in various cancers, including gastric adenocarcinoma and embryonic carcinoma, while it is limited to embryonic development in normal tissues.Citation27 It was demonstrated that ASP1650 binds specifically to claudin-6 without cross-reactivity with claudin-3, −4, and −9 and induces antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) in claudin-6-expressing cells in vitro.Citation28 However, clinical efficacy and safety have not been reported in published articles. Monoclonal antibodies against cancer-specific claudins are appropriate for the treatment of cancer; however, cancer-specific claudins have not been found, and the development of antibodies is difficult because each claudin is highly homologous with others. Thus far, only antibodies targeting claudin-6 and −18.2 have been used in clinical trials for cancer treatment.
Roles of claudin-18.2 in individual cancer types
In this section, we describe the function, localization, and expression level of claudin-18.2, and its correlation with intracellular signaling pathways, clinicopathological factors, and prognosis in several cancers. Claudin-18.2 has been actively studied owing to its potential value in the diagnosis of malignant tumors and the application of zolbetuximab, a monoclonal antibody against claudin-18.2.
Gastric cancer
Claudin-18 has two alternatively spliced variants: claudin-18.1 in the lung and claudin-18.2 in the stomach ( and ).Citation29 The expression of claudin-18.2 in normal tissues is limited to differentiated epithelial cells of the gastric mucosa. Claudin-18.2 is retained in primary gastric cancers and their metastases and can also be found in pancreatic, esophageal, ovarian, lung, and colitis-associated colorectal tumors.Citation6,Citation30 Claudin-18.2 of the epithelium in the stomach regulates cell lineage differentiation and blocks paracellular gastric acid leakage from the gastric lumen into the submucosal space.Citation31,Citation32 Paracellular H+ leakage contributes to the progression of gastritis.Citation31 Helicobacter pylori (H. pylori) infection attenuates claudin-18 expression in mice by 6 months post-infection, and its expression decreases over time.Citation32 The loss of claudin-18.2 contributes to paracellular H+ leakage and promotes gastric tumor formation with and without H. pylori infection in mice.Citation32,Citation33 Furthermore, the expression levels of C-X-C motif chemokine 5 (CXCL5), Toll-like receptor 2 (TLR2), and CD44 splice variants increased during gastric tumorigenesis in claudin-18 knockout mice.Citation33 Another analysis of claudin-18 knockout mice demonstrated that claudin-18 activates cellular signaling pathways, including Wnt/beta-catenin downstream effectors (CD44, EFNB1, EFNB2, and EPHB2) and the Yes-associated protein (YAP)/Hippo signaling pathway in gastric tumorigenesis.Citation32 Notably, these studies revealed that claudin-18.2 knockout mice expressed claudin-18.1, which localized to tight junctions in the gastric mucosa.Citation32,Citation33 This suggests that a different claudin-18 variant to claudin-18.2 is expressed and plays a role in protecting the stomach tissue.
Figure 1. Amino acid sequences of claudin-18.1 and claudin-18.2.
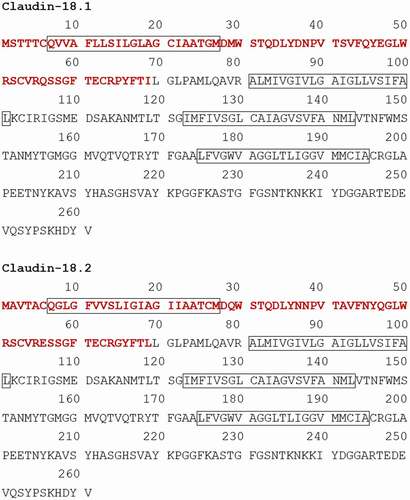
Figure 2. Claudin-18.2 expression in gastric cancer.
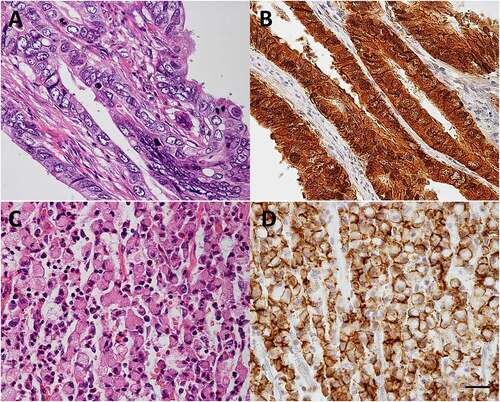
Claudin-18.2 of gastric cancer cells is regulated by the methylation status of CLDN18.2 promoter regionCitation6 and by the protein kinase C (PKC)/mitogen-activated protein kinase/activator protein 1-dependent pathway in vitro.Citation34 Furthermore, claudin-18.2 expression positively correlated with other adherens junction molecules, such as E-cadherin and Rho GTPase-activating protein (RhoGAP), which contribute to the organization of actin and microtubule cytoskeletons.Citation35 Gene fusion between CLDN18 and ARHGAP (the gene encoding RhoGAP) was observed in 15.1% of young adult patients with gastric cancer and 13.8% of “genomically stable” gastric cancer patients in The Cancer Genome Atlas dataset.Citation36 “Genomically stable” was defined as the absence of microsatellite and chromosomal instabilities. Fusion between CLDN18 and ARHGAP26 in gastric cancer cells resulted in the loss of their epithelial phenotype and enhanced migration capacity,Citation37 increased invasiveness, impaired barrier properties, and reduced cell-cell and cell-extracellular matrix adhesion.Citation38 In addition, CLDN18-ARHGAP fusion predicted distant organ metastasisCitation39 and poor prognosis in patients.Citation36,Citation37
For the development of the cancer treatment using the anti-claudin-18.2 antibody, it is important to identify which gastric cancer types highly retain claudin-18.2. It has been found that claudin-18.2 is not expressed in all gastric cancers; however, its expression is dependent on cancer cell types and individuals. Most reports investigating the expression rate of claudin-18.2 in gastric cancer are based on immunostaining results using antibodies with an epitope on the C-terminal side common to claudin-18.1 and claudin-18.2 (). According to the results, the expression rate of claudin-18 (claudin-18.2) in gastric cancer varies in the range of 53.0–87.0%.Citation6,Citation40–43 Depending on the selected antibody, the delineation rate varies in the range of 55.6–77.3% using polyclonal antibodies,Citation6,Citation43 53.0–87.0% using monoclonal antibodies clone 43–14A and 34H14L15 targeting the C-terminus of Claudin-18,Citation40–42 and 6.0–42.2% using clone EPR19202, a monoclonal antibody targeting amino acids 1–100, specific to claudin-18.2.Citation40,Citation44 These expression rates include everything from weak to strong expression. The definition of strong expression has not been defined generally, so the criteria vary among publications. In the MONO study, patients with moderate to strong membrane expression in ≥50% of tumor cells (inclusion criteria) were 26.7% and in ≥70% were 14.4% of the 261 enrolled patients.Citation45
The expression rate of claudin-18.2 varies depending on which region the patient is from. In a study using the same monoclonal antibody (clone 43–14A), the rate was 53.0% in Germany,Citation40 compared to 87.0% in Japan.Citation41 Nevertheless, only a few reports regarding the expression rate using the same antibody on regional differences exist, and future investigations are needed.
There have been various reports on the relationship between claudin-18.2 expression status and clinicopathological factors or the prognosis of gastric cancer patients. Several studies have shown that claudin-18.2 is retained in gastric cancer of diffuse-type rather than intestinal-typeCitation6,Citation41,Citation42,Citation46 Low expression of claudin-18 in an immunohistochemistry (IHC) staining was closely associated with nerve invasionCitation43and high levels of proliferation and invasion.Citation32,Citation47 and indicated a poor prognosis of gastric cancer patients.Citation43,Citation46,Citation48 In metastatic diffuse-type gastric cancer, claudin-18.2 expression was reduced in patients with peritoneal metastasis, however, it increased in patients with bone metastasis.Citation35 Alternatively, increased expression of claudin-18.2 detected with clone 43–14A was not associated with histomorphological subtype, tumor localization, TNM stage, or the overall survival rate in a large Caucasian cohort.Citation40 In a recently published meta-analysis, no significant correlations were found between the claudin-18.2 expression level and the overall survival of patients or their clinicopathological features such as TNM stage; Lauren classification; HER2 expression; and gastric cancer grade.Citation49
In summary, it seems that approximately half of the patients develop claudin-18.2-positive gastric cancer; however, we should consider the impact of antibody clones such as 43–14A, EPR19202, and 34H14L15, which have a large impact on the claudin-18.2 detection rate in gastric tissue. Furthermore, patient race may affect the positive rate of claudin-18.2. Ongoing clinical trials, involving patients from a wide range of regions globally, may reveal racial differences in claudin-18.2 expression rates ().Citation7–9 In addition, they may clarify correlations between prognostic and pathological factors and claudin-18.2. These studies will finally identify appropriate candidates for zolbetuximab. Clinical trials with zolbetuximab will be discussed in a later section.
Table 1. Phase II and III clinical trials with results using zolbetuximab and CAR T cells (accessed on 29 March, 2021)
Another therapeutic approach for claudin-18.2 is chimeric antigen receptor (CAR) T immunotherapy. CAR T cells can recognize tumor-associated antigens and promote T cell proliferation, cytotoxicity, and the transcription of genes encoding cytokines, resulting in antitumor activity.Citation52 The CAR T cells developed by Jiang et al. achieved partial or complete tumor elimination in a claudin-18.2-positive patient-derived tumor xenograft model of gastric cancer.Citation53 The CAR T cells had no obvious off-target effects, including that on gastric tissue, even though the expression level of murine claudin-18.2 in the gastric mucosa was similar to that of human claudin-18.2 in gastric cancer cell lines. The authors noted two possible reasons for the absence of adverse events in the normal gastric mucosa: first, claudin-18.2 of cancer cells is located in the tight junction fraction and on the basolateral membrane, and second, the microenvironment of the normal tissue protects it from cytotoxic effects of CAR T cells.Citation53 The ongoing clinical phase I trial (NCT03159819) using CAR T cells against claudin-18.2 in advanced gastric or pancreatic adenocarcinoma also reported no severe gastric toxicity or cytokine release syndromes.Citation54 Furthermore, the total objective response rate (ORR) was 33.3%, and as of 2018, one of 11 study participants with gastric cancer achieved complete remission. Currently, there is one clinical phase I/II trial with CAR T cells (NCT04581473) (). It is expected to elucidate the mechanism of CAR T cell-specific adverse events through in vivo experiments, and the benefits and toxicity risks for enrolled patients.
Pancreatic cancer
Claudin-18.2 is highly expressed in pancreatic adenocarcinoma and pancreatic cancer metastases. IHC analysis showed that 59.2% of primary pancreatic adenocarcinoma, 69.4% of metastatic lymph nodes, and 65.7% of liver metastases are claudin-18.2 positive. In contrast, only 20% of neuroendocrine neoplasias are claudin-18.2-positive.Citation55 In addition, claudin-18.2 is expressed in atypical and cystic lesions such as pancreatic intraepithelial neoplasia, intraductal papillary mucinous neoplasm, and mucinous cystic neoplasm.Citation56–59 As claudin-18.2 expression is pronounced in well-differentiated pancreatic cancers, patients with high expression levels of claudin-18 survive longer than those with low claudin-18 expression.Citation56
Claudin-18.2 is regulated at the transcriptional level via PKC signaling pathways in human gastric cancer,Citation34 pancreatic cancer, and normal pancreatic duct epithelial cells (HPDEs).Citation59 Claudin-18.2 mRNA and claudin-18 protein are markedly induced by the PKC activator 12-O-tetradecanoylphorbol-13-acetate (TPA) in well- and moderately differentiated human pancreatic cancer cell lines and HPDEs transfected with the human telomerase reverse transcriptase (hTERT) gene. In pancreatic cancer cell lines, TPA-induced claudin-18 expression is localized on apical and basolateral cell surfaces. The PKC family contains at least 12 different isozymes. Activation of PKCα, PKCδ, and PKCε has been reported to be associated with the upregulation of claudin-18 in human pancreatic cancer cell lines, whereas PKCα, PKCδ, and PKCθ activation has been associated with the upregulation of claudin-18 in hTERT-transfected HPDEs.Citation59 Furthermore, the increase in TPA-induced claudin-18 expression is enhanced by DNA demethylation. Consequently, the regulation of claudin-18.2 is correlated with genomic hypomethylation of promoter CpG islands.Citation6
Türeci et al. revealed that zolbetuximab induced ADCC and CDC against human pancreatic cancer cells in ex vivo models using human peripheral blood mononuclear cells and serum as effectors.Citation45 They also showed that zolbetuximab suppressed tumor development and lung metastasis formation in human pancreatic cancer cell lines transduced with lentiviral claudin-18.2 in mouse xenograft models.Citation60 Notably, claudin-18.2 expression on the cell surface was increased by gemcitabine or 5-fluorouracil (chemotherapy agents) administration in vitro. This phenomenon means that even if pancreatic cancer cells are not killed by chemotherapy, the patients can be newly eligible for zolbetuximab therapy, owing to the increased expression of claudin-18.2. It is unclear why this phenomenon occurred, and there is no mention of it in previous literature; however, it could be useful information for zolbetuximab use in the treatment of pancreatic cancer patients.
Cholangiocarcinoma
The upregulation of claudin-18.2 in cholangiocarcinoma is thought to have potential clinical use in the detection of cancer cells because it is difficult to diagnose benign or malignant types based on small preoperative bile duct specimens. The expression level of claudin-18 increased in intra- and extrahepatic cholangiocarcinoma and biliary intraepithelial neoplasia.Citation61,Citation62 Claudin-18 IHC assays were useful to distinguish the neoplastic region from the non-neoplastic region in surgical specimens and preoperative biopsies.Citation62 The assay also improved the sensitivity of the cytological diagnosis of pancreaticobiliary adenocarcinoma.Citation63
As noted in the previous section, claudin-18.2 in pancreatic cancer is an indicator of well-differentiated cancer types and good prognosis,Citation56 however, it was shown to correlate with poor overall survival and lymph node metastasis in intrahepatic cholangiocarcinoma.Citation61 Claudin-18 induced growth and invasiveness of bile duct cancer cell lines.Citation64 Notably, wound edges of confluent tumor cells showed strong claudin-18 expression in the wound healing assay. The authors demonstrated that claudin-18 in cancer cells activated ERK1/2, suggesting that claudin-18 plays a role in biliary carcinogenesis.Citation64 Claudin-18 was absent in the normal epithelium of the gallbladder; however, high expression of claudin-18 was detected in almost all metaplastic cells and half of the cancer cells.Citation65 Its expression was associated with metaplastic change, which was indicated by SOX2 positivity; however, it was not associated with pathological T stage and histological differentiation.
Lung cancer
The role of claudin-18.2 in lung cancer is still not well defined. Therefore, in this section, we will focus on the lung-specific isoform claudin-18.1. Claudin-18.1 is highly expressed in the lung alveolar epithelium and is not detectable in normal airways and lung endothelium.Citation29,Citation66 Claudin-18 was shown to play a role in the epithelial barrier function of lung alveolar epithelium in claudin-18 knockout mice.Citation67,Citation68 Claudin-18.1 was expressed at lower levels in asthmatic patients than in healthy controls.Citation69 In mice, claudin-18 knockout caused enlargement of the lung and other organs, increased the proliferation of alveolar epithelial type II (AT2) cells, which are lung progenitor cells, and tumorigenesis via YAP activation. Thus, claudin-18.1 has been suggested to regulate the proliferation of AT2 cells in normal lung epithelial cells and promote regeneration following lung injury by disrupting tight junctions.Citation70
Claudin-18.1 expression in human lung adenocarcinoma was also downregulated with cancer progression,Citation70 and a minority of non-small cell lung cancers showed claudin-18.2 expression (30 of 73 cancer specimens).Citation6 Claudin-18 expression was lower in lung cancer than in normal lung tissue, regardless of whether it was small cell or non-small cell carcinoma.Citation71 Claudin-18 suppressed the proliferation and motility of lung adenocarcinoma cells by inhibiting the PI3K/PDK1/Akt signaling pathwayCitation71 and regulating ZO-2 and matrix metalloproteinase 2.Citation72 Notably, the latter two investigations did not differentiate between claudin-18.1 and −18.2.
Clinical advances using monoclonal antibodies targeting claudin-18.2
Clinical trials have shown the efficacy of monoclonal antibodies against claudin-18.2, such as zolbetuximab (also known as IMAB362 and claudiximab).Citation50 Zolbetuximab is a structurally chimeric IgG1 monoclonal antibody that specifically binds to the first extracellular loop of claudin-18.2 on the tumor cell surface. This antibody causes ADCC and CDCCitation45,Citation73 and these cytotoxic effects induce apoptosis and inhibit proliferation of tumor cells, resulting in beneficial effects for cancer patients.Citation74 This section will focus on the results of recent clinical trials using zolbetuximab, viz. the MONO and the FAST studies, and summarize their main findings.
In the MONO study, a phase II investigation using zolbetuximab as a single agent in patients with metastatic or advanced gastric/gastroesophageal junction/esophageal adenocarcinoma, patients with moderate or strong claudin-18.2 membrane staining intensity in ≥50% of tumor cells were included.Citation45 The treatment showed a 9% ORR (the proportion of patients with complete and partial response in terms of tumor size) and 23% clinical benefit rate (partial response and stable disease).Citation45 Moreover, in a subgroup of patients with claudin-18.2 expression in ≥70% of tumor cells, the ORR increased up to 14%.
Recently published results of the FAST study, a phase II investigation with zolbetuximab plus epirubicin, oxaliplatin, and capecitabine (EOX) vs. EOX alone, demonstrated that zolbetuximab had antitumor activity in patients with claudin-18.2-positive advanced gastric/gastroesophageal junction/esophageal adenocarcinoma.Citation50 Claudin-18.2 positivity was defined as ≥40% of tumor cells with moderate or strong staining intensity in the CLAUDETECT™18.2 IHC assay. The
CLAUDETECT 18.2 Kit (developed by Ganymed and now acquired by Astellas) was introduced for in vitro diagnosis of a semi-quantitative IHC assay for claudin-18 protein expression.Citation45,Citation49,Citation73,Citation75 The authors defined three arms: arm 1, EOX alone; arm 2, zolbetuximab + EOX (loading dose 800 mg/m2, then 600 mg/m2); arm 3, zolbetuximab + EOX (1000 mg/m2). Of the 686 patients, 334 (49%) had claudin-18.2-positive tumor cells. In arms 1 and 2, 70.2% and 74.0% of the tumors had ≥70% cells with claudin-18.2 staining, respectively. Between arms 1 and 2, both progression-free survival (hazard ratio [HR], 0.44; 95% confidence interval [CI], 0.29–0.67; P < .0005) and overall survival (HR, 0.55; 95% CI, 0.39–0.77; P < .0005) were significantly improved with zolbetuximab + EOX compared to EOX alone. This significant benefit of progression-free survival was retained in patients with claudin-18.2 expression in ≥70% of tumor cells (HR, 0.38; 95% CI, 0.23–0.62; P < .0005). However, in patients with 40–69% claudin-18.2 expression in tumor cells, progression-free survival and overall survival were not significantly different between the arms (HR, 0.78; 95% CI, 0.40–1.49; P = .401). Therefore, we speculate that the inclusion criteria for current clinical trials examining the effects of zolbetuximab are “the expression of claudin-18.2 in ≥75% of tumor cells”.Citation7–9,Citation50 Moreover, high-dose zolbetuximab (arm 3) showed no significant improvement in overall survival compared with arm 1 (HR, 0.68; 95% CI, 0.44–1.05; P = .1285). The addition of zolbetuximab to EOX was associated with significant increases in ORR (39.0% vs. 25.0%; P = .034), response duration (32.6 months vs. 21.7 months; P = .023), and time to progression (39.1 months vs. 31.3 months; P = .0017), compared to EOX alone in the overall population. The authors suggest that these outcomes may be related to tumor inhibition and cytotoxic activity in tumor cells through ADCC and CDC.Citation50
The most common adverse events related to zolbetuximab plus EOX were nausea (81.8%), vomiting (67.5%), anemia (45.5%), and neutropenia (44.2%). The zolbetuximab plus EOX group showed no increase in the frequency of gastric hemorrhages, and the addition of zolbetuximab did not increase adverse events of any grade. In the zolbetuximab plus EOX arm, the incidence of vomiting was lower in patients who previously underwent total or partial gastrectomy (38.1%) than in patients who did not undergo gastrectomy (78.6%), whereas such a difference was not observed in the EOX alone arm. The profile of adverse events in the FAST study was similar to that in the MONO study.Citation45
The results of the MONO and FAST studies indicate that zolbetuximab has an antitumor effect on gastric cancer and has little effect on the normal gastric mucosa, despite the expression of claudin-18.2 in normal gastric mucosal and cancer cells. Zolbetuximab resulted in tumor shrinkage, prolonged survival duration, and a low incidence of adverse events such as gastric ulcer and perforation. It is speculated that most anti-claudin-18.2 antibodies cannot bind to normal gastric mucosal cells because the expression of claudin-18.2 in cancer cells is abundant on the membrane including the basolateral region, while its expression in normal mucosal cells is limited to inside the tight junction complex.Citation50 Two possible mechanisms may be responsible for this. One is that targets on the apical membrane are further from the microvasculature and are more difficult to reach than targets on the basolateral membrane, and the other is that IgG antibodies have difficulty binding to the target which is surrounded by several tight junction components. However, these speculations have not been confirmed. To confirm this hypothesis, further molecular experiments are essential and will aid to further our understanding of anti-cancer treatment using zolbetuximab.
Based on currently available results of phase I/II studies including that of MONO and FAST, two phase II studies (NCT03816163 for metastatic pancreatic cancerCitation51 and NCT03505320 for advanced G/GEJ cancer, ILUSTROCitation7) and two phase III studies (NCT03504397, SPOTLIGHTCitation8 and NCT03653507, GLOWCitation9 for advanced G/GEJ cancer) are ongoing to investigate the efficacy of zolbetuximab (). shows ongoing phase II/III clinical trials with the main characteristics and results using zolbetuximab and CAR T cells. Claudin-18.2 staining in cancer cells has been increased to ≥75% in the inclusion criteria of recent studies, as opposed to 40–50% in the MONO and FAST trials. Thus far, the trials have only included patients in Europe; however, they will now include patients from all parts of the world. As mentioned in the “Gastric Cancer” section, the detection rate of the claudin 18.2 protein varies depending on the antibody clone and race. The clones of antibodies used to evaluate the expression of claudin-18.2 in ongoing studies are not currently listed. Evaluating the claudin-18.2 expression is also important in determining the patient’s indication for the future treatment.Citation49
Conclusion
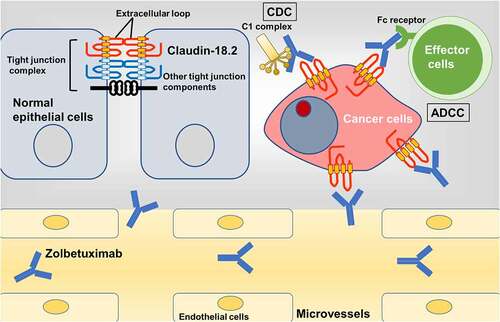
Normally, claudin-18.2 is strictly expressed at the surface of differentiated epithelial cells of the gastric mucosa and prevents the development of gastritis,Citation31,Citation32 whereas claudin-18.1 is expressed in lung alveolar epithelium and is thought to be responsible for regeneration during lung injury.Citation70 Claudin-18.2 in gastric cancer cells assists cancer progression, fusing with ARHGAP26.Citation37,Citation38 Furthermore, the expression of claudin-18.2 was transcriptionally upregulated in several malignancies.Citation6 Upregulated claudins in cancer cells are appropriate targets for anti-cancer treatment because of their dysregulated location and abundant expression. Carcinogenesis alters their localization from the apical side to the whole membrane, resulting in the loss of polarity. Antibodies or drugs targeting claudins in the bloodstream cannot reach the apical side or the tight junctions of normal cells. In cancer cells, claudins on the basolateral membrane are not bound to neighboring tight junction proteins; thus, they could be good targets for directly binding therapeutics. Unlike other claudins, claudin-18.2 is a promising target in several types of cancer because of its absence in normal tissues, except the non-neoplastic gastric mucosa, and its overexpression on the surface of cancer cells. By expanding the application of the anti-claudin-18.2 antibody to treat other cancers including pancreatic, esophageal, ovarian, and lung cancer,Citation6 we will have more therapeutic options for incurable diseases. Anti-claudin antibodies will damage cancer cells through multiple mechanisms, including the induction of the complement system and immune cell aggregation, and the inhibition of intracellular signals required for cancer progressionCitation6 (). To understand the dynamics of cancer cells following antibody binding, we need to carefully observe the results of basic molecular and clinical studies. Further investigation of claudins, which are highly and specifically expressed in cancer cells, will lead to the development of powerful new treatment options for advanced cancer patients with poor prognoses.
Figure 3. Action of zolbetuximab on claudin-18.2 of cancer cells.
Author contributions
D.K. wrote the manuscript. All authors reviewed and revised the manuscript.
Acknowledgments
The authors would like to thank Editage for their assistance with English language review.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Zihni C, Mills C, Matter K, Balda MS. Tight junctions: from simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol. 2016;17(9):64–79. doi:https://doi.org/10.1038/nrm.2016.80.
- Kyuno D, Takasawa A, Kikuchi S, Takemasa I, Osanai M, Kojima T. Role of tight junctions in the epithelial-to-mesenchymal transition of cancer cells. Biochim Biophys Acta Biomembr. 2021;1863(3):183503. doi:https://doi.org/10.1016/j.bbamem.2020.183503.
- Brunner J, Ragupathy S, Borchard G. Target specific tight junction modulators. Adv Drug Deliv Rev. 2021;171:266–288. doi:https://doi.org/10.1016/j.addr.2021.02.008.
- Schlingmann B, Molina SA, Koval M. Claudins: gatekeepers of lung epithelial function. Seminars in Cell & Developmental Biology 2015; 42:47–57. doi: https://doi.org/10.1016/j.semcdb.2015.04.009.
- Kyuno D, Zhao K, Bauer N, Ryschich E, Zoller M. Therapeutic targeting cancer-initiating cell markers by exosome miRNA: efficacy and functional consequences exemplified for claudin7 and EpCAM. Transl Oncol. 2019;12:191–199. doi:https://doi.org/10.1016/j.tranon.2018.08.021.
- Sahin U, Koslowski M, Dhaene K, Usener D, Brandenburg G, Seitz G, Huber C, Türeci Ö. Claudin-18 splice variant 2 is a pan-cancer target suitable for therapeutic antibody development. Clin Cancer Res. 2008;14(23):7624–7634. doi:https://doi.org/10.1158/1078-0432.CCR-08-1547.
- Klempner SJ, Ajani JA, Al-Batran S-E, Bang Y-J, Catenacci DVT, Enzinger PC, Ilson DH, Kim S, Lordick F, Shah MA, et al. Phase II study of zolbetuximab plus pembrolizumab in claudin 18.2: positive locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma (G/GEJ)—ILUSTRO Cohort 3. J Clin Oncol. 2021;39: TPS260–TPS. doi:https://doi.org/10.1200/JCO.2021.39.3_suppl.TPS260.
- Yamaguchi K, Shitara K, Al-Batran SE, Bang YJ, Catenacci D, Enzinger P, Ilson D, Kim S, Lordick F, Shah M, et al. SPOTLIGHT: comparison of zolbetuximab or placebo + mFOLFOX6 as first-line treatment in patients with claudin18.2+/HER2– locally advanced unresectable or metastatic gastric or gastroesophageal junction adenocarcinoma (GEJ): a randomized phase III study. Annals of Oncology. 2019;30:ix66–ix7. doi:https://doi.org/10.1093/annonc/mdz422.074.
- Xu RH, Ajani JA, Al-Batran SE, Bang YJ, Catenacci D, Enzinger PC, Ilson DH, Kim S, Lordick F, Shitara K, et al. 195TiP GLOW: phase III study of first-line zolbetuximab + CAPOX versus placebo + CAPOX in Claudin18.2⁺/HER2⁻ advanced/metastatic gastric or gastroesophageal junction adenocarcinoma (G/GEJ). Annals of Oncology. 2020;31:S1315–S6. doi:https://doi.org/10.1016/j.annonc.2020.10.459.
- Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2(4):285–293. doi:https://doi.org/10.1038/35067088.
- Zeisel MB, Dhawan P, Baumert TF. Tight junction proteins in gastrointestinal and liver disease. Gut. 2019;68(3):547–561. doi:https://doi.org/10.1136/gutjnl-2018-316906.
- Suh Y, Yoon CH, Kim RK, Lim EJ, Oh YS, Hwang SG, An S, Yoon G, Gye MC, Yi J-M, et al. Claudin-1 induces epithelial-mesenchymal transition through activation of the c-Abl-ERK signaling pathway in human liver cells. Oncogene. 2013;32(41):4873–4882. doi:https://doi.org/10.1038/onc.2012.505.
- Gowrikumar S, Ahmad R, Uppada SB, Washington MK, Shi C, Singh AB, Dhawan P. Upregulated claudin-1 expression promotes colitis-associated cancer by promoting beta-catenin phosphorylation and activation in Notch/p-AKT-dependent manner. Oncogene. 2019;38(26):5321–5337. doi:https://doi.org/10.1038/s41388-019-0795-5.
- Dhawan P, Ahmad R, Chaturvedi R, Smith JJ, Midha R, Mittal MK, Krishnan M, Chen X, Eschrich S, Yeatman TJ, et al. Claudin-2 expression increases tumorigenicity of colon cancer cells: role of epidermal growth factor receptor activation. Oncogene. 2011;30(29):3234–3247. doi:https://doi.org/10.1038/onc.2011.43.
- Che J, Yue D, Zhang B, Zhang H, Huo Y, Gao L, Zhen H, Yang Y, Cao B. Claudin-3 inhibits lung squamous cell carcinoma cell epithelial-mesenchymal transition and invasion via suppression of the Wnt/β-catenin signaling pathway. Int J Med Sci. 2018;15(4):339–351. doi:https://doi.org/10.7150/ijms.22927.
- Jiang L, Yang Y-D, Fu L, Xu W, Liu D, Liang Q, Zhang X, Xu L, Guan X-Y, Wu B, et al. CLDN3 inhibits cancer aggressiveness via Wnt-EMT signaling and is a potential prognostic biomarker for hepatocellular carcinoma. Oncotarget. 2014;5(17):7663–7676. doi:https://doi.org/10.18632/oncotarget.2288.
- Günzel D, Yu ASL. Claudins and the modulation of tight junction permeability. Physiol Rev. 2013;93(2):525–569. doi:https://doi.org/10.1152/physrev.00019.2012.
- Kyuno D, Kojima T, Yamaguchi H, Ito T, Kimura Y, Imamura M, Takasawa A, Murata M, Tanaka S, Hirata K, et al. Protein kinase Calpha inhibitor protects against downregulation of claudin-1 during epithelial-mesenchymal transition of pancreatic cancer. Carcinogenesis. 2013;34(6):1232–1243. doi:https://doi.org/10.1093/carcin/bgt057.
- Bhat AA, Uppada S, Achkar IW, Hashem S, Yadav SK, Shanmugakonar M, Al-Naemi HA, Haris M, Uddin S, et al. Tight junction proteins and signaling pathways in cancer and inflammation: a functional crosstalk. Front Physiol. 2018;9:1942. doi:https://doi.org/10.3389/fphys.2018.01942.
- Bednarz-Misa I, Fortuna P, Diakowska D, Jamrozik N, Krzystek-Korpacka M. Distinct local and systemic molecular signatures in the esophageal and gastric cancers: possible therapy targets and biomarkers for gastric cancer. Int J Mol Sci. 2020;21(12):21. doi:https://doi.org/10.3390/ijms21124509.
- Yang H, Park H, Lee YJ, Choi JY, Kim T, Rajasekaran N, Lee S, Song K, Hong S, Choi J-S, et al. Development of human monoclonal antibody for Claudin-3 overexpressing carcinoma targeting. Biomolecules. 2019;10(1):51. doi:https://doi.org/10.3390/biom10010051.
- Singh AB, Sharma A, Dhawan P. Claudin family of proteins and cancer: an overview. J Oncol. 2010;2010:541957. doi:https://doi.org/10.1155/2010/541957.
- Hewitt KJ, Agarwal R, Morin PJ. The claudin gene family: expression in normal and neoplastic tissues. BMC Cancer. 2006;6(1):186. doi:https://doi.org/10.1186/1471-2407-6-186.
- Hagen SJ. Non-canonical functions of claudin proteins: beyond the regulation of cell-cell adhesions. Tissue Barriers. 2017;5(2):e1327839. doi:https://doi.org/10.1080/21688370.2017.1327839.
- Sahin U, Jaeger D, Marme F, Mavratzas A, Krauss J, De Greve J, Vergote I, Tureci O, et al. First-in-human phase I/II dose-escalation study of IMAB027 in patients with recurrent advanced ovarian cancer (OVAR): preliminary data of phase I part. J Clin Oncol. 2015;33:5537. doi:https://doi.org/10.1200/jco.2015.33.15_suppl.5537.
- Adra N, Vaughn DJ, Einhorn L, Hanna NH, Rosales M, Arozullah A, Feldman DR, et al. A phase II study assessing the safety and efficacy of ASP1650 in male patients with incurable platinum refractory germ cell tumors. J Clin Oncol. 2020;38: TPS424–TPS. doi:https://doi.org/10.1200/JCO.2020.38.6_suppl.TPS424.
- Micke P, Mattsson JS, Edlund K, Lohr M, Jirstrom K, Berglund A, Botling J, Rahnenfuehrer J, Marincevic M, Pontén F, et al. Aberrantly activated claudin 6 and 18.2 as potential therapy targets in non-small-cell lung cancer. Int J Cancer. 2014;135(9):2206–2214. doi:https://doi.org/10.1002/ijc.28857.
- Türeci Ö, Kreuzberg M, Walter K, Wöll S, Schmitt R, Mitnacht-Kraus R, Nakajo I, Yamada T, Sahin U, et al. Abstract 882: the anti-claudin 6 antibody, IMAB027, induces antibody-dependent cellular and complement-dependent cytotoxicity in claudin 6-expressing cancer cells. Cancer Res. 2018;78:882. doi:https://doi.org/10.1158/1538-7445.Am2018-882.
- Niimi T, Nagashima K, Ward JM, Minoo P, Zimonjic DB, Popescu NC, Kimura S. Claudin-18, a novel downstream target gene for the T/EBP/NKX2.1 homeodomain transcription factor, encodes lung- and stomach-specific isoforms through alternative splicing. Mol Cell Biol. 2001;21(21):7380–7390. doi:https://doi.org/10.1128/MCB.21.21.7380-7390.2001.
- Iwaya M, Hayashi H, Nakajima T, Matsuda K, Kinugawa Y, Tobe Y, Tateishi Y, Iwaya Y, Uehara T, Ota H, et al. Colitis-associated colorectal adenocarcinomas frequently express claudin 18 isoform 2: implications for claudin 18.2 monoclonal antibody therapy. Histopathology. 2021;79(2):227–237. doi:https://doi.org/10.1111/his.14358.
- Hayashi D, Tamura A, Tanaka H, Yamazaki Y, Watanabe S, Suzuki K, Suzuki K, Sentani K, Yasui W, Rakugi H, et al. Deficiency of claudin-18 causes paracellular H+ leakage, up-regulation of interleukin-1beta, and atrophic gastritis in mice. Gastroenterology. 2012;142(2):292–304. doi:https://doi.org/10.1053/j.gastro.2011.10.040.
- Hagen SJ, Ang LH, Zheng Y, Karahan SN, Wu J, Wang YE, Caron TJ, Gad AP, Muthupalani S, Fox JG, et al. Loss of tight junction protein Claudin 18 promotes progressive neoplasia development in mouse stomach. Gastroenterology. 2018;155(6):1852–1867. doi:https://doi.org/10.1053/j.gastro.2018.08.041.
- Suzuki K, Sentani K, Tanaka H, Yano T, Suzuki K, Oshima M, Yasui W, Tamura A, Tsukita S. Deficiency of stomach-type Claudin-18 in mice induces gastric tumor formation independent of H pylori infection. Cell Mol Gastroenterol Hepatol. 2019;8(1):119–142. doi:https://doi.org/10.1016/j.jcmgh.2019.03.003.
- Yano K, Imaeda T, Niimi T. Transcriptional activation of the human claudin-18 gene promoter through two AP-1 motifs in PMA-stimulated MKN45 gastric cancer cells. Am J Physiol Gastrointest Liver Physiol. 2008;294(1):G336–43. doi:https://doi.org/10.1152/ajpgi.00328.2007.
- Kim SR, Shin K, Park JM, Lee HH, Song KY, Lee SH, Kim B, Kim S-Y, Seo J, Kim J-O, et al. Clinical significance of CLDN18.2 expression in metastatic diffuse-type gastric cancer. J Gastric Cancer. 2020;20(4):408–420. doi:https://doi.org/10.5230/jgc.2020.20.e33.
- Nakayama I, Shinozaki E, Sakata S, Yamamoto N, Fujisaki J, Muramatsu Y, Hirota T, Takeuchi K, Takahashi S, Yamaguchi K, et al. Enrichment of CLDN18-ARHGAP fusion gene in gastric cancers in young adults. Cancer Sci. 2019;110:1352–1363. doi:https://doi.org/10.1111/cas.13967.
- Yang H, Hong D, Cho SY, Park YS, Ko WR, Kim JH, Hur H, Lee J, Kim S-J, Kwon SY, et al. RhoGAP domain-containing fusions and PPAPDC1A fusions are recurrent and prognostic in diffuse gastric cancer. Nat Commun. 2018;9(1):4439. doi:https://doi.org/10.1038/s41467-018-06747-4.
- Yao F, Kausalya JP, Sia YY, Teo AS, Lee WH, Ong AG, Zhang Z, Tan JH, Li G, Bertrand D, et al. Recurrent fusion genes in gastric cancer: CLDN18-ARHGAP26 induces loss of epithelial integrity. Cell Rep. 2015;12:272–285. doi:https://doi.org/10.1016/j.celrep.2015.06.020.
- Tanaka A, Ishikawa S, Ushiku T, Yamazawa S, Katoh H, Hayashi A, Kunita A, Fukayama M. Frequent CLDN18-ARHGAP fusion in highly metastatic diffuse-type gastric cancer with relatively early onset. Oncotarget. 2018;9(50):29336–29350. doi:https://doi.org/10.18632/oncotarget.25464.
- Arnold A, Daum S, von Winterfeld M, Berg E, Hummel M, Rau B, Stein U, Treese C. Prognostic impact of Claudin 18.2 in gastric and esophageal adenocarcinomas. Clin Transl Oncol. 2020;22(12):2357–2363. doi:https://doi.org/10.1007/s12094-020-02380-0.
- Rohde C, Yamaguchi R, Mukhina S, Sahin U, Itoh K, Tureci O. Comparison of Claudin 18.2 expression in primary tumors and lymph node metastases in Japanese patients with gastric adenocarcinoma. Jpn J Clin Oncol. 2019;49(9):870–876. doi:https://doi.org/10.1093/jjco/hyz068.
- Coati I, Lotz G, Fanelli GN, Brignola S, Lanza C, Cappellesso R, Pellino A, Pucciarelli S, Spolverato G, Guzzardo V, et al. Claudin-18 expression in oesophagogastric adenocarcinomas: a tissue microarray study of 523 molecularly profiled cases. Br J Cancer. 2019;121:257–263. doi:https://doi.org/10.1038/s41416-019-0508-4.
- Lu Y, Wu T, Sheng Y, Dai Y, Xia B, Xue Y. Correlation between Claudin-18 expression and clinicopathological features and prognosis in patients with gastric cancer. J Gastrointest Oncol. 2020;11(6):1253–1260. doi:https://doi.org/10.21037/jgo-20-463.
- Dottermusch M, Kruger S, Behrens HM, Halske C, Rocken C. Expression of the potential therapeutic target claudin-18.2 is frequently decreased in gastric cancer: results from a large Caucasian cohort study. Virchows Arch. 2019;475(5):563–571. doi:https://doi.org/10.1007/s00428-019-02624-7.
- Tureci O, Sahin U, Schulze-Bergkamen H, Zvirbule Z, Lordick F, Koeberle D, Thuss-Patience P, Ettrich T, Arnold D, Bassermann F, et al. A multicentre, phase IIa study of zolbetuximab as a single agent in patients with recurrent or refractory advanced adenocarcinoma of the stomach or lower oesophagus: the MONO study. Ann Oncol. 2019;30:1487–1495. doi:https://doi.org/10.1093/annonc/mdz199.
- Sanada Y, Oue N, Mitani Y, Yoshida K, Nakayama H, Yasui W. Down-regulation of the claudin-18 gene, identified through serial analysis of gene expression data analysis, in gastric cancer with an intestinal phenotype. J Pathol. 2006;208(5):633–642. doi:https://doi.org/10.1002/path.1922.
- Oshima T, Shan J, Okugawa T, Chen X, Hori K, Tomita T, Fukui H, Watari J, Miwa H. Down-regulation of claudin-18 is associated with the proliferative and invasive potential of gastric cancer at the invasive front. PLoS One. 2013;8(9):e74757. doi:https://doi.org/10.1371/journal.pone.0074757.
- Jun KH, Kim JH, Jung JH, Choi HJ, Chin HM. Expression of claudin-7 and loss of claudin-18 correlate with poor prognosis in gastric cancer. Int J Surg. 2014;12:156–162. doi:https://doi.org/10.1016/j.ijsu.2013.11.022.
- Ungureanu BS, Lungulescu C-V, Pirici D, Turcu-Stiolica A, Gheonea DI, Sacerdotianu VM, Liliac IM, Moraru E, Bende F, Saftoiu A, et al. Clinicopathologic relevance of Claudin 18.2 expression in gastric cancer: a meta-analysis. Front Oncol. 2021;11:643872. doi:https://doi.org/10.3389/fonc.2021.643872.
- Sahin U, Tureci O, Manikhas G, Lordick F, Rusyn A, Vynnychenko I, Dudov A, Bazin I, Bondarenko I, Melichar B, et al. FAST: a randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma. Ann Oncol. 2021doi:https://doi.org/10.1016/j.annonc.2021.02.005.
- Park W, O’Reilly EM, Furuse J, Kunieda F, Jie F, Kindler HL. Phase II, open-label, randomized study of first-line zolbetuximab plus gemcitabine and nab-paclitaxel (GN) in Claudin 18.2–positive metastatic pancreatic cancer (mPC). J Clin Oncol. 2020;38: TPS4667–TPS. doi:https://doi.org/10.1200/JCO.2020.38.15_suppl.TPS4667.
- Bebnowska D, Grywalska E, Niedzwiedzka-Rystwej P, Sosnowska-Pasiarska B, Smok-Kalwat J, Pasiarski M, Góźdź S, Roliński J, Polkowski W. CAR-T cell therapy-an overview of targets in gastric cancer. J Clin Med. 2020;9(6):1894. doi:https://doi.org/10.3390/jcm9061894.
- Jiang H, Shi Z, Wang P, Wang C, Yang L, Du G, Zhang H, Shi B, Jia J, Li Q, et al. Claudin18.2-specific chimeric antigen receptor engineered T cells for the treatment of gastric cancer. J Natl Cancer Inst. 2019;111:409–418. doi:https://doi.org/10.1093/jnci/djy134.
- Zhan X, Wang B, Li Z, Li J, Wang H, Chen L, Jiang H, Wu M, Xiao J, Peng X, et al. Phase I trial of Claudin 18.2-specific chimeric antigen receptor T cells for advanced gastric and pancreatic adenocarcinoma. J Clin Oncol. 2019;37(15_suppl):2509. doi:https://doi.org/10.1200/JCO.2019.37.15_suppl.2509.
- Woll S, Schlitter AM, Dhaene K, Roller M, Esposito I, Sahin U, Tureci O, et al. Claudin 18.2 is a target for IMAB362 antibody in pancreatic neoplasms. Int J Cancer. 2014;134:731–739. doi:https://doi.org/10.1002/ijc.28400.
- Karanjawala ZE, Illei PB, Ashfaq R, Infante JR, Murphy K, Pandey A, Schulick R, Winter J, Sharma R, Maitra A, et al. New markers of pancreatic cancer identified through differential gene expression analyses: claudin 18 and annexin A8. Am J Surg Pathol. 2008;32(2):188–196. doi:https://doi.org/10.1097/PAS.0b013e31815701f3.
- Lee JH, Kim KS, Kim TJ, Hong SP, Song SY, Chung JB, Park SW. Immunohistochemical analysis of claudin expression in pancreatic cystic tumors. Oncol Rep. 2011;25:971–978. doi:https://doi.org/10.3892/or.2011.1132.
- Soini Y, Takasawa A, Eskelinen M, Juvonen P, Karja V, Hasegawa T, Murata M, Tanaka S, Kojima T, Sawada N. Expression of claudins 7 and 18 in pancreatic ductal adenocarcinoma: association with features of differentiation. J Clin Pathol. 2012;65:431–436. doi:https://doi.org/10.1136/jclinpath-2011-200400.
- Ito T, Kojima T, Yamaguchi H, Kyuno D, Kimura Y, Imamura M, Takasawa A, Murata M, Tanaka S, Hirata K, et al. Transcriptional regulation of claudin-18 via specific protein kinase C signaling pathways and modification of DNA methylation in human pancreatic cancer cells. J Cell Biochem. 2011;112(7):1761–1772. doi:https://doi.org/10.1002/jcb.23095.
- Tureci O, Mitnacht-Kraus R, Woll S, Yamada T, Sahin U. Characterization of zolbetuximab in pancreatic cancer models. Oncoimmunology. 2019;8(1):e1523096. doi:https://doi.org/10.1080/2162402X.2018.1523096.
- Shinozaki A, Shibahara J, Noda N, Tanaka M, Aoki T, Kokudo N, Fukayama M. Claudin-18 in biliary neoplasms. Its significance in the classification of intrahepatic cholangiocarcinoma. Virchows Arch. 2011;459:73–80. doi:https://doi.org/10.1007/s00428-011-1092-z.
- Keira Y, Takasawa A, Murata M, Nojima M, Takasawa K, Ogino J, Higashiura Y, Sasaki A, Kimura Y, Mizuguchi T. An immunohistochemical marker panel including claudin-18, maspin, and p53 improves diagnostic accuracy of bile duct neoplasms in surgical and presurgical biopsy specimens. Virchows Arch. 2015;466:265–277. doi:https://doi.org/10.1007/s00428-014-1705-4.
- Tokumitsu T, Sato Y, Yamashita A, Moriguchi-Goto S, Kondo K, Nanashima A, Asada Y. Immunocytochemistry for Claudin-18 and Maspin in biliary brushing cytology increases the accuracy of diagnosing pancreatobiliary malignancies. Cytopathology. 2017;28(2):116–121. doi:https://doi.org/10.1111/cyt.12368.
- Takasawa K, Takasawa A, Osanai M, Aoyama T, Ono Y, Kono T, Hirohashi Y, Murata M, Sawada N. Claudin-18 coupled with EGFR/ERK signaling contributes to the malignant potentials of bile duct cancer. Cancer Lett. 2017;403:66–73. doi:https://doi.org/10.1016/j.canlet.2017.05.033.
- Espinoza JA, Riquelme I, Sagredo EA, Rosa L, Garcia P, Bizama C, Apud-Bell M, Leal P, Weber H, Benavente F, et al. Mucin 5B, carbonic anhydrase 9 and claudin 18 are potential theranostic markers of gallbladder carcinoma. Histopathology. 2019;74(4):597–607. doi:https://doi.org/10.1111/his.13797.
- Ohta H, Chiba S, Ebina M, Furuse M, Nukiwa T. Altered expression of tight junction molecules in alveolar septa in lung injury and fibrosis. Am J Physiol Lung Cell Mol Physiol. 2012;302(2):L193–205. doi:https://doi.org/10.1152/ajplung.00349.2010.
- Li G, Flodby P, Luo J, Kage H, Sipos A, Gao D, Ji Y, Beard LL, Marconett CN, DeMaio L. Knockout mice reveal key roles for claudin 18 in alveolar barrier properties and fluid homeostasis. Am J Respir Cell Mol Biol. 2014;51:210–222. doi:https://doi.org/10.1165/rcmb.2013-0353OC.
- LaFemina MJ, Sutherland KM, Bentley T, Gonzales LW, Allen L, Chapin CJ, okkam D, Sweerus KA, Dobbs LG, Ballard PL, et al. Claudin-18 deficiency results in alveolar barrier dysfunction and impaired alveologenesis in mice. Am J Respir Cell Mol Biol. 2014;51:550–558. doi:https://doi.org/10.1165/rcmb.2013-0456OC.
- Sweerus K, Lachowicz-Scroggins M, Gordon E, LaFemina M, Huang X, Parikh M, Kanegai C, Fahy JV, Frank JA. Claudin-18 deficiency is associated with airway epithelial barrier dysfunction and asthma. J Allergy Clin Immunol. 2017;139:72–81 e1. doi:https://doi.org/10.1016/j.jaci.2016.02.035.
- Zhou B, Flodby P, Luo J, Castillo DR, Liu Y, Yu FX, McConnell A, Varghese B, Li G, Chimge NO, et al. Claudin-18-mediated YAP activity regulates lung stem and progenitor cell homeostasis and tumorigenesis. J Clin Invest. 2018;128:970–984. doi:https://doi.org/10.1172/JCI90429.
- Shimobaba S, Taga S, Akizuki R, Hichino A, Endo S, Matsunaga T, Watanabe R, Yamaguchi M, Yamazaki Y, Sugatani J, et al. Claudin-18 inhibits cell proliferation and motility mediated by inhibition of phosphorylation of PDK1 and Akt in human lung adenocarcinoma A549 cells. Biochim Biophys Acta. 2016;1863(6):1170–1178. doi:https://doi.org/10.1016/j.bbamcr.2016.02.015.
- Akizuki R, Eguchi H, Endo S, Matsunaga T, Ikari A. ZO-2 suppresses cell migration mediated by a reduction in matrix metalloproteinase 2 in Claudin-18-expressing lung adenocarcinoma A549 cells. Biol Pharm Bull. 2019;42:247–254. doi:https://doi.org/10.1248/bpb.b18-00670.
- Sahin U, Schuler M, Richly H, Bauer S, Krilova A, Dechow T, Jerling M, Utsch M, Rohde C, Dhaene K, et al. A phase I dose-escalation study of IMAB362 (Zolbetuximab) in patients with advanced gastric and gastro-oesophageal junction cancer. Eur J Cancer. 2018;100:17–26. doi:https://doi.org/10.1016/j.ejca.2018.05.007.
- Zhang J, Dong R, Shen L. Evaluation and reflection on claudin 18.2 targeting therapy in advanced gastric cancer. Chin J Cancer Res. 2020;32(2):263–270. doi:https://doi.org/10.21147/j.1000-9604.2020.02.13.
- Singh P, Toom S, Huang Y. Anti-claudin 18.2 antibody as new targeted therapy for advanced gastric cancer. J Hematol Oncol. 2017;10:105. doi:https://doi.org/10.1186/s13045-017-0473-4.
