ABSTRACT
Antimicrobial resistance (AMR) is fast becoming a medical crisis affecting the entire global population. The bacterial membrane is the first layer of defense for the bacteria against antimicrobial agents (AMA), specifically transporters in the membrane efflux these AMA out of the bacteria and plays a significant role in the AMR development. Understanding the structure and the functions of these efflux transporters is essential to overcome AMR. This review discusses efflux transporters (primary, secondary, and tripartite), their domain architectures, substrate specificities, and efflux pump inhibitors (EPI). Special emphasis on nosocomial ESKAPEE (Enterococcus faecium., Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, Enterobacter spp. and Escherichia coli) pathogens, their multidrug efflux targets and inhibitors are discussed. Deep knowledge about the functioning of efflux pumps and their structural aspects will open up opportunities for developing new EPI, which could be used along with AMA as combination therapy to overcome the emerging AMR crisis.
1. Introduction
The microbial universe was discovered and introduced to the world by the legends Robert Hooke and Antoni van Leeuwenhoek through serendipity in the 16th century.Citation1Particularly, the discovery of bacteria by Leeuwenhoek was one of the greatest contributions to science and biology, which changed our vision of the biome we live in. Later discoveries identified that some of the microbes are beneficial to the host and some are pathogenic causing diseases in the host. Pathogenic microbes had played a crucial role in the survival history of mankind.Citation2 For instance, Plague or black death (165–180 AD, killed one-third of the world population in the 13th century), malaria (18th century), Spanish influenza (pandemic 1918–1920), tuberculosis (TB in 19th century), smallpox (1972), HIV pandemic (the early 1980s), SARS (21st century), and SARS-COV-2 (late 2019s) outbreaks have resulted in millions of death and affected the livelihood of many worldwide.Citation3–6
To treat these deadly infectious diseases, humans have used both natural product-derived agents (e.g.: quinine from cinchona tree for Malaria) and modern medicines (antibacterial, antibiotics, antiviral, vaccines, etc.) successfully during the past century.Citation4,Citation7–9 Particularly, the discovery of several antimicrobial agents (AMA) in last two centuries had helped to overcome many of the microbial diseases.Citation9,Citation10 However, misuse and/or overuse of AMA without discretions have resulted in the rapid selection of drug-resistant microbes that leads to life-threatening diseases such as tuberculosis, HIV, urinary tract infection and malaria.Citation11 At present, the probable likelihood of (mis)using antibiotics is around 44% for the treatment of viral infections instead of bacterial infections, in many of the countries.Citation12–15
Antimicrobial resistance (AMR) refers to microbes having the ability to neutralize or tolerate the effect of antimicrobial agents (AMA) as well as consistently withstand and proliferate in the presence of AMA.Citation16 The emergence of AMR in both developed and developing countries toward many clinically relevant AMA is troubling.Citation16,Citation17 WHO (World Health Organization) statistics show that globally 0.7 million people are dying every year due to the emergence of antibacterial resistance (ABR), and by 2050, the expected lives lost will be 10 million per year.Citation18 In the year 2017, WHO published a list of antibiotic-resistant “priority pathogens” – a catalog of 12 families of bacteria that pose the greatest threat to human health.Citation19 Among them, potential drug-resistant nosocomial ESKAPEE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, Enterobacter spp. and Escherichia coli) pathogens cause infections in critically ill and immunocompromised individuals.Citation20
In addition to the increasing threat of AMR, multiple analysis has also shown that big-ticket investments are not happening for the development of new AMA’s in the pharmaceutical sectors.Citation21 In the last 10 years, very few AMA has reached the market such as fidaxomicin (2011), ceftazidime/avibactam (2015), ceftobiprole (2015), dalbavancin (2015), oritavancin (2015), solithromycin (2015), tedizolid (2015), plazomicin (2018–19), eravacycline (2018–19), sarecycline (2018–19), omadacycline (2018–19), rifamycin (2018–19), imipenem/cilastatin/relebactam (2019), pretomanid (2019), lefamulin (2019), cefiderocol (2019), and omadacycline (2019).Citation22,Citation23 In the absence of significant investments in the development of new antibiotics, improving the efficacy of existing antibiotics is one of the key actions that could be taken-up to curb the spread of AMR. One can achieve this objective by using appropriate drug/adjuvants to overcome the resistance for already existing AMA. Hence, there is a need to focus on the development of new drugs/adjuvants to overcome AMR.
The bacterial membrane plays a crucial role in the development of resistance to AMA. Specifically, the membrane proteins and channels define and regulate the molecular traffic across the membrane.Citation24 They define and shape the cell and cellular organelles for cell–cell communication, aggregation, transportation, adhesion, cell signaling, and biofilm formation through active and passive transport to disrupt and cross the host tissue barrier.Citation25 Hence, the bacterial outer membrane and its proteins are important for deeper invasion of host tissues and also in evolving antibiotic resistance.Citation26 The role of bacterial membrane and its proteins in the resistance mechanism is discussed in detail in bacterial resistome and effluxome sections.
This review discusses the most common multi-drug resistant mechanism reported in microbes, with an incisive focus on the bacterial effluxome. Details of various efflux transporters involved in AMR of both Gram-positive and Gram-negative bacteria, along with their structural biology has been described lucidly in this review. Further, details of ESKAPEE pathogens, efflux transporters reported in them and the available drugs/inhibitors to overcome the efflux transportation of AMA and their role in biofilm formation are discussed. The review could help in imparting new ideas for the development of bacterial effluxome targeting agents, which is highly desirable for overcoming AMR.
2. Bacterial resistome
In the beginning, single cells were the only living being on earth. By the process of natural selection, from a single cell to anaerobes, anaerobes to aerobes, aerobes to multicellular organisms emerged slowly.Citation27–30 Between anaerobes to aerobes era, various races, for instance, aerotolerant, facultative, obligate bacteria, and so on have emerged.Citation30,Citation31 In the race for “survival of the fittest” or in the “war of defence”, microbes start competing with each other including multicellular organisms (animals including humans). Microbes use various armaments in their defense for survival. To propagate within the host, microbes first use only a few knacks like colonization, adherence and drug-metabolizing enzymes. Later on, they use other tricks such as lenience, frameshift mutations, rapid reproduction to attain the power of “resistance”, leading to better survival.
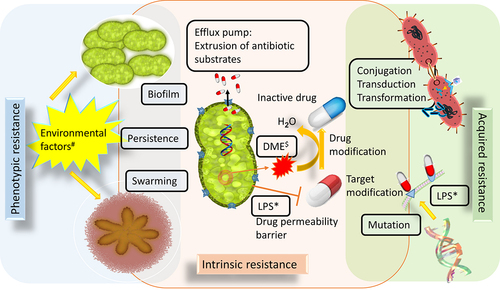
When looking into the origins of resistance of an organism, it is clear that some resistances are intrinsic (from birth), some are acquired during the process of evolution and some are developed from the surrounding environments. As stated by Wright 2007, resistome is “a collection of all the antimicrobial resistant genes (ARGs) and their precursors in pathogenic and non-pathogenic bacteria”.Citation32 The occurrence of bacterial resistome is due to a combination of intrinsic, acquired, and phenotypic characteristics. The three different modes of bacterial resistome are depicted in . Citation32–35
(i) Intrinsic resistance mechanisms are like an innate immunity of the bacteria. Enzymatic inactivation, target bypassing, drug-metabolizing enzymes, antibiotic degrading genes (subsistome), drug/substrate effluxing and preventing drugs through permeability (membrane) barrier, protect the cells from harmful enzymes and phagocytosis through surface layer (s-layer), virulent protein export through twin-arginine translocation (Tat) system and secretion (sec) system are few intrinsic resistance mechanisms.Citation36 For example, the cluster of blaCTX-M genes derived from different strains of Kluyvera (environmental strains are a rich source of resistance genes) found to be the first enzyme to hydrolyze expanded spectrum cephalosporins.Citation35,Citation37
(ii) Acquired resistance mechanisms: acquired antibiotic-resistant genes are frequently present within the mobile genetic elements that are capable of translocation from one part of a genome to another or between genomes by conjugative plasmids or conjugative transposons, transformation as well as horizontal gene transfer, mutation, gene acquisition, transduction, cell-to-cell transfer of plasmid and R plasmid maintenance.Citation35,Citation36 For example, through crossing over mechanisms such as homologous recombination or transposition the naked DNA is taken up by certain bacterial species (e.g.: Neisseria) and assimilated into their host genome.Citation38
(iii) Phenotypic resistance mechanisms are non-inherited and are mainly associated with specific processes such as biofilm formation, development of persistence, swarming and cell-cell communication under certain environmental conditions (temperature, pH, pressure).Citation39
Figure 1. Various resistance mechanisms developed by bacteria to overcome antibacterial agents. $DME-Drug Metabolizing Enzymes (e.g., β-lactamase); *LPS-Lipopolysaccharides; #Environmental factors (e.g., high or low temperature, pH and nutrients).
As stated by Julian, “if the resistance is biochemically possible, it will occur”.Citation40 In the end as a token of victory, the resistant microbes with boosted morbidity and mortality will emerge as ‘superbugs’.Citation41 “Superbugs” are not only limited to bacteria, it also encompasses fungi, algae, viruses (HIV and influenza) and parasites (malaria). Addressing all the modes of AMR is beyond the scope of this review. This review delves into one of the common drug-resistant mechanisms in bacteria, namely, the multidrug effluxome system.
3. Bacterial effluxome
As discussed above, there are many defense/resistant mechanisms in the bacterial system. However, the first line of defense against toxins is the permeability (membrane) barrier, which acts like an “ozone layer” for a bacterial system.Citation42 To reach their targets, the toxins (compounds/substrates/dyes/solutes) need to pass through this protective layer/cell membrane. Besides, the composition of cell membrane is different in Gram-positive and Gram-negative bacteria wherein the presence of extra outer membrane (lipopolysaccharide and protein) along with a thin peptidoglycan layer is seen in Gram-negative bacteria, whereas in Gram-positive bacteria only a thick peptidoglycan layer with no outer lipid membrane is present.Citation43 Passage through this membrane is controlled by many factors, such as membrane permeability barriers, s-layer, Tat system, sec system, lipid A modifications (that affects the binding of antimicrobial agents to the immune receptors), porins (that regulate the transport of hydrophilic molecules including antibiotics across the membrane), extracellular polymeric substances (biofilm formation), receptors (pass the signal to change the intracellular and intercellular pathways/mechanism) and adhesions (host cell colonization). In addition, a certain membrane protein plays a vital role to limit/expel the toxins in and out of the cells, which are called “efflux transporters”.Citation44 Consequently, the entire set of the bacterial efflux transporter expressed in response to environmental perturbations that contribute to the survival by the removal of toxic chemicals from the bacteria are collectively called as bacterial effluxome”.Citation45 In particular, drug-specific efflux transporters efflux the drugs out of the cell and consequently reduce the internal concentration of the drug, thereby decreasing the drug efficacy that leads to AMR. Evolutionarily, the emergence of drug efflux transporters is associated with the existence of “drug-metabolizing enzymes (DME-enzymatic inactivation)”, which was predicted to be present in all domains (eukaryotes, prokaryotes, and Archaea) of life about 2.5 billion years ago.Citation46 Rapid natural selection of DME triggers xenobiotic metabolism and consequent efflux of accumulated xenobiotic metabolites by the efflux pumps. Eventually, repeated modulation of the substrate specificities in the efflux transporters over a period of time resulted in the emergence of “multidrug efflux pumps”. Apart from the drug/compound transport, the efflux transporters are also involved in pathogenicity, transporting toxins, other virulence factors, lipid transport, cell–cell communication, and biofilm formation.Citation47,Citation48
The first described multidrug efflux pump is the ABC transporter P-glycoprotein (ATP as a driving force) from a mammalian cell, which provides resistance to a broad spectrum of compounds including anticancer chemotherapeutic agents.Citation41 In the late 1980s and early 1990s, it became apparent that multidrug efflux pumps were also widespread among microbes. There are around six efflux systems that are widespread in prokaryotes, which are encoded either by chromosomes or plasmids.Citation49 They are ATP-binding cassette (ABC) family, Major Facilitator Superfamily (MFS), Multidrug and Toxin Extrusion (MATE) family, Small Multidrug Resistance (SMR) family, Resistance-Nodulation-cell Division (RND) superfamily and Proteobacterial Antimicrobial Compound Efflux (PACE) family. These efflux systems extrude many biocides, dyes, and antibiotics through force/energy-dependent mechanisms. Previously, it was believed that drug-specific efflux pumps were encoded by plasmids and multidrug resistance (MDR) pumps by chromosomes.Citation47,Citation49 However, subsequent discoveries showed that plasmid can also encode MDR pumps; for instance, QacA and QacC from Staphylococcus aureus, Bmr from Bacillus subtilis, and EmrB from Escherichia coli are plasmid-driven MDR pumps.Citation47
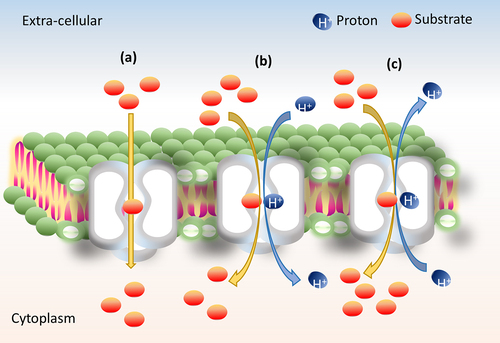
The six families of efflux transporters belong to either primary (ATP dependent) or secondary transporters (proton motif force (PMF) dependent). Primary transporters use adenosine triphosphate (ATP) as a driving force.Citation50,Citation51 Secondary transporters are facilitated by three major mechanisms of transport such as uniport (single molecule transport), symport (molecules move in the same direction; i.e., a substrate with coupling ion (sugar/H+)) and antiport (molecules move in the opposite direction; i.e., a substrate with co-substrate/coupling ion ()).Citation52 The binding of a substrate is dependent on the prior release of another substrate or the coupling ion H+ except in uniporter (facilitated diffusion requires no energy).Citation53 The other two efflux transporters, namely, the symporter and antiporters are active transporters that use energy to facilitate the transport of the substrates through conformational changes.Citation54 The efflux transporters switch from an inward open (cleft toward cytoplasmic membrane to access substrates), to occluded (cleft at either side of the membrane to access the substrates) to an outward open (cleft toward extracellular membrane to expel the substrates) states using the driving agents such as a proton (H+) or a sodium (Na+) gradients, which was first explained by Jardetzky in 1966 and named as ‘alternative access mechanism’.Citation55,Citation56
Figure 2. Illustration of basic transport mechanism. (a) Uniport, (b) symport and (c) antiport transporters.
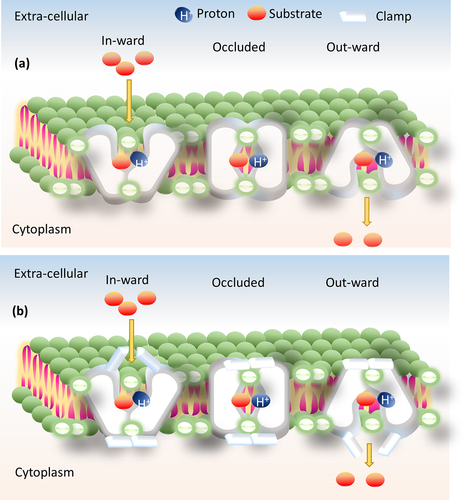
There are many models under alternative access mechanisms, such as “rocker switch” (inward and outward-facing with single substrate binding site) and “clamp-and-switch” (fully/partially inward and outward-facing occluded)” models (). The gating residues (residues involved in conformational change) play a major role, which defines where exactly the substrate binds in the rocker-switch model. Whereas, in the clamp-switch model, these gating residues are in two states: one is the thin gate (small conformational change during substrate binding) and another one is the thick gate (large conformational change during substrate binding).Citation57 To validate these conformational changes, various biochemical and biophysical experiments are there, such as site-directed alkylation, site-directed cross-linking, tryptophan fluorescence, single-molecule FRET (Förster or fluorescence resonance energy transfer), and DEER (double electron–electron resonance).Citation58 The general energy coupling alternative access mechanisms found in almost all the MDR secondary transporters are antiporters, for instance MFS (MdfA, PDB_ID: 4ZOW),Citation59 MATE (PfMATE, PDB_ID: 3W4T),Citation60 SMR (EmrE, PDB_ID: 3B5D),Citation61 and RND (AcrB, PDB_ID: 4DX5).Citation62
Figure 3. Illustration of alternative access mechanism models. (a) Rocker-switch model and (b) clamp-switch model.
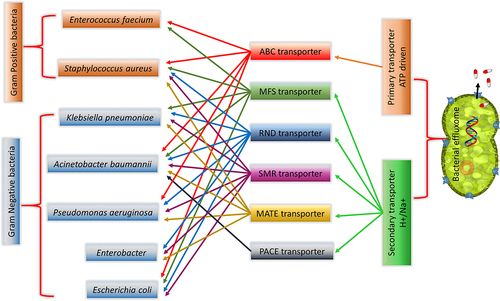
Disabling efflux pumps in the bacterial system affect the survival, biofilm formation, host colonization, and virulence by increasing the accumulation of antimicrobial agents (AMA)/drugs/toxins in the system.Citation63 When compared to Gram-positive bacteria, Gram-negative bacteria are much more resistant to antibiotics, and also they are responsible for ~80% of the nosocomial infections.Citation64 The extra lipid outer membrane not only helps to distinguish bacterial strains but also plays a significant role in the pathogenicity of the organism. The tripartite efflux pump assembly is present only in the Gram-negative bacteria and is completely absent in Gram-positive bacteria. The extra lipid outer membrane in Gram-negative bacteria necessitates the tripartite assembly to connect/traverse the three barriers (inner membrane, periplasmic space, peptidoglycan layer, and outer membrane) to drive the drugs and other compounds across these barriers (). Citation43 Not all the six classes of transporters are presented in the bacterial system as tripartite efflux assembly, one can observe such assembly only in three classes of transporters, such as RND, MFS, and ABC. Some of the transporters work as a part (single-component efflux pumps) and efflux the drugs/compounds to periplasmic space then outward through the tripartite assembly. In Gram-positive bacteria, the efflux pump transporters ABC, MFS, SMR, and MATE alone span the inner membrane and expel the antimicrobial agents/drugs/toxins out of the cell.Citation16 summarizes the characteristics of six bacterial multi-drug efflux systems whereas provide the domain-level architecture and provides a pictorial representation of both the single component and tripartite efflux pump systems. The following section discusses the structure and the mode of drug efflux in detail for the individual efflux systems.
Table 1. Comparative information about the major classes of drug efflux transporters present in bacteria.
Figure 4. Schematic presentation of multidrug transporters in Gram-positive and Gram-negative bacteria. The tripartite assembly is present only in Gram-negative bacteria. ATP-binding cassette (ABC) family, Major Facilitator Superfamily (MFS), Multidrug and Toxin Extrusion (MATE) family, Small Multidrug Resistance (SMR) family and Resistance-Nodulation-cell Division (RND) superfamily.
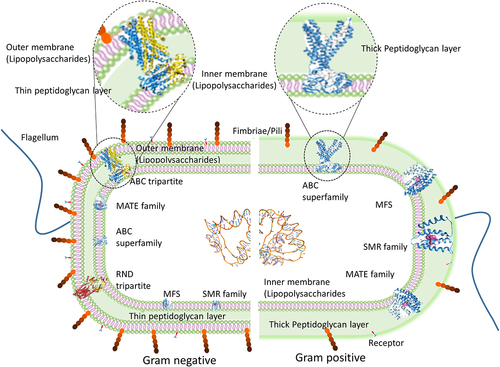
Figure 5. Domain architecture of MFS, RND and SMR efflux transporters. (a) 12 TMH of MFS multidrug efflux pump EmrD-3 from Vibrio cholera, with motif A found between TMD 2 and 3 and Motif C found within TMD 5. (b) 14 TMH of MFS multidrug efflux pump LmrS from Staphylococcus aureus with Motif A and C. (c) Four subdomains (PN1, PN2, PC1, and PC2) of RND transporters form two proximal and distal substrate-binding pockets, separated by Phe loop. The Phe loop forms the boundary between proximal and distal pocket and controls the access of substrates to the distal pocket. (d) Four transmembrane helix of SMR with conserved GLU residue.
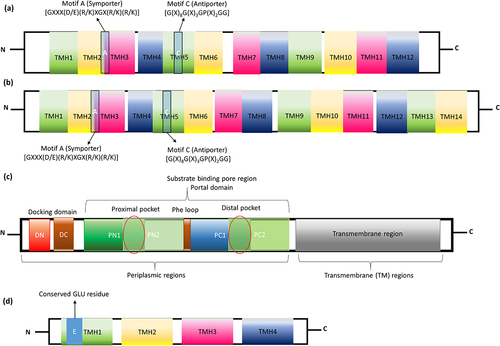
Figure 6. Pictorial representation of (a) single component efflux system and (b) tripartite assemblies with their substrate specificities. (a) LmrS MFS efflux transporter, (b) QacC SMR efflux transporter, (c) NorM MATE efflux transporter, (d) Sav1866 ABC efflux transporter, (e) AceI PACE efflux transporter, (f) EmrAB-TolC MFS tripartite assembly, (g) AdeABC RND tripartite assembly and (h) MacAB-TolC ABC tripartite transporter systems.
3.1. Major facilitator superfamily (MFS) efflux transporters
Major facilitator superfamily (MFS) transporters are single-polypeptide secondary carriers capable of transporting solute molecules.Citation65 In 1961, it was recognized for the transport of only solutes (glucose) in red blood cells of humans and named as solute carrier (SLC) mechanism.Citation66 Later, it was found to be involved in many physiological processes for transporting small as well as large molecules with conserved DRXGRR/K motif and named as “Major facilitator superfamily”.Citation67 Furthermore, there are many families (SLC15, SLC16, SLC21 and SLC22 families) under MFS and SLC mechanisms involved in the transport of a wide range of toxins and drugs.Citation68 Majority of these transporters are present in Gram-positive bacteria compared to Gram-negative bacteria, and it is involved in the process of transport of nutrients, metabolites, and substrates between the cells and intracellular compartments. Usually, it acts as a pipeline or inlet for nutrients uptake and outlet for toxic compounds release in both eukaryotes (e.g., intestinal nutrient absorption and renal and hepatic clearance)Citation69 and prokaryotes (e.g., effluxing of deleterious compounds).Citation70 The widely studied MSF transporters are SLC2 (e.g., the uniporter glucose transporter GLUT1, PDB_ID: 4PYP; dysfunction leads to type 2 diabetes) from humans,Citation71 lactose permease (e.g., the symporter LacY, PDB ID: 1PV7, lactose/H+),Citation57 and SLC37 (e.g., the antiporter glycerol-3-phosphate/phosphate transporter, GlpT, PDB ID: 1PW4) from E. coli (1st crystal structures of MFS, 2003).Citation72
The evolutionary basis of MFS’s structure is still controversial as the number of the transmembrane helix (TMH) can be either 12 or 14 with 400–700 amino acids comprising N- and C-terminal domains.Citation73 The drug-specific antiporters (drug/H+ antiporters also called “DHA”) are broadly classified into four families (DHA1, 2, 3, and 4) containing Motif A, Motif B, Motif C, and Motif D.Citation74 Among them, Motif B “[R112xxQG]” (capital letters denote the highly conserved residues, more than 90%), superscripts denote the mutation position, “x” denotes any residues and small letters denotes less conserved residue/position (less than 65%) and Motif D “[E26fxxY30ianD34miqP38g]” is present in TMH4 and TMH1, respectively.Citation75 The study by Kakarla et al., 2017, predicted that the Motifs A and C are highly conserved in solute transporters of the MFS and also shared common structural motifs (motif A between TMH 2 and 3 and motif C within TMH 5) (), three-dimensional structures and evolutionary origins.Citation76 Motif A “[GXXX(D/E)(R/K)XGX(R/K)(R/K)]” usually acts as a symporter (e.g. emrD-3 from Gram-negative Vibrio cholerae), while the motif C “[G(X)8G(X)3GP(X)2GG]” act as an antiporter (e.g. LmrS from Gram-positive Staphylococcus aureus (); MdfA (PDB_ID: 4ZP0) from Gram-negative E. coli).Citation77 However, Motif B (RxxqG” in TM4) acts as both symporter (KgtP, ShiA, YdfJ, and YhjE from E. coli) and antiporter (EmrB, EmrY, HsrA, MdfA, MdtD, MdtL, MdtM, YajR, and YebQ from E. coli).Citation78 MFS transporter of both Gram-positive and Gram-negative bacteria confers resistance to a wide range of antibiotics. For instance, LmrS (S. aureus) () and Bcr (E. coli) transporters efflux oxazolidinone, phenicols, aminoglycosides, macrolides, QACs, detergents, dyes and bicyclomycin.Citation79
As discussed earlier, tripartite transporters have three components: inner membrane (cytoplasm), outer membrane (extracellular) and periplasmic space protein components. Periplasmic space protein/adopter proteins are the ones which connect the inner and outer membrane protein components. The first identified (in 1992) tripartite transporter operon of MFS is EmrAB from E. coli operon and codes for the well-studied tripartite EmrAB-TolC efflux system (pictorially represented in ). It is a multidrug-resistant tripartite transporter that confers resistance to CCCP (carbonyl-cyanide m-chlorophenylhydrazone), nalidixic acid, and thiolactomycin.Citation80 EmrB is a 14 TMH inner membrane protein that recognizes the substrates and traverses the cytoplasm/inner membrane. These TMHs aid in substrate binding and co-transport coupling due to the presence of substrate-binding pockets surrounded by hydrophobic residues.Citation81 These hydrophobic residues are protonated during the process of substrate binding and disrupt the interface between TMH and periplasmic adopter protein. This allows further tilting of the N- and C-terminal domains to expose the substrate-binding site to the periplasmic space. The binding of the substrate to the pocket changes the conformation followed by the release of the substrate to the cytoplasm in the inward-facing conformation or substrate to the extracellular membrane through the adopter protein in the outward-facing conformation. This same mechanism is followed in all types of MFS; however, the substrate specificity varies due to key residue differences at the substrate-binding site.Citation82 While, EmrA (PDB_ID: 4 TKO) is the adopter protein that traverses the periplasm and connects the EmrB and TolC in a tip-to-tip manner. TolC is the outlet channel present in the extracellular membrane, which extrudes a wide range of substrates out of the cell.Citation83 The first single-particle EM structure of the EmrAB-TolC tripartite assembly from E. coli shows that the 3D architecture of this tripartite system is similar to RND (AcrAB-TolC; ) and ABC (MacAB-TolC; ) tripartite efflux transporter assemblies.Citation84 Its overexpression, due to either induction or by a repressor mutation, confers increased resistance to nalidixic acid, thiolactomycin, nitroxoline, and hydrophobic proton uncouplers.Citation85
3.2. Resistance nodulation and cell division (RND) efflux transporters
The membrane proteins involved in the transport of heavy metals (resistance), nodulation and cell division are collectively described as resistance nodulation and cell division (RND) superfamily. RND transporters are commonly seen in Gram-postive bacteria, e.g. CzcA protein is the first identified RND transporter protein from Ralstonia metallidurans. Initially. it was discovered to efflux the heavy metals, hence considered as “the first layer heavy metal resistance”. Later it was also found to be involved in the transport of hydrophobic compounds, amphiphiles, nodulation factors and protein transport in prokaryotes and sterol transport in eukaryotes. RND transporters are classified under seven sub-families such as the heavy metal efflux (HME; e.g., CusA, PDB_ID: 3KSO from E. coli), the hydrophobe/amphiphile efflux-1 (HAE-1; e.g., AcrB, PDB_ID: 2I6W), the nodulation factor exporter family (NFE; e.g., NolG), the protein-export membrane protein family (e.g., SecDF, PDB_ID: 5XAP), the hydrophobe/amphiphile efflux-2 (HAE-2; e.g., MmpL1, 2, 4, 5, 8, 10 and 12), the eukaryotic sterol homeostasis (ESH; e.g., Npc1, PDB_ID: 5U74), and finally the hydrophobe/amphiphile efflux-3 (HAE-3; e.g., MmpL3, PDB_ID: 6N40; MmpL7 and MmpL11 (PDB_ID: 4Y0L)). HME, HAE-1, NFE are specific to Gram-negative bacteria, SecDF is present in both Gram-positive and Gram-negative bacteria along with archaea, HAE-2 is specific to Gram-positive bacteria, ESH, as the name implies, is specific to eukaryotes and HAE-3 is present in archaea and spirochetes. RND transporter being a secondary transporter uses PMF as the active driving force for the efflux process.Citation86
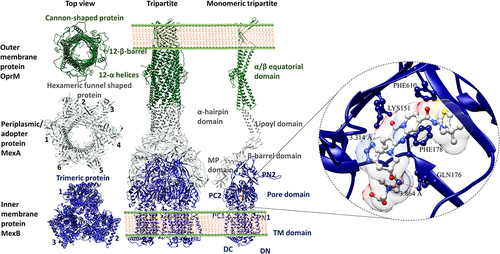
The conjoined tripartite efflux system () is also formed by the RND efflux pump. Among them, the well-established and most important structure is the inner-membrane protein, usually found as a homotrimer with a complex topology. For instance, AcrB (PDB_ID: 1IWG), MexB (PDB_ID: 6IIA), and AdeB (PDB_ID: 6OWS) have three important domain components, namely: (i) 12 TMH in the cytoplasmic inner membrane, (ii) docking domain and (iii) substrate-binding pore region (portal domain) in the periplasmic region (). The docking domain has DN and DC subdomains, while the portal domain consists of PN1, PN2, PC1, and PC2 subdomains. Among these domains, PN2 is highly conserved along with TM2, TM3, TM4, and mediates proton translocation; therefore, a mutation in these domains leads to complete loss of drug efflux.Citation87
The portal domain forms the distal (tight state) and proximal binding (loose stage) pockets separated by a switch loop or PHE loop, which controls the access of substrates to the distal pocket. The proximal and distal pockets are connected by a narrow channel, which is situated below this loop. Here, the substrate-binding pocket is surrounded by aromatic, polar, and charged amino acid residues along with a hydrophobic trap. Moreover, both pockets showed poly-substrate specificities. The homotrimeric structure forms a channel for the conduit of substrates from the cytoplasm to the extracellular region. Each monomer has three entrances and one large exit port for the movement of substrates from the cytoplasm and periplasm to the outside of the cell and periplasm as well (). The access form of the proximal site undergoes conformational changes to become a binding form so that it will efflux the bound substrate by a dynamic process called “peristaltic movement”. This movement is controlled by three conformational states of the inner membrane proteins such as loose (L-inward), tight (T-occluded) and open (O-outward). L to T and T to O states direct the substrates (e.g., Tigecycline, Omadacycline, and Eravacycline) from cytoplasm and periplasmic space to the outer membrane without leaking back into the periplasmic space.Citation87–89
3.3. Small multidrug resistance (SMR) efflux transporters
A small hydrophobic peptide spans the integral inner membrane responsible for drug resistance specifically to quaternary ammonium compounds (Qac) and other lipophilic cations in a distant or unrelated organism and is called a small multidrug resistance (SMR) transporter. A single-component efflux system of this type of transporters is common to both Gram-positive and Gram-negative bacteria and helps in moving drugs from cytoplasm/inner leaflet to periplasmic/extracellular space. The first identified SMR protein was from Staphylococcus aureus and hence named sau-SMR which is also known as Ebr or QacC/D (). Based on the co-expression, function, and comprehensive phenotypic information, it have three subclasses: (i) small multidrug pumps (SMP), (ii) Suppressors of groEL mutation proteins (SUG), and (iii) paired SMR (PSMR). SMPs are encoded by a plasmid, chromosome, as well by an integron and these SMPs mainly confer resistance to drugs and are present in prokaryotes, e.g., EmrE from E. coli (PDB_ID: 3B61) and Smr from Staphylococcus aureus and archaea (Hsmr from Halobacterium salinarum). SUG proteins are generally plasmid-encoded homologs to eukaryotic heat shock protein 60 (HSP60), They influx and efflux Qac and lipophilic dyes, e.g., SugE. PSMR are pair of homologs co-expressed SMR protein that confers resistance to drugs e.g., YdgF and YdgE.Citation90
As the name infers, it is the smallest transporter (around 100–170 amino acids) among all the transporters with a novel dual topology; i.e., the protein homo/hetero dimers often occur in anti-parallel orientation and rarely in parallel orientation, e.g., EmrE (PDB_ID: 1S7B and 2F2M) from E. coli. The best part is this transporter was found to be coded in both plasmid and transposable elements/mobile genetic elements with four amphipathic alpha-TMH. TMH1 to TMH3 contributes to substrate-binding pocket and the TMH4 is involved in the dimerization interaction. Upon substrate binding, the loops between TMH3 and TMH4 undergo structural changes to form a β-strand, which holds TMH4 close to the substrate-binding pocket. The substrate-binding site of TMH1 has a high affinity toward lipophilic cationic drugs (like cetylpredinium, acriflavin/profavin, tetraphenyl phosphonium, ethidium, and benzalkonium) due to the presence of conserved glutamate residue (Glu) (). Moreover, this transporter also uses PMF for effluxing drugs through an alternative access mechanism (inward and outward-facing conformation) and hence coming under the category of drug/H+ antiporter (DHA). Mutation in substrate-binding pocket results in altered SMR drug specificity.Citation89
3.4. Multi-drug and toxic compound extrusion (MATE) transporter
The membrane proteins involved in the binding of a wide variety of xenobiotic compounds and metabolic waste and extrude them out of the cell are called multidrug and toxic compound extrusion transporters (MATE). MATE transporters are ambiguous and first identified in Vibrio parahaemolyticus that conferred resistance to antibiotic Norfloxacin.Citation91 Based on the phylogenetic analysis, MATE is divided into three families with 14 subgroups. Family 1 is classified as bacterial MATE transporter (NorM) and the remaining two families belong to eukaryotes (eMATE) and archaea (DinF).Citation92 MATE recognizes various hydrophobic, cationic drugs, such as fluoroquinolones, aminoglycosides, cimetidine, metformin, procainamide, cephalexin, and acyclovir, effluxing them using H+ or Na+ as the driving force thereby referred to as cationic antiporters.Citation93
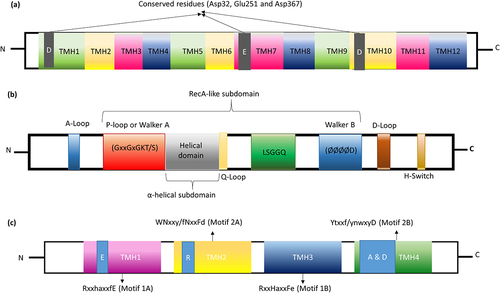
Typically, less than 40% sequence similarity was observed among the MATE proteins, and they had 400–700 amino acids residues comprising 12 TMH.Citation94 TMH1 to TMH6 forms N-terminal domain and TMH7 to TMH12 forms the C-terminal domains (). In addition, the mammalian transporter has extra TMH13. The acidic amino acid residue Asp32 from TMH1, Glu251 from TMH7, and Asp367 from TMH10 are highly conserved in bacterial NorM (). MATE protein is involved in the Na+-dependent drug transport process. C-terminal domain is the typical substrate-binding pocket, and the side chains of Glu255 and Asp371 from TMH6 to TMH12 are the putative cation-binding pockets. Mutation at the conserved amino acid Asp367 (D367A and D367N) leads to a complete loss of drug effluxing function.Citation92
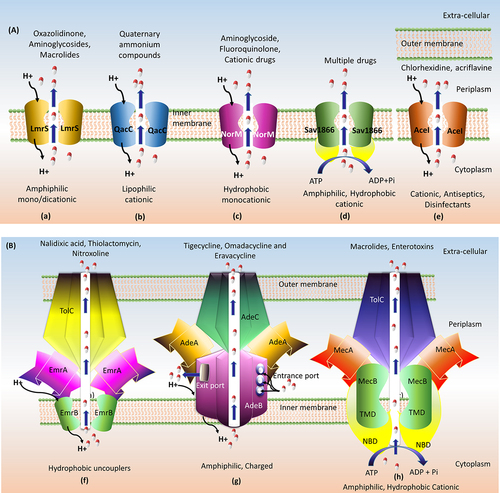
Figure 7. Domain architecture of MATE, ABC and PACE efflux transporters. (a) The charged amino acid (Asp32, Glu251 and Asp367) residues conserved in the transmembrane domain of MATE family. (b) The relative assignment of functional motifs in ABC transporter in the NBD. A-loop tyrosine amino acid; P-loop phosphate binding loop; Q-Loop forming the active site where Mg-ATP binds; LSGGQ-signature sequence; D-loop forms the ATP hydrolysis site; H-Switch is the histidine-switch; Ø hydrophobic amino acid. (c) In PACE family, the upper case residues in motif 1A; RxxhaxxfE (TMH1) are conserved in more than 90% of proteins and lower case residues are conserved in at least 65% of proteins and are very similar to motif 1B; RxxHaxxFe (TMH3). Likewise, the motif 2A, WNxxy/fNxxFd (TMH2) is very similar to motif 2B; Ytxxf/ynwxyD (TMH4).
3.5. ATP-binding cassette (ABC) efflux transporters
ABC (ATP-binding cassette) transporter is one of the largest and oldest known membrane proteins present in all three domains of life from viruses to animals.Citation95 In the 1970s, it was identified as an importer (nutrient uptake) in bacteria.Citation96 Later, in 1976, in eukaryotes p-glycoprotein (p-gp) also referred to as multidrug resistance protein 1 (MDR1) or ABCB1 was discovered.Citation97 As these transporters use adenosine triphosphate (ATP) as an energy source, they were named ABC (ATP-binding cassette) transporter. The first crystal structure of multidrug-resistant ABC transporters from the bacteria S. aureus (Sav1866; PDB_ID: 2HYD; ) was reported in the year 2006Citation98 and the first crystal structure of an intact ABC transporter, the vitamin B12 importer BtuCD from Escherichia coli (PDB_ID: 2QI9) was reported in 2007.Citation99
In bacteria, ABC transporters act as an importer (e.g., nutrients) and exporters (e.g., antimicrobial agents, toxic compounds, ions, sugar, and heavy metals) following an alternative access mechanism. According to Crow et al. (2017), there are seven different ABC transporters, which are broadly classified into importer (Class I, e.g.: ModABC, PDB_ID: 2ONK; Class II, e.g.: BtuCDF, PDB_ID: 2QI9; and Class III, e.g.: EcfAST, PDB_ID: 4 HUQ), exporters (Class IV, e.g.: Sav1866, PDB_ID: 2HYD; and Class V, e.g.: ABCG5/G8, PDB_ID: 5DO7) and mechano-transducers (Class VI, e.g.: LptBFG, PDB_ID: 5X5Y; and ClassVII, e.g.: MacB, PDB_ID: 5LIL). This review focuses only on efflux transporters of drug/antibiotics, which is under exporters (Class IV and Class V) including tripartite efflux system.Citation100
In general, ABC transporters are 2100 amino acids long and have a fusion of two transmembrane domains (TMD) and two nucleotide-binding domains (NBD). Furthermore, each TMD exists as a six transmembrane helix; two out of six TMH are fused with NBD through tight interface binding and form head-to-tail interaction both in the homodimeric or heterodimeric form. The homodimeric NBDs with symmetric ATP binding sites use ATP hydrolysis (converted to ADP and orthophosphate) as a driving force. In contrast, heterodimeric NBDs have a degenerate binding site (asymmetric-binding site) and do not involve in the complete ATP hydrolysis. NBD has one larger domain and one smaller domain. The larger domain is called the catalytic core domain comprising 2 β-strands and 6 α-helixes with RecA-like ATPase subdomain and motifs. And the smaller domain comprises an α-helical subdomain with conserved motifs. There are two conserved motifs in the RecA-like subdomain; one is the Walker A motif, a so-called P-loop and another one is the walker B-motif, a site for nucleotide-binding (). Amid these motifs, a glutamine-rich Q-loop is positioned which connects the NBD to TMD and acts as the sensor of γ- and the β-phosphate groups of ATP. Followed by walker B-motif, D-loop is located; conformational change in this loop affects the catalytic site and helps in ATP hydrolysis site formation. The α-helical subdomain has a peptide linker, LSGGQ signature sequence, or C motif. Residues of these signature sequences coordinate the phosphates for stable binding of ATP to NBD. Adjacent to the C-terminal NBD, the hinge region is formed by the conserved histidine loop (H-loop) involved in the interaction of NBD with ATP. During the substrate-binding at TMD, Q-loop recognizes the γ- and the β-phosphate of ATP and it is coordinated by the signature sequence “LSGGQ” for ATP hydrolysis. TMD is the key region where the substrates (dyes, effluxing substrates, drugs, antibiotics) bind. TMD has a variable number of transmembrane helixes ranging from 5–12 with the conserved binding pocket residues namely asparagine, aspartic acid and arginine, which can recognize and accommodate a variety of substrates.Citation101,Citation102
The well-studied tripartite system of ABC transporters is MacAB-TolC () involved in the efflux of macrolide antibiotics and certain enterotoxins. The periplasmic alpha-helical domain of MacA interlock with the outer membrane alpha-helical domain of TolC. The MacA tip regions make slightly different interactions with the intra-protomer and inter-protomer grooves of TolC. Similarly, the beta-barrel inner membrane domain of MacB interlocks with the periplasmic domain of MacA. Among 6 TMH of MacB, TMH1 and TMH2 play a major role to connect the NBD and periplasmic protein. Specifically, the N-terminal connecting helix present ahead of TMH1 connects the NBD where the ATP hydrolysis takes place. Mutation in the connecting residues results in complete loss of assembly leading to loss of efflux function of the tripartite system.Citation103
3.6. Proteobacterial antimicrobial compound efflux (PACE) transporters
A specific type of efflux transporters is common among proteobacterium phylum named proteobacterial antimicrobial compound efflux (PACE) family. Like other secondary transporters, PACE transporters also use the proton-coupled transport mechanism as a driving force. The PACE has emerged as chlorhexidine, acriflavine, dequalinium, benzalkonium, and proflavine-specific efflux pump.Citation104 Basic understanding about PACE efflux pump is from the study of AdeAB efflux transporters of Gram-negative pathogen Acinetobacter treated with chlorhexidine. Along with AdeAB, another transporter Acinetobacter chlorhexidine efflux protein I (AceI) () effluxes chlorhexidine by 10-fold. As mentioned earlier, they are inherited as a chromosomally encoded common gene in proteobacterial lineages (Pseudomonas, Klebsiella, Enterobacter, Yersinia pestis, Francisella tularensis Salmonella and Burkholderia) excluding E. coli lineage. AceI efflux pump has originated as a prototype for the PACE family with a distinct efflux transporter capability.Citation105 A recent study by Hassan et al., showed that PACE efflux transporter expression is controlled by the regulator LysR family. The single-component efflux system of this particular transporter delivers the substrates into periplasmic space and then expelling them through outer membrane proteins. Targeting the PACE efflux transporter is important because of its specificity toward antiseptics (chlorhexidine and acriflavine) used in wound dressings, hand washes and mouthwashes.Citation106,Citation107 At the secondary structure level, it has four transmembrane helixes (TMH) with two tandem bacterial transmembrane pairs (BTP; one each at N- and C-terminal). It has four universally conserved residues in TMH1 (Glu), TMH2 (Arg) and TMH4 (Ala & Asp) with two motifs between the N- and C-terminal BTP (motif 1A, RxxhaxxfE in TMH1; motif 1B, RxxHaxxFe in TMH3; motif 2A, WNxxy/fNxxFd in TMH2 and motif 2B, Ytxxf/ynwxyD in TMH4). The amino acid sequence in TMH1 motif (motif 1A; RxxhaxxfE) is very similar to the motif in TMH3 (motif 1B; RxxHaxxFe). Similarly, the motif in TMH2 (motif 2A, WNxxy/fNxxFd) is very similar to the motif in TMH4 (motif 2B; Ytxxf/ynwxyD) (). Though N-terminal motifs are important for substrate specificity, C-terminal motifs are highly conserved and distributed within the Proteobacteria.Citation104 More information about the domain-level architecture of PACE is only possible when X-ray crystal/cryo-EM structures are available. The sequence conserved between the N- and C-terminal suggest that PACE transporters evolved by duplication events. Deletion of this particular (AceI) transporter in Acinetobacter ADP1 belonging to proteobacterial lineage leads to a complete loss of resistance toward chlorhexidine.Citation107
4. Efflux transporters of ESKAPEE pathogens and their inhibitors
The group of nosocomial pathogens capable of escape from all known antibiotics irrespective of bacterial type (belonging to both Gram-positive and Gram-negative bacteria) are collectively referred as ESKAPEE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, Enterobacter Spp. and Escherichia coli) pathogens.Citation108 They commonly reside in the skin, nose, intestine and are categorized as Multi-drug resistant (MDR), Pan-drug resistant (PDR) and Extremely drug-resistant (EDR) pathogens based on their resistance profiles.Citation109 Comprehensive understanding of these pathogens may lead to innovative strategies to facilitate multidisciplinary approaches for the development of new antimicrobial agents or agents that can potentiate the existing ones. Efflux pump inhibitors (EPI) can work in many ways to halt the antimicrobial drug efflux: (i) inhibition of efflux pump activity (ii) alter the efflux gene regulation/expression (iii) compromising the efflux pump assembly (iv) affect the transmembrane potential/energy.Citation110 These EPI can be either of natural or of synthetic origin. The resistant mechanisms of ESKAPEE pathogens with a focus on their drug efflux transporters and the reported inhibitors that can overcome such resistance are described in the following section ( and Supplementary Table S1).
4.1. Enterococcus faecium
Enterococcus faecium is a Gram-positive sporadically associated opportunistic pathogens (Lactobacillus) which belongs to the Phylum Firmicutes. It is usually present in the gastrointestinal tract of animals, soil, water, food, and hospital environment. Endogenous contamination by patient’s body fluids and contamination of surgical equipment are common modes responsible for hospital outbreaks. For the past five decades, E. faecium has emerged as one of the leading infectious bacteria as it is accountable for most of the nosocomial infections and the case/fatality ratio was between 12–68% and 4–50% for enterococcal bacteremia and enterococcal sepsis, respectively.Citation111–113 An intensive Care Unit (ICUs) 2016 surveillance reported that E. faecium is responsible for common healthcare-associated infections such as urinary tract infection, bloodstream infection, pneumonia, surgical site infections and is the leading antimicrobial-resistant pathogen from Enterococcus species.Citation114,Citation115 Several virulent factors are involved in the pathogenicity to promote Enterococcus resistance; for e.g., cell-wall-anchored pilus-specific housekeeping sortases (LPXTG surface proteins, adhesins), biofilm-associated pili (Ebp), enterococcal surface protein (esp), membrane proteins (efflux pumps), aggregation substance (asa1), cytolysin (cyl), gelatinase (gelE), hyaluronidase (hyl) and E. faecium endocarditis antigen (efaAfm).Citation116,Citation117
The drug efflux mechanisms in E. faecium are the major reason for its emergence as one of the clinically relevant multidrug-resistant pathogen and confers resistance to a wide range of antimicrobial agents, such as glycopeptides, β-lactams, aminoglycosides, and fluoroquinolones.Citation118 In particular, the emergence of vancomycin-resistant enterococci (VRE) is a worrisome development and now VRE has emerged as a leading cause of hospital-acquired infections worldwide.Citation119 MFS (Tet(K), Tet(L), EfmA), ABC (MsrC and EfrAB) are the commonly identified efflux genes that confer resistance to a wide range of antibiotics/substrates (tetracycline, erythromycin, aminoglycoside, macrolide, gentamicin, norfloxacin, vancomycin, ethidium bromide and chloramphenicol) and they play a key role in the resistance mechanism of E. faecium (Supplementary Table S1).Citation120
Though E. faecium has emerged as an important MDR species, there is no specific EPI found so far except ethylenediamine tetra acetic acid (EDTA; ). EDTA is an organic compound used as a food preservative. Lerma et. al., reported that EDTA (3 mM) can increase the accumulation of various antibiotics, such as gentamicin, streptomycin, chlorhexidine, and triclosan within cells of E. faecium. Greater than 8-fold reduction in the minimum inhibitory concentration (MIC) of these antibacterial agents was observed and correlated with the downregulation of EfrAB (ABC) expression (10–140 folds) in MDR E. faecium strains.Citation120
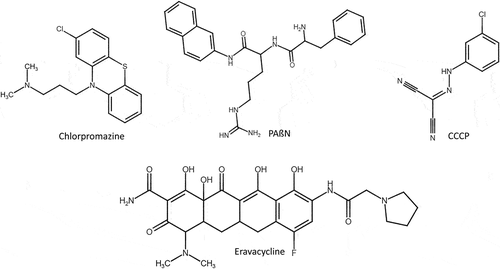
Figure 9. Synthetic efflux pump inhibitors reported to overcome resistance in S. aureus, K. pneumoniae, A. baumannii and P. aeruginosa. Cyanide 3-chlorophenylhydrazone (CCCP) and chlorpromazine are specific NorA efflux pump inhibitors of S. aureus. Phenylalanine arginine β-naphthylamide (PAβN) is specific to AcrAB efflux pump of K. pneumoniae and AdeABC efflux pump of A. baumannii. Tetracycline based eravacycline antibiotic is specific to AdeJ efflux pump of A. baumannii. Chlorpromazine is specific to MexAB-Oprm efflux pump of P. aeruginosa.
4.2. Staphylococcus aureus
Staphylococcus aureus, is one of the major bacterial pathogens categorized under Gram-positive type of bacteria causing mild to life-threatening infections and various kinds of nosocomial infections.Citation121 Similar to E. faecium, S. aureus also belongs to the phylum Firmicutes and is well studied among the Gram-positive bacteria. Due to its asymptomatic colonizing ability, it acts as a commensal bacterium and is commonly present in the upper respiratory tract, armpit, gut mucosa, nose, groin, and skin. They show remarkable diversity in the resistance mechanisms toward antimicrobial and antiseptic agents.Citation122 For instance, several types are categorized for their resistance toward certain antibiotics such as methicillin-resistant S. aureus (MRSA), vancomycin-intermediate resistant S. aureus (VISA), and vancomycin-resistant S. aureus (VRSA).Citation123 Among these, MRSA predominantly causes bloodstream infections (BSIs) and affects ~20% population globally.Citation124 Apart from BSIs, it can cause skin infections, bacteremia, wound infections, septic arthritis, staphylococcal endocarditis, infective endocarditis, and pneumonia.Citation125,Citation126
The most diverse genetic region of S. aureus is oriC (origin of replication), where many transposons and insertion sequences are present and this region is critical for the development of MDR and survival.Citation127 In different environmental conditions, this region maintains only the genes needed for survival through deletion, recombination and crossing over. In S. aureus, MFS (NorA, NorB, NorC, MdeA, SdrM, LmrS, QacA, QacB, TetA(K)/Tet(K)/TetK), RND (FarE), SMR (QacC, QacD, Ebr, QacG, QacH, QacJ), MATE (MepA) and ABC (LsaE, MsrA, Sav1866, AbcA, VgaA, VgaB) transporters are reported that provides increased resistance to a wide range of common antibiotics and antiseptics (Supplementary Table S1).Citation128
Thus, S. aureus expresses almost all types of efflux pump transporters and thereby confers resistance to a wide variety of antibiotics. Hence, there is an urgent need to develop new EPI to overcome the MDR of S. aureus. Except for ABC transporters, majority of the transporters are proton-driven pumps and EPIs that targets the PMF pumps inhibits almost all type of transporters. Glenn et. al., reported that prochlorperazine and trans(E)-flupentixo can reduce the transmembrane potential (PMF) of S. aureus. In the same study, reserpine (natural compound) and chlorpromazine (a synthetic compound) were found to decrease the MIC for tetracycline (4 to8 fold) by affecting TetA(K)/tetK/tet(K) (MFS) efflux pump. Many studies reported that carbonyl cyanide m-chlorophenylhydrazone (CCCP), curcumin, reserpine, piperine, piperic acid, chlorpromazine, and phenothiazines as EPIs of S. aureus NorA (MFS) efflux pump that effluxes hydrophilic fluoroquinolones, monocationic compounds and antiseptics ( and Supplementary Table S1).Citation129
Figure 10. Natural efflux pump inhibitors reported to overcome resistance in E. faecium, S. aureus, A. baumannii, P. aeruginosa and E. coli. EDTA (ethylenediamine tetra acetic acid) is a synthetic EPI specific to EfrAB efflux pump of MDR clinical isolate E. faecium. Reserpine, 2-Phenylquinoline, curcumin and piperine are EPI specific to NorA efflux pump of MDR clinical isolate S. aureus. NMP and reserpine are EPI specific to AdeABC efflux pump of A. baumannii. Conessine and pyridopyrimidine derivative is specific to MexB efflux pump of P. aeruginosa and lysergol is specific to YojI and pyranopyridines derivatives (MBX2319) is specific to AcrB from E. coli, respectively.
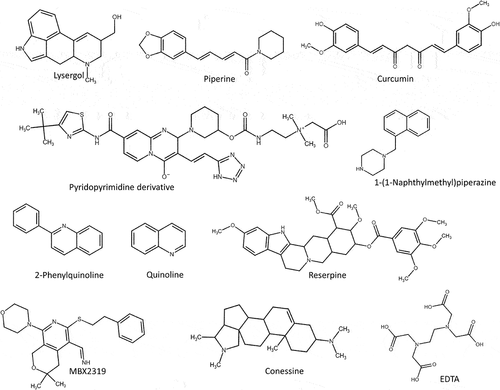
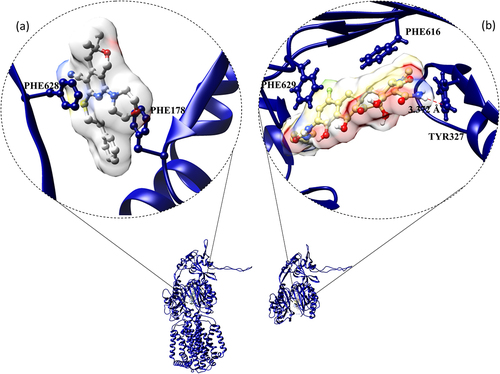
Figure 11. Efflux pump inhibitors bound to RND inner membrane efflux transporters from nosocomial pathogens. (a) Eravacylcine bound AdeJ RND (PDB_ID: 7M4P) pump from A. baumannii, and (b) pyranopyridine derivative (MBX2319) bound structure of AcrB efflux pump (PDB_ID: 5ENO) from E. coli. Red dotted lines represent H-bonds.
In addition, Tommaso et. al., in 2018 reported that 2-phenylquinoline derivatives are potential EPIs to restore the efficacy of ciprofloxacin by inhibiting NorA in different strains of S. aureus.Citation130 Further, a recent study by Marina et al., in 2021, reported that the chalcones (2E)-1-(4′-aminophenyl)-3-(phenyl)‑prop-2-en-1-one (APCHAL), and (2E)-1-(4′-aminophenyl)-3-(4-chlorophenyl)‑prop-2-en-1-one (ACLOPHENYL) were effective against S. aureus and reduces the MIC of gentamicin up to 70%. In the same investigation, in silico docking study showed that the binding region of norfloxacin in NorA was occupied by the chalcones, which could result in the accumulation of norfloxacin in S. aureus.Citation131
Zimmermann et. al., in 2019, illustrated the presence of two distinct binding sites in NorA transporter. One is an internal cavity present in the transmembrane region surrounded by the residues Ile15, Ile19, Gly18, Ile23, Phe47, Gln51, Gly111, Leu115, Tyr131, Ser138, Ile244, and Tyr292 where the EPIs dihydroergotamine, ergoloid, azelastine, doxazosin, and telmisartan binds. And the other binding site is the “groove” present at the top of the NorA efflux pump situated outside of the TM region embedded in the phospholipid bilayer of the cytoplasmic membrane. This site was formed by the residues Val281, Leu285, Leu286, PHE-288, GLU-222, and ILE-355, which connect to the internal cavity of the transporter by a tiny channel where the two EPIs nilotinib and reserpine binds.Citation132 Figueredo et. al., in 2020 reported that hydroxylamine-derived lapachol and norlachol derivatives significantly reduce the MICs of norfloxacin by modulating the efflux pump NorA. Further, molecular docking studies showed that the hydroxylamine-derived compounds and PaβN are occupying similar active sites with the same 3D pharmacophore features such as one hydrogen bond acceptor, one positive charge and two aromatic rings. The complex between the ligands and NorA was stabilized by the interactions with hydrophobic residues (Met23, Gly56, Leu30, Ile53, TYR55, Phe27, Phe142 and Phe146), hydrogen bond interaction (Gln59 and Gln345) and sulfur-π interactions (Met26).Citation133
4.3. Klebsiella pneumoniae
Klebsiella pneumoniae is a Gram-negative non-motile, facultative anaerobic, rod-shaped intestinal bacterium resistant to beta-lactam antibiotics.Citation134 Like other ESKAPEE pathogens, it also causes various kinds of nosocomial infections such as bacteremia, catheter-related infections, ventilator-associated pneumonia (VAP), bloodstream infections, meningitis, wound and surgical site infections and urinary tract infections in immune-compromised patients, particularly in chronic alcoholics, diabetes mellitus, or chronic pulmonary obstruction patients. Its clinical significance has increased over a period of time as it is held responsible for causing neonatal sepsis and device-related infections in intensive care unit (ICU) settings.Citation135 Infection due to K. pneumoniae is more common in men and geriatric people.Citation136 Noticeably, the gene blaKPC encodes the carbapenemase that confers resistance to carbapenem antibiotics and emerges as the carbapenemase-producing K. pneumoniae (KPC).Citation137 Center for Disease Control and Prevention (CDC), USA reported that 7,900 infections and 520 deaths each year is due to KPC.Citation138
There are many factors and genes involved in the pathogenicity, virulence and drug resistance of K. pneumoniae. Some examples for pathogenicity and virulence genes are, namely, fim, mrk, ent, K-locus, O-locus, ybt, clb, iuc, iro and rmpA/A2.Citation139 Drug resistance genes in KPC are bla gene (ampicillin), 16S-RMTase genes (aminoglycosides), gyrA, gyrB, parC and parE genes (fluoroquinolones), acrR (phenicols), sul genes (sulfonamides), RamA (tetracyclines), dfr-genes (trimethoprim), extended-spectrum beta-lactamases (ESBLs), blaIMP-1 and blaNDM-1 genes (carbapenems).Citation140 Genes encoding MDR efflux pumps are activated by several stress factors including environmental conditions and mutations. MFS (KpnGH, KmrA and SmvA), RND (OqxAB, AcrAB KexEF EefABC and KexC or KexD), SMR (KpnEF and KdeA) and MATE (KetM) are the major efflux pump transporters expressed in K. pneumoniae that confers resistance to a wide range of substrates such as antibiotics, dyes, detergents and disinfectants (Supplementary Table S1).Citation141–143
Thus far, to overcome the resistance very few EPIs were found and tested against K. pneumoniae such as phenylalanine arginine β-naphthylamide (PAβN),Citation144 cyanide 3-chlorophenylhydrazone (CCCP),Citation145 quinoline derivativesCitation146 and theobromine.Citation147 The EPI, theobromine and PAβN are known to target AcrAB-TolC efflux pump.Citation147 According to the studies of Padilla et al., and Howard et. al., the susceptibility to carbapenems is increased in AcrAB knockout or TolC knockout strains and such carbapenems susceptibility is observed in EPI (e.g. PAβN) treated strains of K. pneumoniae (). Citation148
4.4. Acinetobacter baumannii
Gramma proteobacteria group comprises a greater number of species that are responsible for nosocomial infections. The bacterium Acinetobacter baumannii is one of them and is consistently designated as the leading WHO priority pathogen.19 A. baumannii is a well-known opportunistic pathogen that causes infections in immune-compromised individuals and the mortality rate was around 68%.Citation149 In addition, it also causes 64% community-acquired infections.Citation149–151 In addition, it is predominately seen in patients under surgical treatment, prolonged hospitalization, ICU admission and in patients who are supported by mechanical ventilators and invasive medical devices. Ventilator-associated infections such as pneumonia, bacteriemia, and catheter-related infections such as meningitis, urinary tract infections, and severe bloodstream infection are some types of infections caused by A. baumannii. Moreover, A. baumannii is seen in patients with a history of alcoholism, cancer, diabetes, and obstructive pulmonary disorders.Citation152
In A. baumannii, the highly expressed chromosomally encoded genes (blaOXA-23, blaOXA-24/40, and blaOXA-58-like gene clusters, lpxA, lpxC, and lpxD, ISAba1 and ISAba125, blaPER-1, QnrA, QnrB, QnrS blaADC, blaOXA-51) are the key factors that confer resistance to a different class of antibiotics such as carbapenem, aminoglycoside, β-lactamases, tigecycline lactams, chloramphenicol, erythromycin, cephalosporins, and tetracycline by reducing their accumulation.Citation152–154 In addition, this pathogen has been reported to express all types of transporters such as, MFS (AbaF, AmvA, SmvA, mdfA, CraA, Tet(A) and Tet(B), CmlA, TetM, FloR), RND (AdeFGH, AdeABC, AdeIJK, AdeXYZ, AdeDE), SMR (AbeS and QacE), MATE (AbeM), ABC (MacAB-TolC) and PACE (AceI)), which are detailed in Supplementary Table S1.Citation155,Citation156
One of the most studied type of efflux pump in A. baumannii is RND tripartite transporters. A. baumannii expresses several Ade (Acinetobacter derived efflux) type RND tripartite transporters such as AdeABC, AdeDE, AdeFGH, AdeIJK and AdeXYZ and some known EPIs for this RND transporters are PAβN, NMP, 2-substituted benzothiazoles, CCCP omeprazole, verapamil and reserpine ().Citation157 Recently, many studies have focused on AdeABC transporter which is controlled by the two component system AdeRS, where adeR is the response regulator and adeS is the sensor histidine kinase. Researchers found that substitution (A94V, D30G, M197I and G200C) or insertion (by IS element ISAbaI- Insertion sequence (IS) of A. baumannii) mutation on AdeS resulted in the overexpression of AdeABC transporter leading to reduced susceptibility to tigecycline.Citation158 In vitro studies by Valentine et. al., in 2008, Mehrdad et. al., in 2015 and Francis et. al., in 2018 reported that the PAβN (10–100 µg/ml) can restore susceptibility to ciprofloxacin (2 to 8-fold improvement), imipenem (4–64 fold), tigecycline (2-fold), fluoroquinolone (2–16 fold), nicotinic acid (16-fold), minocycline (≥ 04-fold) and it also reduces the MIC of trimethoprim, clindamycin and chloramphenicol in MDR strains of A. baumannii.Citation157,Citation159,Citation160
A computational study by Shirin et al., in 2017, comprehended the binding potential of EPI PAβN to AdeB monomer. PAβN was found to be bound at the multi-substrate binding site of the distal binding pocket of AdeB under the Phe loop (Phe136, Phe178, Phe569, Phe612, Phe623, and Phe669). This Phe loop governs the substrate binding by forming the boundary between proximal and distal binding pockets. To prevent the peristaltic movement of the tripartite assembly pump, PAβN could bind to the gate of the distal binding pocket to keep it in the binding state followed by the rapid conformational change from access to the binding form. The hydrophobic moieties (Met570 and Phe612) in the pocket strengthen the PAβN-adeB complex by forming van der walls interactions with the phenyl and naphthyl rings. In addition, the PAβN perfectly fitted in between the key loops of binding pocket such as phenyl (Phe612) and serine (Ser134) and the guanidinium group of PAβN is surrounded by Gln42 and Glu665 residues. Ser134, Glu665, Thr668, and Gln42 form H-bonds with PAβN. Thereby, PAβN closed the access stage of this pump to block the drug/substrate efflux (). Citation88 The cryo-EM structure of a fully synthetic tetracycline-based antibiotics Eravacycline bound AdeJ (PDB_ID: 7M4P) RND transporter was deposited recently in the PDB (protein data bank) by Zhang, et al., .Citation161 The protein-ligand interaction in the complex is elucidated through the protein-ligand interaction profiler.Citation162 The binding site in this protein is lined by hydrophobic residues specifically phenylalanine (Phe178 and Phe629). Further, the complex of eravacycline-AdeJ is strengthened by H-bond (Tyr327), π-π (Phe616) and π-cation (Phe629) interactions ().
4.5. Pseudomonas aeruginosa
Pseudomonas aeruginosa, one of the enteric pathogens causing infections in immune-compromised patients (ventilator-associated pneumonia and various sepsis syndromes), is also found in soil, water and as a part of skin flora.Citation163 Like, other ESKAPEE pathogens, it also possesses intrinsic resistance with low antibiotic susceptibility and permeability. It develops acquired resistance either by mutation in drug target or horizontal gene transfer of resistance encoding genes. Usually, P. aeruginosa propagates in the skin, lungs, throat, urinary tract, stool, bone joints, blood, kidneys, and in surgical sites. According to CDC, the estimated P. aeruginosa infection in US is 51,000 every year with the death rate of 13–55%; among them, 13% are infected by MDR P. aeruginosa.Citation164,Citation165
There are many virulent factors and antibiotic resistance genes responsible for its pathogenicity. Examples include endotoxins, exotoxins A, hemolysin, proteases A, surface factors (lipopolysaccharide, and polysaccharide slime, O-antigen, flagellum and pili, outer membrane vesicles), types of secretion system, LasR/RhlR (transcriptional activators), cytotoxins, quorum sensing regulatory system proteins, elastase, phospholipase C, phenazines and pigment production.Citation166,Citation167 RND (MexAB-OprM, MexCD-OprJ, MexEF-OprN, MexJK‐OprM MexVW-OprM, MexAB-OprM, MexGHI‐OpmD, MexXY-OprM and CzrCBA), ABC (NppA1A2BCD) and MATE (PmpM) transporters are found exclusively in this pathogen to confer resistance to numerous antibiotics (Supplementary Table S1).Citation41,Citation168
RND tripartite Mex efflux transporters (MexAB-OprM, MexCD-OprJ, MexEF-OprN and MexXY-OprM) are regulated by the genes such as mexR, nfxB, nfxC, and mexZ.Citation169 Mutation (insertion, deletion, or substitution) in the Mex regulator genes, increases the expression of these transporters.Citation170 Like, A. baumannii RND efflux pumps, these P. aeruginosa RND Mex pumps are also inhibited by PAßN (50 mg/L) with a 4-fold decrease in the MIC of gentamicin (). Citation171 In addition, Siriyong et al., in 2017 reported that the plant-based alkaloid conessine (20 mg/L) as a potential EPI for MexAB-Oprm efflux pump of this pathogen (). Citation172
As discussed earlier in the RND transporter section, the tripartite structure of MexAB-Oprm (PDB_ID: 6IOK) has cannon shaped outer membrane with α/β equatorial domain, a hexameric funnel-shaped periplasmic protein with α-hairpin domain, lipoyl domain, β-barrel domain and membrane periplasmic (MP) domain and homotrimeric inner membrane protein with pore and transmembrane (TM) domain. The monomeric tripartite structure with the above-described domain details are illustrated in . Citation173 The crystal structure of MaxB with the inhibitor pyridopyrimidine derivative (ABI-PP, ) (PDB_ID: 3W9J) was deposited in the PDB by Sakurai, et. al., in 2013 describing the hydrophobic trap surrounded by phenylalanine (Phe136, Phe178, Phe573, Phe610, Phe615 and Phe628) along with H-bond (Lys151 and Gln176), electrostatic (Arg128) and π-stacking (Phe178 and Phe610) interactions (). The hydrophilic tetrazole ring of ABI-PP interacts with Asp274, Agr620 and Lys151 residues and piperidine aceto-amino ethylene ammonia-acetate (PAEA) moiety interacts with the charged (ionic) residues and extends to the residue Arg128. They also observed that the entire molecule of n-dodecyl-β-D-maltoside (DDM) partially overlaps with the ABI-PP in the distal multi-binding pocket.Citation174
Figure 12. Efflux pump inhibitors bound to RND inner membrane efflux transporters from P. aeruginosa. MexAB-OprM (PDB_ID: 6IOK) outer, periplasmic and outer membrane top-view, tripartite, and monomeric tripartite embedded membrane and MexB (inner membrane) with inhibitor pyridopyrimidine derivative (PDB_ID: 3W9J) from P. aeruginosa.
4.6. Enterobacter
Enterobacter is the genera that comprises motile bacteria (except Enterobacter asburiae) that belong to the large family of Enterobacteriacae. Several species in this genera are clinically relevant as they cause infections such as bacteremia, surgical site infections, lower respiratory tract infections, food poisoning, diarrhea, endocarditis, intra-abdominal infections, urinary tract infections, central nervous system infections, septic arthritis, osteomyelitis, and ophthalmic infections.Citation175 CDC reported that in 2017 Carbapenem-resistant Enterobacterales (CRE) and ESBL (extended-spectrum beta-lactamases)-producing Enterobacterales infected around 13,100 and 197,400 patients with a mortality rates of 8.3% and 4.6%, respectively.Citation176–178 Enterobacter aerogenes, Enterobacter cloacae, Enterobacter agglomerans, and Enterobacter sakazakii are the common disease-causing subspecies under Enterobacter.Citation179 All these pathogens are isolated from the respiratory tract and differentiated by many biochemical tests, such as lysine and ornithine decarboxylase, D-sorbitol, and arginine dihydrolase. Among these, E. cloacae and E. aerogenes are the most frequently encountered nosocomial pathogens under this genus.Citation180
Enterotoxins, α-hemolysin, thiol-activated pore-forming cytotoxins and porins (OmpD and OmpF) are the crucial virulence factors in Enterobacter.Citation181 Presence of different sets of enzymes plays a vital role in the resistance mechanism of these pathogens such as ß-lactamase enzymes, aminoglycoside-inactivating enzymes, aminoglycoside-acetylating enzymes (AAC (3)-II, AAC (69), AAC (3)-III, AAC (3)-I, and AAC (3)-V) and aminoglycoside-nucleotidylating enzymes (ANT (20)).Citation182 RND (AcrAB-TolC), SMR (SugE), and MATE (EmmdR) are the efflux transporters reported in E. cloacae which confers resistance to many antibiotics, cationic surfactants, detergents and dyes (Supplementary Table S1).Citation183 As reported by Abdallah et. al., and Ghisalberti et. al., in 2006, quinoline derivatives obtained from plant Ruteceus and PAßN showed a remarkable effect on AcrAB-TolC (RND) efflux pump.Citation137 The plant-derived quinolines () reduce the MIC of chloramphenicol up to 8-fold in different strains of E. aerogenes by blocking the drug affinity sites of AcrB.Citation184,Citation185
4.7. Escherichia coli
Escherichia coli is a Gram-negative facultative anaerobic motile coliform bacterium, belonging to the Enterobacteriaceae family. Entero-pathogenic strains of E. coli bacteria, are the well-known causative agent for traveler’s diarrhea, which amounts to 0.1% of human gut microbiota. Enterovirulent E. coli pathotypes are varied and they are classified based upon their mode of virulence – like enterotoxigenic E. coli (ETEC), enteropathogenic E. coli (EPEC), enterohemorrhagic E. coli (EHEC), enteroinvasive E. coli (EIEC), enteroaggregative E. coli (EAEC), and diffusely adherent E. coli (DAEC). They are commonly involved in foodborne illnesses, urinary tract infections, neonatal meningitis, hemorrhagic colitis, and Crohn’s disease, severe abdominal cramps, diarrhea, vomiting, bowel necrosis, hemolytic-uremic syndrome, peritonitis, mastitis, sepsis, pneumonia, and gastroenteritis.Citation186,Citation187 Shiga toxin-producing E. coli (STEC) leads to foodborne infections and WHO estimated that STEC infections in 2010 is about 1 million (~13,000 infections and 100 deaths per day).Citation188
The resistance mechanism of ETEC is mainly due to the overexpression of efflux pumps. MFS (EmrKY‐TolC, QepA, EmrD, Dep, Bcr, EmrAB‐TolC, Mef(B), Fsr, AraJ), RND (MdtABC‐TolC, AcrAD‐TolC, MdtEF‐TolC, OqxAB‐TolC, YhiUV‐TolC, MdfA, AcrEF‐TolC, AaeX, AcrAB-TolC, CusCFBA), SMR (TehAB, SugE, EmrE, MdtJI) and ABC (YojI, YhdX, LsrA) transporter confer resistance to a variety of antibiotics/substrates (Supplementary Table S1).Citation47,Citation189,Citation190 Basically, the efflux pump inhibitors PAβN and NMP inhibit RND efflux pumps; while, CCCP is the commonly used efflux pump inhibitor against all PMF-driven efflux pumps. Winfried et. al., in 2006 showed that NMP (at 100 μg/mL) reduced the MIC of levofloxacin, linezolid, oxacillin, rifampin, chloramphenicol, clarithromycin, and ethidium bromide up to 4-fold in the AcrAB and AcrEF pumps overexpressing strains. NMP resistant mutant enables the extrusion of linezolid and interestingly this type of resistant mutant were observed only in the presence of linezolid.Citation191 In silico study by Vargiu et, al., in 2012, reported that EPIs PAβN and NMP bound near the hydrophobic trap (Phe136, Phe178, Phe615, and Phe628) of distal pocket by interfering with the movement of the switch-loop (phenyl loop or G-loop). Thereby narrowing down the multi-substrate binding distal pocket to block the drug efflux. ().Citation192
In addition, Maurya et al., in 2013 reported that lysergol (10 µg/mL) was a potential EPI for the YojI ABC transporter of this pathogen (). Citation193 In the same year Opperman et al., reported that pyranopyridines (MBX2319) increased intracellular accumulation of the dye Hoechst 33,342 and nitrocefin. Later, Sjuts et al., in 2015 deposited the MBX2319 bound x-ray crystal structure of the AcrB efflux pump (PDB_ID: 5ENO) in PDB. The binding site is mainly lined by a list of hydrophobic residues (Val139, Phe178, Ile227, Ala279, Tyr327, Phe610, Val612 and Phe628). There was a strong π–π stacking interaction between the pyridine ring of MBX2319 and the aromatic side chain of Phe628. Particularly, the derivatives of MBX2319 such as MBX3132 and MBX3135 showed complete inhibition even at 0.1 μM, i.e., 500-fold reduction than the classical inhibitors like PAβN and 10 to 20-fold better activity than the MBX2319 in checkerboard assay. Further, the phenyl and morpholinyl moieties of MBX2319 interact with Phe178 and Phe615, respectively, and the dimethyl moiety interacts with the side chain of Tyr327 and Met573. The Phe610 side chain is lying at right angles to the di-methylene sulfide moiety of MBX2319 that connects the pyranopyridine core to the phenyl group. Computational molecular dynamic simulation confirms that Phe178 and Phe628 residues were involved in rigid binding ().Citation194
The crystal complex compounds (minocycline, rhodamine 6 G and doxorubicin) and in silico studies of adeB inhibitors, such as peptidomimetic EPIs (PAβN and C-capped dipeptide analogs), 1-(1-Naphthylmethyl)-Piperazine (NMP) and the aryl piperazine analogs, pyridopyrimidine (D13–9001), and pyranopyridines (MBX2319) reveal that the EPIs share a similar binding site on the AcrB, confirming that the hydrophobic trap is a likely target for EPIs.Citation191 In a recent study by Grimsey et al., in 2020, chlorpromazine and amitriptyline were also reported to occupy the same hydrophobic trap. Thus, Switch-loop movement interference by EPIs and conformational change in the hydrophobic trap of the distal pocket by EPIs are the two possible ways to block the effluxing of drug.Citation195
5. Role of efflux pumps in biofilm formation
Apart from effluxing the antimicrobial agents, efflux pumps have also been implicated to have alternative roles, such as increasing virulence and biofilm formation. Biofilms are defined as ‘aggregates of sessile microorganisms in which cells are irreversibly embedded in an autogenous matrix of extracellular polymeric substances (EPS) that usually aid intracellular communications. This communication between bacteria is also referred as quorum sensing (QS).Citation196 While bringing the role of efflux pump in biofilm, it leads to two entirely different roles; one is promoting biofilm formation and another one is inhibiting the biofilm formation. There have been increasing evidences to suggest that efflux pumps play a very crucial role in biofilm formation.Citation197 Such functions are shown particularly in some of the important pathogens such as A. baumannii, E. coli, P. aeruginosa and S. aureus. Interestingly, all these pathogens are in the list of ESKAPEE pathogens and having propensity to develop extreme drug resistance. Many studies have shown that efflux pumps such as mdeA, norB and norC of S. aureus, acrA, emrB, oqxA, and qacEΔ1 in K. pneumonia, adeFGH of A. baumannii, mexAB-oprM and mexCD-oprJ, MexGHI-OpmD, mexG and mexH of P. aeruginosa, and AceAB-tolC, araJ, ddpD, emrK, gltK, ycbO, yhdX, mdtL, mdtG, setB, emrY, fsr, ydeA and yqgA of E. coli, play crucial role in biofilm formation and are significantly up-regulated.Citation110,Citation197,Citation198 Mutants strains overexpressing the AdeABC, AdeFGH and AdeIJK efflux pump resulted in reduced biofilm formation. These efflux pump stimulating the biofilm aggregation by effluxing the molecules such as glucoses, L-amino acids and arabinose. For instance, SetB, YhdWXYZ, ydeA, acrD, acrE, mdtE, emrD, emrK and emrE genes of E. coli is involved in the efflux of glucose, L-amino acids, sugar metabolites and arabinose, mutation in these genes reflect the fall in biofilm formation.Citation199
Though efflux pump inhibitors (EPIs) are known to inhibit the activity of effluxing, very few EPIs are known to disrupt biofilm. Several cysteine-based compounds that are putative EPIs are capable of antagonizing the QS circuit and thereby affecting biofilm formation in Gram-negative bacteria especially P. aeruginosa. According to Kasper et al., 2016, RNA-seq analysis of P. aeruginosa strain exposed to cysteine-based compounds such as S-phenyl-L-cysteine sulfoxide shown to down regulate phenazine biosynthesis and T4SS genes.Citation200 This compound also activates MexT regulon that includes MDR efflux pumps encoded genes such as MexEF-oprN, which was previously shown to inhibit QS and pathogenicity of P. aeruginosa .Citation201 More investigation on this compound revealed that it has structural similarity with kymurenine, a precursor of antranilate that is known to be critical for virulence of P. aeruginosa. Additional studies revealed that S-phenyl-L-Cysteine sulfoxide competitively inhibits the kynureninase activity of P. aeruginosa in vitro and it is also capable of reducing PQS (Pseudomonas quinolone signal) production in vivo .Citation200 On S-phenyl-L-cysteine sulfoxide treatment there is elevation of expression of MexEF-oprN from endogenous levels, which is shown to inhibit QS. Perturbance of tight regulation of MexEF-oprN efflux pump on exposure to cysteine based compound shown to bring in reduction in PQS leading to QS inhibition, though the exact mechanism is not clearly understood. P. aeruginosa MexAB-OprM tripartite efflux pump selects certain acyl-HSLs QS molecules that has to be extruded out of cell thereby regulating QS in this pathogen. Deletion mutant of MexAB-OprM or use of EPI such as ABI ([[2- ({[((3 R)-1-{8-{[(4-tert-butyl-1,3-thiazol-2-yl) amino]carbonyl}- 4-oxo-3-[(E)-2-(1 H-tetrazol-5-yl)vinyl]-4H-pyrido [1,2-a]pyrimidin-2-yl} piperidin-3-yl)oxy]carbonyl}amino) ethyl](dimethyl)ammonio] acetate) inhibits the efflux of acyl-HSLs thereby hampering the QS. These results demonstrate yet another QS regulation mechanism via the efflux system MexAB-OprM in P. aeruginosa .Citation202
The known EPI’s CCCP, NMP, thioridazine and PAβN also have a variable effect on QS signaling in many of the nosocomial pathogens such as A. baumannii, E. coli and P. aeruginosa and pyocyanin production in strain-specific manner.Citation203,Citation204 In the study by El Shaer et al., four virulent strains were investigated for PAβN activity that showed reduction in QS signaling molecules without affecting bacterial viability. This study also revealed that at the transcription level, PAβN significantly reduced the relative expression of QS cascade genes (lasI, lasR, rhlI, rhlR, pqsA and pqsR) and QS regulated-type II secretory genes lasB (elastase) and toxA (exotoxin A), when compared with the untreated control isolates. Further, PAβN eliminated virulence factors such as elastase, protease, pyocyanin and bacterial motility in the virulent strains. Such results imply that the inhibition of efflux pumps by PAβN has a cascading effect leading to reduction of the extracellular accumulation of QS signals resulting in the blockade of the QS circuit. Similar observations were made in case of another EPI, baicalin, an inhibitor of MsrA efflux pump in Staphylococcus wherein there was a drastic down regulation of mRNA transcription levels of QS system regulators such as agrA and agrC .Citation205 Nonetheless, PAβN or any other EPIs should be tested on large panels of ESKAPEE pathogen isolates obtained from different types of infection to check their anti-QS properties. Any successful EPI candidate having anti-QS traits could be a potential dual effect compound that could diminish the resistance and pathogenicity of nosocomial pathogens.
6. Summary
Many factors are influencing antimicrobial resistance in bacteria; among them, the drug efflux transportation mechanism is an important contributor. Efflux transporters compromise the effectiveness of the antibacterial agents by effluxing them out of the microbe. Ongoing efforts at developing new efflux pump inhibitors (EPIs) for combination chemotherapy is an effective approach to target the efflux transporters, thereby re-sensitizing the drug-resistant microbes to antimicrobial agents. Currently, there are no known EPIs approved for overcoming antimicrobial resistance. In recent years, it is evident that the key to a successful new drug design project is to understand the structural biology aspects of the drug targets (using techniques such as X-ray, NMR and Cryo-EM). Information gathered about the active site (size and shape of the active site, the type of residues present) and the interaction with a ligand/drug molecule (2D/3D interaction of macromolecule-ligand) could help in identifying new lead molecules or help us in the lead optimization/designing of new analogs for the target under investigation.
In this review, a systematic approach to understand the structural biology aspects of various classes of drug transporters present in clinically important pathogens was detailed, with a particular emphasis on the efflux transporters and EPIs for ESKAPEE pathogens. Plant-based compounds (alkaloids, phenolics and terpenes) are potential inhibitors for a majority of the efflux pumps reported in these pathogens. The synthetic EPI, Phenylalanine arginine β-naphthylamide (PAβN) is one of the most commonly reported EPIs for most of the pathogens regardless of the bacterial type. Also, it is interesting to note that in RND transporters, the inner membrane hydrophobic trap is a suitable target site for developing new EPIs and also reported to have anti-quorum sensing properties. Investigations on key structural features of efflux transporters will undoubtedly aid in the structure-based drug design of novel analogs that can help the abrogation of efflux transporters and rejuvenate the search for antimicrobial chemotherapy in the future.
Abbreviations
| AMR | = | Anti-Microbial Resistance |
| ABR | = | Anti-Bacterial Resistance |
| ABC | = | ATP-binding cassette |
| BSI | = | Bloodstream infections |
| CDC | = | Center for Disease Control and Prevention |
| MFS | = | Major Facilitator Superfamily |
| MATE | = | Multidrug and Toxin Extrusion |
| SMR | = | Small Multidrug Resistance |
| RND | = | Resistance-Nodulation-cell Division |
| PACE | = | Proteobacterial Antimicrobial Compound Efflux |
| CRE | = | Carbapenem-resistant Enterobacterales |
| ESBL | = | Extended-spectrum beta-lactamases)-producing Enterobacterales |
| EREC | = | enterotoxigenic E. coli |
| EPEC | = | Enteropathogenic E. coli |
| EHEC | = | Enterohemorrhagic E. coli |
| EIEC | = | Enteroinvasive E. coli |
| EAEC | = | Enteroaggregative E. coli |
| DAEC | = | Diffusely adherent E. coli |
| DME | = | Drug-metabolizing enzymes |
| PMF | = | proton motif force |
| FRET | = | Förster or fluorescence resonance energy transfer |
| DEER | = | double electron-electron resonance |
| MDR | = | Multi-drug resistance |
| CCCP | = | Carbonyl-cyanide m-chlorophenylhydrazone |
| ICU | = | Intensive care unit |
| QAC | = | Quaternary ammonium compounds |
| TMD | = | Transmembrane domains |
| NBD | = | Nucleotide-binding domains |
| TMH | = | Transmembrane helix |
| MDR | = | Multi-drug resistant |
| PDR | = | Pan-drug resistant |
| EDR | = | Extremely drug-resistant |
| EPI | = | Efflux pump inhibitors |
| MIC | = | minimum inhibitory concentration |
| STEC | = | Shiga toxin-producing E. coli |
| VRE | = | Vancomycin-resistant enterococci |
| MRSA | = | Methicillin-resistant S. aureus |
| VISA | = | Vancomycin-intermediate resistant S. aureus |
| VRSA | = | Vancomycin-resistant S. aureus |
| VAP | = | Ventilator-associated pneumonia |
| KPC | = | Carbapenemase-producing K. pneumoniae |
Supplemental Material
Download PDF (105.6 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website
Additional information
Funding
References
- Gest H. The discovery of microorganisms by Robert Hooke and Antoni van Leeuwenhoek, fellows of the Royal Society. Notes Rec. R. Soc. 2004;58(2):1–34. doi:10.1098/rsnr.2004.0055.
- Lane N. The unseen World: reflections on Leeuwenhoek (1677) ‘Concerning little animals. Philos. Trans. R. Soc. B Biol. Sci. 2015;370(1666):20140344. doi:10.1098/rstb.2014.0344.
- Huremović D. Brief history of pandemics (pandemics throughout history). Psychiatry Pandemics A Ment Heal Response to Infect Outbreak. 2019:7–35. doi:10.1007/978-3-030-15346-5_2.
- Dagen M. History of malaria and its treatment. In: Antimalarial agents. 1-48. United Kingdom: Elsevier Ltd. 2020. doi:10.1016/b978-0-08-101210-9.00001-9.
- Daniel TM. The history of tuberculosis. Respir. Med. 2006;100(11):1862–1870. doi:10.1016/j.rmed.2006.08.006.
- Acuti Martellucci C, Flacco ME, Cappadona R, Bravi F, Mantovani L, Manzoli L. SARS-CoV-2 pandemic: an overview. Adv. Biol. Regul. 2020;77:1–11. doi:10.1016/j.jbior.2020.100736.
- Permin H, Norn S, Kruse E, Kruse PR. On the history of Cinchona bark in the treatment of malaria. Dan Medicinhist Arbog. 2016;44:9–30 https://pubmed.ncbi.nlm.nih.gov/29737660/.
- Davies J. Origins and evolution of antibiotic resistance. Microbiologia. 1996;12:9–16 doi:10.1128/MMBR.00016-10.
- Aminov RI. A brief history of the antibiotic era: lessons learned and challenges for the future. Front Microbiol. 2010;1:1–7. doi:10.3389/fmicb.2010.00134.
- Gaynes R. The discovery of Penicillin—New insights after more than 75 years of clinical use. Emerg. Infect. Dis. 2017;23(5):849–853. doi:10.3201/eid2305.161556.
- Nikaido H. Multidrug resistance in bacteria. Annu. Rev. Biochem. 2009;78(1):119–146. doi:10.1146/annurev.biochem.78.082907.145923.
- Hersh AL, Jackson MA, Hicks LA; DISEASES, the C. O. N. I. Principles of judicious antibiotic prescribing for upper respiratory tract infections in pediatrics. Pediatrics. 2013;132(6):1146–1154. doi:10.1542/peds.2013-3260.
- Luyt C-E, Bréchot N, Trouillet J-L, Chastre J. Antibiotic stewardship in the intensive care unit. Crit Care. 2014;18(5):480. doi:10.1186/s13054-014-0480-6.
- Centers for Disease Control and Prevention. CDC: 1 in 3 antibiotic prescriptions unnecessary. 3 May 2016. https://www.cdc.gov/media/releases/2016/p0503-unnecessary-prescriptions.html.
- Llor C, Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014;5(6):229–241. doi:10.1177/2042098614554919.
- Sun J, Deng Z, Yan A. Bacterial multidrug efflux pumps: mechanisms, physiology and pharmacological exploitations. Biochem. Biophys. Res. Commun. 2014;453(2):254–267. doi:10.1016/j.bbrc.2014.05.090.
- Dhingra S, Rahman NAA, Peile E, Rahman M, Sartelli M, Hassali MA, Islam T, Islam S, Haque M. Microbial resistance movements: an overview of global public health threats posed by antimicrobial resistance, and how best to counter. Frontiers in Public Health. 2020;8:531 doi:10.3389/fpubh.2020.535668.
- de Kraker MEA, Stewardson AJ, Harbarth S. Will 10 million people die a year due to antimicrobial resistance by 2050? PLoS Med. 2016;13(11):e1002184. doi:10.1371/journal.pmed.1002184.
- World Health Organization. WHO publishes list of bacteria for which new antibiotics are urgently needed https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed. (2017)
- Asokan GV, Ramadhan T, Ahmed E, Sanad H. WHO global priority pathogens list: a bibliometric analysis of medline-PubMed for knowledge mobilization to infection prevention and control practices in Bahrain. Oman Med. J. 2019;34(3):184–193. doi:10.5001/omj.2019.37.
- Plackett B. Why big pharma has abandoned antibiotics. Nature. 2020;586(7830):S50–S52. doi:10.1038/d41586-020-02884-3.
- Oniga O, Nastasă C, Ionuț I, Stana A, Oniga S, Tiperciuc B. Recent developments in antibacterial antibiotics. Rev Farm a Mold. 2016:42–50. doi:10.3390/ma9070599
- Andrei S, Droc G, Stefan G. FDA approved antibacterial drugs: 2018–2019. Discov. Craiova, Rom. 2019;7(4):e102–e102. doi:10.15190/d2019.15.
- Nelson DL, Lehninger AL, Cox MM. Lehninger principles of biochemistry. United States: Macmillan doi:10.1007/978-3-662-08289-8; 2008.
- Ribet D, Cossart P. How bacterial pathogens colonize their hosts and invade deeper tissues. Microbes Infect. 2015;17(3):173–183. doi:10.1016/j.micinf.2015.01.004.
- Amidon GL, Sadée W. Membrane transporters as drug targets. United States: Springer Science & Business Media doi:10.1007/b112928; 2006. Vol. 12.
- Kumala M. The Never-Ending Story—The Origin and Diversification of Life. Evol Educ Outreach. 2013;6(1):1–21. doi:10.1007/s12052-010-0278-1.
- Libby E, Ratcliff WC. Ratcheting the evolution of multicellularity. Science. 2014;346(80):426–427. doi:10.1126/science.1262053.
- Pennisi E. The momentous transition to multicellular life may not have been so hard after all. Science. 2018;28(80):6. doi:10.1126/science.aau5806.
- Thannickal VJ. Oxygen in the evolution of complex life and the price we pay. Am. J. Respir. Cell Mol. Biol. 2009;40(5):507–510. doi:10.1165/rcmb.2008-0360PS.
- Martin WF, Sousa FL. Early Microbial Evolution: the Age of Anaerobes. Cold Spring Harb. Perspect. Biol. 2015;8(2):a018127–a018127. doi:10.1101/cshperspect.a018127.
- Wright GD. The antibiotic resistome: the nexus of chemical and genetic diversity. Nat. Rev. Microbiol. 2007;5(3):175–186. doi:10.1038/nrmicro1614.
- Nikaido H. Antibiotic resistance caused by gram-negative multidrug efflux pumps. Clin. Infect. Dis. 1998;27(Suppl s1):S32–41. doi:10.1086/514920.
- Perry JA, Westman EL, Wright GD. The antibiotic resistome: what’s new? Curr Opin Microbiol. 2014;21:45–50. doi:10.1016/j.mib.2014.09.002.
- Munita JM, Arias CA, Kudva IT, Zhang Q. Mechanisms of antibiotic resistance. Microbiol Spectr. 2016:4. doi:10.1128/9781555819286.ch17
- Reygaert WC. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018;4(3):482–501. doi:10.3934/microbiol.2018.3.482.
- Bonnet R. Growing group of extended-spectrum beta-lactamases: the CTX-M enzymes. Antimicrob. Agents Chemother. 2004;48(1):1–14. doi:10.1128/AAC.48.1.1-14.2004.
- van Hoek AH, Mevius D, Guerra B, Mullany P, Roberts AP, Aarts HJ. Acquired antibiotic resistance genes: an overview. Frontiers in Microbiology. 2011;(2): 203. doi:10.3389/fmicb.2011.00203.
- Corona F, Martinez JL. Phenotypic resistance to antibiotics. Antibiot (Basel, Switzerland). 2013;2:237–255 doi:10.3390/antibiotics2020237.
- Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010;74(3):417–433. doi:10.1128/MMBR.00016-10.
- Blanco P, Hernando-Amado S, Reales-Calderon J, Corona F, Lira F, Alcalde-Rico M, Bernardini A, Sanchez M, Martinez J. Bacterial Multidrug efflux pumps: much more than antibiotic resistance determinants. Microorganisms. 2016;4(1):14. doi:10.3390/microorganisms4010014.
- Zhou G, Shi Q-S, Huang X-M, Xie X-B. The three bacterial lines of defense against antimicrobial agents. Int. J. Mol. Sci. 2015;16(9):21711–21733. doi:10.3390/ijms160921711.
- Silhavy TJ, Kahne D, Walker S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010;2(5):a000414–a000414. doi:10.1101/cshperspect.a000414.
- Delmar JA, Su -C-C, Yu EW. Bacterial multidrug efflux transporters. Annu Rev Biophys. 2014;43:93–117. doi:10.1146/annurev-biophys-051013-022855.
- Ioannidis A, Magana M, Bologa CG, Oprea TI, Paulsen IT, Tegos GP. Defining the microbial effluxome in the content of the host-microbiome interaction. Front. Pharmacol. 2015;6:31. doi:10.3389/fphar.2015.00031.
- Nebert DW, Dieter MZ. The evolution of drug metabolism. Pharmacology. 2000;61(3):124–135. doi:10.1159/000028393.
- Li X-Z, Nikaido H. Efflux-mediated drug resistance in bacteria: an update. Drugs. 2009;69(12):1555–1623. doi:10.2165/11317030-000000000-00000.
- Löscher W, Potschka H. Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx. 2005;2(1):86–98. doi:10.1602/neurorx.2.1.86.
- Sharma A, Gupta VK, Pathania R. Efflux pump inhibitors for bacterial pathogens: from bench to bedside. Indian J. Med. Res. 2019;149(2):129–145. doi:10.4103/ijmr.IJMR_2079_17.
- Spengler G, Kincses A, Gajdács M, Amaral L. New roads leading to old destinations: efflux pumps as targets to reverse multidrug resistance in bacteria. Molecules. 2017;22(3):468. doi:10.3390/molecules22030468.
- Kabra R, Chauhan N, Kumar A, Ingale P, Singh S. Efflux pumps and antimicrobial resistance: paradoxical components in systems genomics. Prog Biophys Mol Biol. 2019;141:15–24. doi:10.1016/j.pbiomolbio.2018.07.008.
- Majumder P, Mallela AK, Penmatsa A. Transporters through the looking glass. An insight into the mechanisms of ion-coupled transport and methods that help reveal them. J. Indian Inst. Sci. 2018;98(3):283–300. doi:10.1007/s41745-018-0081-5.
- Griffith J, Sansom C. The transporter factsbook. United States: Elsevier doi:10.1016/B978-0-12-303965-1.X5000-8; 1997.
- Cherak SJ, Gugala N, Turner RJ. Membrane transport Basic biochemistry (Austin). https://austinpublishinggroup.com/ebooks/basic-biochemistry/; 2019.
- Weyand S, Shimamura T, Beckstein O, Sansom MSP, Iwata S, Henderson PJF, Cameron AD. The alternating access mechanism of transport as observed in the sodium-hydantoin transporter Mhp1. J. Synchrotron Radiat. 2011;18(1):20–23. doi:10.1107/S0909049510032449.
- Du D, van Veen HW, Murakami S, Pos KM, Luisi BF. Structure, mechanism and cooperation of bacterial multidrug transporters. Curr Opin Struct Biol. 2015;33:76–91. doi:10.1016/j.sbi.2015.07.015.
- Quistgaard EM, Löw C, Guettou F, Nordlund P. Understanding transport by the major facilitator superfamily (MFS): structures pave the way. Nat. Rev. Mol. Cell Biol. 2016;17(2):123–132. doi:10.1038/nrm.2015.25.
- Kaback HR, Smirnova I, Kasho V, Nie Y, Zhou Y. The alternating access transport mechanism in LacY. J. Membr. Biol. 2011;239(1–2):85–93. doi:10.1007/s00232-010-9327-5.
- Nagarathinam K, Nakada-Nakura Y, Parthier C, Terada T, Juge N, Jaenecke F, Liu K, Hotta Y, Miyaji T, Omote H, Iwata S, . Outward open conformation of a Major Facilitator Superfamily multidrug/H+ antiporter provides insights into switching mechanism. Nat. Commun. 2018;9(1):1–9. doi:10.1038/s41467-018-06306-x.
- Kim J, Cater RJ, Choy BC, Mancia F. Structural insights into transporter-mediated drug resistance in infectious diseases. J Mol Biol. 2021;167005 16 doi:10.1016/j.jmb.2021.167005 .
- Padariya M, Kalathiya U, Baginski M. Structural and dynamic changes adopted by EmrE, multidrug transporter protein—Studies by molecular dynamics simulation. Biochim Biophys Acta (BBA)-Biomembranes. 2015;1848(10):2065–2074. doi:10.1016/j.bbamem.2015.05.014.
- Eicher T, Cha H-J, Seeger MA, Brandstatter L, El-Delik J, Bohnert JA, Kern WV, Verrey F, Grutter MG, Diederichs K, et al. Transport of drugs by the multidrug transporter AcrB involves an access and a deep binding pocket that are separated by a switch-loop. Proc. Natl. Acad. Sci. 2012;109(15):5687–5692. doi:10.1073/pnas.1114944109.
- Pasqua M, Bonaccorsi di Patti MC, Fanelli G, Utsumi R, Eguchi Y, Trirocco R, Prosseda G, Grossi M, Colonna B. Host-Bacterial pathogen communication: the Wily role of the multidrug efflux pumps of the MFS family. Front Mol Biosci. 2021;8:709. doi:10.3389/fmolb.2021.723274.
- Kaye KS, Pogue JM. Infections caused by resistant gram‐negative bacteria: epidemiology and management. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2015;35(10):949–962. doi:10.1002/phar.1636.
- Pao SS, Paulsen IT, Saier MH Jr. Major facilitator superfamily. Microbiol. Mol. Biol. Rev. 1998;62(1):1–34. doi:10.1128/MMBR.62.1.1-34.1998.
- Wright EM, Loo DDF, Hirayama BA. Biology of human sodium glucose transporters. Physiol. Rev. 2011;91(2):733–794. doi:10.1152/physrev.00055.2009.
- Reddy VS, Shlykov MA, Castillo R, Sun EI, Saier MH Jr. The major facilitator superfamily (MFS) revisited. FEBS J. 2012;279(11):2022–2035. doi:10.1111/j.1742-4658.2012.08588.x.
- Bai X, Moraes TF, Reithmeier RAF. Structural biology of solute carrier (SLC) membrane transport proteins. Mol. Membr. Biol. 2017;34(1–2):1–32. doi:10.1080/09687688.2018.1448123.
- Zhao R, Matherly LH, Goldman ID. Membrane transporters and folate homeostasis: intestinal absorption and transport into systemic compartments and tissues. Expert Rev Mol Med. 2009;11:e4–e4. doi:10.1017/S1462399409000969.
- Li P, Gu Y, Li J, Xie L, Li X, Xie J. Mycobacterium tuberculosis major facilitator superfamily transporters. J. Membr. Biol. 2017;250(6):573–585. doi:10.1007/s00232-017-9982-x.
- Navale AM, Paranjape AN. Glucose transporters: physiological and pathological roles. Biophys. Rev. 2016;8(1):5–9. doi:10.1007/s12551-015-0186-2.
- Huang Y, Lemieux MJ, Song J, Auer M, Wang D-N. Structure and mechanism of the glycerol-3-phosphate transporter from Escherichia coli. Science. 2003;301(80):616–620. doi:10.1126/science.1087619.
- Kumar S, Lekshmi M, Parvathi A, Ojha M, Wenzel N, Varela MF. Functional and structural roles of the major facilitator superfamily bacterial multidrug efflux pumps. Microorganisms. 2020;8(2):266. doi:10.3390/microorganisms8020266.
- Abdel-Motaal H, Meng L, Zhang Z, Abdelazez AH, Shao L, Xu T, Meng F, Abozaed S, Zhang R, Jiang J, et al. An uncharacterized major facilitator superfamily transporter from Planococcus maritimus exhibits dual functions as a Na+ (Li+, K+)/H+ antiporter and a multidrug efflux pump. Front. Microbiol. 2018;9:1601. doi:10.3389/fmicb.2018.01601.
- Heng J, Zhao Y, Liu M, Liu Y, Fan J, Wang X, Zhao Y, Zhang XC. Substrate-bound structure of the E. coli multidrug resistance transporter MdfA. Cell Res. 2015;25(9):1060–1073. doi:10.1038/cr.2015.94.
- Kakarla P, Ranjana KC, Shrestha U, Ranaweera I, Mukherjee MM, Willmon TM, Hernandez AJ, Barr SR, Varela MF, . Functional roles of highly conserved amino acid sequence motifs A and C in solute transporters of the major facilitator superfamily. In Drug resistance in bacteria, fungi, malaria, and cancer. Switzerland: Springer; 2017. p. 111–140 doi:10.3390/microorganisms8020266.
- Andersen JL, He G-X, Kakarla P, Kc R, Kumar S, Lakra W, Mukherjee M, Ranaweera I, Shrestha U, Tran T, et al. Multidrug efflux pumps from Enterobacteriaceae, Vibrio cholerae and Staphylococcus aureus bacterial food pathogens. Int J Environ Res Public Health. 2015;12(2):1487–1547. doi:10.3390/ijerph120201487.
- Zhang XC, Zhao Y, Heng J, Jiang D. Energy coupling mechanisms of MFS transporters. Protein Sci. 2015;24(10):1560–1579. doi:10.1002/pro.2759.
- Saidijam M, Bettaney KE, Leng D, Ma P, Xu Z, Keen JN, Rutherford NG, Ward A, Henderson PJ, Szakonyi G, Ren Q, . The MFS efflux proteins of gram-positive and gram-negative bacteria. Adv Enzymol Relat Areas Mol Biol. 2011;77:147–166 doi:10.1002/9780470920541.ch4.
- Tanabe M, Szakonyi G, Brown KA, Henderson PJF, Nield J, Byrne B. The multidrug resistance efflux complex, EmrAB from Escherichia coli forms a dimer in vitro. Biochem. Biophys. Res. Commun. 2009;380(2):338–342. doi:10.1016/j.bbrc.2009.01.081.
- Zgurskaya HI. Multicomponent drug efflux complexes: architecture and mechanism of assembly. Future Microbiology. 2009;4(7):919–932. doi:10.2217/fmb.09.62.
- Drew D, North RA, Nagarathinam K, Tanabe M. Structures and general transport mechanisms by the Major Facilitator Superfamily (MFS). Chem. Rev. 2021;121(9):5289–5335. doi:10.1021/acs.chemrev.0c00983.
- Hinchliffe P, Greene NP, Paterson NG, Crow A, Hughes C, Koronakis V. Structure of the periplasmic adaptor protein from a major facilitator superfamily (MFS) multidrug efflux pump. FEBS Lett. 2014;588(17):3147–3153. doi:10.1016/j.febslet.2014.06.055.
- Alav I, Kobylka J, Kuth MS, Pos KM, Picard M, Blair JMA, Bavro VN. Structure, assembly, and function of tripartite efflux and type 1 secretion systems in Gram-Negative bacteria. Chem. Rev. 2021;121(9):5479–5596. doi:10.1021/acs.chemrev.1c00055.
- Puértolas-Balint F, Warsi O, Linkevicius M, Tang P-C, Andersson DI. Mutations that increase expression of the EmrAB-TolC efflux pump confer increased resistance to nitroxoline in Escherichia coli. J. Antimicrob. Chemother. 2020;75(2):300–308. doi:10.1093/jac/dkz434.
- Nies DH. Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol. Rev. 2003;27(2–3):313–339. doi:10.1016/S0168-6445(03)00048-2.
- Du D, Wang Z, James NR, Voss JE, Klimont E, Ohene-Agyei T, Venter H, Chiu W, Luisi BF. Structure of the AcrAB-TolC multidrug efflux pump. Nature. 2014;509(7501):512–515. doi:10.1038/nature13205.
- Jamshidi S, Sutton JM, Rahman KM. Computational study reveals the molecular mechanism of the interaction between the efflux inhibitor PAβN and the AdeB transporter from Acinetobacter baumannii. ACS Omega. 2017;2(6):3002–3016. doi:10.1021/acsomega.7b00131.
- Du D, Wang-Kan X, Neuberger A, van Veen HW, Pos KM, Piddock LJV, Luisi BF. Multidrug efflux pumps: structure, function and regulation. Nat. Rev. Microbiol. 2018;16(9):523–539. doi:10.1038/s41579-018-0048-6.
- Bay DC, Rommens KL, Turner RJ. Small multidrug resistance proteins: a multidrug transporter family that continues to grow. Biochim Biophys Acta (BBA)-Biomembranes. 2008;1778(9):1814–1838. doi:10.1016/j.bbamem.2007.08.015.
- Moriyama Y, Hiasa M, Matsumoto T, Omote H. Multidrug and toxic compound extrusion (MATE)-type proteins as anchor transporters for the excretion of metabolic waste products and xenobiotics. Xenobiotica. 2008;38(7–8):1107–1118. doi:10.1080/00498250701883753.
- Kusakizako T, Miyauchi H, Ishitani R, Nureki O. Structural biology of the multidrug and toxic compound extrusion superfamily transporters. Biochim Biophys Acta (Bba)-biomembranes. 2020;1862(12):183154. doi:10.1016/j.bbamem.2019.183154.
- Kuroda T, Tsuchiya T. Multidrug efflux transporters in the MATE family. Biochim Biophys Acta (BBA)-Proteins Proteomics. 2009;1794(5):763–768. doi:10.1016/j.bbapap.2008.11.012.
- Qiao C, Yang J, Wan Y, Xiang S, Guan M, Du H, Tang Z, Lu K, Li J, Qu C. A genome-wide survey of MATE transporters in Brassicaceae and unveiling their expression profiles under abiotic stress in rapeseed. Plants (Basel, Switzerland). 2020;9:1072 doi:10.3390/plants9091072.
- Higgins CF. ABC transporters: from microorganisms to man. Annu. Rev. Cell Biol. 1992;8(1):67–113. doi:10.1146/annurev.cb.08.110192.000435.
- Theodoulou FL, Kerr ID. ABC transporter research: going strong 40 years on. Biochem. Soc. Trans. 2015;43(5):1033–1040. doi:10.1042/BST20150139.
- Callaghan R, Luk F, Bebawy M. Inhibition of the multidrug resistance P-glycoprotein: time for a change of strategy? Drug Metab. Dispos. 2014;42(4):623–631. doi:10.1124/dmd.113.056176.
- Dawson RJP, Locher KP. Structure of a bacterial multidrug ABC transporter. Nature. 2006;443(7108):180–185. doi:10.1038/nature05155.
- Hvorup RN, Goetz BA, Niederer M, Hollenstein K, Perozo E, Locher KP. Asymmetry in the structure of the ABC transporter-binding protein complex BtuCD-BtuF. Science (80). 2007;317(5843):1387–1390. doi:10.1126/science.1145950.
- Crow A, Greene NP, Kaplan E, Koronakis V. Structure and mechanotransmission mechanism of the MacB ABC transporter superfamily. Proc Natl Acad Sci. 2017;114:12572–12577. doi:10.1073/pnas.1712153114.
- Schneider E, Hunke S. ATP-binding-cassette (ABC) transport systems: functional and structural aspects of the ATP-hydrolyzing subunits/domains. FEMS Microbiol. Rev. 1998;22(1):1–20. doi:10.1111/j.1574-6976.1998.tb00358.x.
- Wilkens S. Structure and mechanism of ABC transporters. F1000Prime Rep. 2015;7:14. doi:10.12703/P7-14.
- Fitzpatrick AWP, Llabrés S, Neuberger A, Blaza JN, Bai X-C, Okada U, Murakami S, van Veen HW, Zachariae U, Scheres SHW, et al. Structure of the MacAB–TolC ABC-type tripartite multidrug efflux pump. Nat. Microbiol. 2017;2(7):1–8. doi:10.1038/nmicrobiol.2017.70.
- Hassan KA, Liu Q, Elbourne LDH, Ahmad I, Sharples D, Naidu V, Chan CL, Li L, Harborne SPD, Pokhrel A, et al. Pacing across the membrane: the novel PACE family of efflux pumps is widespread in Gram-negative pathogens. Res. Microbiol. 2018;169(7–8):450–454. doi:10.1016/j.resmic.2018.01.001.
- Hassan KA, Elbourne LDH, Li L, Gamage HKAH, Liu Q, Jackson SM, Sharples D, Kolstø A-B, Henderson PJF, Paulsen IT, et al. An ace up their sleeve: a transcriptomic approach exposes the AceI efflux protein of Acinetobacter baumannii and reveals the drug efflux potential hidden in many microbial pathogens. Front. Microbiol. 2015;6:333. doi:10.3389/fmicb.2015.00333.
- Hassan KA, Jackson SM, Penesyan A, Patching SG, Tetu SG, Eijkelkamp BA, Brown MH, Henderson PJF, Paulsen IT. Transcriptomic and biochemical analyses identify a family of chlorhexidine efflux proteins. Proc. Natl. Acad. Sci. U. S. A. 2013;110(50):20254–20259. doi:10.1073/pnas.1317052110.
- Hassan KA, Liu Q, Henderson PJF, Paulsen IT, Bush K. Homologs of the Acinetobacter baumannii AceI transporter represent a new family of bacterial multidrug efflux systems. MBio. 2015;6(1):e01982–14. doi:10.1128/mBio.01982-14.
- Santajit S, Indrawattana N. Mechanisms of antimicrobial resistance in ESKAPE pathogens. Biomed Res Int. 2016;2016:2475067. doi:10.1155/2016/2475067.
- Magiorakos A-P, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012;18(3):268–281. doi:10.1111/j.1469-0691.2011.03570.x.
- Shriram V, Khare T, Bhagwat R, Shukla R, Kumar V. Inhibiting bacterial drug efflux pumps via Phyto-Therapeutics to combat threatening antimicrobial resistance. Front Microbiol. 2018;9:2990. doi:10.3389/fmicb.2018.02990.
- Gao W, Howden BP, Stinear TP. Evolution of virulence in Enterococcus faecium, a hospital-adapted opportunistic pathogen. Curr Opin Microbiol. 2018;41:76–82. doi:10.1016/j.mib.2017.11.030.
- Jett BD, Huycke MM, Gilmore MS. Virulence of enterococci. Clin. Microbiol. Rev. 1994;7(4):462–478. doi:10.1128/CMR.7.4.462.
- Willems RJL, Hanage WP, Bessen DE, Feil EJ. Population biology of Gram-positive pathogens: high-risk clones for dissemination of antibiotic resistance. FEMS Microbiol. Rev. 2011;35(5):872–900. doi:10.1111/j.1574-6976.2011.00284.x.
- European Centre for Disease Prevention and Control. Surveillance of antimicrobial resistance in Europe 2018. https://www.ecdc.europa.eu/en/publications-data/surveil lance-antimicrobial-resistance-europe-2018; (2019).
- Haque M, Sartelli M, McKimm J, Abu Bakar M. Health care-associated infections - an overview. Infect Drug Resist. 2018;11:2321–2333. doi:10.2147/IDR.S177247.
- Soheili S, Ghafourian S, Sekawi Z, Neela V, Sadeghifard N, Ramli R, Hamat RA. Wide distribution of virulence genes among Enterococcus faecium and Enterococcus faecalis clinical isolates. Sci. World J. 2014;2014:1–6. doi:10.1155/2014/623174.
- Fiore E, Van Tyne D, Gilmore MS, Fischetti VA, Novick RP, Ferretti JJ, Portnoy DA, Braunstein M, Rood JI. Pathogenicity of Enterococci. Microbiol Spectr. 2019;7(4). doi:10.1128/microbiolspec.GPP3-0053-2018.
- Miller WR, Munita JM, Arias CA. Mechanisms of antibiotic resistance in enterococci. Expert Rev. Anti. Infect. Ther. 2014;12(10):1221–1236. doi:10.1586/14787210.2014.956092.
- Ahmed MO, Baptiste KE. Vancomycin-resistant enterococci: a review of antimicrobial resistance mechanisms and perspectives of human and animal health. Microb. Drug Resist. 2018;24(5):590–606. doi:10.1089/mdr.2017.0147.
- Lerma LL, Benomar N, Sánchez Valenzuela A, Casado Muñoz MDC, Gálvez A, Abriouel H. Role of EfrAB efflux pump in biocide tolerance and antibiotic resistance of Enterococcus faecalis and Enterococcus faecium isolated from traditional fermented foods and the effect of EDTA as EfrAB inhibitor. Food Microbiol. 2014;44:249–257. doi:10.1016/j.fm.2014.06.009.
- Bergdoll MS. Staphylococcus aureus. J. Assoc. Off. Anal. Chem. 1991;74:706–710 doi:10.1093/jaoac/74.4.706.
- David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin. Microbiol. Rev. 2010;23(3):616–687. doi:10.1128/CMR.00081-09.
- Chambers HF. The changing epidemiology of Staphylococcus aureus? Emerg. Infect. Dis. 2001;7(2):178. doi:10.3201/eid0702.010204.
- Di Franco S, Alfieri A, Pace MC, Sansone P, Pota V, Fittipaldi C, Fiore M, Passavanti MB. Blood stream infections from MDR Bacteria. Life. 2021;11(6):575. doi:10.3390/life11060575.
- Lipsky ZW, Marques CNH, German GK. Lipid depletion enables permeation of Staphylococcus aureus bacteria through human stratum corneum. Tissue Barriers. 2020;8(2):1754706. doi:10.1080/21688370.2020.1754706.
- Yasmin M, El Hage H, Obeid R, El Haddad H, Zaarour M, Khalil A. Epidemiology of bloodstream infections caused by methicillin-resistant Staphylococcus aureus at a tertiary care hospital in New York. Am J Infect Control. 2016;44(1):41–46. doi:10.1016/j.ajic.2015.08.005.
- Hiramatsu K, Ito T, Tsubakishita S, Sasaki T, Takeuchi F, Morimoto Y, Katayama Y, Matsuo M, Kuwahara-Arai K, Hishinuma T, Baba T, . Genomic basis for Methicillin resistance in Staphylococcus aureus. Infect. Chemother. 2013;45(2):117–136. doi:10.3947/ic.2013.45.2.117.
- Costa SS, Viveiros M, Amaral L, Couto I. Multidrug efflux pumps in Staphylococcus aureus: an update. Open Microbiol. J. 2013;7(1):59–71. doi:10.2174/1874285801307010059.
- Kaatz GW, Moudgal VV, Seo SM, Kristiansen JE. Phenothiazines and Thioxanthenes inhibit multidrug efflux pump activity in Staphylococcus aureus. Antimicrob. Agents Chemother. 2003;47(2):719–726. doi:10.1128/AAC.47.2.719-726.2003.
- Felicetti T, Cannalire R, Pietrella D, Latacz G, Lubelska A, Manfroni G, Barreca ML, Massari S, Tabarrini O, Kieć-Kononowicz K, et al. 2-Phenylquinoline S. aureus NorA efflux pump inhibitors: evaluation of the importance of Methoxy group introduction. J Med Chem. 2018;61(17):7827–7848. doi:10.1021/acs.jmedchem.8b00791.
- Ferreira MKA, Da Silva AW, Silva FCO, Holanda CLA, Barroso SM, Lima JDR, Vieira Neto AE, Campos AR, Bandeira PN, Dos Santos HS, et al. Anxiolytic-like effect of chalcone N-{(4′-[(E)-3-(4-fluorophenyl)-1-(phenyl) prop-2-en-1-one]} acetamide on adult zebrafish (Danio rerio): involvement of the GABAergic system. Behav. Brain Res. 2019;374:111871. doi:10.1016/j.bbr.2019.03.040.
- Zimmermann S, Klinger-Strobel M, Bohnert JA, Wendler S, Rödel J, Pletz MW, Löffler B, Tuchscherr L. Clinically approved drugs inhibit the Staphylococcus aureus multidrug NorA efflux pump and reduce biofilm formation. Front. Microbiol. 2019;10:2762. doi:10.3389/fmicb.2019.02762.
- Figueredo FG, Ramos IT, Paz JA, Silva TM, Câmara CA, de Morais Oliveira-Tintino CD, Tintino SR, de Farias PA, de Menezes IR, Coutinho HD, Fonteles MM, . Effect of hydroxyamines derived from lapachol and norlachol against Staphylococcus aureus strains carrying the NorA efflux pump. Infect Genet Evol. 2020;84:104370. doi:10.1016/j.meegid.2020.104370.
- Selden R, Lee S, Wang WLL, Bennett JV, Eickhoff TC, Lee S, Wang WLL, Bennett JV, Eickhoff TC, Wang WLL, et al. Nosocomial Klebsiella infections: intestinal colonization as a reservoir. Ann. Intern. Med. 1971;74(5):657–664. doi:10.7326/0003-4819-74-5-657.
- Monegro AF, Muppidi V, Regunath H. Hospital Acquired Infections Statpearls. https://www.ncbi.nlm.nih.gov/books/NBK441857/; 2017.
- Stupka JE, Mortensen EM, Anzueto A, Restrepo MI. Community-acquired pneumonia in elderly patients. Aging Health. 2009;5(6):763–774. doi:10.2217/ahe.09.74.
- Codjoe FS, Donkor ES. Carbapenem Resistance: a review. Med Sci (Basel, Switzerland). 2017;6:1 doi:10.3390/medsci6010001.
- Calfee DP. Recent advances in the understanding and management of Klebsiella pneumoniae. F1000Research. 2017;6:1760. doi:10.12688/f1000research.11532.1.
- Wyres KL, Lam MMC, Holt KE. Population genomics of Klebsiella pneumoniae. Nat. Rev. Microbiol. 2020;18(6):344–359. doi:10.1038/s41579-019-0315-1.
- Ferreira RL, Da Silva BCM, Rezende GS, Nakamura-Silva R, Pitondo-Silva A, Campanini EB, Brito MCA, Da Silva EML, Freire CCDM, Cunha AFD, et al. High prevalence of multidrug-resistant Klebsiella pneumoniae Harboring several virulence and β-Lactamase encoding genes in a Brazilian Intensive Care Unit. Front Microbiol. 2019;9:3198. doi:10.3389/fmicb.2018.03198.
- Srinivasan VB, Singh BB, Priyadarshi N, Chauhan NK, Rajamohan G. Role of novel multidrug efflux pump involved in drug resistance in Klebsiella pneumoniae. PLoS One. 2014;9:e96288 doi:10.1371/journal.pone.0096288.
- Srinivasan VB, Rajamohan G. KpnEF, a new member of the Klebsiella pneumoniae cell envelope stress response regulon, is an SMR-type efflux pump involved in broad-spectrum antimicrobial resistance. Antimicrob. Agents Chemother. 2013;57(9):4449–4462. doi:10.1128/AAC.02284-12.
- Ni RT, Onishi M, Mizusawa M, Kitagawa R, Kishino T, Matsubara F, Tsuchiya T, Kuroda T, Ogawa W. The role of RND-type efflux pumps in multidrug-resistant mutants of Klebsiella pneumoniae. Sci. Rep. 2020;10(1):1–10. doi:10.1038/s41598-020-67820-x.
- Vera-Leiva A, Carrasco-Anabalón S, Lima CA, Villagra N, Domínguez M, Bello-Toledo H, González-Rocha G. The efflux pump inhibitor phenylalanine-arginine β-naphthylamide (PAβN) increases resistance to carbapenems in Chilean clinical isolates of KPC-producing Klebsiella pneumoniae. J. Glob. Antimicrob. Resist. 2018;12:73–76. doi:10.1016/j.jgar.2017.12.003.
- Osei Sekyere J, Amoako DG. Carbonyl cyanide m-chlorophenylhydrazine (CCCP) reverses resistance to colistin, but not to carbapenems and tigecycline in multidrug-resistant Enterobacteriaceae. Front Microbiol. 2017;8:228. doi:10.3389/fmicb.2017.00228.
- Mahamoud A, Chevalier J, Alibert-Franco S, Kern WV, Pagès J-M. Antibiotic efflux pumps in Gram-negative bacteria: the inhibitor response strategy. J. Antimicrob. Chemother. 2007;59(6):1223–1229. doi:10.1093/jac/dkl493.
- Piddock LJV, Garvey MI, Rahman MM, Gibbons S. Natural and synthetic compounds such as trimethoprim behave as inhibitors of efflux in Gram-negative bacteria. J. Antimicrob. Chemother. 2010;65(6):1215–1223. doi:10.1093/jac/dkq079.
- Padilla E, Llobet E, Doménech-Sánchez,A, Martínez-Martínez L, Bengoechea JA, Albertí S . Klebsiella pneumoniae AcrAB efflux pump contributes to Antimicrobial resistance and virulence. Antimicrob. Agents Chemother. 2010;54(1):177–183. doi:10.1128/AAC.00715-09.
- Morris FC, Dexter C, Kostoulias X, Uddin MI, Peleg AY. The mechanisms of disease caused by Acinetobacter baumannii. Front Microbiol. 2019;10:1601. doi:10.3389/fmicb.2019.01601.
- Howard A, O’Donoghue M, Feeney A, Sleator RD. Acinetobacter baumannii: an emerging opportunistic pathogen. Virulence. 2012;3(3):243–250. doi:10.4161/viru.19700.
- Gonzalez-Villoria AM, Valverde-Garduno V. Antibiotic-Resistant Acinetobacter baumannii increasing success remains a challenge as a Nosocomial pathogen. J Pathog. 2016;2016:7318075. doi:10.1155/2016/7318075.
- Ayoub Moubareck C, Hammoudi Halat D. Insights into Acinetobacter baumannii: a review of microbiological, virulence, and resistance traits in a threatening Nosocomial pathogen. Antibiot (Basel, Switzerland). 2020;9:119 doi:10.3390/antibiotics9030119.
- Lopes BS, Amyes SGB. Role of ISAba1 and ISAba125 in governing the expression of bla ADC in clinically relevant Acinetobacter baumannii strains resistant to cephalosporins. J. Med. Microbiol. 2012;61(8):1103–1108. doi:10.1099/jmm.0.044156-0.
- Zhao Y, Smet H, Prat I, Sands A, Urassa W, Fransen K, Crucitti T. Outbreak of carbapenem-resistant Acinetobacter baumannii carrying the carbapenemase OXA-23 in ICU of the eastern Heilongjiang Province, China. BMC Infect. Dis. 2019;19(1):1–7. doi:10.1186/s12879-018-3567-x.
- Abdi SN, Ghotaslou R, Ganbarov K, Mobed A, Tanomand A, Yousefi M, Asgharzadeh M, Kafil HS. Acinetobacter baumannii efflux pumps and antibiotic resistance. Infect. Drug Resist. 2020;13:423–434. doi:10.2147/IDR.S228089.
- Coyne S, Courvalin P, Périchon B. Efflux-Mediated antibiotic resistance in Acinetobacter spp. Antimicrob. Agents Chemother. 2011;55(3):947–953. doi:10.1128/AAC.01388-10.
- Temgoua FTD, Wu L. Mechanisms efflux pumps of Acinetobacter baumannii (MDR): increasing resistance to antibiotics. J Biosci Med. 2018;7:48–70 doi:10.4236/jbm.2019.71006.
- Sun JR, Perng CL, Chan MC, Morita Y, Lin JC, Su CM, Wang WY, Chang TY, Chiueh TS . A truncated AdeS kinase protein generated by IS Aba1 insertion correlates with tigecycline resistance in Acinetobacter baumannii. PLoS One. 2012;7(11):e49534. doi:10.1371/journal.pone.0049534.
- Valentine SC, Contreras D, Tan S, Real LJ, Chu S, Xu HH. Phenotypic and molecular characterization of Acinetobacter baumannii clinical isolates from Nosocomial outbreaks in Los Angeles County, California. J. Clin. Microbiol. 2008;46(8):2499–2507. doi:10.1128/JCM.00367-08.
- Gholami M, Hashemi A, Hakemi-Vala M, Goudarzi H, Hallajzadeh M. Efflux Pump Inhibitor Phenylalanine-Arginine Β-Naphthylamide effect on the minimum inhibitory concentration of imipenem in Acinetobacter baumannii strains isolated from hospitalized patients in Shahid Motahari Burn Hospital, Tehran, Iran. Jundishapur J. Microbiol. 2015;8(10):e19048–e19048. doi:10.5812/jjm.19048.
- Zhang Z, Morgan CE, Bonomo RA, Yu EW, Projan SJ. Cryo-EM determination of Eravacycline-Bound structures of the Ribosome and the multidrug efflux pump AdeJ of Acinetobacter baumannii. MBio. 2021;12(3):e01031–21. doi:10.1128/mBio.01031-21.
- Adasme MF, Linnemann KL, Bolz SN, Kaiser F, Salentin S, Haupt V, Schroeder M. expanding the scope of the protein–ligand interaction profiler to DNA and RNA. Nucleic Acids Res. 2021 PLIP;49(W1):W530–W534. doi:10.1093/nar/gkab294.
- Iglewski BH. Pseudomonas. In Med. Microbiol. 4th ed. Galvest. Univ. Texas Med. Branch Galvest. https://www.ncbi.nlm.nih.gov/books/NBK8326/; 1996.
- Hirsch EB, Tam VH. Impact of multidrug-resistant Pseudomonas aeruginosa infection on patient outcomes. Expert Rev. Pharmacoecon. Outcomes Res. 2010;10(4):441–451. doi:10.1586/erp.10.49.
- Moradali MF, Ghods S, Rehm BHA. Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Front Cell Infect Microbiol. 2017;7:39. doi:10.3389/fcimb.2017.00039.
- Alonso B, Fernández-Barat L, Di Domenico EG, Marín M, Cercenado E, Merino I, de Pablos M, Muñoz P, Guembe M . Characterization of the virulence of Pseudomonas aeruginosa strains causing ventilator-associated pneumonia. BMC Infect Dis. 2020;20:1–8. 10.1186/s12879-020-05534-1
- Newman JW, Floyd RV, Fothergill JL. The contribution of Pseudomonas aeruginosa virulence factors and host factors in the establishment of urinary tract infections. FEMS Microbiol. Lett. 2017;364(15). doi:10.1093/femsle/fnx124.
- Dreier J, Ruggerone P. Interaction of antibacterial compounds with RND efflux pumps in Pseudomonas aeruginosa. Front Microbiol. 2015;6:660. doi:10.3389/fmicb.2015.00660.
- Lister PD, Wolter DJ, Hanson ND. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin. Microbiol. Rev. 2009;22(4):582–610. doi:10.1128/CMR.00040-09.
- Maseda H, Sawada I, Saito K, Uchiyama H, Nakae T, Nomura N. Enhancement of the mexAB-oprM Efflux Pump Expression by a Quorum-Sensing Autoinducer and its cancellation by a regulator, MexT, of the mexEF oprN efflux pump Operon in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2004;48(4):1320–1328. doi:10.1128/AAC.48.4.1320-1328.2004.
- Lamers RP, Cavallari JF, Burrows LL, Webber MA. The efflux inhibitor phenylalanine-arginine beta-naphthylamide (PAβN) permeabilizes the outer membrane of gram-negative bacteria. PLoS One. 2013;8(3):e60666. doi:10.1371/journal.pone.0060666.
- Siriyong T, Srimanote P, Chusri S, Yingyongnarongkul B-E, Suaisom C, Tipmanee V, Voravuthikunchai SP. Conessine as a novel inhibitor of multidrug efflux pump systems in Pseudomonas aeruginosa. BMC Complement. Altern. Med. 2017;17(1):405. doi:10.1186/s12906-017-1913-y.
- Tsutsumi K, Yonehara R, Ishizaka-Ikeda E, Miyazaki N, Maeda S, Iwasaki K, Nakagawa A, Yamashita E. Structures of the wild-type MexAB–OprM tripartite pump reveal its complex formation and drug efflux mechanism. Nat. Commun. 2019;10(1):1–10. doi:10.1038/s41467-019-09463-9.
- Nakashima R, Sakurai K, Yamasaki S, Hayashi K, Nagata C, Hoshino K, Onodera Y, Nishino K, Yamaguchi A . Structural basis for the inhibition of bacterial multidrug exporters. Nature. 2013;500(7460):102–106. doi:10.1038/nature12300.
- Ramirez D, Giron M. Enterobacter infections. StatPearls [Internet]. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK559296/; 2020.
- Jacob JT, Klein E, Laxminarayan R, Beldavs Z, Lynfield R, Kallen AJ, Ricks P, Edwards J, Srinivasan A, Fridkin S, Rasheed JK . Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR Morb Mortal Wkly Rep. 2013;62:165 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4604788/.
- Dong LT, Espinoza HV, Espinoza JL. Emerging superbugs: the threat of Carbapenem Resistant Enterobacteriaceae. AIMS Microbiol. 2020;7(3):176–182. doi:10.3934/microbiol.2020012.
- Evins CW, Sutton CM, Withers ST, Grier JT, Schammel CMG, Fiester SE. Community-Acquired, Extended-Spectrum β-Lactamase-Producing and extensively drug-resistant Escherichia coli in a 28-year-old Pyelonephritis patient lacking risk factors. Antibiotics. 2021;10(5):533. doi:10.3390/antibiotics10050533.
- Wu W, Wei L, Feng Y, Xie Y, Zong Z. Precise species identification by whole-genome sequencing of Enterobacter bloodstream infection, China. Emerg. Infect. Dis. 2021;27(1):161. doi:10.3201/eid2701.190154.
- Davin-Regli A, Lavigne J-P, Pagès J-M. Enterobacter spp.: update on taxonomy, clinical aspects, and emerging antimicrobial resistance. Clin. Microbiol. Rev. 2019;32(4):e00002–19. doi:10.1128/CMR.00002-19.
- Krzymińska S, Mokracka J, Koczura R, Kaznowski A. Cytotoxic activity of Enterobacter cloacae human isolates. FEMS Immunol. Med. Microbiol. 2009;56(3):248–252. doi:10.1111/j.1574-695X.2009.00572.x.
- Vatopoulos AC, Tsakris A, Tzouvelekis LS, Legakis NJ, Pitt TL, Miller GH, Shaw KJ, Antreou M, Nikolopoulou M, Komninou Z . Diversity of aminoglycoside resistance in Enterobacter cloacae in Greece. Eur. J. Clin. Microbiol. Infect. Dis. 1992;11(2):131–138. doi:10.1007/BF01967064.
- Slipski CJ, Zhanel GG, Bay DC. Biocide Selective TolC-Independent Efflux Pumps in Enterobacteriaceae. J. Membr. Biol. 2018;251(1):15–33. doi:10.1007/s00232-017-9992-8.
- Mahamoud A, Chevalier J, Davin-Regli A, Barbe J, Pages J-M. Quinoline derivatives as promising inhibitors of antibiotic efflux pump in multidrug resistant Enterobacter aerogenes isolates. Curr Drug Targets. 2006;7(7):843–847. doi:10.2174/138945006777709557.
- Ghisalberti D, Mahamoud A, Chevalier J, Baitiche M, Martino M, Pages J, Barbe J. Chloroquinolines block antibiotic efflux pumps in antibiotic-resistant Enterobacter aerogenes isolates. Int J Antimicrob Agents. 2006;27(6):565–569. doi:10.1016/j.ijantimicag.2006.03.010.
- Takahashi N, Sulijaya B, Yamada-Hara M, Tsuzuno T, Tabeta K, Yamazaki K. Gingival epithelial barrier: regulation by beneficial and harmful microbes. Tissue Barriers. 2019;7(3):e1651158. doi:10.1080/21688370.2019.1651158.
- Jafari A, Aslani MM, Bouzari S. Escherichia coli: a brief review of diarrheagenic pathotypes and their role in diarrheal diseases in Iran. Iran. J. Microbiol. 2012;4:102–117 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3465535/.
- World Health Organization. Shiga Toxin-producing Escherichia Coli (STEC) and Food: Attribution, Characterization and Monitoring. https://apps.who.int/iris/handle/10665/272871; 2019.
- Anes J, McCusker MP, Fanning S, Martins M. The ins and outs of RND efflux pumps in Escherichia coli. Front Microbiol. 2015;6:587. doi:10.3389/fmicb.2015.00587.
- Kurgan G, Panyon LA, Rodriguez-Sanchez Y, Pacheco E, Nieves LM, Mann R, Nielsen DR, Wang X. Bioprospecting of native efflux pumps to enhance furfural tolerance in ethanologenic Escherichia coli. Appl. Environ. Microbiol. 2019;85(6):e02985–18. doi:10.1128/AEM.02985-18.
- Opperman TJ, Nguyen ST. Recent advances toward a molecular mechanism of efflux pump inhibition. Front Microbiol. 2015;6:421. doi:10.3389/fmicb.2015.00421.
- Vargiu AV, Nikaido H. Multidrug binding properties of the AcrB efflux pump characterized by molecular dynamics simulations. Proc. Natl. Acad. Sci. 2012;109(50):20637–20642. doi:10.1073/pnas.1218348109.
- Maurya A, Dwivedi GR, Darokar MP, Srivastava SK. Antibacterial and synergy of Clavine Alkaloid Lysergol and its derivatives against Nalidixic acid-resistant Escherichia coli. Chem. Biol. Drug Des. 2013;81(4):484–490. doi:10.1111/cbdd.12103.
- Sjuts H, Vargiu AV, Kwasny SM, Nguyen ST, Kim H-S, Ding X, Ornik AR, Ruggerone P, Bowlin TL, Nikaido H, et al. Molecular basis for inhibition of AcrB multidrug efflux pump by novel and powerful pyranopyridine derivatives. Proc. Natl. Acad. Sci. 2016;113(13):3509–3514. doi:10.1073/pnas.1602472113.
- Grimsey EM, Fais C, Marshall RL, Ricci V, Ciusa ML, Stone JW, Ivens A, Malloci G, Ruggerone P, Vargiu AV, et al. Chlorpromazine and amitriptyline are substrates and inhibitors of the AcrB multidrug efflux pump. MBio. 2020;11(3):e00465–20. doi:10.1128/mBio.00465-20.
- Papenfort K, Bassler BL. Quorum sensing signal–response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016;14(9):576–588. doi:10.1038/nrmicro.2016.89.
- Alav I, Sutton JM, Rahman KM. Role of bacterial efflux pumps in biofilm formation. J. Antimicrob. Chemother. 2018;73(8):2003–2020. doi:10.1093/jac/dky042.
- Tang M, Wei X, Wan X, Ding Z, Ding Y, Liu J. The role and relationship with efflux pump of biofilm formation in Klebsiella pneumoniae. Microb. Pathog. 2020;147:104244. doi:10.1016/j.micpath.2020.104244.
- Matsumura K, Furukawa S, Ogihara H, Morinaga Y. Roles of multidrug efflux pumps on the biofilm formation of Escherichia coli K-12. Biocontrol Sci. 2011;16(2):69–72. doi:10.4265/bio.16.69.
- Kasper SH, Bonocora RP, Wade JT, Musah RA, Cady NC. Chemical inhibition of kynureninase reduces Pseudomonas aeruginosa quorum sensing and virulence factor expression. ACS Chem. Biol. 2016;11(4):1106–1117. doi:10.1021/acschembio.5b01082.
- Olivares J, Alvarez-Ortega C, Linares JF, Rojo F, Köhler T, Martínez JL. Overproduction of the multidrug efflux pump MexEF‐OprN does not impair Pseudomonas aeruginosa fitness in competition tests, but produces specific changes in bacterial regulatory networks. Environ. Microbiol. 2012;14(8):1968–1981. doi:10.1111/j.1462-2920.2012.02727.x.
- Minagawa S, Inami H, Kato T, Sawada S, Yasuki T, Miyairi S, Horikawa M, Okuda J, Gotoh N. RND type efflux pump system MexAB-OprM of Pseudomonas aeruginosa selects bacterial languages, 3-oxo-acyl-homoserine lactones, for cell-to-cell communication. BMC Microbiol. 2012;12(1):1–10. doi:10.1186/1471-2180-12-70.
- El-Shaer S, Shaaban M, Barwa R, Hassan R. Control of quorum sensing and virulence factors of Pseudomonas aeruginosa using phenylalanine arginyl β-naphthylamide. J. Med. Microbiol. 2016;65(10):1194–1204. doi:10.1099/jmm.0.000327.
- Rampioni G, Pillai CR, Longo F, Bondì R, Baldelli V, Messina M, Imperi F, Visca P, Leoni L. Effect of efflux pump inhibition on Pseudomonas aeruginosa transcriptome and virulence. Sci. Rep. 2017;7(1):1–14. doi:10.1038/s41598-017-11892-9.
- Wang J, Jiao H, Meng J, Qiao M, Du H, He M, Ming K, Liu J, Wang D, Wu Y, et al. Baicalin inhibits biofilm formation and the quorum-sensing system by regulating the MsrA drug efflux pump in Staphylococcus saprophyticus. Front. Microbiol. 2019;10:2800. doi:10.3389/fmicb.2019.02800.