ABSTRACT
Background
Sodium arsenite is a dangerous bio-accumulative poison affecting a large number of people as well as animals throughout the world. It is used clinically in the treatment of certain medical conditions, but due to its harmful damage to different tissues and mainly the cardiotoxicity, its medical application is limited.
Aim
This study was conducted to investigate the protective effects of spirulina on cardiotoxicity induced by sodium arsenite biochemically and histologically.
Methods
30 young adult male albino rats were randomly equally divided into three groups 10 animals each. Group I (control), Group II Arsenic intoxicated (10 mg/kg/day/ 4 weeks), Group III spirulina protected animals (concomitant sodium arsenite 10 mg/kg/day/ 4 weeks and spirulina 200 mg/kg/day/ 4 weeks).
Results
It was evident from the study that arsenic exposure exerted a significant increase in cardiac enzyme levels, serum creatine kinase MB (CKMB) and troponin. Concomitant treatment with spirulina is considerably recovered their serum levels. Histological alterations associated with arsenite treated animals are significantly decreased after using spirulina.
Conclusions
The results of the present study showed that use of spirulina could alleviate the toxic effects on the heart following exposure to arsenic toxicity.
INTRODUCTION
Heavy metal arsenic is considered as a ubiquitous trace element and the 52nd most common element in the earth’s crust. Along with the useful effects, it is well known to be a powerful toxic heavy metal. Arsenic toxicity is a worldwide health problem affecting millions of persons worldwide through environmental and occupational exposure, as well as intended suicide and homicide attempts. Arsenic contamination happens due to its industrial usage in the manufacturing of agricultural insecticides, wood preservatives, and glass production and in medicine.Citation1–6 Arsenic trioxide is frequently used for the treatment of patients with acute promyelocytic leukemia since the U.S. Food and Drug Administration authorized it in the 1970s, but its applicability has been limited due to its cardiotoxic effects. Some earth rocks are rich in this element, and the excessive requests of agricultural insecticides and rodenticides maximize arsenic contamination in ground water.Citation7–9 Pollution of arsenic in the soil results in arsenic aggregation in the food products and thus enters the food chain. Rice is a common food in many countries, it is at a higher risk for arsenic accumulation since it is capable of accumulating various heavy metals as well as metalloids existent in the soil, out of which, arsenic is the most common.Citation10–12 In broiler farms, arsenic in the forms of roxarsone and arsenilic acid is used as chemical in the feed which is used as controller of protozoan parasites and to increase weight gain. If the recommended levels of arsenic in broiler feed are not observed strictly, then it can accumulate in broiler flesh, which might be detrimental to the consumers.Citation13,Citation14 The industrial contamination of arsenic causes tragic changes in freshwater systems. Thus, arsenic contamination can also disturb the structure of lake food webs through trophic transfer.Citation15
Chronic arsenic poisoning can cause serious health problems including cancers, hyperkeratosis, restrictive lung disease, and ischemic heart disease, noncancerous skin lesions, bronchitis, hepatomegaly, neuropathy, peripheral vascular diseases, and cardiovascular disease. The increased production of free radicals yielding to oxidative stress has been assumed as one of the major mechanisms behind arsenic-induced poisoning in different organs. The high production of free radicals via oxidative stress has been implicated as one of the numerous mechanisms related to arsenic-induced toxic effects in different organs. The potential of dietary antioxidants to reduce the arsenic burden in human by increasing its metabolism has drawn attention in recent years. Vitamins such as A, E, C routine with arsenic-free water were shown to be successful in improving arsenic-induced melanosis and keratosis. Some investigators suggested that the effectiveness of retinol, β-carotene, ascorbic acid, selenium, tocopherol, alpha lipoic acid, spinach, for the reduction of body burden of chronic arsenic toxicity. Spirulina extract plus zinc was found to be advantageous in patients of chronic arsenic poisoning.Citation16–21
Plants and herbs have been used throughout the world as treatment agents. The Spirulina is a filamentous blue-green alga that is normally found in tropical and subtropical areas in warm alkaline water. Spirulina platensis (SP) is a cyanobacterium, which has a mounting attention for of its nutritional value and pharmacological properties. It is characterized by high nutritional value where it contains high protein content (60–70% by dry weight), plenty of vitamins, amino acids, gamma-linoleic acid, and minerals. Spirulina has protective effects against oxidative stress and this effect is associated to C-phycocyanin. Phycocyanin reduced ischemia-reperfusion of cardiac dysfunction by its antioxidant and anti-apoptotic activities. It also has neuroprotective effects. Limited studies have stated the therapeutic implications of Spirulina for the treatment of neurodegenerative disorders.Citation22–24 The intake of Spirulina as a diet supplement has health benefits in preventing or managing hypercholesterolemia, hyperglucagonemia, obesity, inflammation, cancer, antidiabetic effect and cardiovascular disease.Citation18 These activities were mainly associated to phycocyanin, a dynamic protein of Spirulina. It has been reported that Phycocyanin has significant antioxidant and radical scavenging properties, offering protection against oxidative stress. Antioxidants can minimize arsenic toxicity via chelating it and scavenging free radicals. It was reported that Phycocyanin could bind with heavy metals; hence, it can chelate and remove them.Citation25–27
One of the most common causes of death worldwide is cardiovascular diseases. The problem of heart disease is a substantial public concern. According to Ministry of Health in Jordan, the top four leading causes of death in Jordan are: Circulatory system diseases (39.1%), Neoplasm’s (16.5%), External causes of mortality (8.2%), Endocrine, nutritional and metabolic diseases (8.1%).Citation28 Furthermore, the prevalence of IHD in Jordan accounts for 54.7 of the annual deaths according to The Institute for Health Metrics and Evaluation (IHME).Citation29 The potential protective effects of Spirulina have been studied in many different tissues;Citation22–24 however, to the best of our knowledge it is the first time to study its beneficial effects on the cardiac tissue. In view of the potential beneficial effects and antioxidant properties of spirulina, the present study was designed to evaluate its effects on arsenic-induced cardiotoxicity in young male albino rats.
MATERIAL & METHODS
Chemicals: Sodium arsenite was purchased from Sigma Chemicals, St Louis, MO, USA. The arsenic compound (NaAsO2) was dissolved in distilled water instantly prior to use. The required amount of NaAsO2 for a day (5 mg/kg BW) was weighted separately for each group of rats and mixed with the drinking water daily immediately before allocation for drinking for that particular group. Generally, 10 ml drinking water per rat was assigned for mixing NaAsO2 to make sure that the full amount of NaAsO2 was taken by the rats. Once finishing the drinking of the NaAsO2 mixed water, normal drinking water was supplemented ad libitum. Spirulina platensis powder was purchased from local herbal market, a product of Siam Algae Company (Thailand). It was administrated at a dose of 200 mg/kg per day by oral gavage for 4 weeks.
Experimental Animals: A total of 30 Inbred mature male albino rat (Sprague Dawley strain), average weight 150–200 g, were obtained from the animal facility of College of Medicine, Mutah University. They were maintained under standard conditions 12:12 light: dark cycle at room temperature 24 ± 1°C, and 50%±10% relative humidity. All animals were kept in wire mesh cages and drinking tap water ad libitum. The experimentations were conducted according to the ethical norms approved by the Faculty Ethics Committee (reference number 17092021).
Experimental Protocol: The rats were randomly distributed in three groups (ten rats in each group): Group I: further subdivided into two subgroups (five rats each): Group Ia received normal diet (rat chow) for 4 weeks. Group Ib received Spirulina platensis at a dose of 200 mg/kg per day by oral gavage for 4 weeks.Citation26 Group II: received sodium arsenite (in a dose of 10 mg/kg body weight/day/oral gavage) for 4 weeks.Citation27 Group III: received (Spirulina platensis at a dose of 200 mg/kg per day by oral gavage concomitant with sodium arsenite 10 mg/kg, oral gavage) for 4 weeks.
Evaluation of the biomarkers: At the end of the experimental period (4 weeks), blood samples were collected from puncturing the retro-orbital venous sinus and immediately centrifuged at 8,000 ×g for 10 min at 4°C to separate serum. Serum creatine kinase MB (CKMB Elisa Kit, ThermoFisher Scientific), and troponin (Troponin Elisa Kit, ThermoFisher Scientific) were measured using available commercial kit and following the kit manufacturer instructions.
Tissue processing for microscopy: After rats were euthanized via overdose sodium pentobarbital (200 mg/kg), the hearts were rapidly excised. Two specimens were excised from the heart. The first specimen processed for light microscope. Briefly, the left ventricle was fixed in 4% paraformaldehyde for 24 h at room temperature. The heart tissues were removed from the fixing fluid, and were then cleared, dehydrated, macerated, and embedded in paraffin. The tissue samples were sectioned at 5-µm thickness. Hematoxylin and eosin were used to stain sections and examined under a light microscope to detect changes within heart tissue according to standard procedures. The second sample was prepared for transmission electron microscope after being immediately fixed in 3% glutaraldehyde solution.Citation30
Statistical analysis: All results were expressed as mean ± SD (Standard Deviation). Statistical analyses were performed using the SPSS version 19.0 statistical analysis package. A one-way ANOVA test. P < .05 was accepted as statistically significant.
RESULTS
Cardiac enzyme: shows the estimated levels of the examined cardiac enzymes (Creatine kinase – MB (CKMB) and troponin) in the three studied groups. Serum CKMB and troponin activities were increased in the arsenic intoxicated group (group II) compared with the Control group (group I). Comparing group II with spirulina treated group (group III) results showed a decrease in CK and troponin activities almost to original levels.
Table 1. The estimated levels of cardiac enzymes in the studied groups
Histological results of light microscopy
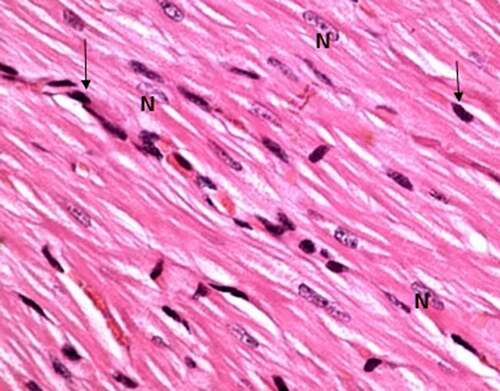
Group I (control group): Light microscopic examination of the histological sections of both negative (received no treatment) and positive control subgroups (received Spirulina platensis) revealed the normal cardiomyocytes architecture; the cardiac muscle fibers appeared to be branched and running in different directions, striated, cylindrical in shape with acidophilic cytoplasm and rounded vesicular centrally located nuclei. In between the muscle fibers, darkly stained nuclei of the connective tissue cells were present in narrow interstitial spaces ().
Figure 1. A photomicrograph of the control rat left ventricle (group I) showing, branching striated cardiac muscle fibers running in different directions with single central oval vesicular nuclei (n) and narrow spaces in between. Notice dark nuclei of connective tissue cells (↑). (H&E stain, X 100).
Group II (arsenic intoxicated group): In comparison with the control group, the following histological alterations were detected in the cardiomyocytes of arsenic intoxicated rats left ventricles (group II). The cardiac muscle fibers were damaged with loss of its continuity, widely separated with widening of the spaces in between, interstitial edema and congested blood vessels. Some of the dilated blood vessels filled with vacuolated materials and associated with cellular infiltration in the form of increased dark nuclei ().
Figure 2. A photomicrograph of treated rat left ventricle of arsenic intoxicated group (group II) showing, widely separated cardiomyocytes by wide spaces (↑), dilated blood vessels (v) some contain vacuolated material (Va), hyalinosis and loss of striation (*). (H&E stain, X 100).
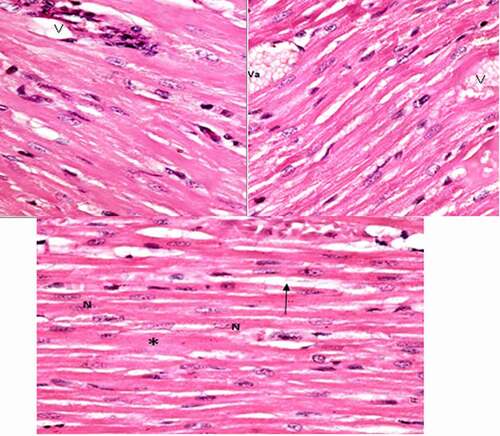
Group III (Protected group): The histological sections of rats' left ventricle of group III (protected group) revealed mild affection of cardiomyocytes than the previous intoxicated group, which appeared dark acidophilic with central oval vesicular nuclei and more or less with normal striation. Focal area showed hyalinosis with loss of striation associated with congested blood vessels containing hyaline material ()
Figure 3. Photomicrographs of rat left ventricle of protected group (group III) showing, very mild affection of cardiomyocytes with normal central oval vesicular nuclei (n) and more or less normal striation and other area showing hyalinosis with loss of striation (*) associated with congested blood vessels. (H&E stain, X 100).
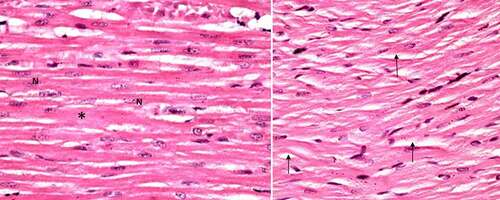
Generally, the morphology of heart sections from the control group had healthy cardiomyocyte structures. Conversely, the sections from the arsenic-induced cardiotoxicity group contained extensive necrosis, inflammatory cell infiltration, cytoplasmic vacuolization, slight fiber swelling, interstitial edema and myofiber loss. Compared with the Control group, it was found that treatment with spirulina significantly improved these findings of arsenic-induced cardiotoxicity, suggesting that spirulina exerted a potent protective effect in this model of cardiac injury.
Ultrastructural results
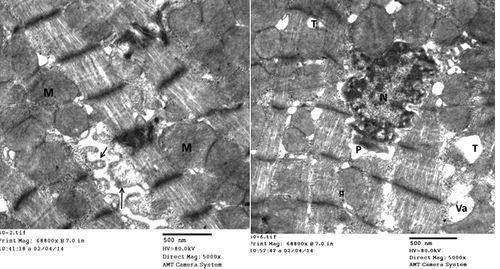
Group I (control group): Electron microscopic examination of rats left ventricle of both negative and positive control subgroups showed the classical architecture of cardiomyocyte. It showed well-organized muscle fibers. The myofibril had normal striation pattern, normal sarcomere with normal A-band, I-band, Z- line which bound the sarcomere. Sarcoplasm contained well-organized rows of normal mitochondria between myofibrils with numerous tightly packed cristae. Cardiac muscle fibers connect to each other through regular and intact intercalated discs formed of both longitudinal and transverse areas; step wise ().
Figure 4. Electromicrograph of the control rat left ventricle showing, normal striation pattern, normal cellular features, and step wise intact intercalated disc. (Uranyl acetate/lead citrate stain).
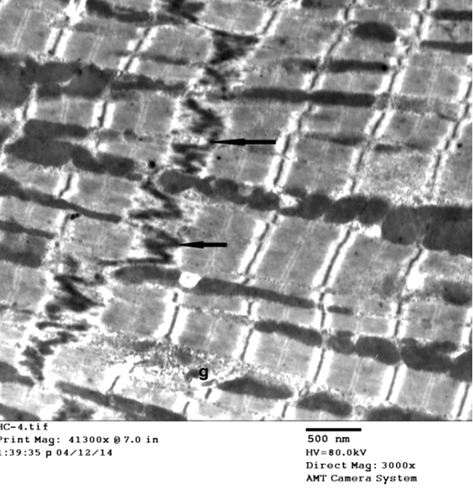
Group II (experimental group): In comparison with the control group, the following ultrastructural changes were detected in the cardiac muscle of arsenic intoxicated rats. Group II revealed, evident changes involving both sarcoplasm and nuclei; destruction, breaking the continuity of myofibrils with loss of striation of cardiomyocytes and widening of intercellular space by edema. Sarcomere lost their regular array, appeared distorted and fragmented widening of perinuclear space. Destruction, disorganization, and abnormal shape mitochondria associated with disruption of intercalated disc ().
Figure 5. Electron micrograph of rat left ventricle (group II) showing, destruction of cardiomyocytes, disorganization, and abnormal shape mitochondria (m), disrupted and discontinuous intercalated disc (↑) and widely separated myofibrils by edema (*). (Uranyl acetate/lead citrate stain).
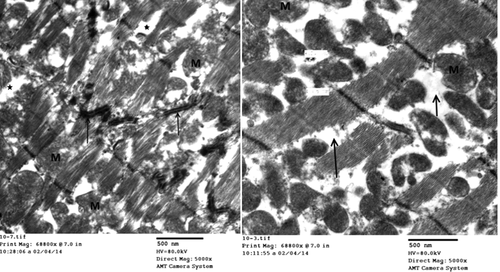
Group III (Protected group): Electron microscopic examination of rat left ventricle of group III (protected group) revealed, milder affection of cardiomyocytes than the previous group as evident by irregular nuclei with widening of perinuclear space. The sarcolemma lost its usual scalloped pattern and appeared lifted away from the underlying sarcomeres with formation of sarcolemma blebs in some areas. T-tubules were dilated with vacuolations, and the sarcoplasm revealed large and swollen mitochondria that appeared with clear matrices ().
DISCUSSION
Cardiovascular diseases are the primary cause of death here in Jordan and globally. Cardiomyopathy is one of the principal causes of cardiovascular disease. Arsenic is a dangerous cardiovascular toxicant associated with many biomarkers of cardiovascular diseases. Arsenic is also a carcinogen; however, arsenic trioxide is one of the treatment options that is used in acute promyelotic leukemia (APL). The therapeutic use of arsenic is restricted due to its severe cardiovascular side effects. Many of its toxic effects are mediated by mitochondrial dysfunction and related to its effect on oxidative stress.Citation31 An increasing body of evidence proposes that antioxidant phytonutrients may improve the toxic effects of arsenic on mitochondria by scavenging free radicals.Citation32 Therefore, we investigated the protective effect of spirulina against arsenic induced cardiovascular dysfunction. CKMB is one of the three isoenzymes of CK, which is a more specific marker of myocardial damage than total CK activity. On the other hand, troponins are a group of proteins found in muscle fibers that regulate muscular contraction. Troponin tests measure the level of troponin in the blood to help detect heart injury, the more damage there is the greater the concentration in the blood. As presented above, the administration of Sodium arsenite (Group II) caused creatine kinase (CKMB) and troponin activities to increase, which is suggestive of disturbed cardiomyocyte function. Those results agree with previous study,Citation33 which showed not only association with the increased levels of cardiac enzymes but also showed strong associations among arsenic administration, oxidative stress, and diastolic function. These findings were improved in the present results after the administration of Spirulina (Group III), via attenuating Sodium arsenite induced toxicity. Presented by decreasing levels of CKMB and troponin release. Numerous reports have showed that many cardiovascular diseases are linked to the release of intracellular reactive oxygen species (ROS), ROS-induced apoptosis and mitochondrial damage, which might be the main reason for arsenic trioxide-induced cardiotoxicity.Citation33–35 Accordingly, those evidence suggest that oxidative stress serves an important role in arsenic induced cardiac damage. Therefore, producing increased release of troponin and CK-MB, especially CK-MB. Arsenic cardiotoxicity influences various physiological processes including cardiac repolarization, calcium homeostasis and causes sarcoplasmic reticulum stress as evident histologically (–6) by dilated transverse tubules.Citation35–37 Conversely, it was suggested by other spirulina scavenges ROS and reserves calcium homeostasis.Citation33–37 The administration of spirulina protects the integrity of cardiac myocytes after exposure to arsenic, thereby decreasing CK-MB, and troponin release, as well as facilitating arsenic efflux.
Our optical histological changes presented above showed widely separated cardiomyocytes, dilated blood vessels with some vacuolated material, hyalinosis and loss of striation in arsenite-intoxicated rats. On ultrastructural level, there was destruction of cardiomyocytes, disorganization, and abnormal shape mitochondria, disrupted and discontinuous intercalated disc and widely separated myofibrils by edema. These findings in agreement of previous studies, which revealed severe histopathological alterations including cellular infiltration, myofibrillar loss, myocardial hemorrhage, cardiomyocytes necrosis and apoptosis.Citation38–44 Arsenic stimulated the inflammatory responses and resulting ultrastructural damage to cardiomyocytes. These inflammatory responses relatively contributed to heavy metal-related cardiotoxicity.Citation32 In addition, arsenite exposure produced intracellular calcium accumulation caused by the release of ROS.Citation41 Numerous evidence propose that oxidative stress serves a main role in arsenic-induced cardiac damage. Apoptosis is a common mechanism of cardiotoxicity, and arsenic triggers apoptosis in cells. The present study also revealed destruction of mitochondria and cellular infiltration observed under arsenite exposure. As reported by others, where there were elevated apoptotic markers, this could be caused by a reduction of both oxygen consumption and ATP production, consequently apoptosis and necrosis.Citation42–45 The present histopathological alterations to the myocardium of Sodium arsenite-treated rats were significantly alleviated by spirulina administration. It was revealed that spirulina treatment concomitant with sodium arsenite efficiently prevented morphological changes. These findings are in agreement with previous study, which revealed the ameliorating effects of spirulina induced by arsenic toxicity in testis, liver and kidney.Citation46 Oxidative stress plays a key role in contributing to cardiotoxicity. Spirulina is a powerful antioxidant that buffers the potential adverse consequences of free radicals.Citation47 Initial arsenite induced myocardial injury by causing overproduction of ROS and subsequent oxidative stress and apoptosis. Conversely, the cardiovascular effects of spirulina ameliorate oxidative stress. Spirulina not only attenuates arsenic-induced cardiotoxicity but also, as reported by others, it reduces arsenic accumulation.Citation47
Recently, many studies have investigated in the role of constituent in dietary supplement in the prevention and treatment of cardiovascular disease. Particularly, arsenite induced cardio myotoxicity, by means of antioxidants, which scavenges the free radicals and ROS produced by arsenic exposure.Citation41 Ascorbic acid, Vitamin A, zinc, iron, spirulina, lipoic acid, ascorbic acid, and α-tocopherol all have got ameliorating role against chronic arsenic poisoning.Citation48 The phytochemical analysis of spirulina platensis has been performed by many researchers.Citation49–54 Those studies revealed that spirulina platensis is an enriched unique blend of nutrients like proteins, B-complex vitamins, minerals, essential fatty acid, γ-linolenic acid, phycocyanin, vitamin E and β-carotene. Most of these components are of super antioxidant potential. Therefore, micronutrients and antioxidant components of spirulina have a positive role in ameliorating the biochemical and histological changes induced by arsenic toxicities.
In conclusion, the present results suggested that spirulina protected cardiomyocytes from arsenic-induced cardiotoxicity. The cardio-protective effect of spirulina is illustrated by ameliorating the induced changes biochemically and histologically. Therefore, spirulina may have medical application value in treating arsenic-induced cardiotoxicity. Further investigation is required to explain the possible mechanisms by which spirulina protects arsenic-induced cardiac injury.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Gibb H, Haver C, Gaylor D, Ramasamy S, Lee JS, Lobdell D, Wade T, Chen C, White P, Sams R. Utility of recent studies to assess the National Research Council 2001 estimates of cancer risk from ingested arsenic. Environ Health Perspect. 2011 Mar;119(3):284-90. doi:https://doi.org/10.1289/ehp.1002427. Epub 2010 Oct 28. PMID: 21030336; PMCID: PMC3059988.
- Abhyankar LN, Jones MR, Guallar E, Navas-Acien A. Arsenic exposure and hypertension: a systematic review. Environ. Health Perspect. 2012;120(4):1–11. doi:https://doi.org/10.1289/ehp.1103988.
- Argos M, Ahsan H, Graziano JH. Arsenic and human health: epidemiologic progress and public health implications. Rev Environ Health. 2012;27(4):191–195. doi:https://doi.org/10.1515/reveh-2012-0021.
- Wang X, Mandal AK, Saito H, Pulliam JF, Lee EY, Ke ZJ, Lu J, Ding S, Li L, Shelton BJ, Tucker T, Evers BM, Zhang Z, Shi X. Arsenic and chromium in drinking water promote tumorigenesis in a mouse colitis-associated colorectal cancer model and the potential mechanism is ROS-mediated Wnt/β-catenin signaling pathway. Toxicol Appl Pharmacol. 2012 Jul 1;262(1):11-21. doi:https://doi.org/10.1016/j.taap.2012.04.014. Epub 2012 Apr 19. PMID: 22552367; PMCID: PMC4429765.
- Chang SI, Jin B, Youn P, Park C, Park J-D, Ryu D-Y. Arsenic-induced toxicity and the protective role of ascorbic acid in mouse testis. Toxicology and Applied Pharmacology. 2007;218(2):196–203. doi:https://doi.org/10.1016/j.taap.2006.11.009.
- Medina-Pizzali M, Robles P, Mendoza M, Torres C. Ingesta de arsénico: el impacto en la alimentación y la salud humana [Arsenic Intake: Impact in Human Nutrition and Health]. Rev Peru Med Exp Salud Publica. 2018 Jan-Mar;35(1):93-102. Spanish. doi:https://doi.org/10.17843/rpmesp.2018.351.3604. PMID: 29924286.
- Zhang T, Lu H, Li W, Hu R, Chen Z. Identification of arsenic direct-binding proteins in acute promyelocytic leukaemia cells. Int. J. Mol. Sci 2015;16:26871–26879.
- Rao Y. Li R and Zhang D: a drug from poison: how the therapeutic effect of arsenic trioxide on acute promyelocytic leukemia was discovered. Sci China Life Sci. 2013;56:495–502.
- Lengfelder E, Hofmann W-K, Nowak D. Impact of arsenic trioxide in the treatment of acute promyelocytic leukemia. Leukemia. 2012;26:433–442.
- Abedi T. Mojiri A Arsenic uptake and accumulation: mechanisms in rice species. Plants. 2020;9(2):129. doi:https://doi.org/10.3390/plants9020129.
- Abedin MJ, Cresser MS, Meharg AA, Feldmann J, Cotter-Howells J. Arsenic accumulation and metabolism in rice (Oryza sativa L.). Environ Sci Technol. 2002a;36:962–996.
- Ali W, Isayenkov SV, Zhao FJ. Maathuis FJ Arsenite transport in plants. Cell Mol Life Sci. 2009;66(14):2329–2339. doi:https://doi.org/10.1007/s00018-009-0021-7.
- Hantson P, Haufroid V, Buchet JP, Mahieu P. Acute arsenic poisoning treated by intravenous dimercaptosuccinic acid (DMSA) and combined extrarenal epuration techniques. J. Toxicol. Clin. Toxicol 2003;41(1):1‒6. doi:https://doi.org/10.1081/CLT-120018263.
- Sharaf R, A Khan, MZ Khan, I Hussain, RZ Abbas, ST Gul, F Mahmood and MK Saleemi,2013. Arsenic induced toxicity in broiler chicks and its amelioration with ascorbic acid: Clinical, hematological and pathological study. Pak Vet J, 33(3): 277-281
- Rodríguez-Lado L, Sun G, Berg M, Zhang Q, Xue H, Zheng Q, Johnson CA. Groundwater arsenic contamination throughout China. Science. 2013 Aug 23;341(6148):866-8.doi:https://doi.org/10.1126/science.1237484.PMID:23970694.
- Yu X, Wang Z, Shu Z, Li Z, Ning Y, Yun K, Bai H. Liu R and Liu W: effect and mechanism of Sorbus pohuashanensis (Hante) Hedl. Flavonoids protect against arsenic trioxide-induced cardiotoxicity. Biomed Pharmacother. 2017;88:1–10. doi:https://doi.org/10.1016/j.biopha.2016.12.130.
- Varghese MV, Abhilash M, Paul MV. Alex M and Nair RH: omega-3 fatty acid protects against arsenic trioxide-induced cardiotoxicity in vitro and in vivo. Cardiovasc Toxicol.;2017,17. 109–119.
- Sfaxi I, Charradi K, Limam F. El May MV and Aouani E: grape seed and skin extract protects against arsenic trioxide induced oxidative stress in rat heart. Can J Physiol Pharmacol 2016;94(2):168–176. doi:https://doi.org/10.1139/cjpp-2015-0088.
- Raghu KG, Cherian OL. Characterization of cytotoxicity induced by arsenic trioxide (a potent anti-APL drug) in rat cardiac myocytes. J Trace Elem Med Biol. 2009;23(1):61–68. doi:https://doi.org/10.1016/j.jtemb.2008.10.001.
- Das J, Ghosh J, Manna P, Sinha M, Sil PC. Taurine protects rat testes against NaAsO2-induced oxidative stress and apoptosis via mitochondrial dependent and independent pathways. Toxicology Letters. 2009;187(3):201–210. doi:https://doi.org/10.1016/j.toxlet.2009.03.001.
- Ramanathan K, Anusuyadevi M, Shila S, Panneerselvam C. Ascorbic acid and α-tocopherol as potent modulators of apoptosis on arsenic induced toxicity in rats. Toxicology Letters. 2005;156(2):297–306. doi:https://doi.org/10.1016/j.toxlet.2004.12.003.
- Hoseini SM, Khosravi-Darani K, Mozafari MR. Nutritional and medical applications of spirulina microalgae. Mini-Reviews in Medicinal Chemistry. 2013;13(8):1231–1237. doi:https://doi.org/10.2174/1389557511313080009.
- Samad N, Rao T, Rehman MHU, Bhatti SA, Imran I. Muhammad Habib Ur Rehman, Sheraz Ahmed Bhatti, Imran Imran Inhibitory Effects of Selenium on Arsenic-Induced Anxiety-/Depression-Like Behavior and Memory Impairment. Biol Trace Elem Res. 2021 Mar 20. doi:https://doi.org/10.1007/s12011-021-02679-1. Online ahead of print.
- Ahmed KA, Korany RMS, El Halawany HA. Ahmed KS Spirulina platensis alleviates arsenic-induced toxicity in male rats: biochemical, histopathological and immunohistochemical studies. Adv Anim Vet Sci. 2019;7:701–710.
- Zhang J, Zhang Y, Wang W. Li C and Zhang Z: double-sided personality: effects of arsenic trioxide on inflammation. Inflammation. 2018;41(4):1128–1134. doi:https://doi.org/10.1007/s10753-018-0775-x.
- Moradi-Kor N, Ghanbari A, Rashidipour H, Yousefi B, Bandegi AR, Rashidy-Pour A. Beneficial effects of Spirulina platensis, voluntary exercise and environmental enrichment against adolescent stress induced deficits in cognitive functions, hippocampal BDNF and morphological remolding in adult female rats. Horm Behav. 2019;112:20–31. doi:https://doi.org/10.1016/j.yhbeh.2019.03.004.
- Biswas S, Anjum A, Banna HU, Rahman M, Siddique AE, Karim Y, Nikkon F, Haque A, Hossain K. Zahangir Alam Saud Manganese attenuates the effects of arsenic on neurobehavioral and biochemical changes in mice co-exposed to arsenic and manganese. Environ Sci Pollut Res Int. 2019 Oct;26(28):29257–29266. doi:https://doi.org/10.1007/s11356-019-06112-y.
- Ministry of Health Jordan; 2015, accessed 28/8/2021 https://www.moh.gov.jo/Echobusv3.0/SystemAssets/f5df91c9-5b2c-42fe-a1d3-379616db9bf0.pdf
- The Institute for Health Metrics and Evaluation (IHME); 2016, accessed 26/8/2021 http://www.healthdata.org/Jordan
- Bancroft JD, Gamble M. Theory and practice of histological techniques. 6th edn ed. London: Churchill Livingstone; 2007. p. 125–138.
- Sodhi KK, Kumar M, Agrawal PK, Singh DK. Singh DK Perspectives on arsenic toxicity, carcinogenicity and its systemic remediation strategies. Environ Technol Innov. 2019;16:100462. doi:https://doi.org/10.1016/j.eti.2019.100462.
- Nakayama H, Chen X, Baines CP, Klevitsky R, Zhang X, Zhang H, Jaleel N, Chua BH, Hewett TE, Robbins J, Houser SR, Molkentin JD. Ca2+- and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. J Clin Invest. 2007 Sep;117(9):2431-44. doi:https://doi.org/10.1172/JCI31060.PMID:17694179;PMCID: PMC1937500.
- Ducas RA, Seftel Md, Ducas J, Seifer C. Monomorphic ventricular tachycardia caused by arsenic trioxide therapy for acute promyelocytic leukaemia. The Journal of the Royal College of Physicians of Edinburgh. 2011;41(2):117–118. doi:https://doi.org/10.4997/JRCPE.2011.204.
- Zhang W, Guo C, Gao R, Ge M, Zhu Y, Zhang Z. The protective role of resveratrol against arsenic trioxide-induced cardiotoxicity. Evidence-based complementary and alternative medicine volume. 2013; Article ID 407839:8.
- Neyrinck MA, Taminiau B, Walgrave H, Daube G, Cani PD, Bindels LB, Delzenne N. Spirulina Protects against Hepatic Inflammation in aging: an effect related to the modulation of the gut microbiota? Nutrients. 2017;9(6):633. doi:https://doi.org/10.3390/nu9060633.
- Pabon MM, Jernberg JN, Morganti J, Contreras J, Hudson CE, Klein RL. Bickford PC A spirulina-enhanced diet provides neuroprotection in an αsynuclein model of Parkinson’s disease, PLoS One. 2012;7(9):e45256.
- Palaniswamy R, Veluchamy C. Therapeutic uses of spirulina. International Journal of Current Innovation Research. 2018;4:975–979.
- Patel S, Goyal A. Current and prospective insights on food and pharmaceutical applications of Spirulina. Curr. Trends Biotechnol. Pharm 2013;7:95–681.
- Szulinska M, Gibas-Dorna M, Miller-Kasprzak E, Suliburska J, Miczke A, WalczakGałezewska M, Stelmach-Mardas M. Walkowiak J and Bogdanski P. Spirulina maxima improves insulin sensitivity, lipid profile, and total antioxidant status in obese patients with well-treated hypertension: a randomized double-blind placebo-controlled study. Eur Rev Med Pharmacol Sci. 2017;21:2473–2481.
- Miao X, Tang Z, Wang Y, Su G, Sun W, Wei W, Li W, Miao L, Cai L. Tan Y and Liu Q: metallothionein prevention of arsenic trioxide-induced cardiac cell death is associated with its inhibition of Mitogen-activated protein kinases activation in vitro and in vivo. Toxicol Lett. 2013;220(3):277–285. doi:https://doi.org/10.1016/j.toxlet.2013.04.025.
- Zhang W, Guo C, Gao R, Ge M, Zhu Y, Zhang Z. The protective role of resveratrol against arsenic trioxide-induced cardiotoxicity. Evidence-based complementary and alternative medicine. 2013; Article ID 407839:8.
- Zhao X, Feng T, Chen H, Shan H, Zhang Y, Lu Y, Yang B. Arsenic trioxide-induced apoptosis in H9c2 cardiomyocytes: implications in cardiotoxicity. Basic Clin Pharmacol Toxico. 2008;102(5):419–425. l2008. doi:https://doi.org/10.1111/j.1742-7843.2007.00150.x.
- Zhang W. Li Y and Ge Z: cardiaprotective effect of crocetin by attenuating apoptosis in isoproterenol induced myocardial infarction rat model. Biomed Pharmacother. 2017;93:376–382. doi:https://doi.org/10.1016/j.biopha.2017.06.032.
- Korany RMS, Ahmed KS, El Halawany HA, Ahmed KA. Pathological and immunohistochemical studies on the ameliorating effect of Spirulina platensis against arsenic induced reproductive toxicity in female albino rats. Inter J Vet Sci. 2019;8:113–119.
- Al-Dalaen SM, Al-Qtaitat AI. Review article: oxidative stress versus antioxidants. American Journal of Bioscience and Bioengineering. 2014;2(5):60–71. doi:https://doi.org/10.11648/j.bio.20140205.11.
- Ahmed KA, Korany RMS, El Halawany HA, Ahmed KS. Spirulina platensis alleviates arsenic-induced toxicity in male rats: biochemical, histopathological and immunohistochemical studies. Adv Anim Vet Sci. 2019;7(8):701–710. doi:https://doi.org/10.17582/journal.aavs/2019/7.8.701.710.
- Ravi M, De SL. Azharuddin S and Paul S. The beneficial effects of Spirulina focusing on its immunomodulatory and antioxidant properties. Nutrition and Dietary Supplements. 2010;2:73–83.
- Korany RMS, Ahmed KS, El Halawany HA, Ahmed KA. Pathological and immunohistochemical studies on the ameliorating effect of Spirulina platensis against arsenic induced reproductive toxicity in female albino rats. Inter. J. Vet. Sci 2019;8:113–119.
- Kulshreshtha A, Zacharia AJ, Jarouliya U, Bhadauriya P, Prasad GB, Bisen PS. Spirulina in health care management. Curr. Pharmaceut. Biotechnol 2008;9(5):400–405. doi:https://doi.org/10.2174/138920108785915111.
- Holman BW, Malau-Aduli AE. Spirulina as a livestock supplement and animal feed. J. Anim. Physiol. Anim. Nutr 2012;97:615–623.
- Mane RS, Chakraborty B. Phytochemical screening of Spirulina platensis extracts from Rankala Lake Kolhapur, India. J. Algal Biomass Utln 2018;9:38–41.
- El-Chaghaby GA, Rashad S, Abdel-Kader SF. Rawash EA and Abdul Moneem M. Assessment of phytochemical components, proximate composition and antioxidant properties of Scenedesmus obliquus, Chlorella vulgaris and Spirulina platensis algae extracts. Egyptian Journal of Aquatic Biology & Fisheries. 2019;23:521–526.
- Chakraborty B, Varsale AR, Singh VK, Mali SS, Parihar PK, Mane RS. Phytochemical analysis, antioxidant and antifungal activity of different solvent extracts of Spirulina platensis collected from Rankala Lake, Kolhapur, Maharashtra. J. Algal Biomass Utln 2019;10:36–42.
- Sayed MA, Gofur MR, Khair A, Awal MA. Protective role of spirulina and vitamin e against arsenic toxicity in rats. Asian J. Anim. Sci 2015;9:330–340.