ABSTRACT
The intestinal barrier orchestrates selective permeability to nutrients and metabolites while excluding noxious stimuli. Recent scientific advances establishing a causal role for the gut microbiota in human health outcomes have generated a resurgent interest toward intestinal permeability. Considering the well-established role of the gut barrier in protection against foreign antigens, there is mounting evidence for a causal link between gut permeability and the microbiome in regulating human health. However, an understanding of the dynamic host–microbiota interactions that govern intestinal barrier functions remains poorly defined. Furthermore, the system-level mechanisms by which microbiome-targeted therapies, such as probiotics and prebiotics, simultaneously promote intestinal barrier function and host health remain an area of active investigation. This review summarizes the recent advances in understanding the dynamics of intestinal permeability in human health and its integration with gut microbiota. We further summarize mechanisms by which probiotics/prebiotics influence the gut microbiota and intestinal barrier functions.
Introduction
There is a growing appreciation for the role of gut permeability in human health. “Google” and “PubMed” searches for the term “gut permeability” returned 19,200,000 and 53,660 results, respectively, as of March 2022. Despite being frequently used analogous to the terms “leaky gut” and “gut barrier dysfunction”, intestinal permeability is a normal functional feature of the gastrointestinal (GI) barrier in a healthy individual that is measured by the flux rates of micro- and macro-molecules across the gut epithelium.Citation1 In contrast, terms like “leaky gut” and “barrier dysfunction” more accurately describe states of “abnormal intestinal permeability” where disturbances in intestinal permeability arise due to functional impairments and diseases.
As with intestinal permeability, there is a growing appreciation of the role of gut microbiota (the collective bacterial, archaeal, viral, and eukaryotic microorganisms occupying the GI tract) in maintaining normal human physiology and pathobiology. Considering the well-established role of the gut barrier in protecting the body from foreign antigens, including those derived from the gut microbiota, there is mounting evidence for a causal link between gut permeability and the microbiome in regulating human health and disease. A comprehensive understanding of the relationships among gut barrier biology, intestinal permeability, and the gut microbiome can therefore provide novel insights into the dynamic balance between human health and disease, which is the focus of this review article.
The intestinal barrier: greater than the sum of its parts
The term “intestinal barrier” traditionally refers to a complex multilayer system consisting of physiochemical, structural, and immunological compartments. The physiochemical and structural compartments consist of a mucus layer and a single layer of epithelium, which cover a surface area of about 400 m2 and require 40% of the body’s energy for their maintenance.Citation2 Importantly, these components combine to serve as a fence that limits the entry of bacteria, luminal antigens, toxins, and metabolites into the underlying mucosa.Citation3 Should this physiochemical barrier be breached, the immunological component – consisting primarily of innate immune cells including macrophages and dendritic cells – senses luminally derived molecular patterns and initiates inflammatory signals to promote antigen destruction and restitution of the intestinal barrier.Citation4
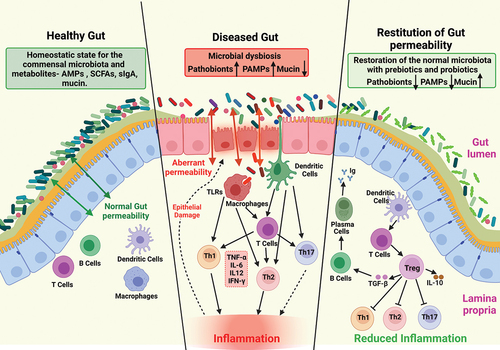
In addition to these structural and immunological compartments, the gut microbiota is now being recognized for its critical contribution to homeostatic regulation and pathogenic disruption of the intestinal barrier. In this capacity, the gut microbiota provides energy to colonocytes, safeguards epithelial integrity by producing bacteriocins (antibacterial molecules), and regulates mucosal immune responses.Citation5 Collectively, these GI compartments create a functional intestinal barrier that facilitates the exchange of nutrients and metabolites between the host and its environment, and simultaneously manages the entry of pathogens, toxins, and foreign antigens into the body (). Unfortunately, there is a general lack of appreciation for the nuances and dynamic nature of gut permeability in health and disease, particularly with respect to the host–microbiota interactions that govern permeability; hence, our focus is on those details in this review.
Figure 1. Changes in intestinal homeostasis under different conditions.
Intestinal mucus: the frontline of the gut barrier
The entire length of the GI tract is coated with a viscous, thick, and jelly-like substance referred to as mucus that is continuously secreted into the intestinal lumen at a rate of ~10 liters/day.Citation6 As an interface between luminal contents and the intestinal epithelium, mucus not only serves as a physical barrier to protect the intestinal epithelial cell (IEC) monolayer from luminal contents but also aids in nutrient digestion and maintaining a homeostatic relationship with the gut microbiota.Citation7,Citation8
Extensive discussions regarding the composition, regulation, and function of the intestinal mucus layer are the subject of multiple excellent reviewsCitation8–10 and will not be covered in detail here. However, the present discussion necessitates a succinct overview of mucus biochemistry to better illustrate the extent of host–microbiota interactions at the mucus interface. In brief, mucus is composed of glycosylated proteins called mucins. Goblet cells, a type of specialized intestinal epithelial cell, are principally responsible for the synthesis and secretion of mucin.Citation10 Mucin consists of a protein core containing three crucial amino acid residues (proline, threonine, and serine) commonly referred to as a PTS domain.Citation11 The serine and threonine residues substantially undergo O-glycosylation, while the proline facilitates the O-glycosylation process in the Golgi complex.Citation12 O-glycosylation is critical for preservation of the protein core from endogenous protease-dependent degradation and enhances the ability of mucin to bind with water molecules to form a gel.Citation13 Mucins can be broadly classified into either gel-forming or transmembrane mucins based on their architectural and operative characteristics. The secreted gel-forming mucins MUC2, MUC5AC, MUC5B, and MUC6 are the key constituents of intestinal mucus, whereas MUC2 is the predominant mucin of the mucus barrier. MUC1, MUC3, MUC4, MUC13, and MUC17 are the key transmembrane mucins of the intestine expressed on the apical surface of epithelial cells and are responsible for creating a protective barrier between the secreted mucins and the underlying epithelial cells.Citation14,Citation15
The mucus layer plays a critical role in defending against microbial infections by working synergistically with commensal organisms.Citation16 The components of mucus not only form a passive physical barrier and act as adhesion decoys for pathogens, but they also actively produce antimicrobial molecules. For example, α-1-4-linked N-acetyl-glucosamine, a mucin O-glycan, protects the host from Helicobacter pylori infection by inhibiting its cell wall biosynthesis.Citation17 Similarly, MUC7 has inherent direct candidacidal activity that protects it from oral candidiasis.Citation18 Host-derived antimicrobial molecules such as secretory α-defensins, secretory enzymes (i.e., lysozyme), and immunoglobulin A (IgA) are also an integral part of the mucus layer.Citation19 However, if mucin synthesis is aberrant and/or secreted mucins are degraded, then the activity of the antimicrobial molecules is impaired. Consequently, their ability to protect against exposure to luminal antigens is limited and disease may ensue.Citation20–23 Indeed, aberrant mucin expression is a hallmark of multiple human intestinal diseases, including inflammatory bowel diseases (IBD), irritable bowel syndrome (IBS), Celiac Disease (CD), and colorectal cancer (CRC), all of which are also associated with changes in the gut microbiota.Citation24–26
Tight junctions: guardians of intestinal barrier function
Underlying the mucus layer is the intestinal epithelial barrier, a single layer of epithelial cells linked by cell–cell adhesions, including tight junctions (TJs). Owing to their physical location, tight junctions, the most apical cell–cell adhesion complex, are considered key regulators of the epithelial barrier function.Citation27 Overall, the TJ is a complex entity composed of a myriad of proteins, including claudins, occludin, zonula occludens (ZO-1, 2, 3), and junctional adhesion molecules (JAM).Citation28
Many excellent reviews have focused on the details of TJ structure/composition.Citation29–32 In brief, ZO proteins are peripheral membrane phosphoproteins with a molecular mass of 220 KDaCitation33 that are expressed in all epithelial and endothelial cells as well as in cell types lacking TJs.Citation34,Citation35 Thus, while ZO proteins are not integral proteins of the TJ, a complex interdependence among these proteins and other TJ proteins, including claudins and barrier integrity, has been reported.Citation36 Similarly, JAM proteins are also involved in the maintenance of intestinal barrier homeostasis, and JAM-A knockout (KO) mice present with increased barrier permeability, increased bacterial translocation into the mucosa, and elevated numbers of colonic lymphocytes.Citation37 Occludin is another integral TJ protein found in both epithelial and endothelial cells that contributes to TJ stabilization and optimal barrier functions. However, occludin KO mice possess intact TJs but display complex histological phenotypes, thus suggesting that occludin could be involved in TJ maturity and possibly indispensable for TJ formation.Citation38 Notably, a recent study provided evidence that occludin may also help regulate apoptosis in intestinal epithelial cells under inflammatory conditions.Citation39 Yet another study has also demonstrated that occludin is required for apoptosis when claudin–claudin interactions are disrupted.Citation40 Such findings point to a complex role for occludin in maintaining the intestinal barrier by promoting cell survival.
Recent studies, including those from our laboratory, have revealed an integral function for the claudin family of proteins in the regulation of TJ structure and function.Citation41–43 Studies manipulating claudin protein expression both in cell culture and in vivo have shown that these proteins are expressed in a cell- and tissue-specific manner and also regulate cellular functions distinct from their role in barrier function. For example, claudin-1 KO mice die postnatally due to the dysregulation of dermal barrier function.Citation44 In contrast, forced claudin-1 overexpression in the intestinal epithelium results in remarkable changes in epithelial cell homeostasis, including goblet cell loss and susceptibility to colitis and colitis-associated cancer.Citation45,Citation46 Together, these findings suggest that optimal expression of claudin-1 is essential for normal mammalian homeostasis and barrier function.
In contrast to the effects of deletion and overexpression of claudin-1, genetic manipulation of the pore-forming claudin protein family member claudin-2, alters trans-epithelial resistance and paracellular permeability of cations, especially Na+ and Ca++ ions.Citation47 Claudin-2 is upregulated in both IBD and CRC.Citation48,Citation49 Interestingly, studies using murine model of claudin-2 overexpression (which is observed in IBD patients), have shown a maladaptive function for claudin-2 in mice when subjected to colitis-induced by an epithelial irritant (Dextran Sodium Sulfate; DSS) and infectious colitis.Citation47 However, when subjected to colitis induced by the adoptive transfer of activated T cells, the effects of claudin-2 expression contrast with the effects from DSS- or C. rodentium-induced colitis, suggesting a rather complex and context-dependent role of claudin-2 in mucosal inflammation.Citation50
In contrast to claudin-2, claudin-3 is abundantly expressed in the gut epithelium and considered to be a barrier-sealing protein as its loss in mice manifests into a more permeable intestinal barrier, upregulates IL-6/Stat3 signaling, and promotes aggressive colon cancer.Citation51,Citation52 Notably, Clostridium perfringens, one of the most common cause of foodborne illness, uses claudin-3 and 4 as receptors for deregulating gut barrier integrity.Citation53 Studies have also demonstrated a key role for claudin-7 in intestinal epithelial homeostasis, and post-embryonic lethality occurs with genetic deletion of claudin-7 expression primarily due to the loss of cell–cell and cell-matrix adhesions.Citation54,Citation55 Likewise, claudin-15 deficiency not only dysregulates the intestinal paracellular Na+ transport but also results in a “mega-intestine” phenotype characterized by increased intestine length and hyperproliferation in addition to glucose malabsorption.Citation56,Citation57 Remarkably, when mice lacked expression of both claudin-2 and 15, they experienced severe defects in paracellular Na+ permeability, which disrupted nutrient absorption and resulted in death due to malnourishment.Citation58 Both the claudin-2 and −15 are paracellular pores for the passage of Na+, and results from this study also highlighted the critical role of gut barrier integral proteins in regulating Na+/glucose transport and thus the luminal milieu.Citation59 Altogether, the aforementioned studies highlight the complexity of the TJ integral and related proteins as well as their complex role in the maintenance of the intestinal barrier integrity and luminal environment, which may be critical in creating appropriate niches for specific members of the gut microbiota.
The gut microbiome and intestinal barrier function: a new frontier
Studies in developmental biology support a role for host–microbiota interactions in intestinal barrier function as early as birth.Citation60 Establishment of the commensal microbiota shortly after birth prompts the rapid development of a functional intestinal barrier and a subsequent decrease in intestinal permeability.Citation61 Furthermore, the expression of claudin proteins during functional maturation of the gut barrier is dynamic, with high expression of claudin-2 and low expression of the barrier-forming claudins, including claudin-3, 4, 7, and 15, at birth. As the gut barrier matures, expression of claudin-2 is downregulated, while expression of the barrier sealing proteins claudin-3, 7, 15 is upregulated.Citation62 Such a pattern suggests a dynamic relationship between claudin proteins and gut barrier functions.
Integration between the gut microbiota and barrier structure/function is also supported by reports using adult animal models. For instance, short-term antibiotic treatment markedly increased intestinal permeability in rats, which was accompanied by the downregulation of tight junction proteins including claudin-1, ZO-1, and occludin.Citation63 Furthermore, the antibiotic-induced increases in intestinal permeability that are accompanied by reduced claudin-3 expression can be corrected following the administration of probiotics.Citation64 Other signaling pathways are also activated following antibiotic administration that confer deleterious effects on TJ proteins and intestinal permeability, further highlighting a connection between the gut microbiome and barrier function. Notably, alterations in the gut microbiome following antibiotic treatment promote intestinal activation of the NLRP3 inflammasome.Citation65 Similarly, stress induced by environmental or psychological factors also induces gut barrier dysregulation and gut microbial dysbiosis.Citation66,Citation67
Taken together, these studies suggest a potential regulatory feedback mechanism between the intestinal barrier and the gut microbiota. A general postulation is that translocation of commensal microbes or their metabolites, due to a breach in the intestinal barrier, stimulates the mucosal immune systemCitation68 which, in turn, contribute to the dysregulation of TJ expression. Other studies indicate that TJ expression may regulate the gut microbiota. For example, JAM-A KO mice harbor significantly greater levels of Desulfovibrionaceae and less Akkermansia in their gut compared to their wild-type counterparts, indicating a potential role for JAM-A in modulating the composition of the gut microbial community.Citation69,Citation70 Notably, JAM-A KO mice also experience increased barrier permeability and elevated levels of bacterial translocation.Citation37,Citation69 Similarly, gut epithelium-specific overexpression of claudin-1 or knockdown of claudin-3 resulted in dysregulated barrier function and changes in gut microbiota composition (Unpublished data). A recent report has demonstrated a similar effect of claudin-7 loss upon gut permeability and gut microbial dysbiosis.Citation71 Although claudin-2 expression is known to increase gut barrier permeability,Citation72 its effects on the gut microbiota remain to be examined.
As with the intestinal barrier and the microbiota, a similar regulatory feedback mechanism also likely exists specifically between the mucus layer and the gut microbiota. As discussed previously in this review, intestinal mucus trap microbes and water-insoluble antigens to limit their contact with the underlying epithelium and immune cells. Additionally, some beneficial, mucolytic microbes have even adapted to the mucus layer where they can heavily utilize glycosylated mucins as metabolic substrates.Citation73 This relationship, however, represents a double-edged sword as insufficient dietary fiber consumption by the host leads to degradation of the mucus layers as mucolytic species encroach upon the epithelium and increase host susceptibility to inflammation.Citation74 A similar relationship has also been highlighted in studies with Muc2-deficient mice, which spontaneously develop colitis soon after birth due to excessive microbial contact with the colonic epithelium.Citation13,Citation75 However, considering the complex interactions between the gut microbiota and IEC, further detailed mechanistic investigations are required that explain and model the dynamic host-microbe interdependent relationship needed to balance microbial entry, recognition, and immune activation.
Neurohormones: potential regulators of gut microbiota-intestinal barrier dynamics
A specialized group of cells in the intestinal epithelium known as Enterochromaffin cells (EC) produce and secrete melatonin, serotonin, cholecystokinin, and somatostatin.Citation76 These neurotransmitters, in turn, play a crucial role in maintaining homeostasis in the human body,Citation77 and mounting evidence supports the existence of bidirectional communication between the gut and the central nervous system, referred to as the gut-brain-axis.Citation78
A burgeoning literature suggests that serotonin and melatonin, in particular, play an important role in gut barrier function in addition to mediating crosstalk with the gut microbiota.Citation79,Citation80 Of note, serotonin has been shown to play a role in several gastrointestinal diseases, including IBS and IBD.Citation81,Citation82 In this regard, expression of serotonin reuptake transporters (SERT) is suppressed in IBD patients, and re-expression of SERT in genetically deficient mice exacerbates colitis.Citation83 The endogenous production of serotonin appears to be dependent on gut microbiota as germ-free mice have lower basal plasma serotonin compared to the conventional mice.Citation84 Moreover, commensal intestinal bacteria such as Escherichia coli, Enterococcus species, Streptococcus species, and Bifidobacterium infantis have all been shown to modulate 5-hydroxytryptamine (5-HT) levels by increasing plasma levels of its precursor, tryptophan.Citation85 Proximity is likely an important factor in this relationship, as epithelial cells in the GI tract serve as the largest reservoir of serotonin – alternatively, 5-HT in the body.Citation86 Small amounts of 5-HT are transcytosed through the intestinal barrier to enter the systemic circulation and are absorbed by platelets for generalized neuronal functions.Citation87 In a case–control study, the oral administration of 5-HTP, an intermediary serotonin substrate, reduced intestinal permeability, upregulated ZO-1, and remodeled the TJ proteins in healthy individuals but not in IBS patients.Citation88 Together, these findings collectively implicate dysregulated serotonergic signaling in the pathophysiology of gastrointestinal disorders associated with increased gut permeability. However, details of the causal association with the commensal gut bacteria in regulating the serotonergic signaling in human health and disease remain to be understood.
The GI tract is also the primary reservoir for melatonin as the gut contains ~400 times more melatonin compared to the pineal gland.Citation89 Melatonin mediates endocrine, paracrine, and autocrine actions and regulates multiple aspects of GI physiology, including intestinal motility, ion transport mechanisms, and mucosal immune responses, possibly through TLR4-dependent signaling mechanisms.Citation80 A recent study demonstrated that exogenous administration of melatonin to DSS-treated mice significantly improved intestinal barrier function, antimicrobial peptide secretion and wound healing responses in wild-type but not in TLR4 deficient mice.Citation80 The gut microbiota may also mediate some of the anti-inflammatory effects of melatonin.Citation90 In this regard, melatonin can influence the structure of the gut microbiota, including richness and diversity,Citation91 and the abundance of specific species such as Akkermansia, Bacteroides, and Faecalibacterium.Citation92 Melatonin can also prevent obesity by modulating the composition of gut microbiota.Citation93 Together, these findings suggest that mechanistic investigations linking gut-derived neurohormones, the gut microbiota, and intestinal permeability are certainly warranted. Also, additional knowledge about the role of the gut-brain axis in regulating intestinal permeability is essential for improving our understanding of human disease processes and developing effective therapies.
Repairing the rampart: diet–microbiota interactions to the rescue
In recent years, the gut microbiota has emerged as an attractive therapeutic modality to target for multiple acute and chronic conditions. Because host diet is a recognized determinant of gut microbiota ecology and function,Citation94,Citation95 many investigators have turned to examine the effects of dietary components on the microbiota in hopes of better understanding the systemic interactions connecting the microbiota to human health.Citation96 However, given the highly personalized nature of both the microbiome and nutrition, rational, microbiome-targeted dietary interventions with predictable effects on the microbiome remain elusive.Citation97
Despite the complex nature of diet–microbiota interactions, an existing body of literature has extensively documented the effects of diet on the regulation of intestinal barrier function, including permeability of the GI tract.Citation98 Therefore, reevaluating the literature through the lens of the gut microbiome’s contribution to those effects may provide novel insights into the reciprocal mechanisms governing the diet-barrier function-microbiota axis. Moreover, studies examining the effects of specific bacteria, namely probiotics, on intestinal permeability can also suggest mechanisms by which microbial organisms may beneficially regulate host barrier function to improve health through interactions with their host.
Probiotics: regulators of intestinal barrier function
Probiotics are defined by an expert consensus as “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host”.Citation99 Probiotic microbes are typically supplemented in the range of 108–1010 colony forming units (CFU) per gram. In general, these bacteria do not become permanent members of the host microbiota, but the transient increase (direct/indirect) in the abundance of a single species can have a significant effect on collective intestinal barrier function.Citation100 However, the precise mechanisms by which probiotics enhance intestinal barrier function are complex, and research supports species- and even strain-specific effects of probiotics on host health.
The capacity of probiotic organisms to participate in communal short chain fatty acid (SCFA) production is one mechanism by which probiotics are generally considered to promote health. Namely, Bifidobacterium and lactic acid bacteria (LAB), such as Lactobacillus and Streptococcus species, provide lactic acid and acetate substrates for butyrate production in a community metabolic reaction referred to as the “bif(id)-shunt”.Citation101,Citation102 Although these species only transiently occupy the GI tract, they maintain much of their metabolic activity during transit through the harsh conditions of the upper GI tract.Citation103 Consequently, their ability to promote SCFA synthesis and butyrogenic gut microbial communities can regulate intestinal barrier functions through mechanisms similar to those mentioned above.
SCFA production alone, however, is insufficient to explain the therapeutic benefits and strain-specific health benefits of probiotic supplementation – molecular organization of the microbial cell surface is also an important aspect of probiotic functions. This concept has been extensively studied for Lactobacillus species and may account for some strain-level differences observed in the probiotic literature.Citation104 For example, lipoteichoic acids, which are anionic, polymeric units linked to peptidoglycan, can direct the organization of intestinal TJ via binding to TLR2 on the apical IEC membrane. TLR2 stimulation in the Caco-2 cell line induced phosphorylation of PKC in a PI3K/Akt/mTORC1-dependent manner, subsequently promoting ZO-1 translocation to TJ.Citation105 A similar effect has also been observed following Lactobacillus treatment in human volunteers.Citation106 In support of such mechanism, one study reported that heat-killed L. rhamnosus was sufficient to prevent DSS-induced intestinal permeability in vivo.Citation107 L. plantarum WCFS1 also promoted organization of TJ in healthy humans and Caco-2 cells.Citation106 Thus, it appears likely that some combination of metabolism and strain-specific variations in cell wall organization may be necessary to explain the benefits of LAB probiotics on intestinal barrier function.
The probiotic, Escherichia coli Nissle 1917 (EcN), has a rich history of use in human gastrointestinal disease that spans a century.Citation108 Several studies support the notion that EcN regulates IEC-specific functions to control intestinal TJ functions. The first in vivo study to examine EcN influence on intestinal TJ reported that germ-free mice mono-colonized with EcN had higher baseline mRNA and protein expression of ZO-1 and that intragastric supplementation of EcN to conventional mice prevented DSS-mediated increases in intestinal permeability.Citation109 Another study found that EcN increased the trans-epithelial resistance (TEER) of intact T84 colon cells and restored their barrier function following infection with a strain of enteropathogenic E. coli (EPEC).Citation110
The specific components of EcN that propagate the signals regulating tight junction components are not yet clear. As with other probiotics, immunomodulatory ligands expressed on the outer cell membrane are likely partially responsible for the barrier function enhancing effects of EcN. For instance, flagellin expressed by EcN promotes the secretion of antimicrobial peptides by IEC in vitro.Citation111 However, the supernatant from a mutant E. coli Nissle strain lacking flagellin had no effect on TJ function in T84 cells.Citation112 Rather, the TIR domain-containing protein encoded by the gene TcpC in E. coli has been identified as at least partially responsible for TJ modulating effects of EcN.Citation112,Citation113 Although the presence of TcpC is generally associated with bacterial pathogens, these studies suggest that the immunogenic effects of TcpC in E. coli may be contextual.Citation114 Furthermore, supernatants or isolated outer membrane vesicles from EcN, of which TcpC may be a component, are sufficient to increase TEER and counteract the effects of EPEC on TJ.Citation112,Citation113,Citation115 In conclusion, these molecular investigations not only increase our understanding of how probiotics interact with IECs to promote barrier function but also provide valuable insights to guide the future design and selection of live biotherapeutics that benefit human health.
Fermentable fibers, the gut microbiota, and intestinal permeability
Fermentation of dietary biomolecules by commensal gut bacteria accumulates a wealth of metabolic byproducts that serve as signaling molecules to direct host-microbiota cross-talk as well as regulate gut microbial ecology and function.Citation116 Although the aforementioned neurohormones and their substrates are among these metabolites, SCFA derived from fermentation of complex carbohydrates (i.e., dietary fiber) are the most abundant and widely studied microbial metabolites. Among their many functions, the SCFA butyrate, propionate, and acetate are important regulators of intestinal barrier function through their direct activity on enterocyte biology as well as peripheral anti-inflammatory effects.
It has been widely reported that the biological activity of microbiota-derived SCFA on colonocytes is linked with activation of the 5’ adenosine monophosphate activated kinase (AMPK). AMPK activation is well-recognized to promote intestinal barrier function, either by inducing the differentiation of intestinal epithelial cells or as mediators of cellular signaling in differentiated enterocytes.Citation117–119 Evidence suggests that the induction of AMPK by SCFA is essential to their barrier-enhancing functions. For example, butyrate has been shown to facilitate the association between transcription factors and the claudin-1 promoter to increase AMPK activity and reduce bacterial translocation. Supplementing butyrate to monolayers of Caco-2 cells also promotes re-localization of ZO-1 to the paracellular junction in a calcium- and AMPK-dependent manner.Citation120 Interestingly, acetate and propionate can also activate AMPK and decrease the paracellular permeability of Caco-2 monolayers in this model.Citation121 However, AMPK inhibitors and siRNA-mediated inhibition of AMPKα only partially abrogated the effects of SCFA on intestinal permeability, suggesting that other AMPK-independent mechanisms contribute to this mechanism.
Independent of AMPK, evidence also suggests that SCFA regulates IEC barrier functions through their respective apical-free fatty acid receptors (FFARs). Three de-orphaned GPCRs – GPR41, GPR43, and GPR109A – have been identified as SCFA receptors and are essential in regulating the biology of IECs.Citation122 A study reported that physiological concentrations of SCFA increased TEER and decreased paracellular permeability of Ussing chamber mounted rat cecal tissues within 10 min of application independently of SCFA transport and metabolism. This finding suggests that intestinal permeability is regulated by SCFA through interactions with membrane-bound, extracellular receptors rather than as a metabolic substrate.Citation123 Furthermore, butyrate has been reported to increase the expression of claudin-3 in Caco-2 cells in a GPR109a-dependent mechanism.Citation124 In line with this finding, others have confirmed that the presence of either GPR43 or GPR109a SCFA receptors on colonocytes is required for the anti-inflammatory benefits of a high-fiber diet in the context of a chemically induced colitis injury, where loss of barrier integrity is a major pathophysiological mechanism.Citation125 The SCFA-mediated activation of AMPK has not yet been investigated in the context of FFAR, thus it is possible that AMPK is activated downstream of FFAR induction. Given the reported global benefits of SCFA for intestinal barrier functions, including the regulation of intestinal permeability, thorough mechanistic investigations are essential for our understanding of the relationship between these gut microbiota-derived metabolites and human health.
Insoluble fibers can also regulate intestinal permeability
Although generally considered non-fermentable, it is worth noting that insoluble fiber can modulate gut microbiome composition and affect host physiology via both microbiome-dependent and -independent mechanisms.Citation126 Dietary insoluble fiber is derived primarily from the structural components of plant cell walls as cellulose and complexed lignin polymers.Citation127 Gut health benefits of dietary cellulose were historically believed to be limited to increasing fecal bulk and accelerating GI transit.Citation128 Although cellulose’s effects on GI transit are microbiome-independent, cellulose-mediated changes in intestinal motility were associated with alterations in the microbiome composition of humanized mice.Citation129 Moreover, microbiome-dependent effects of cellulose on intestinal health have now been reported in the literature, often conferring some health benefits in such experiments.Citation130–132 In one notable study of septicemic mice, feeding a high cellulose diet (20% w/w) increased expression of claudin-1 by two-fold and occludin by more than three-fold compared to feeding a low cellulose diet (5% w/w) prior to the onset of severe IEC apoptosis.Citation130 The authors further noted that mice consuming a high cellulose diet experienced an increase in the abundance of Akkermansia muciniphilia in their feces. An expansion of A. muciniphilia was also observed in C57BL6/J mice fed with 30% cellulose diet for 12 weeks. In this case, the high cellulose diet protected mice from DSS colitis when compared to low-cellulose (5% w/w) and normal chow (6% crude fiber) diets.Citation132 In both studies, treating animals with antibiotics abolished these effects, thereby supporting a microbiome-dependent health effect for cellulose consumption. Furthermore, the association among cellulose, A. muciniphilia, and TJ protein regulation is novel, and certainly challenges preconceived notions concerning the functional activities of dietary cellulose.
The claims of beneficial and microbiome-dependent effects of cellulose consumption may be considered controversial, as cellulose is widely considered “inert” as a fermentative substrate and is frequently used as a control in experimental nutrition research where dietary fiber is involved.Citation133 This controversy is compounded by reports from several groups in which mice consuming experimental diets devoid of fermentable fiber develop an abnormal gut physiology characterized by severely atrophied colons and ceca as well as an increased susceptibility to DSS colitis.Citation134–138 Moreover, mice fed high cellulose diets secrete fewer antimicrobial peptides, thereby promoting encroachment of bacteria toward epithelial cells.Citation135 Still others have reported that mice consuming a high cellulose (15% w/w) diet for only one-week experienced increased intestinal permeability compared to mice consuming a high soluble fiber diet (15% psyllium fiber, w/w) and concluded that this effect was microbiome-dependent.Citation139 These discordant results collectively suggest a failure to recognize the totality of the physiological effects of cellulose consumption and that future nutrition studies would benefit from considering the influence of insoluble fiber in their models.
Conclusions and future directions
Mounting evidence supports that regulation of GI homeostasis is complex and dynamic. In current review article, we have specifically discussed the regulation of intestinal barrier functions and the likely critical role of the gut microbiota in maintaining intestinal homeostasis (). In addition, we have discussed the role of the diet in regulating these attributes in the context of human health and disease. In discussing these key relationships, we have also stressed upon the canonical and non-canonical functions of the barrier integral and associated proteins and possible impact of such functions upon gut barrier integrity and microbiota. However, during these deliberations, we have also noted the limitations of the current knowledge regarding gut barrier dysfunction and microbiota-related therapeutic interventions, due primarily to the plasticity of observed associated changes. For example, despite the well-recognized role of increased gut permeability in promoting inflammation, defined probes to accurately gauge the extent of gut permeability of pathological significance are lacking. The same is true for the gut microbiota since there are distinct microbiota colonization patterns specific to the various portions of GI tract that are not likely reflected in the stool samples frequently used to assess microbiota changes. Similarly, studies performed in mice or other mammals regarding the effects of diet upon intestinal homeostasis may not recapitulate outcomes in human studies due to differences in dietary habits and genotypes.
Thus, a closer (and deeper) look at the dynamics of gut microbiome changes in causal association with epithelial permeability is needed, which requires refined animal models and tools. However, animal models where host-microbe interdependence can be studied/modeled in a dynamic manner remains a major bottleneck in such an endeavor, though mice modified for specific barrier integral protein/s expression in a gut epithelium-specific manner hold great promise to provide novel insights into these causal relationships. Nonetheless, the genetic manipulation of animal models of barrier protein function carries its own caveats depending on the nature of gene manipulation (constitutive/inducible) and fails to offer an opportunity to examine any compensatory changes in other barrier proteins and/or epithelial or immune homeostasis.
As noted in this review, diet, including consumption of prebiotics and probiotics, has a tremendous impact upon both gut microbiome composition and barrier function, and thus offers an additional line of investigation into novel approaches to influencing interactions between gut microbes and the intestinal barrier. However, the utility of probiotics as a therapeutic modality also remains uncertain as these supplements are currently regulated by the FDA. Moreover, the question remains whether dietary control alone can serve as a therapy or play a supporting role in maintaining GI homeostasis. In our current times of fast-changing dietary habits and notable increases in the incidence of colon cancer and other intestinal diseases, particularly in young adults,Citation140 a deeper examination of the causal relationships between diet, the gut microbiome, and intestinal permeability is clearly warranted as a way to provide valuable knowledge for a healthier future.
Acknowledgments
Biorender.com was used to create the illustration.
Disclosure statement
There are no potential conflicts of interest to disclose.
Additional information
Funding
References
- Karl JP, Margolis LM, Madslien EH, Murphy NE, Castellani JW, Gundersen Y, Hoke AV, Levangie MW, Kumar R, Chakraborty N, et al. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress. Am J Physiol Gastrointest Liver Physiol. 2017;312(6):97–113. doi:10.1152/ajpgi.00066.2017.
- Bischoff SC, Barbara, G., Buurman, W., Ockhuizen, T., Schulzke, J.D., Serino, M., Tilg, H., Watson, A. and Wells, J.M, et al. Intestinal permeability–a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189. doi:10.1186/s12876-014-0189-7.
- Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9(11):799–809. doi:10.1038/nri2653.
- Dave M, Papadakis KA, Faubion WA Jr. Immunology of inflammatory bowel disease and molecular targets for biologics. Gastroenterol Clin North Am. 2014;43(3):405–424. doi:10.1016/j.gtc.2014.05.003.
- Hayes CL, Dong J, Galipeau HJ, Jury J, McCarville J, Huang X, Wang X-Y, Naidoo A, Anbazhagan AN, Libertucci J, et al. Commensal microbiota induces colonic barrier structure and functions that contribute to homeostasis. Sci Rep. 2018;8(1):14184. doi:10.1038/s41598-018-32366-6.
- Ensign LM, Cone R, Hanes J. Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv Drug Deliv Rev. 2012;64(6):557–570. doi:10.1016/j.addr.2011.12.009.
- Schneider H, Pelaseyed T, Svensson F, Johansson MEV. Study of mucin turnover in the small intestine by in vivo labeling. Sci Rep. 2018;8(1):5760. doi:10.1038/s41598-018-24148-x.
- Herath M, Hosie, S., Bornstein, J.C., Franks, A.E and Hill-Yardin, E.L, et al. The role of the gastrointestinal mucus system in intestinal homeostasis: implications for neurological disorders. Front Cell Infect Microbiol. 2020;10:248. doi:10.3389/fcimb.2020.00248.
- Cornick S, Tawiah A, Chadee K. Roles and regulation of the mucus barrier in the gut. Tissue Barriers. 2015;3(1–2):e982426. doi:10.4161/21688370.2014.982426.
- Birchenough GM, Johansson ME, Gustafsson JK, Bergström JH, Hansson GC. New developments in goblet cell mucus secretion and function. Mucosal Immunol. 2015;8(4):712–719. doi:10.1038/mi.2015.32.
- Lang T, Hansson GC, Samuelsson T. Gel-forming mucins appeared early in metazoan evolution. Proc Natl Acad Sci U S A. 2007;104(41):16209–16214. doi:10.1073/pnas.0705984104.
- Sommer F, Adam N, Johansson MEV, Xia L, Hansson GC, Bäckhed F. Altered mucus glycosylation in core 1 O-glycan-deficient mice affects microbiota composition and intestinal architecture. PLoS One. 2014;9(1):e85254. doi:10.1371/journal.pone.0085254.
- Macierzanka A, Mackie AR, Krupa L. Permeability of the small intestinal mucus for physiologically relevant studies: impact of mucus location and ex vivo treatment. Sci Rep. 2019;9(1):17516. doi:10.1038/s41598-019-53933-5.
- Ermund A, Schütte A, Johansson MEV, Gustafsson JK, Hansson GC. Studies of mucus in mouse stomach, small intestine, and colon. I. Gastrointestinal mucus layers have different properties depending on location as well as over the peyer’s patches. Am J Physiol Gastrointest Liver Physiol. 2013;305(5):G341–7. doi:10.1152/ajpgi.00046.2013.
- Fontaine N, Meslin JC, Lory S, Andrieux C. Intestinal mucin distribution in the germ-free rat and in the heteroxenic rat harbouring a human bacterial flora: effect of inulin in the diet. Br J Nutr. 1996;75(6):881–892. doi:10.1079/BJN19960194.
- Willemsen LE, Koetsier, M.A., Van Deventer, S.J.H and Van Tol, E.A.F, et al. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E(1) and E(2) production by intestinal myofibroblasts. Gut. 2003;52(10):1442–1447. doi:10.1136/gut.52.10.1442.
- Kawakubo M, Ito Y, Okimura Y, Kobayashi M, Sakura K, Kasama S, Fukuda MN, Fukuda M, Katsuyama T, Nakayama J. Natural antibiotic function of a human gastric mucin against helicobacter pylori infection. Science. 2004;305(5686):1003–1006. doi:10.1126/science.1099250.
- Gururaja TL, Levine JH, Tran DT, Naganagowda GA, Ramalingam K, Ramasubbu N, Levine MJ. Candidacidal activity prompted by N-terminus histatin-like domain of human salivary mucin (MUC7)1. Biochim Biophys Acta. 1999;1431(1):107–119. doi:10.1016/S0167-4838(99)00034-5.
- Muniz LR, Knosp C, Yeretssian G. Intestinal antimicrobial peptides during homeostasis, infection, and disease. Front Immunol. 2012;3:310. doi:10.3389/fimmu.2012.00310.
- Li H, Limenitakis JP, Fuhrer T, Geuking MB, Lawson MA, Wyss M, Brugiroux S, Keller I, Macpherson JA, Rupp S, et al. The outer mucus layer hosts a distinct intestinal microbial niche. Nat Commun. 2015;6:8292. doi:10.1038/ncomms9292.
- Pereira RT, Nebo C, Paula Naves L, Fortes‐Silva R, Regina Cardoso de Oliveira I, Paulino RR, Drummond CD, Rosa PV. Distribution of goblet and endocrine cells in the intestine: a comparative study in Amazonian freshwater Tambaqui and hybrid catfish. J Morphol. 2020;281(1):55–67. doi:10.1002/jmor.21079.
- Johansson ME, Larsson JM, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci U S A. 2011;108 Suppl 1:4659–4665. doi:10.1073/pnas.1006451107.
- Johansson ME, Jakobsson H, Holmén-Larsson J, Schütte A, Ermund A, Rodríguez-Piñeiro A, Arike L, Wising C, Svensson F, Bäckhed F, et al. Normalization of host intestinal mucus layers requires long-term microbial colonization. Cell Host Microbe. 2015;18(5):582–592. doi:10.1016/j.chom.2015.10.007.
- Moehle C, Ackermann N, Langmann T, Aslanidis C, Kel A, Kel-Margoulis O, Schmitz-Madry A, Zahn A, Stremmel W, Schmitz G, et al. Aberrant intestinal expression and allelic variants of mucin genes associated with inflammatory bowel disease. J Mol Med (Berl). 2006;84(12):1055–1066. doi:10.1007/s00109-006-0100-2.
- Fang J, Wang H, Zhou Y, Zhang H, Zhou H, Zhang X. Slimy partners: the mucus barrier and gut microbiome in ulcerative colitis. Exp Mol Med. 2021;53(5):772–787. doi:10.1038/s12276-021-00617-8.
- Coleman OI, Haller D. Microbe-Mucus interface in the pathogenesis of colorectal cancer. Cancers (Basel). 2021;13(4):616. doi:10.3390/cancers13040616.
- Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375–412. doi:10.1083/jcb.17.2.375.
- Heinemann U, Schuetz A. Structural features of tight-junction proteins. Int J Mol Sci. 2019;20(23):6020. doi:10.3390/ijms20236020.
- Vermette D, Hu P, Canarie MF, Funaro M, Glover J, Pierce RW. Tight junction structure, function, and assessment in the critically ill: a systematic review. Intensive Care Med Exp. 2018;6(1):37. doi:10.1186/s40635-018-0203-4.
- Alizadeh A, Akbari P, Garssen J, Fink-Gremmels J, Braber S. Epithelial integrity, junctional complexes, and biomarkers associated with intestinal functions. Tissue Barriers. 2021;1996830. doi:10.1080/21688370.2021.1996830.
- Tsukita S, Tanaka H, Tamura A. The claudins: from tight junctions to biological systems. Trends Biochem Sci. 2019;44(2):141–152. doi:10.1016/j.tibs.2018.09.008.
- Van Itallie CM, Anderson JM. Architecture of tight junctions and principles of molecular composition. Semin Cell Dev Biol. 2014;36:157–165. doi:10.1016/j.semcdb.2014.08.011.
- Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986;103(3):755–766. doi:10.1083/jcb.103.3.755.
- Petrov T, Howarth AG, Krukoff TL, Stevenson BR. Distribution of the tight junction-associated protein ZO-1 in circumventricular organs of the CNS. Brain Res Mol Brain Res. 1994;21(3–4):235–246. doi:10.1016/0169-328X(94)90254-2.
- Howarth AG, Hughes MR, Stevenson BR. Detection of the tight junction-associated protein ZO-1 in astrocytes and other nonepithelial cell types. Am J Physiol. 1992;262(2 Pt 1):C461–9. doi:10.1152/ajpcell.1992.262.2.C461.
- Van Itallie CM, Gambling TM, Carson JL, Anderson JM. Palmitoylation of claudins is required for efficient tight-junction localization. J Cell Sci. 2005;118(Pt 7):1427–1436. doi:10.1242/jcs.01735.
- Khounlotham M, Kim W, Peatman E, Nava P, Medina-Contreras O, Addis C, Koch S, Fournier B, Nusrat A, Denning T, et al. Compromised intestinal epithelial barrier induces adaptive immune compensation that protects from colitis. Immunity. 2012;37(3):563–573. doi:10.1016/j.immuni.2012.06.017.
- Saitou M, Furuse M, Sasaki H, Schulzke J-D, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11(12):4131–4142. doi:10.1091/mbc.11.12.4131.
- Kuo WT, Shen L, Zuo L, Shashikanth N, Ong MLDM, Wu L, Zha J, Edelblum KL, Wang Y, Wang Y, et al. Inflammation-induced occludin downregulation limits epithelial apoptosis by suppressing caspase-3 expression. Gastroenterology. 2019;157(5):1323–1337. doi:10.1053/j.gastro.2019.07.058.
- Beeman N, Webb PG, Baumgartner HK. Occludin is required for apoptosis when claudin-claudin interactions are disrupted. Cell Death Dis. 2012;3:e273. doi:10.1038/cddis.2012.14.
- Morita K, Furuse M, Fujimoto K, Tsukita S. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc Natl Acad Sci U S A. 1999;96(2):511–516. doi:10.1073/pnas.96.2.511.
- Tsukita S, Furuse M. The structure and function of claudins, cell adhesion molecules at tight junctions. Ann N Y Acad Sci. 2000;915:129–135. doi:10.1111/j.1749-6632.2000.tb05235.x.
- Turner JR, Rill BK, Carlson SL, Carnes D, Kerner R, Mrsny RJ, Madara JL. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am J Physiol. 1997;273(4):C1378–85. doi:10.1152/ajpcell.1997.273.4.C1378.
- Tokumasu R, Yamaga K, Yamazaki Y, Murota H, Suzuki K, Tamura A, Bando K, Furuta Y, Katayama I, Tsukita S, et al. Dose-dependent role of claudin-1 in vivo in orchestrating features of atopic dermatitis. Proc Natl Acad Sci U S A. 2016;113(28):E4061–8. doi:10.1073/pnas.1525474113.
- Pope JL, Ahmad R, Bhat AA, Washington MK, Singh AB, Dhawan P. Claudin-1 overexpression in intestinal epithelial cells enhances susceptibility to adenomatous polyposis coli-mediated colon tumorigenesis. Mol Cancer. 2014;13:167. doi:10.1186/1476-4598-13-167.
- Singh AB, Sharma A, Dhawan P. Claudin-1 expression confers resistance to anoikis in colon cancer cells in a Src-dependent manner. Carcinogenesis. 2012;33(12):2538–2547. doi:10.1093/carcin/bgs275.
- Ahmad R, Chaturvedi R, Olivares-Villagómez D, Habib T, Asim M, Shivesh P, Polk DB, Wilson KT, Washington MK, Van Kaer L, et al. Targeted colonic claudin-2 expression renders resistance to epithelial injury, induces immune suppression, and protects from colitis. Mucosal Immunol. 2014;7(6):1340–1353. doi:10.1038/mi.2014.21.
- Dhawan P, Ahmad R, Chaturvedi R, Smith JJ, Midha R, Mittal MK, Krishnan M, Chen X, Eschrich S, Yeatman TJ, et al. Claudin-2 expression increases tumorigenicity of colon cancer cells: role of epidermal growth factor receptor activation. Oncogene. 2011;30(29):3234–3247. doi:10.1038/onc.2011.43.
- Randall K, Henderson, N., Reens, J., Eckersley, S., Nyström, A.C., South, M.C., Balendran, C.A., Böttcher, G., Hughes, G. and Price, S.A, et al. Claudin-2 expression levels in ulcerative colitis: development and validation of an in-Situ hybridisation assay for therapeutic studies. PLoS One. 2016;11(9):e0162076. doi:10.1371/journal.pone.0162076.
- Raju P, Shashikanth N, Tsai P-Y, Pongkorpsakol P, Chanez-Paredes S, Steinhagen PR, Kuo W-T, Singh G, Tsukita S, Turner JR, et al. Inactivation of paracellular cation-selective claudin-2 channels attenuates immune-mediated experimental colitis in mice. J Clin Invest. 2020;130(10):5197–5208. doi:10.1172/JCI138697.
- Thuijls G, Derikx JP, de Haan JJ, Grootjans J, de Bruine A, Masclee AA, et al. Urine-based detection of intestinal tight junction loss. J Clin Gastroenterol. 2010;44(1):e14–9. doi:10.1097/MCG.0b013e31819f5652.
- Ahmad R, Kumar B, Chen Z, Chen X, Müller D, Lele SM, Washington MK, Batra SK, Dhawan P, Singh AB, et al. Loss of claudin-3 expression induces IL6/gp130/Stat3 signaling to promote colon cancer malignancy by hyperactivating Wnt/beta-catenin signaling. Oncogene. 2017;36(47):6592–6604. doi:10.1038/onc.2017.259.
- Romanov V, Whyard TC, Waltzer WC, Gabig TG. A claudin 3 and claudin 4-targeted Clostridium perfringens protoxin is selectively cytotoxic to PSA-producing prostate cancer cells. Cancer Lett. 2014;351(2):260–264. doi:10.1016/j.canlet.2014.06.009.
- Lopardo T, Lo Iacono N, Marinari B, Giustizieri ML, Cyr DG, Merlo, G. Claudin-1 is a p63 target gene with a crucial role in epithelial development. PLoS One. 2008;3(7):e2715. doi:10.1371/journal.pone.0002715.
- Khairallah H, El Andalousi J, Simard A, Haddad N, Chen Y-H, Hou J, Ryan AK, Gupta IR. Claudin-7, −16, and −19 during mouse kidney development. Tissue Barriers. 2014;2(4):e964547. doi:10.4161/21688362.2014.964547.
- Tamura A, Kitano Y, Hata M, Katsuno T, Moriwaki K, Sasaki H, Hayashi H, Suzuki Y, Noda T, Furuse M, et al. Megaintestine in claudin-15-deficient mice. Gastroenterology. 2008;134(2):523–534. doi:10.1053/j.gastro.2007.11.040.
- Tamura A, Hayashi H, Imasato M, Yamazaki Y, Hagiwara A, Wada M, Noda T, Watanabe M, Suzuki Y, Tsukita S, et al. Loss of claudin-15, but not claudin-2, causes Na+ deficiency and glucose malabsorption in mouse small intestine. Gastroenterology. 2011;140(3):913–923. doi:10.1053/j.gastro.2010.08.006.
- Wada M, Tamura A, Takahashi N, Tsukita S. Loss of claudins 2 and 15 from mice causes defects in paracellular Na+ flow and nutrient transport in gut and leads to death from malnutrition. Gastroenterology. 2013;144(2):369–380. doi:10.1053/j.gastro.2012.10.035.
- Thorsen K, Drengstig T, Ruoff P. Transepithelial glucose transport and Na+/K+ homeostasis in enterocytes: an integrative model. Am J Physiol Cell Physiol. 2014;307(4):C320–37. doi:10.1152/ajpcell.00068.2013.
- Reyman M, van Houten MA, van Baarle D, Bosch AATM, Man WH, Chu MLJN, Arp K, Watson RL, Sanders EAM, Fuentes S, et al. Impact of delivery mode-associated gut microbiota dynamics on health in the first year of life. Nat Commun. 2019;10(1):4997. doi:10.1038/s41467-019-13014-7.
- Rojo D, Méndez-García C, Raczkowska BA, Bargiela R, Moya A, Ferrer M, Barbas C. Exploring the human microbiome from multiple perspectives: factors altering its composition and function. FEMS Microbiol Rev. 2017;41(4):453–478. doi:10.1093/femsre/fuw046.
- Hill DR, Huang S, Nagy MS, Yadagiri VK, Fields C, Mukherjee D, Bons B, Dedhia PH, Chin AM, Tsai YH, Thodla S, et al. Bacterial colonization stimulates a complex physiological response in the immature human intestinal epithelium. Elife. 2017;6. e29132
- Tulstrup MV, Christensen EG, Carvalho V, Linninge C, Ahrné S, Højberg O, Licht TR, Bahl MI. Antibiotic treatment affects intestinal permeability and gut microbial composition in Wistar rats dependent on antibiotic class. PLoS One. 2015;10(12):e0144854. doi:10.1371/journal.pone.0144854.
- Patel RM, Myers LS, Kurundkar AR, Maheshwari A, Nusrat A, Lin PW. Probiotic bacteria induce maturation of intestinal claudin 3 expression and barrier function. Am J Pathol. 2012;180(2):626–635. doi:10.1016/j.ajpath.2011.10.025.
- Feng Y, Huang Y, Wang Y, Wang P, Song H, Wang F. Antibiotics induced intestinal tight junction barrier dysfunction is associated with microbiota dysbiosis, activated NLRP3 inflammasome and autophagy. PLoS One. 2019;14(6):e0218384. doi:10.1371/journal.pone.0218384.
- Pearce SC, Mani V, Boddicker RL, Johnson JS, Weber TE, Ross JW, Rhoads RP, Baumgard LH, Gabler NK, et al. Heat stress reduces intestinal barrier integrity and favors intestinal glucose transport in growing pigs. PLoS One. 2013;8(8):e70215. doi:10.1371/journal.pone.0070215.
- Madison A, Kiecolt-Glaser JK. Stress, depression, diet, and the gut microbiota: human-bacteria interactions at the core of psychoneuroimmunology and nutrition. Curr Opin Behav Sci. 2019;28:105–110. doi:10.1016/j.cobeha.2019.01.011.
- Stacy A, Andrade-Oliveira V, McCulloch JA, Hild B, Oh JH, Perez-Chaparro PJ, Sim CK, Lim AI, Link VM, Enamorado M, et al. Infection trains the host for microbiota-enhanced resistance to pathogens. Cell. 2021;184(3):615–627 e17. doi:10.1016/j.cell.2020.12.011.
- Laukoetter MG, Nava P, Lee WY, Severson EA, Capaldo CT, Babbin BA, Williams IR, Koval M, Peatman E, Campbell JA, et al. JAM-A regulates permeability and inflammation in the intestine in vivo. J Exp Med. 2007;204(13):3067–3076. doi:10.1084/jem.20071416.
- Rahman K, Desai C, Iyer SS, Thorn NE, Kumar P, Liu Y, Smith T, Neish AS, Li H, Tan S, et al. Loss of junctional adhesion molecule a promotes severe steatohepatitis in mice on a diet high in saturated fat, fructose, and cholesterol. Gastroenterology. 2016;151(4):733–746 e12. doi:10.1053/j.gastro.2016.06.022.
- Ding Y, Wang K, Xu C, Hao M, Li H, Ding L. Intestinal Claudin-7 deficiency impacts the intestinal microbiota in mice with colitis. BMC Gastroenterol. 2022;22(1):24. doi:10.1186/s12876-022-02100-8.
- Krug SM, Schulzke JD, Fromm M. Tight junction, selective permeability, and related diseases. Semin Cell Dev Biol. 2014;36:166–176. doi:10.1016/j.semcdb.2014.09.002.
- Derrien M, van Passel MWJ, van de Bovenkamp JHB, Schipper R, de Vos W, Dekker J. Mucin-bacterial interactions in the human oral cavity and digestive tract. Gut Microbes. 2010;1(4):254–268. doi:10.4161/gmic.1.4.12778.
- Desai MS, Seekatz AM, Koropatkin NM, Kamada N, Hickey CA, Wolter M, Pudlo NA, Kitamoto S, Terrapon N, Muller A, et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 2016;167(5):1339–1353 e21. doi:10.1016/j.cell.2016.10.043.
- Fishman JE, Levy G, Alli V, Zheng X, Mole DJ, Deitch EA. The intestinal mucus layer is a critical component of the gut barrier that is damaged during acute pancreatitis. Shock. 2014;42(3):264–270. doi:10.1097/SHK.0000000000000209.
- Braun T, Voland P, Kunz L, Prinz C, Gratzl M. Enterochromaffin cells of the human gut: sensors for spices and odorants. Gastroenterology. 2007;132(5):1890–1901. doi:10.1053/j.gastro.2007.02.036.
- Mittal R, Debs LH, Patel AP, Nguyen D, Patel K, O’Connor G, Grati MH, Mittal J, Yan D, Eshraghi AA, Deo SK, et al. Neurotransmitters: the critical modulators regulating gut-brain axis. J Cell Physiol. 2017;232(9):2359–2372.
- Carabotti M, Scirocco, A., Maselli, M.A and Severi, C, et al. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28(2):203–209.
- Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011;108(7):3047–3052. doi:10.1073/pnas.1010529108.
- Kim SW, Kim S, Son M, Cheon JH, Park YS. Melatonin controls microbiota in colitis by goblet cell differentiation and antimicrobial peptide production through Toll-like receptor 4 signalling. Sci Rep. 2020;10(1):2232. doi:10.1038/s41598-020-59314-7.
- Crowell MD. Role of serotonin in the pathophysiology of the irritable bowel syndrome. Br J Pharmacol. 2004;141(8):1285–1293. doi:10.1038/sj.bjp.0705762.
- den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud D-J, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54(9):2325–2340. doi:10.1194/jlr.R036012.
- Haub S, Ritze Y, Bergheim I, Pabst O, Gershon MD, Bischoff SC. Enhancement of intestinal inflammation in mice lacking interleukin 10 by deletion of the serotonin reuptake transporter. Neurogastroenterol Motil. 2010;22(7):826–34, e229. doi:10.1111/j.1365-2982.2010.01479.x.
- De Vadder F, Grasset E, Mannerås Holm L, Karsenty G, Macpherson AJ, Olofsson LE, Bäckhed F. Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc Natl Acad Sci U S A. 2018;115(25):6458–6463. doi:10.1073/pnas.1720017115.
- Yano JM, Yu K, Donaldson G, Shastri G, Ann P, Ma L, Nagler C, Ismagilov R, Mazmanian S, Hsiao E, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161(2):264–276. doi:10.1016/j.cell.2015.02.047.
- Mawe GM, Hoffman JM. Serotonin signalling in the gut–functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10(8):473–486. doi:10.1038/nrgastro.2013.105.
- Gwynne RM, Clarke AJ, Furness JB, Bornstein JC. Both exogenous 5-HT and endogenous 5-HT, released by fluoxetine, enhance distension evoked propulsion in Guinea-pig ileum in vitro. Front Neurosci. 2014;8:301. doi:10.3389/fnins.2014.00301.
- Keszthelyi D, Troost FJ, Jonkers DM, van Eijk HM, Lindsey PJ, Dekker J, Buurman WA, Masclee AAM. Serotonergic reinforcement of intestinal barrier function is impaired in irritable bowel syndrome. Aliment Pharmacol Ther. 2014;40(4):392–402. doi:10.1111/apt.12842.
- Kvetnoy IM, Ingel IE, Kvetnaia TV, Malinovskaya NK, Rapoport SI, Raikhlin NT, Trofimov AV, Yuzhakov VV. Gastrointestinal melatonin: cellular identification and biological role. Neuro Endocrinol Lett. 2002;23:121–132.
- Zhu D, Ma Y, Ding S, Jiang H, Fang J. Effects of Melatonin on Intestinal Microbiota and Oxidative Stress in Colitis Mice. Biomed Res Int. 2018;2018:2607679. doi:10.1155/2018/2607679.
- Xu P, Wang, J., Hong, F., Wang, S., Jin, X., Xue, T., Jia, L and Zhai, Y, et al. Melatonin prevents obesity through modulation of gut microbiota in mice. J Pineal Res. 2017;62(4). e12399
- Gao T, Wang, Z., Dong, Y., Cao, J., Lin, R., Wang, X., Yu, Z. and Chen, Y., et al. Role of melatonin in sleep deprivation-induced intestinal barrier dysfunction in mice. J Pineal Res. 2019;67(1):e12574. doi:10.1111/jpi.12574.
- Porcelli P, Leoci C, Guerra V. A prospective study of the relationship between disease activity and psychologic distress in patients with inflammatory bowel disease. Scand J Gastroenterol. 1996;31(8):792–796. doi:10.3109/00365529609010354.
- Muegge BD, Kuczynski J, Knights D, Clemente JC, González A, Fontana L, Henrissat B, Knight R, Gordon JI. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science. 2011;332(6032):970–974. doi:10.1126/science.1198719.
- David LA, Maurice, C.F., Carmody, R.N., Gootenberg, D.B., Button, J.E., Wolfe, B.E., Ling, A.V., Devlin, A.S., Varma, Y., Fischbach, M.A and Biddinger, S.B, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–563. doi:10.1038/nature12820.
- Sonnenburg JL, Backhed F. Diet-microbiota interactions as moderators of human metabolism. Nature. 2016;535(7610):56–64. doi:10.1038/nature18846.
- Johnson AJ, Vangay P, Al-Ghalith GA, Hillmann BM, Ward TL, Shields-Cutler RR, Kim AD, Shmagel AK, Syed AN, Walter J, et al. Daily sampling reveals personalized diet-microbiome associations in humans. Cell Host Microbe. 2019;25(6):789–802 e5. doi:10.1016/j.chom.2019.05.005.
- Gent AE, Hellier, M.D., Grace, R.H., Swarbrick, E.T and Coggon, D, et al. Inflammatory bowel disease and domestic hygiene in infancy. Lancet. 1994;343(8900):766–767. doi:10.1016/S0140-6736(94)91841-4.
- Hill C, Guarner, F., Reid, G., Gibson, G.R., Merenstein, D.J., Pot, B., Morelli, L., Canani, R.B., Flint, H.J., Salminen, S. and Calder, P.C, et al. Expert consensus document. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–514. doi:10.1038/nrgastro.2014.66.
- Gareau MG, Sherman PM, Walker WA. Probiotics and the gut microbiota in intestinal health and disease. Nat Rev Gastroenterol Hepatol. 2010;7(9):503–514. doi:10.1038/nrgastro.2010.117.
- de Vries W, Stouthamer AH. Pathway of glucose fermentation in relation to the taxonomy of bifidobacteria. J Bacteriol. 1967;93(2):574–576. doi:10.1128/jb.93.2.574-576.1967.
- Markowiak-Kopec P, Slizewska K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients. 2020;12(4):1107. doi:10.3390/nu12041107.
- Haller D, Colbus H, Gänzle MG, Scherenbacher P, Bode C, Hammes WP. Metabolic and functional properties of lactic acid bacteria in the gastro-intestinal ecosystem: a comparative in vitro study between bacteria of intestinal and fermented food origin. Syst Appl Microbiol. 2001;24(2):218–226. doi:10.1078/0723-2020-00023.
- Sengupta R, Altermann E, Anderson RC, McNabb WC, Moughan PJ, Roy NC. The role of cell surface architecture of lactobacilli in host-microbe interactions in the gastrointestinal tract. Mediators Inflamm. 2013;2013:237921. doi:10.1155/2013/237921.
- Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology. 2004;127(1):224–238. doi:10.1053/j.gastro.2004.04.015.
- Karczewski J, Troost FJ, Konings I, Dekker J, Kleerebezem M, Brummer RJM, Wells JM. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am J Physiol Gastrointest Liver Physiol. 2010;298(6):G851–9. doi:10.1152/ajpgi.00327.2009.
- Miyauchi E, Morita H, Tanabe S. Lactobacillus rhamnosus alleviates intestinal barrier dysfunction in part by increasing expression of zonula occludens-1 and myosin light-chain kinase in vivo. J Dairy Sci. 2009;92(6):2400–2408. doi:10.3168/jds.2008-1698.
- Sonnenborn U, Robertson L. Escherichia coli strain nissle 1917—from bench to bedside and back: history of a special Escherichia coli strain with probiotic properties. FEMS Microbiol Lett. 2016;363(19):fnw212. doi:10.1093/femsle/fnw212.
- Ukena SN, Singh A, Dringenberg U, Engelhardt R, Seidler U, Hansen W, Bleich A, Bruder D, Franzke A, Rogler G, et al. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS One. 2007;2(12):e1308. doi:10.1371/journal.pone.0001308.
- Zyrek AA, Cichon C, Helms S, Enders C, Sonnenborn U, Schmidt MA. Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKCzeta redistribution resulting in tight junction and epithelial barrier repair. Cell Microbiol. 2007;9(3):804–816. doi:10.1111/j.1462-5822.2006.00836.x.
- Wehkamp J, Harder J, Wehkamp K, Meer BWV, Schlee M, Enders C, Sonnenborn U, Nuding S, Bengmark S, Fellermann K, et al. NF-κB- and AP-1-mediated induction of human beta defensin-2 in intestinal epithelial cells by Escherichia coli Nissle 1917: a novel effect of a probiotic bacterium. Infect Immun. 2004;72(10):5750–5758. doi:10.1128/IAI.72.10.5750-5758.2004.
- Hering NA, Richter JF, Fromm A, Wieser A, Hartmann S, Günzel D, Bücker R, Fromm M, Schulzke JD, Troeger H, et al. TcpC protein from E. coli Nissle improves epithelial barrier function involving PKCzeta and ERK1/2 signaling in HT-29/B6 cells. Mucosal Immunol. 2014;7(2):369–378. doi:10.1038/mi.2013.55.
- Alvarez CS, Badia J, Bosch M, Giménez R, Baldomà L. Outer membrane vesicles and soluble factors released by probiotic Escherichia coli Nissle 1917 and commensal ECOR63 enhance barrier function by regulating expression of tight junction proteins in intestinal epithelial cells. Front Microbiol. 2016;7:1981. doi:10.3389/fmicb.2016.01981.
- Nagarjuna D, Dhanda RS, Gaind R, Yadav M. tcpC as a prospective new virulence marker in blood Escherichia coli isolates from sepsis patients admitted to the intensive care unit. New Microbes New Infect. 2015;7:28–30. doi:10.1016/j.nmni.2015.05.002.
- Alvarez CS, Giménez R, Cañas M-A, Vera R, Díaz-Garrido N, Badia J, Baldomà L. Extracellular vesicles and soluble factors secreted by Escherichia coli Nissle 1917 and ECOR63 protect against enteropathogenic E. coli-induced intestinal epithelial barrier dysfunction. BMC Microbiol. 2019;19(1):166. doi:10.1186/s12866-019-1534-3.
- Shapiro H, Thaiss CA, Levy M, Elinav E. The cross talk between microbiota and the immune system: metabolites take center stage. Curr Opin Immunol. 2014;30:54–62. doi:10.1016/j.coi.2014.07.003.
- Sun X, Yang Q, Rogers CJ, Du M, Zhu M-J. AMPK improves gut epithelial differentiation and barrier function via regulating Cdx2 expression. Cell Death Differ. 2017;24(5):819–831. doi:10.1038/cdd.2017.14.
- Rowart P, Wu J, Caplan M, Jouret F. Implications of AMPK in the formation of epithelial tight junctions. Int J Mol Sci. 2018;19(7):2040. doi:10.3390/ijms19072040.
- Zhu MJ, Sun X, Du M. AMPK in regulation of apical junctions and barrier function of intestinal epithelium. Tissue Barriers. 2018;6(2):1–13. doi:10.1080/21688370.2018.1487249.
- Peng L, Li Z-R, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. 2009;139(9):1619–1625. doi:10.3945/jn.109.104638.
- Elamin EE, Masclee AA, Dekker J, Pieters H-J, Jonkers DM. Short-chain fatty acids activate AMP-activated protein kinase and ameliorate ethanol-induced intestinal barrier dysfunction in Caco-2 cell monolayers. J Nutr. 2013;143(12):1872–1881. doi:10.3945/jn.113.179549.
- Kimura I, Ichimura A, Ohue-Kitano R, Igarashi M. Free fatty acid receptors in health and disease. Physiol Rev. 2020;100(1):171–210. doi:10.1152/physrev.00041.2018.
- Suzuki T, Yoshida S, Hara H. Physiological concentrations of short-chain fatty acids immediately suppress colonic epithelial permeability. Br J Nutr. 2008;100(2):297–305. doi:10.1017/S0007114508888733.
- Feng W, Wu Y, Chen G, Fu S, Li B, Huang B, Wang D, Wang W, Liu J. Sodium butyrate attenuates diarrhea in weaned piglets and promotes tight junction protein expression in colon in a GPR109A-dependent manner. Cell Physiol Biochem. 2018;47(4):1617–1629. doi:10.1159/000490981.
- Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong S, Maruya M, Ian Mckenzie C, Hijikata A, Wong C, et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun. 2015;6:6734. doi:10.1038/ncomms7734.
- Cai Y, Folkerts J, Folkerts G, Maurer M, Braber S. Microbiota-dependent and -independent effects of dietary fibre on human health. Br J Pharmacol. 2020;177(6):1363–1381. doi:10.1111/bph.14871.
- O’Sullivan AC. Cellulose: the structure slowly unravels. Cellulose. 1997;4(3):173–207. doi:10.1023/A:1018431705579.
- Anderson JW, Baird, P., Davis, R.H., Ferreri, S., Knudtson, M., Koraym, A., Waters, V. and Williams, C.L., et al. Health benefits of dietary fiber. Nutr Rev. 2009;67(4):188–205. doi:10.1111/j.1753-4887.2009.00189.x.
- Kashyap PC, Marcobal A, Ursell LK, Larauche M, Duboc H, Earle KA, Sonnenburg ED, Ferreyra JA, Higginbottom SK, Million M, et al. Complex interactions among diet, gastrointestinal transit, and gut microbiota in humanized mice. Gastroenterology. 2013;144(5):967–977. doi:10.1053/j.gastro.2013.01.047.
- Di Caro V, Alcamo AM, Cummings JL, Clark RSB, Novak EA, Mollen KP, Morowitz MJ, Aneja RK. Effect of dietary cellulose supplementation on gut barrier function and apoptosis in a murine model of endotoxemia. PLoS One. 2019;14(12):e0224838. doi:10.1371/journal.pone.0224838.
- Berer K. Dietary non-fermentable fiber prevents autoimmune neurological disease by changing gut metabolic and immune status. Sci Rep. 2018;8(1):10431. doi:10.1038/s41598-018-28839-3.
- Kim Y, Hwang SW, Kim S, Lee Y-S, Kim T-Y, Lee S-H, Kim SJ, Yoo HJ, Kim EN, Kweon M-N, et al. Dietary cellulose prevents gut inflammation by modulating lipid metabolism and gut microbiota. Gut Microbes. 2020;11(4):944–961. doi:10.1080/19490976.2020.1730149.
- Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123(11):1939–1951. doi:10.1093/jn/123.11.1939.
- Pellizzon MA, Ricci MR. Choice of laboratory rodent diet may confound data interpretation and reproducibility. Curr Dev Nutr. 2020;4(4):nzaa031. doi:10.1093/cdn/nzaa031.
- Zou J, Chassaing B, Singh V, Pellizzon M, Ricci M, Fythe MD, Kumar MV, Gewirtz AT. Fiber-mediated nourishment of gut microbiota protects against diet-induced obesity by restoring il-22-Mediated colonic health. Cell Host Microbe. 2018;23(1):41–53 e4. doi:10.1016/j.chom.2017.11.003.
- Miles JP, Zou, J., Kumar, M.V., Pellizzon, M., Ulman, E., Ricci, M., Gewirtz, A.T. and Chassaing, B., et al. Supplementation of low- and high-fat diets with fermentable fiber exacerbates severity of DSS-induced acute colitis. Inflamm Bowel Dis. 2017;23(7):1133–1143. doi:10.1097/MIB.0000000000001155.
- Carvalho FA, Koren O, Goodrich J, Johansson MV, Nalbantoglu I, Aitken J, Su Y, Chassaing B, Walters W, González A, et al. Transient inability to manage proteobacteria promotes chronic gut inflammation in TLR5-deficient mice. Cell Host Microbe. 2012;12(2):139–152. doi:10.1016/j.chom.2012.07.004.
- Rutten AA, de Groot AP. Comparison of cereal-based diet with purified diet by short-term feeding studies in rats, mice and hamsters, with emphasis on toxicity characteristics. Food Chem Toxicol. 1992;30(7):601–610. doi:10.1016/0278-6915(92)90194-P.
- Llewellyn SR, Britton GJ, Contijoch EJ, Vennaro OH, Mortha A, Colombel J-F, Grinspan A, Clemente JC, Merad M, Faith JJ, et al. interactions between diet and the intestinal microbiota alter intestinal permeability and colitis severity in mice. Gastroenterology. 2018;154(4):1037–1046 e2. doi:10.1053/j.gastro.2017.11.030.
- Crosbie AB, Roche LM, Johnson LM, Pawlish KS, Paddock LE, Stroup AM. Trends in colorectal cancer incidence among younger adults-Disparities by age, sex, race, ethnicity, and subsite. Cancer Med. 2018;7(8):4077–4086. doi:10.1002/cam4.1621.
