ABSTRACT
Fistula treatment represents a major unmet medical need in the therapy of Crohn’s disease (CD). Current medical therapies, such as anti-TNF antibody treatments, are often insufficient and do not achieve permanent fistula closure. Previously published data point toward a critical role for metalloproteinase-9 (MMP-9)/gelatinase B in fistula pathogenesis. The aim of this project was to investigate in detail MMP-9 expression in different fistula types and to confirm that MMP-9 is a potential target for fistula therapy in CD patients.
Immunohistochemistry for total and active MMP-9, Cytokeratin 8 (CK-8) and co-staining of active MMP-9/CK-8 was performed in specimen derived from perianal fistulas, entero-enteric fistulas and fistulas from patients not responding to anti-TNF therapy. In addition, fistulas from the xenograft mouse model (anti-TNF treated or untreated) were analyzed.
Total and active MMP-9 protein was detectable in cells lining the tracts of perianal and entero-enteric fistulas. Of note, total and active MMP-9 was also expressed in fistulas of CD patients non-responding to anti-TNF treatment. Interestingly, we detected considerable co-staining of active MMP-9 and CK-8 in particular in cells lining the fistula tract and in transitional cells around the fistulas. Furthermore, total and active MMP-9 are detectable in both anti-TNF treated and untreated xenograft fistulas.
Taken together, our data suggest that MMP-9 is involved in fistula pathogenesis in CD patients, in fistulas of different origins and particularly in patients non-responding to anti-TNF therapy. Our xenograft fistula model is suitable for in vivo studies investigating a possible therapeutic role for MMP-9 targeting as fistula therapy.
Introduction
There is a high unmet medical need for the treatment of fistulizing Crohn’s disease (CD), which affects about 1/3 of CD patients.Citation1 The present day medical treatment is often insufficient, frequently requiring surgery. However, even after surgery relapses are still common.Citation2 In particular, anti-TNF therapy results in fistula closure in only 30–35% of treated patients, and these fistulas tend to re-appear after the treatment is stopped.Citation3
Complete fistula healing is important for patients, since draining fistulas significantly reduce quality of life. Newly developed mesenchymal stem cell therapy is successful in more than 60% of fistulas, particularly in combination with anti-TNF treatment. However, even more efficient fistula therapies are urgently needed, since mesenchymal stem cell therapy is very expensive and a significant number of patients does not respond to therapy.Citation4–6
80% of CD patients with fistulizing disease present with inflammation and fibrosis.Citation7 Previous studies demonstrated that epithelial to mesenchymal transition (EMT) plays an important role in the pathogenesis of inflammatory bowel disease (IBD)Citation8,Citation9 and is particularly a central mechanism of fistula formation and maintenance.Citation10–15 EMT processes can be detected at the origin of the fistula as well as along the penetrating fistula tract.Citation10,Citation11 In fact, epithelial cells with mesenchymal cell phenotype were positive for the epithelial markers cytokeratin 8 (CK-8), CK-20 (but not Vimentin)Citation10 and for EMT markers (e.g. SNAIL).Citation12 Those particular cells undergoing EMT appear histologically as forming a line along the fistula tract and are thus called transitional cells (TC). It is believed that they critically contribute to fistula formation.Citation10 MMP-9 has also been associated to IBD pathogenesisCitation16 and proposed as a biomarker for CD activity.Citation17 Also, MMP-9 expression has been detected in the vicinity of fistulas in CD patients.Citation18 The MMP-9 expression pattern observed in immunohistochemistry (IHC) staining of fistula from CD patients suggested that the TC undergoing EMT co-localize with MMP-9.Citation7,Citation18,Citation19 Fistula formation/maintenance and turnover are continuous dynamic processes in active disease. An association of MMP-9 expression with TC would allow for reasonable speculations on anti-MMP-9 treatment efficacy during both, fistula development and maintenance. Of note, anti-MMP-9 treatment might also have an effect on promoting tissue-remodeling.Citation19
Furthermore, patients with active fistulizing CD (Montreal B3 penetrating phenotype) display intestinal inflammation and fibrosis together with elevated levels of serum C3M, which is a circulating biomarker of MMP-9 activity. Patients with perianal fistula (B3p group) show less inflammation and lower serum C3M levels.Citation19 A further study has demonstrated that pro-C3/C3M ratios are superior in separating penetrating CD from inflammatory or stricturing CD.Citation20 Those aspects suggest anti-MMP-9 treatment as a target for fistula therapy.
However, the role of MMP-9 in IBD is controversial. In vivo studies comparing wild type and MMP-9 knock-out mice treated with different models of acute and chronic colitis mimicking ulcerative colitis and CD did not show significant differences between phenotypes.Citation21 Also, the inhibition of MMP-9 with peptides was not beneficial in experimental colitis. Furthermore, a phase 2 randomized, placebo-controlled trial assessing safety and efficacy of Andecaliximab, a synthetic monoclonal MMP-9 inhibitor, in patients with moderately to severe active CD showed no differences between the treatment groups.Citation22 Here, CD patients receiving subcutaneously anti-MMP-9 antibody at different doses and treatment regimen did not achieve better clinical outcome than patients receiving placebo after 8 weeks. However, fistulizing CD was not in the focus of this study (only 17.6% of the patients had fistulas at screening). On the other hand, a recent publication with induced colitis in mice treated with thalidomide, showed an amelioration of intestinal fibrosis, diminished inflammatory infiltration and in particular a down-regulation of MMP-9 expression.Citation23 This result would suggest a potential beneficial effect of MMP-9 inhibition in the setting of colitis. Taken together, the implication of MMP-9 in fistula formation and maintenance still needs further investigations. Particularly, previous studies did not examine the effect of MMP-9 inhibition in the context of fistulizing disease in CD patients. Therefore, to assess if MMP-9 might be a possible target for fistula therapy in CD patients, we investigated tissue protein levels of total and active MMP-9 in different fistula types from CD patients. We found that total and active MMP-9 are expressed in perianal as well as in entero-enteric fistulas from CD patients even under anti-TNF treatment.
Ethical considerations
Patient data
To assess the expression of MMP-9 in fistulizing CD, immunohistochemistry (IHC) staining was performed on tissues from the following patient groups: CD patients with perianal fistula (n = 7, no anti-TNF treatment); CD patients with entero-enteric fistula (n = 6, no anti-TNF treatment) and CD patients (with perianal or entero-enteric fistula) under anti-TNF treatment at surgery or who terminated treatment within 3 months (n = 9). Specifications regarding anti-TNF treatment are indicated in . All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the ethical approval was obtained from the Cantonal Ethics Committee of the Canton Zürich, Switzerland (EK-1317).
Table 1. Anti-TNF treatment in patients with perianal or entero-enteric fistula
Xenograft mouse model
In our previously published animal model, we studied formalin-fixed and paraffin-embedded intestinal fistula samples from our human gut xenograft mouse model for intestinal fistula (n = 6).Citation24 In addition, we analyzed xenograft fistulas of mice injected intraperitoneally once with the TNF inhibitor Humira® (adalimumab) (10 mg/kg) (n = 4). Animal experiments were performed under the license MD-12-13427-4, MD-14-14164-3, MD-15-14631-4, MD-17-15204-5 from the Ethics Committee for Animal Experimentation, Hebrew University of Jerusalem; and 5–31.03.06 from the Helsinki Ethics Committee of the Hadassah University Hospital following the guidelines for the Care and Use of Laboratory Animals of the Israel Ministry of Health in accordance with Israeli law.
Materials and methods
Immunohistochemistry staining
For IHC, we used two different antibodies to detect MMP-9/gelatinase B in the tissue samples: (1) anti-human pro-MMP-9 (Abcam, USA (ab137867), a rabbit monoclonal [EP1255Y] antibody that recognizes the C-terminal domain of MMP-9 present in the latent (proenzyme) and the active form of MMP-9, hereafter referred to as total MMP-9); (2) anti-human active MMP-9 (Novus Biologicals, Germany (NBP2-13173), a mouse monoclonal (4A3) antibody recognizing the N-terminus of human MMP-9, specific for the active MMP-9 but not for MMP-9 proenzyme and not for active and proenzyme forms of MMP2/gelatinase A), according to the manufacturers, respectively.
Staining of human pro-MMP-9 and active MMP-9 was performed by the horseradish peroxidase method with diaminobenzidine (DAB) (brown substrate reaction product) and staining of human CK-8 with VIP (purple chromogen). First, the slides with paraffin-embedded 3 to 5 μm tissue sections were hydrated (2 times 10 min in xylol, 2 times 3 min in 100% ethanol, 8 min in 96% ethanol and 8 min in 70% ethanol solutions respectively). Slides were shortly rinsed in double distilled water and phosphate-buffered saline (PBS) before antigen retrieval treatment: sections were boiled in 10 mM citrate buffer, pH 6.0 (Target Retrieval Solution, pH 6.0, Dako, Agilent, US (S1699) in a water bath at 98°C for 45 min and slowly cooled to room temperature (RT). Endogenous peroxidase activity was inhibited by an incubation in 0.9% hydrogen peroxide solution for 15 min. After each of these treatments, sections were rinsed twice for 10 min in PBS, pH 7.2 while shaking. Tissue sections were incubated in blocking buffer (1:1 3% BSA, 2.5% normal horse serum) for 1 h at RT.
The following primary antibodies diluted in blocking buffer were used for the overnight incubation at 4°C: anti-human pro-MMP-9 (Abcam, USA (ab137867), rabbit monoclonal (EP1255Y), dilution 1:1000); anti-human active MMP-9 (Novus Biologicals, Germany (NBP2-13173), mouse monoclonal (4A3), dilution 1:200); anti-human CK-8 (Novus Biologicals, Germany (NBP1-79068), rabbit monoclonal, dilution 1:200); IgG1 Kappa (Novus Biologicals, Germany (NBP2-27787), mouse monoclonal, dilution 1:200) as an isotype control for active MMP-9.
After rinsing the slides twice for 10 min in PBS, the secondary antibody incubation with ImmPRESS HRP Anti-Mouse IgG (Peroxidase) Polymer Detection Kit (Vector Laboratories, MP-7402) or with ImmPRESS™ HRP Anti-Rabbit IgG (Peroxidase) Polymer Detection Kit (MP-7401) was performed for 1 h at RT. After 2 times 10 min washing in PBS, antibody binding was visualized using ImmPACT DAB Peroxidase (HRP) Substrate (Vector Laboratories, SK-4105) for pro-MMP-9, active MMP-9 and isotype IgG1Kappa antibodies and ImmPACT VIP Peroxidase (HRP) Substrate (Vector Laboratories, SK-4605) for CK-8 detection. After rinsing the slides in tap water and PBS for 10 min each, sections were counterstained with hematoxylin prior dehydration (incubation in 70% ethanol, 96% ethanol, 2 times in 100% ethanol for 1 min each and 2 times 5 min in xylol), mounting and drying overnight. The absence of the primary antibody was used as a negative control for each primary antibody. IgG1 Kappa served as an isotype control for active MMP-9.
For the double staining with active MMP-9 and CK-8, the above-mentioned antibodies and reagents were used in a one-day protocol, where incubation for the first primary antibody was 1 h at RT, for the first secondary antibody for 20 min. After DAB product was developed according to the manufacturer’s protocols to detect MMP-9 expression, sections were washed twice for 10 min in tap water and PBS respectively. The second primary and secondary antibodies were incubated 1 h and 20 min respectively, followed by VIP substrate incubation for CK-8 expression.
For histology, one of the serially sectioned slides was stained with hematoxylin and eosin (H & E) after dehydration and rehydration following standard protocols. The sections were examined with the Imager Z2 microscope (Zeiss) and AxioVision software (Zeiss).
Results
MMP-9 expression in perianal fistulas
We studied protein expression of total as well as active MMP-9 along and around perianal fistula tracts from patients currently not under anti-TNF treatment by IHC. Both, total and active protein showed a strong staining in both locations. They were both also clearly detectable in cells lining the fistula tract and in the tissue surrounding the fistula. Higher magnifications demonstrate that total and active MMP-9 are detectable in immune cells surrounding the fistula tract ()). Our findings are summarized in . As a negative control for IHC, we used isotype control IgG1 Kappa instead of active MMP-9. No signal was detected (Supplemental Figure 1a-c). Also as a control for unspecific staining, we omitted the primary antibodies and added only the secondary antibodies (data not shown).
Table 2. Summary of IHC findings
Figure 1. Immunohistochemistry staining showing representative images for active (a-c), total MMP-9 (d-f) and co-staining for active MMP-9 and CK-8 (g-i) in CD perianal fistulas (n = 7). There is a strong expression of total and active MMP-9 along and around the fistula tract. Since the sections were counterstained with hematoxylin, the nuclei of MMP-9 and CK-8 negative cells are stained in blue. Active MMP-9/CK-8 double-stained cells are also found in abnormal crypts (Supplemental figs S1D-I). A, D and G are consecutive sections and B-C, E-F and H-I are magnifications of the respective quadrants. Dashed line indicates the fistula tract. Small arrows indicate enlargements.
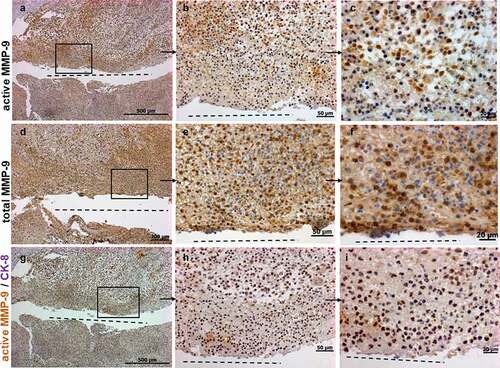
To further investigate whether MMP-9 is expressed by TC cells that are characteristic for fistula formation, we performed IHC co-staining for active MMP-9 and the epithelial marker CK-8. We found a considerable number of cells positive for both, active MMP-9 and CK-8 ()). This demonstrates that TC penetrating into deeper tissue layers express active MMP-9 and points toward a critical role for MMP-9 in fistula formation.
Of note, we also detected a strong positive staining for active MMP-9 and CK-8 in cells of aberrant crypts beside the fistula tracts (Supplemental Figure 1d-i). This observation is in line with our previous findings describing a strong staining for EMT transcription factors SNAIL1 and SLUG in cells of aberrant crypts near the fistula tracts.Citation12 The expression patterns of active MMP-9, CK-8, SNAIL1 and SLUG clearly point toward the fact that the epithelial cells of morphologically aberrant crypts alongside fistula tracts in CD patients are somehow affected by EMT and might critically contribute to fistula pathogenesis.
MMP-9 expression in entero-enteric fistulas
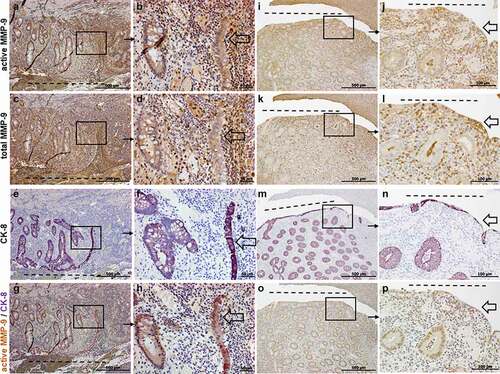
We next investigated the expression of total and active MMP-9 in entero-enteric fistulas resected from CD patients (without anti-TNF treatment). As shown in ), both total and active MMP-9 were detectable in cells lining the fistula tract as well as in cells surrounding the fistulas, similar to the findings in perianal fistulas. We detected a strong staining for total and active MMP-9 in cells lining the surface of the entero-enteric fistulas, as well as in cells of tissue layers below the surface of the fistula tract. To further characterize the cell type expressing active MMP-9 along and around entero-enteric fistulas, we performed a co-staining for active MMP-9 and CK-8. Here, again, we detected a significant number of positive cells for active MMP-9 and CK-8 in the tissue surrounding the fistula, including also abnormal crypt structures as well as TC lining the fistula tract (, 2 M-P).
Figure 2. Representative images of immunohistochemistry staining results for active (a, b, i, j), total MMP-9 (c, d, k, l), CK-8 (e, f, m, n) and active MMP-9 and CK-8 co-staining (g, h, o, p) in CD entero-enteric fistulas (n = 6). Figures A-H show an entero-enteric fistula with abnormal crypts and myofibroblast-like single cells and TC (transitional cells: epithelial cells with mesenchymal cell phenotype) entering tissue. In Figures I-P, a different representative entero-enteric specimen, myofibroblast-like single cells and TC are found lining the fistula tract. Those cells as well as abnormal crypts are positively stained for active MMP-9 and CK-8. Total MMP-9 expression is found in some scattered myofibroblast-like cells and TC cells (c, d, k, l). From 2 specimen, A, C, E, G and I, K, M, O are consecutive sections and B, D, F, H and J, L, N, P are magnifications of the respective quadrants. Dashed line indicates the fistula tract. Small arrows indicate enlargements. Large arrows indicate TC.
MMP-9 expression in fistulas of CD patients under anti-TNF treatment
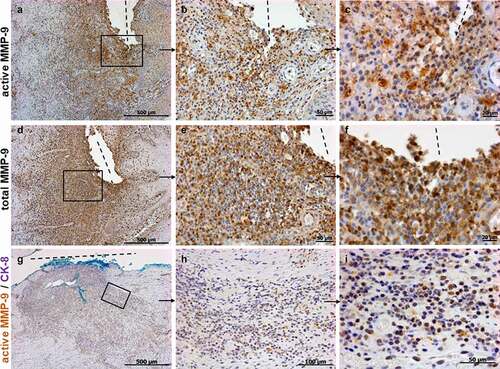
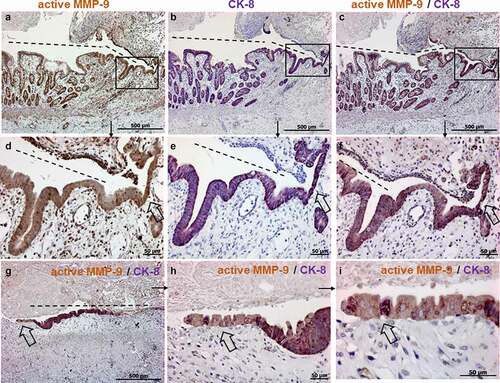
Next, we studied, whether total and active MMP-9 are expressed along fistulas in patients under anti-TNF treatment. Patients requiring fistula resection despite anti-TNF therapy for more than 3 months were defined as non-responders. shows a strong staining for total and active MMP-9 along the tract and in the surrounding tissue of a fistula in non-responder to anti-TNF therapy. This is of particular interest since it indicates that active MMP-9 is still detectable and biologically active in and around fistulas in CD patients even after anti-TNF therapy. Co-staining for both, active MMP-9 and CK-8 validated that active MMP-9 is also expressed in TC along fistula and in cells around the fistula in patients under anti-TNF therapy ().
Figure 3. Immunohistochemistry staining showing representative images for active (a-c), total MMP-9 (d-f) and co-staining for active MMP-9 and CK-8 (g-i) in CD perianal or entero-enteric fistulas in non-responder patients under anti-TNF therapy (n = 9). Strong expressions of active and total MMP-9 are detected along and around the fistula. In a different specimen, double positive cells for active MMP-9 and CK-8 are also present along and around the fistula (H, I magnifications of G). The light blue staining is due to the dye used by the surgeons during resection. A and D are consecutive sections with higher magnifications found in B, C and E, F respectively. Dashed line indicates the fistula tract. Small arrows indicate enlargements.
MMP-9 expression in human xenograft fistulas
After demonstrating that total and active MMP-9 are expressed in CD patients along the fistula tracts as well as in the surrounding tissue of perianal fistulas and entero-enteric fistulas from both, patients responding and non-responding to anti-TNF therapy, we next aimed at studying the anti-MMP-9 therapy for fistula treatment in an in vivo mouse model. In our previously published xenograft fistula mouse model, we transplanted 12–18 weeks old human fetal small intestine or colon segments subcutaneously onto the back of a SCID mouse.Citation24 Grafts develop in situ and look similar to typical small and large intestinal tissue.Citation25 As previously reported, < 20% of fully developed subcutaneous human gut xenografts spontaneously developed enterocutaneous fistulas, revealing striking histopathological similarities with CD fistula specimens. They resemble human fistula from CD patients in all aspects such as inflammatory cells (hCD45+ and CD3+ cells) and other observed markers of EMT such as CK-8.Citation24 Animals with fistulizing human gut xenografts were systemically treated with a single intraperitoneal injection of Humira® (10 mg/kg) (n = 4) and compared to non-treated xenografts (n = 6).
As illustrated in , we detected strong staining for total and active MMP-9 in cells lining the fistula tract of the human xenograft and in abnormal crypts. We found also some, but clearly less intense staining for total and active MMP-9 in cells around the fistula tract. This finding was confirmed by IHC co-staining for active MMP-9 and CK-8. We detected a strong staining for active MMP-9, CK-8 as well as co-staining for active MMP-9 and CK-8, particularly in cells lining the fistula tract (). The co-staining clearly demonstrated that those cells represent TC. By histologic analysis we observed that the typical cylindric intestinal epithelial cells at the base of the fistula tract became more and more cubic with fistula invasion into the tissue; at the tip of the fistula, co-staining was even found in flat, mesenchymal-shaped cells ( and 5(a-i))). Similar protein expression patterns of active MMP-9 were also found in xenograft fistulas of mice treated once with anti-TNF (Data not shown).
Figure 4. Representative images of immunohistochemistry staining results for active (A-C, G), total MMP-9 (d-f), double staining active MMP-9/CK-8 (h) and single staining for CK-8 (i) in human xenograft fistulas (n = 6). The histology of the human xenograft fistula is similar to the human fistula. Total and active MMP-9 positive cells are found around the fistula tract (a, b, d, e) and abnormal crypts (c, f). Epithelial cells in abnormal crypts are CK-8 positive (I) and myofibroblast-like single cells and TC are double positive when co-stained with MMP-9/CK-8 (h). Figures A, D, H show consecutive sections with higher magnifications found in B, E, G. Figures C, F and E show also consecutive sections from a different specimen. Dashed line indicates the fistula tract. Small arrows indicate enlargements. Large arrows indicate TC.
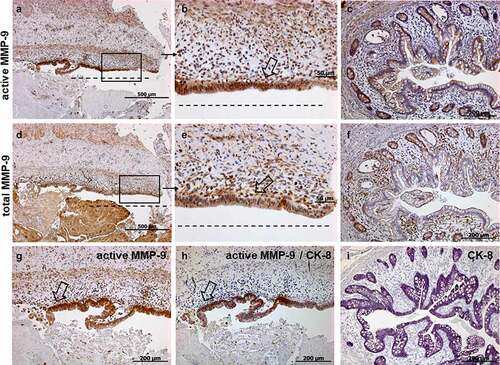
Figure 5. Representative images of immunohistochemistry staining results for active (a, d), CK-8 (b, e) and double staining for active MMP-9/CK-8 (C, F-I) in human xenograft fistulas (n = 6). The histology of the human xenograft fistula is similar to the human fistula. TC are found positive in the single staining for MMP-9 (a) and CK-8 (b) and in the co-staining MMP-9/CK-8 (c, g) at the tip of the fistula. Figures A, B, C are consecutive sections with higher magnifications found in D-F. Figure G shows TC from a different specimen with enlargements shown in H, I. Single stained and doubled stained cells with nuclei stained with hematoxylin are recognizable (i). Dashed line indicates the fistula tract. Small arrows indicate enlargements. Large arrows indicate TC.
The detection of active MMP-9 in xenograft fistulas indicates that it probably also critically contributes to fistula development in the in vivo model. This lays the foundation for the use of this model to further investigate anti-MMP-9 therapy as a possible treatment option for fistulizing CD.
Discussion
Our data demonstrate that total and active MMP-9 are expressed in perianal as well as in entero-enteric fistulas from CD patients, even in tissues derived from patients under anti-TNF treatment.
Anti-TNF antibodies are currently the standard-of-care for medical fistula treatment in CD patients, however long-term response rates are still unsatisfying.Citation3 With respect to a possible therapeutic targeting of MMP-9 in fistulizing CD, our most important finding was that active MMP-9 is still expressed along and around CD fistula tracts in patients non-responding to anti-TNF therapy. This persistent expression indicates that targeting MMP-9 is a promising therapeutic approach in such patients. In particular, antibodies targeting MMP-9 could be applied to patients non-responding to anti-TNF therapy. Given the role for MMP-9 in tissue remodeling and tissue destructionCitation26 as well as considering its expression around CD fistulasCitation18 and in different fistulas shown in this work in more details, we anticipate that sustained MMP-9 activity contributes to fistula pathogenesis in CD patients.Citation7 Therefore, inhibiting MMP-9 activity by pharmacological approaches, e.g. by a specific antibody treatment, might provide a novel tool for fistula therapy.
Based on our current hypothesis on fistula formation, we anticipated that MMP-9 is located downstream of TNF and TGFβ in the event cascade that leads to fistula formation.Citation3,Citation7,Citation27 We expect that TNF is one of the most upstream mediators of fistula formation (and in addition to TGFβ contributes to EMT and wound healing during chronic intestinal inflammation). TNF and TGFβ subsequently induce EMT as well as, in a second step, molecules that are associated with invasive cell growth, such as MMP-9 and β-6-integrin. This step would explain the transformation of the beneficial process of EMT/wound healing into a pathologic process of fistula formation/uncontrolled matrix degeneration/uncontrolled EMT. However, though our findings reveal a still strongly detectable expression of MMP-9 in patient tissue even after anti-TNF treatment, this observation does not prove whether MMP-9 expression would be directly controlled by TNF in this setting. The cell-cell interactions and signaling cascades leading to fistula formation still need to be elucidated. Of note, it seems plausible that also colonic epithelial cells may respond to a surrounding, inflammatory milieu by inducing myofibroblast functions in fistulizing CD similar to those observed during intestinal fibrosis.Citation28
Human fistula studies are limited by the fact that fistulas can only be examined once they have definitely developed. To date there is no indication to predict the location of a fistula development, which is why this process and the mechanisms eventually leading to fistula formation cannot be studied. All of the studies performed so far were executed using human fistula material. Therefore, the studied molecular events were those present once a fistula has already been formed. Nevertheless, our research provides important insights into fistula pathophysiology, since it reveals events occurring in fistulas. Further, one must recognize that fistula formation is a constantly ongoing process in which fistulas invade surrounding tissue layers, e.g. penetrating from the rectum through the skin, inflammation occurs and a constant tissue remodeling around the fistulas takes place.
In order to study developing fistulas, the identification of a reliable in vivo animal model for fistula is essential. Unlike the human CD patient setting, our recently published human xenograft model allows a detailed study of fistula development.Citation24 Of note, in this model we found a strong staining of total and active MMP-9 in cells lining the xenograft fistulas, similar to the staining observed in CD fistulas. This finding lays the premises for the use of our xenograft model as an in vivo model for investigating the efficacy and safety of anti-MMP-9 treatment in the fistula therapy. Interestingly, it appears that there was active MMP-9 expression in intestinal epithelial cells with a morphology preceding that of TC, as shown in , suggesting that indeed MMP-9 is active during fistula development.
Conclusion
Taken together, our data suggest that MMP-9 is involved in fistula pathogenesis in CD patients and that our novel xenograft fistula model is suitable for in vivo studies investigating a possible role of MMP-9 targeting for fistula therapy. Further studies will be needed to investigate whether pharmacologic inhibition of MMP-9 is a potential therapeutic approach for fistula therapy in CD patients.
Author contributions
MS conceived, designed and supervised the research. CM performed the experiments. RB and NYS were involved in preparing the samples from the xenograft mouse model. MS, CM, GR and YC interpreted the data. MT, AR and DCW provided the patient specimen. All authors edited the manuscript and approved the final version.
Summary
MMP-9 is involved in fistula pathogenesis in CD patients, also in patients non-responding to anti-TNF therapy. The new human xenograft mouse model is suitable for in vivo studies investigating a possible therapeutic role by targeting MMP-9.
Non-standard Abbreviation: TC: transitional cells
Supplemental Material
Download TIFF Image (1.5 MB)Disclosure statement
Michael Scharl received an unrestricted research grant from Calypso SA for performing the presented study.
Data availability statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Schwartz DA, Loftus EV, Tremaine WJ, Panaccione R, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of fistulizing Crohn’s disease in Olmsted County, Minnesota. Gastroenterology. 2002;122(4):1–12. doi:https://doi.org/10.1053/gast.2002.32362.
- Gecse KB, Sebastian S, Hertogh G, Yassin NA, Kotze PG, Reinisch W, Spinelli A, Koutroubakis IE, Katsanos KH, Hart A, et al. Results of the Fifth Scientific Workshop of the ECCO [II]: clinical aspects of perianal fistulising Crohn’s disease—the unmet needs. J Crohns Colitis. 2016;10(7):758–765. doi:https://doi.org/10.1093/ecco-jcc/jjw039.
- Scharl M, Rogler G, Biedermann L. Fistulizing Crohn’s disease. Clin Transl Gastroenterol. 2017;8(7):e106. doi:https://doi.org/10.1038/ctg.2017.33.
- Panés J, García-Olmo D, Van Assche G, Colombel JF, Reinisch W, Baumgart DC, Dignass A, Nachury M, Ferrante M, Kazemi-Shirazi L, et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial. Lancet. 2016;388(10051):1281–1290. doi:https://doi.org/10.1016/S0140-6736(16)31203-X.
- Molendijk I, Bonsing BA, Roelofs H, Peeters KCMJ, Wasser MNJM, Dijkstra G, van der Woude CJ, Duijvestein M, Veenendaal RA, Zwaginga -J-J, et al. Allogeneic bone marrow–derived mesenchymal stromal cells promote healing of refractory perianal fistulas in patients with Crohn’s disease. Gastroenterology. 2015;149(4):918–27 e6. doi:https://doi.org/10.1053/j.gastro.2015.06.014.
- Panes J, Garcia-Olmo D, Van Assche G, Colombel JF, Reinisch W, Baumgart DC, Dignass A, Nachury M, Ferrante M, Kazemi-Shirazi L, et al. Long-term efficacy and safety of stem cell therapy (cx601) for complex perianal fistulas in patients with crohn’s disease. Gastroenterology. 2018;154(5):1334–42 e4. doi:https://doi.org/10.1053/j.gastro.2017.12.020.
- Siegmund B, Feakins RM, Barmias G, Ludvig JC, Teixeira FV, Rogler G, Scharl M. Results of the fifth scientific workshop of the ecco (ii): pathophysiology of perianal fistulizing disease. J Crohns Colitis. 2016;10:377–386. doi:https://doi.org/10.1093/ecco-jcc/jjv228.
- Lovisa S, Genovese G, Danese S. Role of epithelial-to-mesenchymal transition in inflammatory bowel disease. J Crohns Colitis. 2019;13(5):659–668. doi:https://doi.org/10.1093/ecco-jcc/jjy201.
- Jiang H, Shen J, Ran Z. Epithelial-mesenchymal transition in crohn’s disease. Mucosal Immunol. 2018;11:294–303. doi:https://doi.org/10.1038/mi.2017.107.
- Bataille F, Klebl F, Rümmele P, Schroeder J, Farkas S, Wild PJ, Fürst A, Hofstädter F, Schölmerich J, Herfarth H, et al. Morphological characterisation of crohn’s disease fistulae. Gut. 2004;53:1314–1321. doi:https://doi.org/10.1136/gut.2003.038208.
- Bataille F, Rohrmeier C, Bates R, Weber A, Rieder F, Brenmoehl J, Strauch U, Farkas S, Fürst A, Hofstädter F, et al. Evidence for a role of epithelial mesenchymal transition during pathogenesis of fistulae in Crohnʼs disease. Inflamm Bowel Dis. 2008;14(11):1514–1527. doi:https://doi.org/10.1002/ibd.20590.
- Scharl M, Weber A, Fürst A, Farkas S, Jehle E, Pesch T, Kellermeier S, Fried M, Rogler G. Potential role for SNAIL family transcription factors in the etiology of Crohnʼs disease-associated fistulae. Inflamm Bowel Dis. 2011;17(9):1907–1916. doi:https://doi.org/10.1002/ibd.21555.
- Scharl M, Frei S, Pesch T, Kellermeier S, Arikkat J, Frei P, Fried M, Weber A, Jehle E, Rühl A, et al. Interleukin-13 and transforming growth factor β synergise in the pathogenesis of human intestinal fistulae. Gut. 2013;62(1):63–72. doi:https://doi.org/10.1136/gutjnl-2011-300498.
- Frei SM, Pesch T, Lang S, Weber A, Jehle E, Vavricka SR, Fried M, Rogler G, Scharl M. A role for tumor necrosis factor and bacterial antigens in the pathogenesis of Crohnʼs disease–associated fistulae. Inflamm Bowel Dis. 2013;19(13):2878–2887. doi:https://doi.org/10.1097/01.MIB.0000435760.82705.23.
- Frei SM, Hemsley C, Pesch T, Lang S, Weber A, Jehle E, Rühl A, Fried M, Rogler G, Scharl M, et al. The role for dickkopf-homolog-1 in the pathogenesis of crohn’s disease-associated fistulae. PLoS One. 2013;8(11):e78882. doi:https://doi.org/10.1371/journal.pone.0078882.
- Baugh MD, Perry MJ, Hollander AP, Davies DR, Cross SS, Lobo AJ, Taylor CJ, Evans GS. Matrix metalloproteinase levels are elevated in inflammatory bowel disease. Gastroenterology. 1999;117(4):814–822. doi:https://doi.org/10.1016/S0016-5085(99)70339-2.
- Faubion WA, Fletcher JG, O’Byrne S, Feagan BG, de Villiers WJ, Salzberg B, Plevy S, Proctor DD, Valentine JF, Higgins PD, et al. EMerging BiomARKers in inflammatory bowel disease (EMBARK) study identifies fecal calprotectin, Serum MMP9, and Serum IL-22 as a novel combination of biomarkers for Crohn’s disease activity: role of cross-sectional imaging. Am J Gastroenterol. 2013;108(12):1891–1900. doi:https://doi.org/10.1038/ajg.2013.354.
- Kirkegaard T, Hansen A, Bruun E, Brynskov J. Expression and localisation of matrix metalloproteinases and their natural inhibitors in fistulae of patients with crohn’s disease. Gut. 2004;53:701–709. doi:https://doi.org/10.1136/gut.2003.017442.
- Goffin L, Fagagnini S, Vicari A, Mamie C, Melhem H, Weder B, Lutz C, Lang S, Scharl M, Rogler G, et al. Anti-mmp-9 antibody: a promising therapeutic strategy for treatment of inflammatory bowel disease complications with fibrosis. Inflamm Bowel Dis. 2016;22(9):2041–2057. doi:https://doi.org/10.1097/MIB.0000000000000863.
- van Haaften WT, Mortensen JH, Karsdal MA, Bay-Jensen AC, Dijkstra G, Olinga P. Misbalance in type III collagen formation/degradation as a novel serological biomarker for penetrating (Montreal B3) Crohn’s disease. Aliment Pharmacol Ther. 2017;46(1):26–39. doi:https://doi.org/10.1111/apt.14092.
- de Bruyn M, Breynaert C, Arijs I, De Hertogh G, Geboes K, Thijs G, Matteoli G, Hu J, Van Damme J, Arnold B, et al. Inhibition of gelatinase b/mmp-9 does not attenuate colitis in murine models of inflammatory bowel disease. Nat Commun. 2017;8(1):15384. doi:https://doi.org/10.1038/ncomms15384.
- Schreiber S, Siegel CA, Friedenberg KA, Younes ZH,Seidler U, Bhandari BR, Wang K, Wendt E, McKevitt M, Zhao S, et al. A phase 2, randomized, placebo-controlled study evaluating matrix metalloproteinase-9 inhibitor, andecaliximab, in patients with moderately to severely active crohn’s disease. J Crohns Colitis. 2018;12:1014–1020.
- Chen H, Xu H, Luo L, Qiao L, Wang Y, Xu M, Li Y, Zhu P, Yang B. Thalidomide prevented and ameliorated pathogenesis of crohn’s disease in mice via regulation of inflammatory response and fibrosis. Front Pharmacol. 2019;10:1486. doi:https://doi.org/10.3389/fphar.2019.01486.
- Bruckner RS, Nissim-Eliraz E, Marsiano N, Nir E, Shemesh H, Leutenegger M, Gottier C, Lang S, Spalinger MR, Leibl S, et al. Transplantation of human intestine into the mouse: a novel platform for study of inflammatory enterocutaneous fistulas. J Crohns Colitis. 2019;13(6):798–806. doi:https://doi.org/10.1093/ecco-jcc/jjy226.
- Nagy N, Marsiano N, Bruckner RS, Scharl M, Gutnick MJ, Yagel S, Arciero E, Goldstein AM, Shpigel NY. Xenotransplantation of human intestine into mouse abdomen or subcutaneous tissue: novel platforms for the study of the human enteric nervous system. Neurogastroenterol Motil. 2018;30(3):e13212. doi:https://doi.org/10.1111/nmo.13212.
- Bauvois B. New facets of matrix metalloproteinases mmp-2 and mmp-9 as cell surface transducers: outside-in signaling and relationship to tumor progression. Biochim Biophys Acta. 2012;1825:29–36.
- Scharl M, Rogler G. Pathophysiology of fistula formation in crohn’s disease. World J Gastrointest Pathophysiol. 2014;5:205–212. doi:https://doi.org/10.4291/wjgp.v5.i3.205.
- Drygiannakis I, Valatas V, Sfakianaki O, Bourikas L, Manousou P, Kambas K, Ritis K, Kolios G, Kouroumalis E. Proinflammatory cytokines induce crosstalk between colonic epithelial cells and subepithelial myofibroblasts: implication in intestinal fibrosis. J Crohns Colitis. 2013;7(4):286–300. doi:https://doi.org/10.1016/j.crohns.2012.04.008.
