ABSTRACT
Stem cells (SC) and amniotic membrane (AM) are recognized for their beneficial impacts on the healing of cutaneous wounds. Thus, this study evaluated the capacity of tissue repair in a skin lesion rat model. Forty Wistar rats were randomized into four groups: group I – control, with full-thickness lesions on the back, without SC or AM; group II–injected SC; group III – covered by AM; group IV–injected SC and covered by AM. Lesion closure was assessed using contraction rate (Cr). Histochemical and immunohistochemical analyses were performed, and collagen, elastic fibers, fibroblast differentiation factor (TGF-β), collagen remodeling (MMP-8), and the number of myofibroblasts and blood vessels (α-SMA) were evaluated. On the 7th postoperative day, Cr 1st-7th day levels were higher in groups III and IV. However, on the 28th day, Cr 1st-28th day were higher in the control group. Picrosirius staining showed that type I collagen was predominant in all groups; however, the SC + AM group obtained a higher average when compared to the control group. Elastic fiber analysis showed a predominance in groups that received treatment. Groups II and IV showed the lowest expression levels of TGF-β and MMP-8, and α-SMA was significantly lower in group IV. The application of SC and AM accelerated the initial healing phase, probably owing to their anti-inflammatory effect that favored early formation of collagen and elastic fibers.
Introduction
The skin is the main protective barrier of an organism and its basic functions are to prevent excessive fluid loss, protect against external agents, maintain body temperature, synthesize vitamin D when exposed to sunlight, act as a sense organ, and participate in homeostasis.Citation1
In Brazil, wounds are a serious public health problem owing to the large number of patients with changes in the integrity of their skin, although the records of these visits are scarce.Citation2 In this context, tissue repair is one of the main challenges of regenerative medicine and the cost of treating wounds is also an important factor.
Some complications can occur in skin repair, as when healing is made impossible by lack of skin elasticity, scarcity of local tissues, infection, association of orthopedic lesions with skin loss, and a demand for a prolonged healing time, required hospitalizations, and preparation for subsequent skin grafting or patchwork, which generates a negative impact on the psychological, social, and economic balance of patients. These treatments often evolve into unsightly and poorly functioning scars.Citation3
Wound healing is a complex process regulated by cell and molecular mechanisms dependent-stages of healing, leading to inflammation, proliferation, matrix formation and remodeling. During these stages occur activation and migration of keratinocytes, inflammatory response, the proliferation of fibroblasts, angiogenesis, and deposition and remodeling of extracellular matrix (ECM).Citation4 These cellular events are fundamental for the repair processes, but the easily may be impaired in hypoxia conditions or hyper inflammation. For example, hyper inflammation leads to an overexpression of metalloproteinases (MMP), degradation of growth factors, and the accumulation of senescent cells, which delay or block the wound healing.Citation5
Various studies have focused on new therapeutic strategies for extensive skin lesions,Citation6,Citation7 to improve the quality of healing and reduce treatment time, factors that interfere with the patients’ quality of life and budget. As such, the use of stem cell (SC) therapy on acute or chronic wounds accelerates the healing process because of their beneficial impact on all stages of healing,Citation8 through its trophic, paracrine, and immunomodulatory properties.Citation9 SC paracrine activity, through the secretion of several bioactive factors, have the most prominent therapeutic effect in several conditions, including wound healing.Citation5 Several studies have indicated that SC accelerate wound healing by regulating inflammatory responses, angiogenesis, increasing migration and proliferation of keratinocytes, fibroblasts and myofibroblast and activating collagen and elastin synthesis by fibroblasts. In addition to the formation of a less evident scar by regulating ECM remodeling.Citation9–11
The amniotic membrane (AM) promotes re-epithelialization, with anti-inflammatory, anti-fibrotic, and anti-angiogenic effects, making it clinically useful in different health areas. Its physical properties, particularly its thickness, smoothness, and transparency, also favor its use.Citation12,Citation13The AM is composed of a monolayer of metabolically active epithelium, a basement membrane, a compact layer, a fibroblast layer, and a spongy layer. A variety of growth factors, such as transforming growth factor-β and epidermal growth factor, as well as cytokines, have been shown to produce the epithelium and stromal matrix. In addition, AM has some components that are also present in ECM, such as collagen, elastin, laminin and fibronectin. Low immunogenicity, good biocompatibility and high affinity for human stem cells are among the reasons that make AM an attractive candidate for tissue repair.Citation14
Thus, studies in tissue engineering have sought the association of different cell types with biomaterials, accelerating the repair process by reducing the time necessary for the patient’s cells to occupy the implanted matrix.Citation15 In this context, it has been shown that the use of matrices, such as AM, associated with SC, accelerates and improves the quality of tissue regeneration.Citation16–18
Therefore, the objective of this study was to evaluate the contraction rate (Cr) and immunohistological analysis of the use of bone marrow mononuclear cells associated or not with AM in skin lesions in Wistar rats.
Materials and methods
Ethical approval
This experimental study was carried out in accordance with the rules of the Brazilian College of Animal Experimentation (COBEA) and approved by the Ethics Committee for the Use of Animals (CEUA) of the Pontifical Catholic University of Paraná (PUCPR), under the approval number 01288 and Research Ethics Committee (CEP) of the PUCPR, under the approval number 01238.
Decellularization of the amniotic membrane
Two term human placentas were donated by patients with normal pregnancy (gestational age greater 38–40 weeks and negative results for HIV-1 and 2, hepatitis B and C, and syphilis) who underwent elective cesarean sections and who signed the Informed Consent Form.
Decellularization was performed using an aseptic technique in a Class II BioSAFE (Veco®) biological safety cabinet, according to the methodology described.Citation19 For this process, the membranes were removed from pH 7.2 saline phosphate buffer (PBS) (Gibco) and treated with 0.01% SDS (sodium dodecyl sulfate) and 0.01% SD (sodium deoxycholate) solution for 24 h at 37°C, with the aid of a mechanical stirrer (stirring table 109 M, Nova Ética Ltda.). They were then preserved in PBS at 4°C.
The AM was then sectioned into appropriately sized pieces (5 × 3 cm), which was similar to the cutaneous lesion induced in the dorsal region of the rats.
Animal model
Male Wistar rats weighing 250–300 g were kept in individual cages and received commercial feed and water ad libitum.
Rat dorsum trichotomy was performed and, with a 5 × 3 cm rectangular mold (stamp), the area where the cutaneous lesion was to be induced in the dorsal region between the two scapulae was delimited, with the largest axis in the longitudinal direction.Citation20
A full-thickness skin segment with an area of 5 × 3 cm was removed. After this procedure, the animals were returned to their individual cages.
Experimental design
Forty Wistar rats were divided into four groups (n = 10 per group). Anesthesia was administered by intramuscular injection of ketamine hydrochloride (Ketamin®/Cristalia, 75 mg/kg) and xylazine hydrochloride (Calmiun® 2%/Agener União – 3 mg/kg).Citation21
In group I (control), a skin lesion was created but it was not treated with SC or AM. In group II (SC), the skin lesion received SC, and in group III (AM), the skin lesion was covered with AM and fixed on the four vertices with simple 5–0 nylon stitches, whereas the lesions in group IV (SC + AM) were treated with SC and AM. The mononuclear stem cells were applied to eight areas (subcutaneously administered 1 × 107 cells/area for eight areas in all wound edges) along the margin of the dorsal wound.Citation22,Citation23
All wounds were covered with a Vaseline rayon dressingCitation24 that was fixed with a simple 5–0 nylon stitch on the four vertices of the lesion to avoid contamination and manipulation by other animals. These dressings were removed after 2 days of evolution.
There was one death in group II on the seventh postoperative day during anesthetic induction.
Harvesting stem cells and insulation/mononuclear fraction
The SC were obtained from the puncture of the autologous bone marrow of the rat iliac crest. The bone marrow (0.5 mL) was collected and separated by density gradient centrifugation (d = 1.077 g/m3 – Ficoll-Hypaque-Sigma, St. Louis, MO, USA) using IMDI culture medium (Iscove’s modified Dulbecco medium) supplemented with antibiotics (1% penicillin and streptomycin), according to the technique described by Böyum.Citation25 SC samples were resuspended and applied subcutaneously to lesions in groups II and IV.
Macroscopic evaluation
The wound area was macroscopically evaluated 1, 7, 14, 21 and 28 days after surgery using the images captured with a digital camera (Sony DSC-W camera, 16.4 megapixels resolution], without a flash and using natural light. Macroscopic morphometry was performed using the software Image-Pro plus 4 software (Media Cybernetics, Silver Spring, Md.) to calculate the area of the wound at the different experimental periods;Citation23 the results were expressed as the percentage of regression of the skin wound, that is, the contraction rate (Cr). The Cr was calculated from expression Cr = a1-a/a1 x 100 (area of the initial wound – area of the final wound/area of the initial wound × 100).Citation26
Histological method
On the 28th postoperative day, all animals were sacrificed by an overdose of anesthesia (100 mg/kg sodium pentobarbital, intraperitoneally) and discarded according to the rules of the Brazilian College of Animal Experimentation (COBEA). The area of skin where the wound was created and the surrounding area (5 mm safety margin) were removed full thickness and fixed in 10% formalin, embedded in paraffin, and sections of 4 µm thickness were obtained for staining by histochemical and immunohistochemical techniques.
The sections were subjected to the histochemical technique with Picrosirius red staining, which colors the collagen protein, making it birefringent under the microscope polarized light,Citation27 and with the Verhoeff staining method, used to demonstrate the presence of elastic fibers.Citation28
The slides stained with Picrosirius red and Verhoeff were quantitatively evaluated via digital image analysis to calculate the area occupied by the deposition of types I and III collagen and elastic fibers in the skin wound. The images were processed using the Image-Pro plus software (Media Cybernetics, Silver Spring, MD, USA), which identified, isolated, and measured the areas occupied by type I and type III collagen and elastic fibers in relation to the total area of the image. All histomorphometric analyses were performed blindly.
Immunohistochemical method
Collagen remodeling was assessed by MMP-8 expression, and the fibroblast differentiation factor was examined by TGF-β expression. The quantification of myofibroblasts and blood vessels was detected by α-SMA expression.
The endogenous peroxidase activity was blocked with a 5% hydrogen peroxide solution in phosphate buffer saline (PBS) for 20 minutes and antigen retrieval was performed in a vaporizer (99°C) for 20 minutes, using an Immuno Retriver pH 6/9. The sections were then incubated with primary antibodies anti-MMP-8 (1:200, clone EP1252Y, Abcam, USA), anti-TGF-β (1:200, clone E11262, Spring Bioscience Corp., USA) and anti- α-SMA (1:600, clone ab5695, Abcam, USA) for overnight at temperature 2–8°C. The sections were then incubated with biotinylated goat antirabbit antibodies (HRP-Conjugate, Abcam, USA) and mouse specifying reagent (Abcam, USA) for 15 minutes. After incubation, the polymer amplification system was applied. In order to reveal the reactions, diaminobenzidine (DAB; Sigma Chemical Co®, St. Louis, MO, USA) and counterstaining with Harris hematoxylin was used.
For staining quantification, sections were analyzed using a Zeiss Axioskop 2 plus microscope (Carl Zeiss, Gottingen, Germany) and 20 fields of the dermis of each slide were randomly captured, covering the entire length of the scar area, enlarged 400 × . All images were analyzed using the Image-Pro plus software (Media Cybernetics, Silver Spring, MD, USA) as previously described.Citation23 The number of immunologically positive cells and total cells in each area was counted, and the percentages of positively-labeled cells were calculated.
Statistical analysis
Quantitative variables were compared using analysis of variance (ANOVA), the Kruskal-Wallis test, or the Friedman non-parametric test, whereas normality was assessed using the Shapiro-Wilk test. For qualitative variables, Fisher or Chi-square tests were performed. A p-value < .05 indicated statistical significance. Data are presented as mean ± standard deviation (SD).
Results
Contraction rate
The skin wounds of the animals were evaluated macroscopically 1, 7, 14, 21 and 28 days after the surgical procedures for the reduction of the wound area.
The wound sizes of the groups that received SC and/or AM were significantly smaller following the initial seven days of the repair process (p = .016) ( and ).
Figure 1. Surgical technique. A cutaneous lesion was induced in the dorsal region between the two scapulae with the largest axis in the longitudinal direction. Mononuclear stem cells were administered subcutaneously at eight points (yellow) along the margins and in the center of the wound.
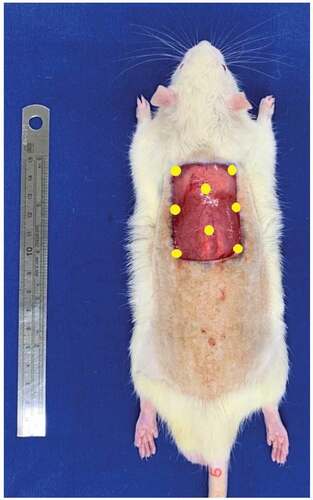
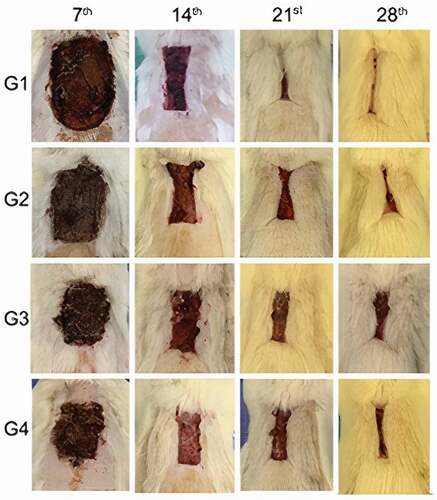
Figure 2. Macroscopic evaluation of wounds. Evaluation of wounds on the 7th, 14th, 21st, and 28th postoperative days for the groups G1 (control), G2 (SC), G3 (AM), and G4 (SC + AM). In the groups that received SC and/or AM, the wounds were significantly smaller after the initial 7 days of the repair process. After 28 days, the wounds in the control group were more contracted than those in the other groups.
On the 7th postoperative day, Cr 1st-7th day was significantly higher in group IV (SC+AM) than in group I (control) (52.3 ± 12.7 versus 34.1 ± 34.3; p = .022). The lowest contraction rates on the 7th-14th and 14th-21st day were in the AM and SC + AM groups (; ).
Table 1. Descriptive statistics of the contraction rates
Table 2. P Values in the comparisons between groups two and two
Figure 3. Graph showing the contraction rate of the groups in the 7th, 14th, 21st, 28th days after surgery.
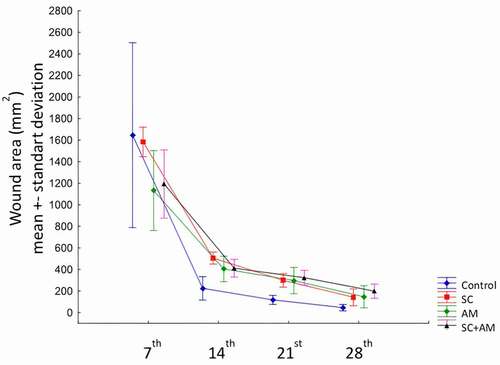
After 28 days, Cr 1st-28th day was the highest in the control group, followed by groups II (SC) and III (AM) (p < .001). Cr 1st-28th day was higher in group I than in group IV (98.2 ± 1.2 versus 92.1 ± 2.6; p < .001), indicating that the control group wounds contracted more quickly (; ).
Considering that a significant difference was found between the groups regarding the areas on days 7, 14, 21 and 28, comparisons were made between groups two by two. shows the p values of these comparisons.
Histological analysis
The dermis is formed by an ECM rich in collagen and elastic fibers, in addition to blood and lymphatic vessels, sebaceous and sweat glands, and nerve endings. Thus, it attributes structural integrity, elasticity and nutrition to the skin. On the 28th day of the experiment, histological analyzes of collagen and elastic fibers were performed using Picrosirius red and Verhoeff.
Descriptive statistics for type I and III collagen showed a predominance of type I collagen in all four groups studied, with SC+AM group had the highest mean (81,5 ± 3,8; p = .057). In relation to type III collagen, the control group had the highest average (29.4 ± 13.5; p = .057) (, ).
Table 3. Descriptive statistics of type I and III collagen and elastic fiber
Figure 4. Photomicrography showing the area of collagen type I and III (400x). Picrossirius Red stains type I collagen in red and type III collagen in green, indicated by the solid yellow and dotted arrows, respectively. Analysis of captured images the polarized light microscope showed a predominance of collagen type I (mature) in all groups at the end of the experiments (28th day).
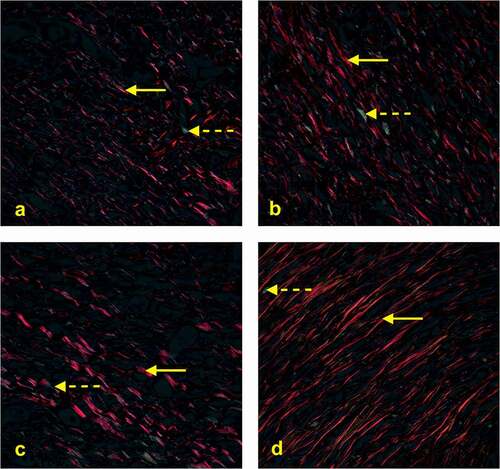
The p value of .057 indicates that there is a tendency for a significant difference between the four groups. When specifically comparing the control and SC+AM groups, a significant difference was found (p = .033; Student’s t-test for independent samples).
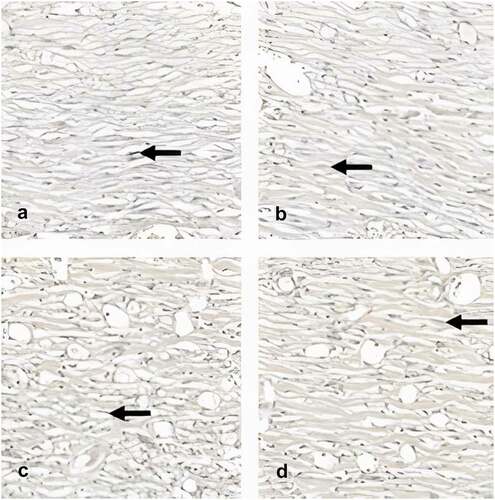
The analysis of elastic fibers showed the highest averages in the groups treated with SC and/or AM (p = .215) (, ).
Figure 5. Photomicrography showing the area of elastic fiber (400x). Black arrows indicate elastic fibers stained black by the Verhoeff technique. After 28 days of the experiments, there was a predominance of elastic fibers in the AM group, followed by the SC + AM, SC and control groups.
Immunohistochemical analysis
MMP-8 expression revealed a lower expression in groups II and IV (SC and SC+AM) (0.344 ± 1.428 and 0.646 ± 0.765; p = .699).
TGF-β expression showed the lowest percentages in groups II and IV (2.18 ± 1.66 and 2.73 ± 3.33; p = .468).
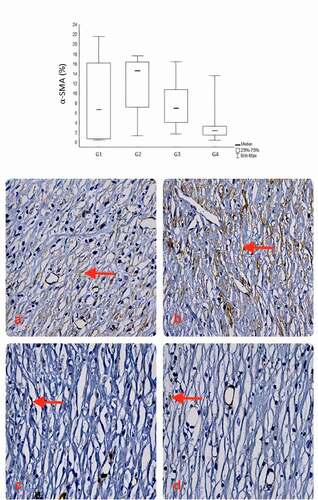
The results showed a lower expression of α-SMA in group IV (3.89 ± 4.45), followed by groups III, I, and II (7.62 ± 4.45; 8.23 ± 7.89; 11.59 ± 5.85; p = .054), respectively.
, and summarizes the immunohistochemical staining of the average percentages of positive-labeled cell expression.
Figure 6. Comparison of groups in relation to the MMP-8. In the analysis of collagen remodeling on the 28th day, the results showed a lower expression of MMP-8 in the SC and SC + AM groups. Red arrows indicate MMP-8 expression stained brown (400X).
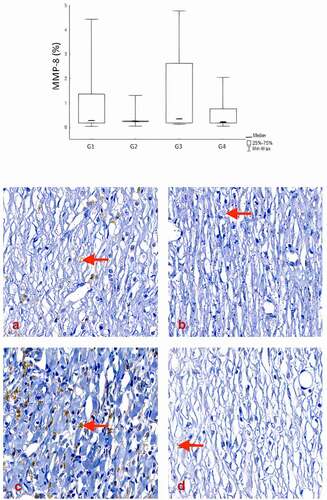
Figure 7. Comparison of groups in relation to the TGF-β. The expression of TGF-β showed the lowest percentages in the SC and SC + AM groups on the 28th day of the experiments. Red arrows indicate TGF-β expression stained brown (400X).
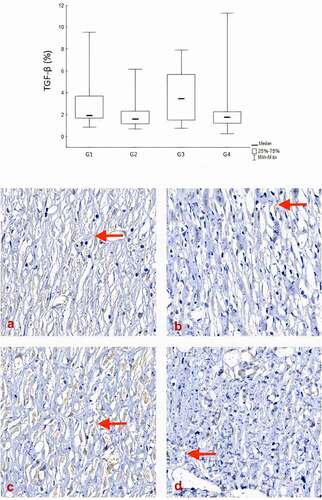
Figure 8. Comparison of groups in relation to the α-SMA. The expression of α-SMA verified the quantification of myofibroblasts and blood vessels, and showed the lowest percentages in the SC + AM group, followed by the AM, Control and SC group at the end of the experiments (28th day). Red arrows indicate expression of α-SMA stained brown (400X).
Discussion
The contraction of a wound is an attempt to reduce the healing time of the injury. There are two theories for the reduction the bloody areas of wounds. In the myofibroblast theory, fibroblasts acquire characteristics of smooth muscle fibers rich in actin microfilaments that, together with the expression of α-SMA, contract the wound. In the second theory, a traction force in the center of the matrix is exerted by fibroblasts, which reorganize collagen fibers, leading to wound contraction.Citation29
In this study, the application of autologous bone marrow mononuclear stem cells and amniotic membrane demonstrated a positive effect by increasing the rate of wound contraction in the first seven days of the repair process. When comparing group I (control) with group IV (SC+AM), we found a Cr 1st-7th day approximately 35% faster in the treated group (p = .022; ).
The contraction rates on the 7th-14th, 14th-21st and 21st-28th day were higher in the control group. After 28 days, Cr 1st-28th day was also higher in the control group, indicating that wounds contracted more quickly, but when we compared this value with those in groups I and IV, wounds closure was 6% faster in the control group (p < .001; ). One of the questions raised by these results was if AM mechanical action would impair wound reduction by maintaining myofibroblasts with less distensibility. However, there are other factors linked to contraction, such as the presence of growth factors and connection genes of the occlusion zone 1,Citation30 which may have influenced this result.
In another hypothesis, this could result from the observation period, since we did not evaluate the lesion until its complete healing. As SC influence all stages of healing (inflammatory, proliferative, and remodeling),Citation8,Citation31 we were unable to confirm the full potential of the treatment. Additionally, SC changes the inflammatory process to a less fibrinogenic process.
In an experimental study published by Longo et al., there was no significant difference in planimetric analyses with the use of human acellular AM for healing third degree thermal burns in rats,Citation32 a conclusion similar to that found in our experimental model.
The results showed that SC and AM application probably reduced the duration of the initial healing phase owing to the anti-inflammatory action, which favored the early onset of collagen fiber formation, as in the remodeling phase, characterized by the replacement of type III collagen for type I collagen. In the present study, collagen fibers of animals in the SC+AM group reached maturity more quickly when compared to the control.
In 2018, Campelo et al. found that treatment with AM accelerated the wound healing process. The anti-inflammatory AM action reduced the duration of the initial healing phase. Another relevant factor is the function of the AM as a protective barrier for injured tissues, which favored the early start of the repair process in protected wounds, in addition to preventing fluid loss and microorganism contamination.Citation26 The results corroborate those of our research on the first 7 days of evolution.
Several studies have demonstrated the beneficial effect promoted by SC treatment derived from bone marrow or adipose tissue by accelerating epithelialization and increasing the formation of granulation tissue and angiogenesis.Citation8,Citation31 SC have a high therapeutic potential to regenerate damaged skin, mainly through paracrine signaling and differentiation.Citation10 Studies suggest that the contribution of SC differentiation is limited owing to insufficient graft and survival at the injury site. Paracrine signaling is probably the primary mechanism for the beneficial effects of SC on wounds, reducing inflammation, promoting angiogenesis, and inducing cell migration and proliferation.Citation31
Histologically, the dermis is composed of dense connective tissue formed mainly by fibroblasts and an extracellular matrix rich in type I and type III collagen and elastic fibers. Then, the dermis attributes structural integrity, elasticity and nutrition to the skin. Images captured by polarized light microscopy showed a predominance of type I (mature) collagen in all groups, with group treated SC+AM had the highest average. In relation to type III collagen (immature), group I had the highest average. When we specifically compared the control and SC+AM groups, a significant difference was found (p = .033).
In 2012, Yun et al. injected SC derived from adipose tissue into skin scars developed after the induction of full-thickness lesions and concluded that SC accelerated scar remodeling.Citation33 Another study, which also corroborates our results, reported that, in the wound healing process in rabbits treated with AM, there was a predominance of mature collagen fibers.Citation34 Therefore, the results confirm the acceleration of the maturity of collagen fibers with AMCitation34 and SCCitation33 use.
The Verhoeff technique showed a predominance of elastic fibers in group III, followed by groups IV, II, and I, evidence of the benefit of AM and/or SC treatment in skin repair. In 2019, Hatzfeld et al. ratified our findings by demonstrating that the healing of full-thickness burns in swine treated with AM stimulated the synthesis of elastic fibers and induced a well-formed network of elastin fibers.Citation35
Recent studies have shown that SC can decrease elastin synthesis or increase the degradation of existing elastic fibers. The molecular mechanisms that guide these events remain poorly understood, and such hypotheses conflict with previous descriptions that demonstrated a positive contribution of SC treatment to changes in elastic fibers, such as aortic aneurysms or chronic obstructive pulmonary disease.Citation36
In 2017, another study confirmed the results of this research, Ochiai et al. studied the healing of linear porcine skin wounds and demonstrated that after 28 days of surgery, the wounds of the control group had few elastic fibers, in contrast to the group treated with bone marrow-derived SC, with elastic fibers and appearance of fibroblasts similar to normal skin.Citation37
The collagen remodeling process, that is, the balance between deposition and catabolism, and the replacement of type III collagen, characteristic of the immature scar, by type I collagen, more resistant and predominant in the mature scar,Citation38,Citation39 are coordinated by proteins called matrix metalloproteinases.
In our study, immunohistochemical evaluation revealed that MMP-8 expression was lower in groups II and IV. This is consistent with the higher percentage of type I collagen in these groups, because MMP-8 is an interstitial collagenase and its increase favors mature collagen breakdown, impairing the repair of lesions.Citation40
TGF-β plays an important role in myofibroblastic differentiation during fibrocontractive and scarring phenomena by regulating the expression of α-AML in these cells. Furthermore, TGF-β is a multifunctional regulator of cell growth and differentiation during development and repair.
Stem cell-treated groups (II and IV) showed the lowest TGF-β expression, probably owing to the anti-inflammatory characteristics of SC that can suppress the activation of mast cells and TGF-β, which play an important role in the formation of scars, being a differentiating factor for fibroblasts.Citation33
Fibroblasts are activated by α-SMA expression, becoming myofibroblasts, which play an essential role in wound contraction/closureCitation41 and secrete extracellular matrix components, such as collagen, glycosaminoglycans, fibronectin, laminin, and elastin, which replace the provisional matrix initially formed.Citation42
α-SMA expression was significantly lower in group IV (SC+AM), which showed the lowest rate of contraction between days 1 and 28 (Cr 1st–28th day), including on the last seven days (Cr 21st–28th day) of the trial period. On the other hand, the greatest expression was found in group II [SC), ratifying the results of Yamaguchi et al., which showed that bone marrow SC, when applied topically to skin wounds, differentiate into myofibroblasts and accelerate wound healing when combined with occlusive dressings.Citation43
The anti-fibrotic effect of the human AM can be explained by the inhibitory action of this tissue on TGF-β expression, which activates fibroblasts that, in turn, are responsible for the healing process. As a result, the fibrotic process is inhibited.Citation12 In this way, the AM can modulate healing through the promotion of tissue reconstruction to the detriment of scar formation.Citation44
In 2012, Yun et al. injected SC derived from adipose tissue into skin scars developed after the induction of full-thickness lesions and concluded that SC accelerated scar remodeling, reduced the activity of mast cells and myofibroblasts, and suppressed the action of TGF-β on fibroblasts, which is in line with our results, in addition to increasing the effects of MMP-1 on collagen remodeling.Citation33
In conclusion, these findings suggest that mononuclear SC and/or AM accelerate the rate of injury contraction in the first seven days of the repair process by reducing inflammatory activity and increasing the maturity of collagen and elastic fibers. SC and AM therapy of skin lesions did not increase the Cr of full-thickness skin lesions in the more advanced stages of healing, but can promote the repair process through collagen remodeling and anti-fibrotic action.
However, additional experiments are still needed to overcome the limitations of our experimental design and to elucidate the mechanism through which SC and AM influence the wound healing process.
Author Contributions
TAKEJIMA, FRANCISCO, SIMEONI, NORONHA, GARBERS, FOLTZ, MACHADO-JUNIOR, SOUZA, PINHO, CARVALHO and GUARITA-SOUZA: Acquisition, analysis, and interpretation of data; drafting and revising the manuscript; final approval of the version to be published
Data Accessibility Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethical Approval
This experimental study was carried out in accordance with the rules of the Brazilian College of Animal Experimentation (COBEA) and approved by the Ethics Committee for the Use of Animals (CEUA) of the Pontifical Catholic University of Paraná (PUCPR), under the approval number 01288, and PUCPR Research Ethics Committee (CEP), under the approval number 01238.
Supplemental Material
Download MS Word (396.5 KB)Acknowledgments
The authors thank the Pontifical Catholic University of Paraná (PUCPR) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) for supporting this work.
Disclosure statement
The authors declare no potential conflict of interest.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Boyko TV, Longaker MT, Yang GP. Laboratory models for the study of normal and pathologic wound healing. Plast Reconstr Surg. 2017;139:1–15. doi:https://doi.org/10.1097/PRS.0000000000003077.
- Brasil, Ministério da Saúde. Secretaria de Políticas de Saúde. Departamento de Atenção Básica. Manual de condutas para úlceras neurotróficas e traumáticas/Ministério da Saúde, Secretaria de Políticas de Saúde. Departamento de Atenção Básica. Brasília, Distrito Federal / Brazil: Ministério da Saúde, 2002. [accessed 2020 July]. http://bvsms.saude.gov.br/bvs/publicacoes/manual_feridas_final.pdf
- Broughton G, Janis JE, Attinger CE. The basic science of wound healing. Plast Reconstr Surg. 2006;117:12S–34S. doi:https://doi.org/10.1097/01.prs.0000225430.42531.c2.
- Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014;6:265sr6. doi:https://doi.org/10.1126/scitranslmed.3009337.
- Lombardi F, Palumbo P, Augello FR, Cifone MG, Cinque B, Giuliani M. Secretome of adipose tissue-derived stem cells (ASCs) as a novel trend in chronic non-healing wounds: an overview of experimental in vitro and in vivo studies and methodological variables. Int J Mol Sci. 2019;20:3721. doi:https://doi.org/10.3390/ijms20153721.
- Karimi H, Soudmand A, Orouji Z, Taghiabadi E, Mousavi SJ. Burn wound healing with injection of adipose-derived stem cells: a mouse model study. Ann Burns Fire Disasters. 2014;27:44–49.
- Pessolato AG, Martins DOS, Ambrósio CE, Mançanares CA, de Carvalho AF. Propolis and amnion reepithelialise second-degree burns in rats. Burns. 2011;37:1192–1201. doi:https://doi.org/10.1016/j.burns.2011.05.016.
- Hocking AM. Mesenchymal stem cell therapy for cutaneous wounds. Adv Wound Care New Rochelle. 2012;1:166–171. doi:https://doi.org/10.1089/wound.2011.0294.
- Xiao S, Xiao C, Miao Y, Wang J, Chen R, Fan Z, Hu Z. Human acellular amniotic membrane incorporating exosomes from adipose-derived mesenchymal stem cells promotes diabetic wound healing. Stem Cell Res Ther. 2021;12:1–16. doi:https://doi.org/10.1186/s13287-021-02333-6.
- Hassan WU, Greiser U, Wang W. Role of adipose-derived stem cells in wound healing. Wound Repair Regener. 2014;22:313–325. doi:https://doi.org/10.1111/wrr.12173.
- Jackson WM, Nesti LJ, Tuan RS. Mesenchymal stem cell therapy for attenuation of scar formation during wound healing. Stem Cell Res Ther. 2012;3:20. doi:https://doi.org/10.1186/scrt111.
- Mamede AC, Carvalho MJ, Abrantes AM, Laranjo M, Maia CJ, Botelho MF. Amniotic membrane: from structure and functions to clinical applications. Cell Tissue Res. 2012;349:447–458.https://doi.org/10.1007/s00441-012-1424-6
- Toda A, Okabe M, Yoshida T, Nikaido T. The potential of amniotic membrane/amnion-derived cells for regeneration of various tissues. J Pharmacol Sci. 2007;105:215–228. doi:https://doi.org/10.1254/jphs.CR0070034.
- Moravvej H, Memariani H, Memariani M, Kabir-Salmani M, Shoae-Hassani A, Abdollahimajd F (2021). Evaluation of fibroblast viability seeded on acellular human amniotic membrane. BioMed research international. 5597758.
- Kamel RA, Ong JF, Eriksson E, Junker JP, Caterson EJ. Tissue engineering of skin. J Am Coll Surg. 2013;217:533–555. doi:https://doi.org/10.1016/j.jamcollsurg.2013.03.027.
- Farzamfar S, Salehi M, Ehterami A, Naseri-Nosar M, Vaez A, Zarnani AH, Aleahmad M, Shokri M-R, Aleahmad M. Promotion of excisional wound repair by a menstrual blood-derived stem cell-seeded decellularized human amniotic membrane. Biomed Eng Lett. 2018;8:393–398. doi:https://doi.org/10.1007/s13534-018-0084-1.
- Kim CH, Kim SS, Shon SK, Kim DH, Song CG, Kim HJ. The effect of human amniotic membrane, epidermal cells and marrow mesenchymal stem cells in healing a skin defect. J Korean Orthop Assoc. 2008;43:276–286. doi:https://doi.org/10.4055/jkoa.2008.43.3.276.
- Kim SS, Song CK, Shon SK, Lee KY, Kim CH, Lee MJ, Wang L. Effects of human amniotic membrane grafts combined with marrow mesenchymal stem cells on healing of full-thickness skin defects in rabbits. Cell Tissue Res. 2009;336:59–66. doi:https://doi.org/10.1007/s00441-009-0766-1.
- Francisco JC, Uemura L, Simeoni RB, da Cunha RC, Mogharbel BF, Simeoni PRB, Guarita-Souza LC, Napimoga MH, Noronha L, Carvalho KAT. Acellular human amniotic membrane scaffold with 15d-PGJ2 nanoparticles in postinfarct rat model. Tissue Eng Part A. 2020;26:1128–1137. doi:https://doi.org/10.1089/ten.tea.2019.0340.
- Calisto FC, Calisto SL, Souza AP, França CM, Ferreira AP, Moreira MB. Use of low-power laser to assist the healing of traumatic wounds in rats. Acta Cirúrgica Brasileira. 2015;30:204–208. doi:https://doi.org/10.1590/S0102-865020150030000007.
- Schanaider A, Silva PC. The use of animals in experimental surgery. Acta Cirúrgica Brasileira, São Paulo. 2004;19:441–447. doi:https://doi.org/10.1590/S0102-86502004000400014.
- Fedato RA, Francisco JC, Sliva G, de Noronha L, Olandoski M, Neto JRF, … Guarita-Souza LC (2019). Stem cells and platelet-rich plasma enhance the healing process of tendinitis in mice. Stem Cells International. 1497898.
- Kuo YR, Wang CT, Cheng JT, Wang FS, Chiang YC, Wang CJ. Bone marrow-derived mesenchymal stem cells enhanced diabetic wound healing through recruitment of tissue regeneration in a rat model of streptozotocin-induced diabetes. Plast Reconstr Surg. 2011;128:872–880. doi:https://doi.org/10.1097/PRS.0b013e3182174329.
- Santos JAF, Dias MB, Oliveira RA, Nicolau RA, Rezende VEA, Arisawa EÂL. Effects of low power light therapy on the tissue repair process of chronical wound in diabetic feet. Photomed Laser Surg. 2018;36:298–304. doi:https://doi.org/10.1089/pho.2018.4455.
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of mononuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89.
- Campelo MBD, Santos JAF, Maia Filho ALM, Ferreira DCL, Sant’Anna LB, de Oliveira RA, Arisawa EÂL, Arisawa EÂL. Effects of the application of the amniotic membrane in the healing process of skin wounds in rats. Acta Cirúrgica Brasileira. 2018;33:144–155. doi:https://doi.org/10.1590/s0102-865020180020000006.
- Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11:447–455. doi:https://doi.org/10.1007/BF01002772
- Jones ML, Bancroft JD, Gamble M. Connective tissues and stains. In: Bancroft JD, Gamble M editors. Theory and practice of histological techniques. 6th. Philadelphia: Churchill Livingstone, Elsevier; 2008. p. 135–160.
- Porter S. The role of the fibroblast in wound contraction and healing. Wounds UK. 2007;3:33–40.
- Rhett JM, Ghatnekar GS, Palatinus JA, O’Quinn M, Yost MJ, Gourdie RG. Novel therapies for scar reduction and regenerative healing of skin wounds. Trends Biotechnol. 2008;26:173–180. doi:https://doi.org/10.1016/j.tibtech.2007.12.007.
- Maxson S, Lopez EA, Yoo D, Danilkovitch-Miagkova A, Leroux MA. Concise review: role of mesenchymal stem cells in wound repair. Stem Cells Transl Med. 2012;1:142–149. doi:https://doi.org/10.5966/sctm.2011-0018.
- Longo B, Orth AFPS, Hubner JZ, Carreiro MM, Junior GSS. The use of human’s acellular amniotic membrane to heal third degree burns in rats. Revista Médica Do Paraná, Curitiba. 2013;71:24–29.
- Yun IS, Jeon YR, Lee WJ, Lee JW, Rah DK, Tark KC, Lew DH. Effect of human adipose derived stem cells on scar formation and remodeling in a pig model: a pilot study. Dermatol Surg. 2012;38:1678–1688. doi:https://doi.org/10.1111/j.1524-4725.2012.02495.x.
- Duarte IGL, Duval-Araujo I. Amniotic membrane as a biological dressing in infected wound healing in rabbits. Acta Cirúrgica Brasileira, São Paulo. 2014;29:334–339. doi:https://doi.org/10.1590/S0102-86502014000500008.
- Hatzfeld AS, Pasquesoone L, Germain N, Danzé P, Drucbert A, Tardivel M, Marchetti P, Duquennoy‐Martinot V, Guerreschi P, Marchetti P. Benefits of cryopreserved human amniotic membranes in association with conventional treatments in the management of full-thickness burns. Int Wound J. 2019;16:1354–1364. doi:https://doi.org/10.1111/iwj.13198.
- Rodrigues HWS, Neto NMA, Silva LDS, Carvalho MAM, Monteiro BS. Stereological and morphometric study of type 3 collagen formation in the cutaneous wounds of diabetic mice treated with mesenchymal stem cells. Acta Scientiae Veterinariae. 2018;46:1553. doi:https://doi.org/10.22456/1679-9216.82619.
- Ochiai H, Kishi K, Kubota Y, Oka A, Hirata E, Yabuki H, Umezawa A, Suzuki H, Umezawa A. Transplanted mesenchymal stem cells are effective for skin regeneration in acute cutaneous wounds of pigs. Regen Therapy. 2017;7:8–16. doi:https://doi.org/10.1016/j.reth.2017.06.003.
- Cheng W, Yan-Hua R, Fang-Gang N, Guo-an Z. The content and ratio of type I and III collagen in skin differ with age and injury. Afr J Biotechnol. 2011;10:2524–2529.
- Wang L, Chen L, Zhou X, Xiong Z, Zhang C, Shehada HMA, Chen L, Song J, Chen L. Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Sci Rep. 2017;7:13321. doi:https://doi.org/10.1038/s41598-017-12919-x.
- Caley MP, Martins VL, O’Toole EA. Metalloproteinases and wound healing. Adv Wound Care. 2015;4:225–234. doi:https://doi.org/10.1089/wound.2014.0581.
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi:https://doi.org/10.1038/nrm809.
- Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol. 2007;25:9–18. doi:https://doi.org/10.1016/j.clindermatol.2006.09.007.
- Yamaguchi Y, Kubo T, Murakami T, Takahashi M, Hakamata Y, Kobayashi E, Itami S, Hosokawa K, Yoshikawa K, Itami S. Bone marrow cells differentiate into wound myofibroblasts and accelerate the healing of wounds with exposed bones when combined with an occlusive dressing. Br J Dermatol. 2005;152:616–622. doi:https://doi.org/10.1111/j.1365-2133.2005.06402.x.
- Manuelpillai U, Tchongue J, Lourensz D, Vaghjiani V, Samuel CS, Liu A, Sievert W, Sievert W. Transplantation of human amnion epithelial cells reduces hepatic fibrosis in immunocompetent CCl4-treated mice. Cell Transplant. 2010;19:1157–1168. doi:https://doi.org/10.3727/096368910X504496.
