ABSTRACT
Intestinal epithelium functions as a tissue barrier to prevent interaction between the internal compartment and the external milieu. Intestinal barrier function also determines epithelial polarity for the absorption of nutrients and the secretion of waste products. These vital functions require strong integrity of tight junction proteins. In fact, intestinal tight junctions that seal the paracellular space can restrict mucosal-to-serosal transport of hostile luminal contents. Tight junctions can form both an absolute barrier and a paracellular ion channel. Although defective tight junctions potentially lead to compromised intestinal barrier and the development and progression of gastrointestinal (GI) diseases, no FDA-approved therapies that recover the epithelial tight junction barrier are currently available in clinical practice. Here, we discuss the impacts and regulatory mechanisms of tight junction disruption in the gut and related diseases. We also provide an overview of potential therapeutic targets to restore the epithelial tight junction barrier in the GI tract.
Introductory note
Tight junction is the key determinant of barrier function in various epithelial cell types. In the intestine, tight junction disruption can cause the leaky gut that is known to be associated with pathogenesis and progression of the gastrointestinal diseases. Therefore, targeting tight junction integrity can be the therapeutic approach for the treatment of related diseases in the gastrointestinal tract. Unfortunately, there is no tight junction-targeted drug in the clinical setting. To discover and develop a such drug that can recover tight junction integrity, insights into the physiological and pathophysiological mechanisms of tight junction regulation are required. In this review, we provide the comprehensive views of evidence regarding the mechanisms of tight junction assembly and integrity as well as related gastrointestinal diseases. In addition, we also discuss about the small molecules and known drugs that can be used for targeting tight junction integrity.
1. A brief history of tight junction discovery
The tight junction (TJ) was first visualized by transmission electron microscopy (TEM) in regions between adjacent epithelial cells in 1963Citation1 and was shown to be related to paracellular transport.Citation2–4 Freeze-fracture electron microscopy demonstrated an anastomosing network of TJ strands that were superficial and deep in “leaky” and “tight” epithelia,Citation5,Citation6 respectively. The morphology of TJ strands was found to be strongly correlated with epithelial barrier function, as indicated by the transepithelial electrical resistance (TER) in the intestinal epithelial cells.Citation7 The lipid micelle model was proposed as the first description of the molecular identity of TJs, in which the exoplasmic leaflets of neighboring cell membranes are partly fused together as lipidic hexagonal cylinders,Citation8–10 establishing a membrane fence. Later, it was reported that fluorescent-tag lipids were unable to diffuse in paracellular space,Citation11 thus refuting this hypothesis.
At present, TJs are known as a group of proteins that seal paracellular space between adjacent cells, near the apical membrane. Zonula occludens-1 (ZO-1), the first TJ protein, was discovered in 1986,Citation12 while cingulin was identified as another peripheral element of TJ in 1989.Citation13 In 1993, occludin was identified as the first transmembrane protein contributing to TJ-related barrier function.Citation14 Other transmembrane proteins acting on TJs, such as claudins, junctional adhesion molecules (JAMs), and MAL and related proteins for vesicle trafficking and membrane link domain-3 (MarvelD3), were later discovered.Citation15–18 In multicellular organisms, there are bicellular and tricellular points of cell–cell contacts. Recently, tricellulin and angulin-1 have been proposed as functional tricellular TJs that can determine the paracellular passaging of macromolecules.Citation19,Citation20
In addition to being a legitimate barrier, some TJ proteins, for example, claudin-2 and claudin-15, can form paracellular pores for the permeability of water and ions in enterocytes.Citation21,Citation22 Enhanced TJ permeability can result from defective integrity of either barrier-forming or activating pore-forming TJs, also known as leak and pore pathways, respectively.Citation23 Importantly, compromised epithelial barrier function can amplify a positive-feedback cycle of immune-associated barrier dysfunction, which is closely postulated with both the pathogenesis and the progression of gastrointestinal diseases. Unfortunately, no drug that can directly modify TJ integrity is currently available. To discover drugs targeting TJ recovery, it is important to obtain mechanistic insights into the regulation of epithelial TJs.
2. Structure and molecular architecture of tight junctions
Tight junctions (TJs) are dynamic structures, which are formed by the aggregation of an assortment of specific proteins at the apical membrane. TJ components can be distinguished by their localization into transmembrane proteins and cytoplasmic scaffolding proteins.
2.1 Transmembrane proteins
The transmembrane proteins of TJs are structurally classified into three groups: 1) single-spanning proteins, including Junctional adhesion molecule (JAM), Crumbs protein homolog 3 (Crb3), and Coxsackievirus and adenovirus receptor (CAR); 2) a tri-spanning protein, Blood vessel epicardial substance (BVES); and 3) tetra-spanning proteins of the claudin and tight junction-associated MARVEL protein (TAMP) families, which include occludin, tricellulin, and MarvelD3 (). JAMs, claudin, occludin, and tricellulin have been extensively studied and shown to be involved in TJ permeability in intestinal epithelial cells.
Figure 1. Cellular architecture of tight junction. Tight junctions can be divided into bi- and tricellular tight junctions based on their localization. For example, tricellulin and angulin are considered as tricellular tight junctions. Most of tight junctions are transmembrane proteins located in bi-cellular point of cell-cell contact including occludin, claudins, and JAMs. Indeed, occludin and claudins consist of four membrane-spanning domains whereas JAM is a single membrane-spanning protein with large extracellular Ig-like domain. On the other hand, ZO-1, afadin, and cingulin are cytosolic scaffolding tight junction proteins that play an important role in arranging tight junction strands. The scaffolding tight junctions consist of a number of binding sites responsible for protein interaction.
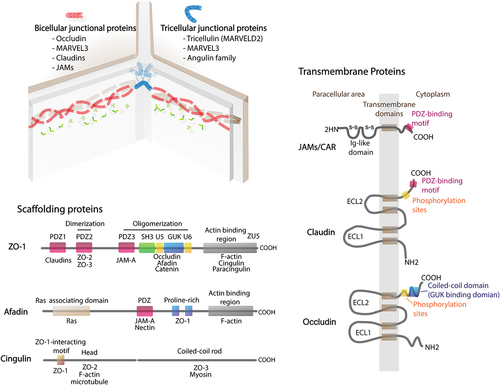
JAMs belong to the immunoglobulin superfamily and are involved in cell–cell adhesion and permeability in the epithelium and endothelium.Citation24 There are four types of JAM, namely, JAM-A, JAM-B, JAM-C, and JAM-4, but the role of JAM-4 in TJs is still unclear.Citation25,Citation26 Two pairs of JAM-A dimers from apposing cell surfaces ensure intercellular interaction through the immunoglobulin-like domain, while their PDZ-binding motif at the C-terminus allows TJ regulation by myriad scaffolding proteins.Citation25,Citation27 JAM-A plays significant roles in apical membrane apposition junctions, regulation of macromolecule permeability, and formation of epithelial permeability.Citation28
laudins are known as fundamental components of TJs involved in modulating the paracellular permeability of ions and molecules.Citation22 At least 26 members of the mammalian claudin family have been identified.Citation29 In TJs, claudins assort themselves into membrane strands via homo- or heteromeric interaction, and two anti-parallel strands connect to form the pore-like structure regulating the paracellular movement of small molecules.Citation22,Citation28,Citation29 This gated permeability and integrity of the claudin strands is largely reported to be sophisticatedly determined by the dynamic expression of pore-forming and siege-forming claudins (i.e., claudin-2 and claudin-4, respectively).Citation22,Citation28,Citation29 Indeed, pore-forming claudins are defined as paracellular ion channels. On the other hand, siege-forming claudins are non-ion channel type of claudins that can form paracellular barriers. The assortment of claudin substitutes in strand and modifications of TJ proteins by cellular signaling have been proposed as factors regulating TJ permeability, which has discussed later.Citation22
Occludin exhibits the MARVEL motif at a bicellular region, which allows the trans-homodimeric apposition of apical membranes.Citation30 The cytosolic C-terminus of occludin is composed of various phosphorylated residues for signaling kinases and a docking site for scaffolds.Citation30,Citation31 Although occludin is considered an important TJ component, depletion of occludin in epithelial cells resulted in intact barrier, unaltered permeability, and increased localization of other TAMPs to the junctional area.Citation31 Occludin may involve in TJ assembly with assistance of scaffolding proteins, i.e., zonula occludens (ZO)-1, but the physiological functions of each single protein are not fully understood.Citation32 Moreover, recent work indicated that ZO-1 was not important for establishment of barrier function, but it played vital roles in intestinal mucosal repair.Citation33
In contrast to occludin, tricellulin, or MARVELD2 is predominantly found and colocalized with MARVELD3 at tricellular contacts where the edges of three cell surfaces are conjoined.Citation15 Recent studies suggested that tricellulin is not only important for controlling macromolecule permeability, but also has prominent roles in the mechanical regulation at bicellular and tricellular regions.Citation34
2.2 Scaffolding proteins
A plaque of scaffolding proteins is incorporated into the TJ area with various domains that bind specifically to motifs of the integral part of TJs, other scaffold proteins, and the cytoskeleton to regulate TJ assembly, junction permeability, and related signaling.
2.2.1 PDZ-containing scaffolding proteins
PDZ-containing proteins form a junctional plaque through binding with TJ integral proteins.Citation35 The evolutionarily conserved, ~90-residue-long PDZ sequence allows the scaffolding proteins to perform two major functions: 1) anchoring the intracellular C-terminus of transmembrane proteins (TJs, ion channels, receptors, etc.) and 2) establishing scaffolding networks via interactions with other PDZ proteins through PDZ–PDZ dimerization.Citation36,Citation37
Zonula occludens (ZO), including ZO-1 and its homologs ZO-2 and ZO-3, is a group of PDZ-related scaffolding proteins that belong to the superfamily of membrane-associated guanylate kinase-like proteins, possessing sites of three tandem PDZ domains at the N-terminusCitation38,Citation39 (). The first PDZ domain interacts with claudins, the binding affinity of which is regulated by phosphorylation at the PDZ-binding motifs of claudins.Citation40 The second PDZ domain establishes dimerization with another PDZ2, while PDZ3 is bound by JAM-A. In addition to PDZ regions, ZO-1 engages with other scaffolding proteins and cytoskeletal components, such as afadin, occludin, and α-catenin, through Src homology 3 (SH3), U5, guanylate kinase-like (GUK) regions, and actin-binding region (ABR).Citation41,Citation42 Moreover, the interactive zone of PDZ3 together with SH3-GUK-U6 facilitates the phase separation of ZO-1 and polymerization of ZO proteins during ZO assembly.Citation43,Citation44
ZO-1 is a fundamental component of TJs that interacts with other scaffolds and F-actin to regulate epithelial barrier permeability.Citation45–47 In mouse epithelia, ZO-1 depletion was shown to cause delayed TJ formation and reassembly with integrity being unaffected, but TJ disruption was not detected in ZO-2-depleted cells.Citation48 However, double deletion of ZO-1 and ZO-2 established leaky monolayers with the complete loss of TJ strands,Citation45 suggesting that ZO-1 and ZO-2 have overlapping functions in anchoring the TJ strand. ZO-1 also plays a key role in conveying the signaling between the TJ structure and the actin cytoskeleton.Citation49 The ABR of ZO-1 normally forms a weak and intermittent interaction with F-actin.Citation50 Either strong binding or no interaction among ZO-1, ABR, and F-actin causes elevated TJ permeability.Citation49,Citation50 Notably, ZO-1 deficiency in intestinal epithelium was demonstrated to lead to the concentration of F-actin and contraction of actomyosin fibers, but did not depend on ABR loss.Citation44 It is suggested that the reorganization of F-actin after change in ZO-1 may indirectly engage ZO-1-binding scaffolds, namely, ZO-2, cingulin, paracingulin, and afadin.Citation41
Other PDZ domain-containing scaffolding proteins, in addition to ZOs, including partitioning defective 3 (Par3), Par6, protein associated with Lin‑7 1 (PALS1), PALS1‑associated tight junction (PATJ), multi-PDZ domain protein 1 (MUPP), and afadin, have been implicated in TJ assembly as well as apico-basal polarity.Citation32,Citation41,Citation51–54 Interestingly, afadin has been identified to bind to ZO-1, JAM-A, actin, and nectin (adherens junction protein).Citation46,Citation55 Afadin plays profound roles in the initiation of TJ assembly and polarization by gathering ZO-1 and JAM-A to the adherens junction area.Citation41,Citation51 Deficiency of afadin in mouse intestine was reported to cause impairments of the TJs and adherens junctions of intestinal crypt cells. In addition, reduction in afadin expression was found to be associated with the progression of colon and pancreatic cancers.Citation56–58
2.2.2 Non-PDZ scaffolding proteins: cingulin and paracingulin
Cingulin and paracingulin homodimers consist of a coiled rod and globular head, which have a shared ZO-1-Interaction-Motif (ZIM) at the N-terminus.Citation59,Citation60 Cingulin was found to bind to TJ components, namely, JAM-A, ZO-1, and ZO-2. Moreover, cingulin exhibits high affinity to actinCitation61 and microtubules,Citation62,Citation63 with this affinity being regulated by AMP-activated protein kinase (AMPK) phosphorylation.Citation62,Citation63 Furthermore, cingulin as well as paracingulin downregulates the activity of Rho family proteins, resulting in the inhibition of claudin-2 expression and cell proliferation.Citation64–66
3. Intestinal epithelium and permeability pathways
3.1 Intestinal epithelial barriers
Mucosal surfaces that are composed by distinct cell types and separate the internal environment from the external milieu are necessities of maintenance of first-line of defense and body homeostasis. There are multiple sites that face the external environment, including mucosal surfaces of the respiratory, urinary, and GI tracts, as well as the skin. Although the skin is the most visible interface, the entire surface of mucosal layers is actually much larger than the whole surface area of the skin.Citation67,Citation68 In the GI tract, epithelium-lined mucosal surfaces form a selective barrier to limit the passage of dietary antigens and virulence factors from the intestinal lumen.Citation23,Citation67–70 The intestinal epithelium also regulates vectorial transport for nutrient and mineral absorptions and secretion of unwanted compounds.Citation71–77 In fact, vectorial transport across epithelial layers and epithelial tissue barriers requires the integrity of tight junction. Increased intestinal permeability can ultimately lead to activation of the mucosal immune system and its sequelae to cause the pathogenesis and progression of inflammation-associated mucosal diseases.Citation21,Citation70,Citation78–85
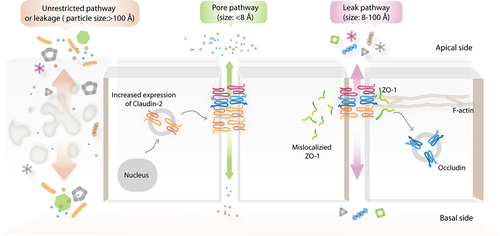
Increased intestinal permeability can be due to tight junction-dependent and tight junction-independent mechanisms. Two tight junction-dependent permeability pathways have been proposed, namely, the leak and pore pathways.Citation23 The pathways can be defined as involving defects of barrier-forming and activation of pore-forming tight junctions, respectively. Meanwhile, increased tight junction-independent unrestricted pathway permeability occurs at sites of apoptosis-induced epithelial damage and tissue erosion.Citation23,Citation68–70,Citation84,Citation86 A comprehensive model of the barrier permeability pathways is illustrated in .
Figure 2. Tight junction-dependent paracellular permeability pathways. There are the well-known paracellular permeability pathways including leak, pore, and unrestricted pathways. Leak and pore are tight junction-dependent paracellular permeability pathways. Indeed, leak pathway is a size-selective permeability whereas pore pathway is size- and charge-selective permeability. Of note, pore-forming tight junction is sometimes called as paracellular ion channel. Conversely, unrestricted pathway occurs at the site of apoptotic cells or tissue damage.
3.2 Leak and unrestricted permeability pathways
The leak pathway is considered to be a low-conductance, size-selective paracellular pathway, which allows the permeability of macromolecules with a hydrodynamic radius of ~8–100 Å.Citation23,Citation68–70,Citation84,Citation86 Defects of barrier-forming tight junctions, for example, ZO-1, occludin, claudin-1, claudin-4, and tricellulin, have been implicated in the leak pathway.Citation32,Citation70,Citation87–96 Among the several proposed signaling pathways, the activation of myosin light-chain kinase (MLCK)-induced, caveolin-1-dependent occludin internalization has been widely accepted as one of the major mechanisms of tumor necrosis factor (TNF)-mediated leak pathway permeability.Citation97–101 Notably, the C-terminal coiled-coil occludin/ELL domain (OCEL) of occludin and its K433 amino sequence are required for TNF-induced leak pathway permeability.Citation102 Three differently sized routes of macromolecular flux have been proposed to contribute to the leak pathway, which are permeable to molecules with hydrodynamic radii of 8, 10, and 60 Å.Citation103–108 In isolated rat small intestine, the capacity for permeability of the upper part of the villi is generally lower than that of the lower part and the crypt is highly permeable to molecules with radii of up to 60 Å.Citation106 In response to activation of Na+/glucose cotransport-1 (SGLT1) in Caco-2 cell monolayers, tight junction permeability was found to be significantly increased, as demonstrated by a decreased TER value.Citation100 Consistent with this, paracellular flux of [3H]mannitol (hydrodynamic radius of 3.6 Å), but not [14C]inulin (hydrodynamic radius of 11.5 Å), was also increased, indicating that a decrease in TER resulted from tight junction-dependent permeability, not epithelial damage.Citation100 This study also provided evidence of the smallest route (hydrodynamic radius of 3.6 Å) of an uncharged, size-selective permeability pathway of macromolecules. Notably, the existence of differently sized routes of leak pathway that are regulated by neither different tight junction components nor the degree of decrease of a similar barrier-forming tight junction has been demonstrated. Upon severe tissue damage, the unrestricted pathway, which is a high-conductance and size/charge-independent pathway, occurs in ulcerated epithelia.Citation70 This nonselective permeability event allows the transmucosal transport of large particles (hydrodynamic radius of >100 Å) such as proteins and bacteria into circulation; it is found in patients with necrotizing enterocolitis and in dextran sulfate sodium (DSS)-treated mice.Citation84,Citation109,Citation110 The 4-kDa fluorescein isothiocyanate-dextran (4-kDa FITC-dextran) is generally used as a probe for investigating leak pathway permeability.Citation111 However, using only one probe, it is not possible to distinguish leak and unrestricted pathways experimentally.Citation112 Both in vitro and in vivo multiplex analyses of size-selective macromolecular permeability assays have recently been developed to enable spontaneous measurements of leak and unrestricted permeability pathways.Citation112,Citation113 These methods are based on the capacity for permeability of a mixture of multi-colored and differently sized fluorescent probes.
3.3 Activation of paracellular ion channel and pore pathway permeability
The pore pathway permeability is defined as a high-conductance, and size- and charge-selective paracellular route that primarily relies on expression of the pore-forming claudins.Citation23,Citation70,Citation114 Notably, the pore pathway accommodates the permeability of small, charged ions with a pore size of ~4–8 Å.Citation21,Citation23,Citation69,Citation80,Citation104,Citation115 Polyethylene glycol (7 Å) and creatinine (6 Å) have been used as probes for the in vivo investigation of pore pathway permeability.Citation80,Citation104 Indeed, 4-kDa FITC-dextran or fluorescein, a smaller uncharged, paracellular fluorescent probe (9 Å diameter), cannot pass through the pore pathway.Citation86 There are many in vitro models that can be used for demonstrating pore pathway permeability, including cultured intestinal epithelial cells (Caco-2 and T84 cell lines) and Madin–Darby canine kidney (MDCK) cells.Citation104,Citation116 Notably, two distinct clones of MDCK, namely, MDCK1 (without expression of pore-forming claudins) and MDCK2 (endogenous expression of pore-forming claudins), can be used for experiments on pore pathway permeability. In enterocytes, claudin-2 and claudin-15 are the dominant paracellular monovalent cation and water channels, with different degrees of expression.Citation78,Citation117–121 In fact, claudin-2 is highly expressed in intestinal tissue at birth, but its expression gradually declines during post-neonatal development until being almost experimentally undetectable in adulthood. In contrast, claudin-15 has a low level of expression in children, but relatively high expression in adults. The expression of claudin-2 and claudin-15 is primarily restricted to crypt epithelia in both rodents and humans.Citation21,Citation78,Citation120 Nevertheless, little is known about the contribution of claudin-15 to pore pathway permeability in GI disease. Conversely, the impact of claudin-2 expression on increased activity of the pore pathway has been extensively investigated and shown to be related to disease progression in experimental colitis and active inflammatory bowel disease (IBD) patients.Citation21,Citation84,Citation86,Citation122,Citation123 Generally, claudin-2-mediated paracellular Na+ conductance can be indirectly measured by dilution and bi-ionic potential assays.Citation23,Citation124 Recently, the trans-tight junction patch clamp approach has been developed to elucidate the gating characteristics of an independent, single claudin-2 channel in Tet-off regulated claudin-2-inducible MDCKI monolayers, which revealed the claudin-2-dependent conductance of ~9 pA.Citation125 This method also identified three main states of non-rectifying claudin-2 gating: open, stable, and transient closed states.Citation125
4. Tight junction dynamics
4.1 Evidence of tight junction mobilization
As proteins related to the structure of the cell, the integrity of tight junctions was thought to provide a static, impermeable barrier at the site of apical intercellular space complexes. However, this hypothesis was proven to be almost entirely incorrect. In fact, the concept of tight junction dynamics has a long history of over 30 years.Citation126 In the last decade, fluorescence recovery after photobleaching (FRAP) revealed different mobile characteristics of fluorescent protein-tagged tight junctions during a steady state.Citation127–129 Although claudin-1 stably localized at the tight junction region, ZO-1 and occludin are differentially exchangeable at subcellular levels. Notably, tight junction-to-cytoplasm switching of ZO-1 was found to occur in an energy-dependent manner, which required activity of the actin-binding region (ABR) and MLCK. Indeed, ZO-1 switching could occur via either MLCK-dependent or MLCK-independent mechanisms, which are slow and fast kinetics, respectively.Citation23 Meanwhile, occludin passively diffuses between apical and lateral membrane.Citation23 Therefore, it is widely accepted that tight junction remodeling can spontaneously occur.Citation127 These kinetic behaviors of three representative tight junction proteins refute the theory of tight junctions having a static architecture ().
Figure 3. Tight junction dynamics. A) Movement of tight junction at steady state. Tight junction is motile as seen from the kinetic active movement of ZO-1 between tight junction area and cytoplasmic pool that requires energy and MLCK in fast-moving state. Occludin always moves along lateral membrane that is passive, no energy required. In contrast, claudin-1 is stable in the tight junction region. B) CK2 is the regulator of tight junction-dependent pore pathway permeability. After being activated by IL-13, claudin-2 is overexpressed and move toward to tight junction region, resulting in trans-homodimerization of claudin-2 to form paracellular cation channel. This formation is partly due to CK2-mediated phosphorylation of S408 of occludin that promotes cis-homodimerization of occludin. Inhibition of CK2 can lead to abolishment of cis-homodimerization of occludin. Therefore, this event can promote ZO-1/occludin/claudin-2 interaction via U5-GUK and PDZ1, leading to prevention of trans-homodimerization of claudin-2. Indeed, CK2 inhibitor is considered as an indirect inhibitor of claudin-2-dependent pore pathway permeability.
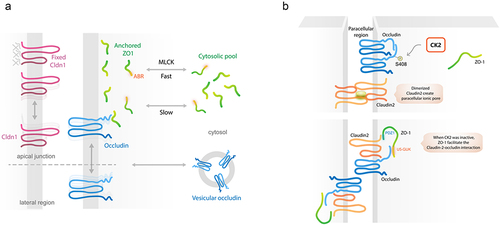
4.2 Roles of casein kinase-2 (CK2) on tight junction interactions
Interaction between each tight junction protein has been reported to regulate barrier function. As a scaffolding protein, ZO-1 has multiple interacting domains, for example, U5-GuK and PDZ1.Citation47,Citation48,Citation130–133 In general, casein kinase-2 (CK2) phosphorylates the S408 amino acid of occludin, resulting in occludin–occludin homodimerization. This in turn allows trans-homodimerization of claudin-2 to form a paracellular cation channel that promotes pore pathway permeability.Citation134 Meanwhile, the inhibition of CK2-mediated phosphorylation of occludin S408 promotes the cis-heterotrimeric complex of ZO-1/occludin/claudin-2. Indeed, ZO-1 can bind to non-phosphorylated occludin and claudin-2 via U5-GuK and PDZ1 domains, respectively. This interaction prevents claudin-2 trans-homodimerization, leading to the attenuation of claudin-2-dependent pore pathway permeability.Citation134 Therefore, CK2 inhibitor has been considered as an indirect inhibitor of claudin-2 ().Citation21
4.3 Integrative functions of bi- and tri-cellular tight junctions
In addition, coordinated functions of bicellular and tricellular tight junctions have been reported. The expression of claudin-1 and claudin-5 enhanced the enrichment of tricellulin and occludin at the intercellular point of cell–cell contacts.Citation135 Moreover, MarvelD3 was reported to interact with occludin and tricellulin at tight junction regions, but no interaction between occludin and tricellulin was observed.Citation135 Tricellulin was found to be capable of localizing on a bi-cellular tight junction in occludin-knockdown cells and occludin-knockout mice to compensate for deficient bi-cellular tight junction-dependent barrier function of occludin.Citation136 Furthermore, recent evidence indicated that double knockout of occludin and tricellulin in MDCKII cells led to decreasing numbers of anastomosing networks and branches compared with those in controls.Citation137 Notably, in occludin/tricellulin double-knockout cells, the rate of paracellular permeability was significantly elevated. It was noted that this effect was not found upon single knockout of either occludin or tricellulin.Citation137 This suggested that occludin and tricellulin play crucial roles in forming barriers to macromolecular passage.
4.4 Claudin–claudin interactions
There are at least two types of claudin–claudin interaction: trans- and cis-interactions.Citation138 Trans-interaction involves the head-to-head binding of claudins between adjacent cells, while cis-interaction involves the lateral interaction between claudins within a single membrane. Tight junction assembly is related to health and diseases as well as being involved in dynamic rearrangements.Citation87,Citation90,Citation139 Recently, using cells transfected with claudin-15 as a model, a spatiotemporally elastic remodeling process of tight junctions has been reported.Citation140 Heterotypic claudin interaction, which can be in trans and cis forms, has been found to contribute to the maintenance of barrier function in many cell types.Citation140–142 Indeed, claudins were originally known as the backbone of the anastomosing network of tight junction strands.Citation141,Citation143–148 Several lines of evidence have indicated that extracellular loops (EL1 and EL2) of claudins are important for both trans- and cis-claudin–claudin interactions.Citation149 Charge of K65 (positive charge) in Claudin-1, E158 (negative charge) in Claudin-3, and the polarity of Q56 and Q62 in Claudin-3 and Q57 in Cldn5 are required for establishing tight junction strands.Citation141 Notably, it has been reported that the first extracellular domains of claudin-2 and claudin-4 are a key determinant of paracellular charge selectivity and barrier function, but these domains are not involved in the formation of tight junction strands.Citation150 Several lines of evidence have indicated homo- and heterodimeric interactions of claudins to form tight junction strands and exert pore-forming activity. In the case of interaction between two barrier-forming claudins, this interaction leads to the establishment of barrier function. However, heterodimeric interaction of barrier-forming and pore-forming claudins has never been fully elucidated. It would be interesting to obtain more insight into this type of interaction since it may act synergistically or antagonistically, which would deepen our understanding of the regulation and restoration of tight junction permeability.
5. Cellular signaling regulators in TJ structures and function
5.1 Myosin light chain kinase
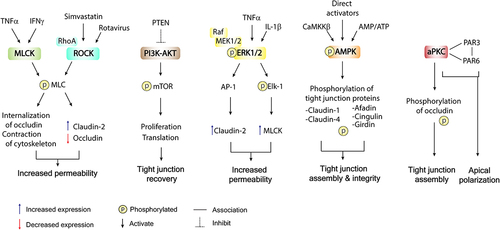
The myosin light-chain kinase (MLCK) is a Ca2+/calmodulin-dependent serine/threonine kinase.Citation151 MLCK orchestrates a series of cytoskeleton-related regulatory proteins and controls actomyosin arrangement and contraction of cardiac, smooth, and skeletal muscle.Citation70,Citation151 In addition, MLCK was previously identified as a diagnostic and drug target for cancer.Citation152 There are at least three different MLCK-encoding genes: MYLK1, MYLK2, and MYLK3. MYLK2 and MYLK3 are skeletal- and cardiac-specific MLCK-encoding genes,Citation153,Citation154 respectively, while MYLK1 encodes long non-muscle isoforms (~210 kDa) including MLCK1 (full-length “long MLCK”) and MLCK2.Citation155 The alternative splicing variants of long MLCK in intestinal epithelia determine the differential functions and localization of MLCK. In the GI tract, MLCK1 localizes mainly in villous epithelium and is highly abundant near the peri-junctional actomyosin ring (PAMR), whereas MLCK2 is expressed ubiquitously along the crypt-villus axis.Citation156 The intimate association between the apical cytoskeleton and intestinal epithelial tight junctions has long been investigated.Citation157–160 MLCK1 expression and recruitment to PAMR have been proposed to contribute to tight junction permeability in physiological and pathological circumstances. Indeed, physiological increase in TJ permeability by MLCK was observed in paracellular nutrient absorption. The activation of Na+-glucose cotransport requires MLCK upregulation to increase paracellular permeability.Citation100 This physiological event could compensate the saturated glucose transporters. Conversely, synergistic activation of IFN-γ and TNF promotes MLCK1 recruitment to PAMR by upregulating TNF receptor 2 to enhance tight junction permeability, contributing to colitis and immune-mediated diarrhea.Citation84,Citation85,Citation161–163 When MLCK1 is recruited to PAMR, it generally phosphorylates the myosin light chain (MLC), resulting in contraction of the cytoskeleton and occludin internalization.Citation100,Citation161,Citation164–166 Therefore, MLCK is widely accepted as a therapeutic target for diseases related to tight junction disruption. Nevertheless, direct enzymatic inhibition of MLCK is not feasible for patients due to its association with severe adverse effects and death.
5.2 Rho-ROCK
The small Rho GTPase family regulates various cellular functions, at least in part via three main G-protein types: Rho, cell division control protein 42 (CDC42), and Rac.Citation167 These signal molecules and the partners with which they interact intracellularly are involved in the regulation of early embryonic development, cell proliferation, adhesion, polarity, cytokinesis, and survival.Citation168 Rho-associated coiled-coil-containing kinase (ROCK), also called Rho kinase, is a downstream effector of RhoA GTPase that appears to regulate several pathways involved in paracellular permeability, for example, extracellular signal-regulated kinase (ERK), NF-κB signaling, myocardin-related transcription factor-A (MRTFA), and MLCK pathways.Citation169–172 Notably, ROCK acts as a key regulator in cytoskeletal rearrangement and actin organization via the suppression of Thr696 and Thr853 phosphorylation of the myosin-binding subunit (MBS).Citation173–175 Several in vitro studies have revealed that ROCK inhibition could prevent neuronal apoptosis,Citation176 decrease intestinal fibrosis and vascular stiffening,Citation177 and enhance E-cadherin stability in necrotizing enterocolitis.Citation177 A previous study also demonstrated that rotavirus disrupted tight junction integrity in polarized MDCK cells via a RhoA/ROCK/MLC-dependent mechanism.Citation178 Simvastatin, a lipid-lowering drug, abrogated the Rho/ROCK signaling pathway, resulting in the amelioration of sepsis-induced intestinal barrier dysfunction, and increasing the expression of tight junction proteins in rats with cecal ligation and puncture.Citation179 Interestingly, ROCK inhibitor was also shown to inhibit lipopolysaccharide (LPS)-induced MLC phosphorylation and occludin downregulation. Furthermore, ROCK was also able to reverse LPS-induced TER decrease and attenuate the paracellular permeability of 4-kDa FITC-dextran in Caco-2 cell monolayers.Citation180 Finally, ROCK inhibitor was reported to suppress the expression of claudin-2 and attenuate disease severity in experimental necrotizing enterocolitis (NEC).Citation181 These findings strongly suggest that the Rho/ROCK pathway is a therapeutic target for restoring disrupted intestinal tight junctions.
5.3 PI3K–PTEN and AKT–mTOR pathway
Phosphoinositide 3-kinase (PI3K) is a signaling molecule involved in cell growth, proliferation, and subcellular trafficking of vesicles. The phosphatase and tensin homolog (PTEN) is known as a tumor suppressor that functionally antagonizes the oncogenic effect of PI3K and vice versa.Citation182 Generally, PI3K-induced generation of phosphatidylinositol (3,4,5)-trisphosphate (PIP3) results in stimulation of the canonical AKT pathway, which promotes the upregulation of mammalian target of rapamycin complex 1 (mTORC1) signaling.Citation182 In the intestinal epithelial cell line Caco-2, LPS were reported to induce intestinal barrier dysfunction by decreasing both mRNA and protein expression of ZO-1 and occludin, with concomitant expression of PTEN.Citation183 Notably, stimulation of the PI3K/AKT pathway was found to antagonize PTEN function to ameliorate LPS-mediated intestinal epithelial barrier disruption.Citation183 Despite the mechanism behind this not being definitively clarified, it is possible that activation of the PI3K pathway indirectly restores tight junctions by promoting proliferative phenotypes and PTEN inhibition may be a plausible target for the treatment of colitis. However, conflicting evidence on this possibility has been reported. For example, the inhibition of PTEN was shown to exacerbate colitis in IL10–/ – mice. Notably, in vivo model is complex, in which increases in the abundance of colitogenic bacteria Akkermansia and Bacteroides in the gut of IL10–/ – mice have been reported in parallel with the administration of PTEN inhibitors.Citation184 These findings revealed the importance of communication between gut microbiota and mucosal intracellular signaling in determining tight junction integrity, although this still requires further investigation.
5.4 ERK1/2
The Raf–MEK–ERK1/2 pathway integrates external stimuli to influence a broad range of cellular processes, including cell survival, proliferation, transcription, and metabolism.Citation185 The association of ERK1/2 on the epithelial barrier and the homeostasis of TJ has been a subject of ongoing investigation. Recent models of inflammation-induced barrier breach demonstrated that ERK1/2 activation underlay the reduction of monolayer integrity, increase in epithelial permeability, and TJ disassembly in several epithelial tissues.Citation186–189 In intestinal epithelial monolayers (), IL-6 or IL-17 exhibited ERK1/2 stimulation, resulting in decreased monolayer integrity as well as increased pore-forming claudin-2 expression.Citation197 The upregulation of claudin-2 upon ERK1/2 activity was also reported in mouse rectal CMT93-II cells.Citation198 Moreover, ERK1/2 enhancement of claudin-2 expression is likely mediated by its downstream transcription factor AP-1, which could act as a transcriptional enhancer of claudin-2.Citation199
Table 1. Roles of ERK pathway in the regulation of tight junction-dependent permeability.
In addition to the elevation of claudin-2, studies have revealed that ERK1/2 induced barrier disruption and increased TJ permeability through the ETS-like-1 protein (Elk)-1–MLCK pathway (). Indeed, activation of ERK1/2 by the hallmark cytokines of IBD, TNFα, or IL-1β, stimulated phosphorylation of the transcription factor Elk-1.Citation190,Citation192 As an MLCK gene enhancer, Elk-1 ultimately augmented the paracellular permeability of Caco-2 monolayers.Citation190,Citation192 In contrast, some findings paradoxically showed that ERK1/2 activation led to protection of the epithelial barrier in distinct tissues and experimental models.Citation196,Citation200 However, the effects of ERK1/2 stimulation on the intestinal epithelial barrier integrity still require further clarification, particularly to distinguish the roles of ERK1/2 in gut epithelial tight junctions under physiological and pathological conditions. ERK1/2 as revealed in intestinal epithelial cells is now known to serve as a mediator of inflammation-induced tight junction disassembly.
5.5 AMP-activated protein kinase
Accumulating evidence of AMP-activated protein kinase (AMPK), the master regulator of cellular energy, has been extensively reported in modulating TJs. Various models of AMPK activation have been established and widely used, including energy deprivation, induction of intracellular calcium, and use of a pharmacological activator to induce phosphorylation of the AMPKα subunit at the Thr-172 residue, leading to enhanced epithelial barrier functions and TJ assembly.Citation91,Citation201–204 Since then, AMPK stimulation has been widely suggested as a therapeutic target for TJ-associated GI diseases, for example, IBDCitation205 and colorectal cancer.Citation206,Citation207 Although definitive findings on the mechanisms by which AMPK promotes TJ assembly and controls permeability have not been obtained, this review classifies the notable modalities by which AMPK regulates TJs into two different features.
First, AMPK activation was shown to increase phosphorylation of claudin, for example, claudin-1 and claudin-4, in mammary gland epithelial cellsCitation208 and saliva gland cells,Citation209 respectively. Indeed, the phosphorylation of claudin-1 protected itself against ubiquitin-mediated destruction, preserving the TJ complex under osmotic stress,Citation208 while claudin-4 phosphorylation enhanced its interaction with occludin, augmenting TJ assembly.Citation209
Interaction with TJ scaffolding proteins appeared to be another mechanism by which AMPK regulates TJ assembly. For instance, Wu et al. found that the phosphorylation of Ser1102 on AF-6 occurs by AMPK’s promotion of afadin-mediated ZO-1 assembly.Citation51 Moreover, AMPK was shown to be required for the homeostasis of TJ–actin–microtubule arrangement and led to cingulin phosphorylation, which improved cingulin–microtubule binding but reduced actin affinity to mitigate TJ permeability.Citation62,Citation63 Furthermore, upon energy deprivation which the Par3–Par6–aPKC polarity complex is inactive,Citation210 activated AMPK tried to maintains the apical polarity complex via the direct phosphorylation of actin-binding protein girdin, maintaining apical junction polarity and integrity during energy stress.Citation211 Taking these findings together, AMPK promotes TJ assembly and preserves TJ integrity in response to cellular stress via the phosphorylation of claudin and TJ scaffolds.
5.6 Atypical PKCs
Atypical PKCs, PKCι/λ, and PKCζ, identified as being functionally insensitive to intracellular Ca2+ or diacylglycerol, have been found to regulate TJ assembly and apico-basal polarization, as reviewed extensively elsewhere.Citation53,Citation212 In short, aPKC undergoes tripartite interaction with Par3–Par6 complex at the apical junction area mediated through PKC-interacting domains on Par3 and Par6, while Par3 is anchored to JAM-A and nectin.Citation41,Citation213,Citation214 Upon embryonic development, Par6 and its colocalized Cdc42 were shown to be initially required for PKCζ activation to induce epithelial polarization.Citation215 Active PKCζ-mediatedPar3 phosphorylation promotes the apical membrane localization of Par3–aPKC complex. However, this complex induces the exclusion of basal-specific proteins, Lethal giant larvae, from the apical complex to promote compartmentalization.Citation216 In addition, PKCζ also facilitated occludin and ZO-1 assembly via the phosphorylation of Thr residues at the C-terminus of occludin.Citation217 The inhibition of aPKCs by either a pseudosubstrate of PKCζ or ATP depletion revealed the dephosphorylation and redistribution of occludin, ZO-1, and Par3 in epithelial cell lines.Citation210,Citation217 The dephosphorylation of occludin might partly occur as a result of protein phosphatase (PP)2A activity. However, the interaction between aPKC and PP2A requires further investigation.Citation218 Taking these findings together, aPKC along with Par3–Par6 has been shown to play a crucial role in promoting epithelial polarization and regulating TJ assembly.
5.7 Noncoding RNAs
Noncoding RNAs (ncRNAs), RNA transcripts without the capacity to produce proteins, have recently emerged as regulators of epigenetics and gene expression.Citation219,Citation220 MicroRNAs (miRNAs) are small ncRNAs of approximately 22 nucleotides in length. Owing to their versatility, they can repress or activate messenger RNAs (mRNAs) to subsequently inhibit or stimulate translation,Citation221,Citation222 respectively. In addition to miRNAs, long noncoding RNAs (lncRNAs) are ncRNAs of at least 200 nucleotides in length. They are also involved in mRNA stability and post-transcriptional regulation, similarly to miRNAs.Citation223,Citation224 Intriguingly, the dysregulation of ncRNAs is associated with gut barrier disruption and inflammation, which are the pathophysiological features of several gastrointestinal diseases. Therefore, the functions and applications of these ncRNAs have received growing attention in research on such diseases.Citation225–228
miRNAs can modulate TJ expression, which is involved in diseases associated with a defective intestinal epithelial barrier. One of the most interesting miRNAs in this context is the evolutionarily conserved miR-21, which was shown to be overexpressed in colonic biopsies of ulcerative colitis patients.Citation229,Citation230 In a model of barrier dysfunction induced by intestinal ischemia–reperfusion injury, the expression of microRNA-21 (miR-21-5p and miR-21-3p) was increased, while that of TJ proteins, including occludin and CLDN1, was decreased.Citation231 Similarly, miR-21-5p was suggested to potentially induce the epithelial permeability of Caco-2 cells due to the downregulation of ADP ribosylation factor 4, which modulates occludin and CLDN4 abundance.Citation232 Profound increases in miR-122a and miR-223 were also identified in IBD models. miR-122a was shown to mediate TNFα-induced barrier leakage, while miR-223 upregulation in enterocytes resulted in the reduction of CLDN8.Citation233,Citation234 Additional miRNAs have recently been found to be involved in other TJ-associated disorders. For example, miR-29a expression was predominantly elevated in intrauterine growth-restricted (IUGR) porcine neonates.Citation235 miR-29a was shown to hamper epithelial integrity and diminish the translation of claudin-1 through binding with the 3, untranslated region (UTR) at the CLDN1 transcript in an intestinal porcine epithelial cell line (IPEC-1).Citation235 In addition, in irritable bowel syndrome (IBS), decreases in miR-16 and miR-125b-5p were identified, leading to increased expression of the CGN and CLDN2 genes, respectively.Citation236
Moreover, accumulating evidence has shown the relationships of lncRNAs to gastrointestinal diseases. lncRNAs are generally known to play crucial roles in the regulation of gene expression, mRNA stabilization, and translational and post-translational modifications,Citation223,Citation224,Citation237 while some serve as competing endogenous RNAs by interacting with certain miRNAs to mitigate mRNA suppression.Citation238 For example, the lncRNA uc.173 was shown to abolish the interaction of miR-29b with CLDN1 mRNA, thus promoting CLDN1 translation and eventually augmenting gut barrier function.Citation239 In IBD colonic biopsies, the expression of the lncRNA colon cancer-associated transcript-1 (CCAT1) was shown to correlate with IBD-specific cytokines.Citation240 CCAT1 disrupted ZO-1 and occludin architecture by concealing the function of miR-185-3p, which normally represses MLCK translation in Caco-2 cells, so it was suggested as a novel MLCK regulator.Citation240 Additionally, upregulated lncRNA H19 in IRI and IBD models promoted permeability by encoding miR-675-5p, which abolished ZO-1 expression.Citation241,Citation242 In contrast, some lncRNAs were found to enhance barrier function. For instance, PlncRNA1 preserved the expression of ZO-1 and occludin by sponging miR-34c in Caco-2 cells.Citation243 In addition, the lncRNA SPRY4 intronic transcript 1 (SPRY4-IT1) similarly enhanced the intestinal epithelial barrier function through augmenting the expression of mRNAs encoding CLDN1, CLDN3, and JAM-1.Citation244 In summary, these studies have revealed the involvement of ncRNAs in gastrointestinal disorders and TJ biology. However, it remains unclear yet whether alteration of ncRNAs essentially causes barrier-associated diseases or it change is a result of pathogenesis and compensatory mechanism. The conclusive models of tight junction regulatory signaling are shown in .
6. The modulators enhancing TJ function in the GI tract
As TJs play key roles in GI barrier disruption and the aforementioned GI diseases, the emerging TJ-modulating agents have attracted substantial attention as enhancers of GI epithelial barrier integrity. Some diet-derived and small-molecule compounds acting on TJ physiology are reviewed here.
6.1 Polyphenols
Polyphenols, the ubiquitous secondary metabolites found in plant-based foods, are primarily classified as flavonoids and non-flavonoids (i.e., phenolic acids, stilbenes, coumarins, and lignans), based on their structures.Citation245,Citation246 They have beneficial activities, such as antioxidant and anti-inflammatory properties.Citation247 Moreover, increasing evidence has shown the additional effect of polyphenols on GI epithelial mucosal homeostasis. Several flavonoids, such as quercetin, epigallocatechin gallate (EGCG), kaempferol, and rutin, have been reported to enhance GI barrier function, characterized by an increase in TER, in in vitro human intestinal epithelial models, including Caco-2 and T84 cell lines. Distinct expression patterns of TJ proteins (CLDN1/3/4, ZO-1/2, and occludin) and protein assembly were reported to be differentially promoted by divergent types of flavonoid.Citation248–251 Besides, polyphenol-rich bee pollen extracts exhibited a protective effect against intestinal barrier dysfunction in Caco-2 cells.Citation252 Moreover, propolis (bee glue) promoted a healthy GI mucosal epithelial barrier through AMPK activation and the ERK 1/2 signaling cascade in Caco-2 cells and rats.Citation253 Grape polyphenols were also revealed to promote the expression of TJ proteins, as well as counteract the production of reactive oxygen species (ROS), mediated by LPS, and exert anti-inflammatory effects in Caco-2 cells.Citation254 Furthermore, catechin and procyanidin B2, the dietary compounds rich in polyphenols, were found to augment the GI epithelial barrier function in co-cultured Caco-2 and HT29-MTX cells.Citation255 Finally, resveratrol, a stilbene compound, also improved gut barrier impairment in mice.Citation256
6.2 Oligosaccharides
Many well-known types of dietary oligosaccharide, including chitosan oligosaccharide, fructooligosaccharide, and galactomannan pentasaccharide, have attracted attention in the nutrition therapy industry. Chitosan, a positively charged linear heteropolysaccharide derived from chitin, which is the second most common natural polysaccharide in the world, is extracted from the shells of shrimp, lobster, and crab.Citation257,Citation258 Chitosan is utilized as an enhancer of oral-based drug delivery.Citation259 However, chitosan was also revealed to upregulate the expression of TJ proteins (e.g., CLDN1, occludin, ZO-1) and strengthen GI barrier function in a mouse model of ulcerative colitis.Citation260 Indeed, chitooligosaccharide (COS), a degraded product of chitosan, also augmented GI barrier integrity in a mouse closed-loop model and mucus-secreting human colonic HT-29 cells.Citation206,Citation261 In addition, fructooligosaccharide (FOS) relieved 5-fluorouracil (5-FU)-induced GI mucosal damage and gut inflammation in BALB/c mice.Citation262 FOS also stimulated TJ assembly in an AMPK-dependent manner in T84 and intestinal epithelial cells.Citation263 Furthermore, pentasaccharide of mannan (MOS5) was recently reported to possess the capacity to enhance tight junction assembly in intestinal epithelial cell monolayers, via a mechanism involving AMPK activation.Citation264
6.3 Short-chain fatty acids
Many short-chain fatty acids (e.g., butyrate, acetate, and propionate) have been revealed to promote the alteration of TJ permeability by activating lipoxygenase (LOX) in the small intestinal epithelium.Citation265,Citation266 Butyrate is commonly found in bacterial fermentation, which is a by-product of dietary fiber in the large intestine.Citation267 Several benefits of butyrate have been reported, including enhancing the permeability of the intestinal barrier through upregulating claudin-1 transcription, which is related to the association between SP1 and claudin-1 promoter.Citation268 In addition, butyrate was shown to protect against Campylobacter jejuni invasion and translocation across Caco-2 cell monolayers.Citation269 Indeed, the mechanism of action of butyrate in facilitating TJ assembly and maintaining the intestinal barrier in Caco-2 cell monolayers was shown to be dependent on AMPK.Citation270 In addition, a recent study showed that short-chain fatty acids can act as ligands of G-protein-coupled receptors (GPCRs), such as GPR41, GPR43, and GPR109A, to activate PKCβ that can in turn phosphorylate ZO-1 in order to increase intestinal barrier function.Citation271
6.4 Small-molecule compounds
As mentioned above, MLCK is widely recognized as a therapeutic target for tight junction disruption-related GI diseases including IBD.Citation70 In addition, MLCK-mediated TJ dysfunction was reported to be associated with IBS.Citation272,Citation273 Consistent with this, a membrane-permeating peptide that inhibits MLC kinase (PIK) was reported to prevent enteropathogenic Escherichia coli (EPEC)-, TNF-, and IFN-γ-induced TER defects and suppress MLC phosphorylation in Caco-2 cell monolayers.Citation274 PIK was also found to be effective for reducing fluid loss in a mouse model of diarrhea induced by T-cell activation.Citation85
However, the inhibition of MLCK is not clinically feasible due to the associated severe toxicity. No approach is currently available that directly targets tight junction integrity without modulating immune function, which always compromises immunity.Citation70 To safely restore the functions of the GI epithelial barrier and protect against cytokine-dependent, MLCK-mediated TJ disruption, a recent study has disclosed the non-enzymatic interruption of MLCK recruitment to perijunctional actomyosin rings as a novel therapeutic.Citation161 A small molecule called divertin, which can bind specifically to the immunoglobulin-like occludin cell adhesion molecule (IgCAM) of epithelial long MLCK1, was shown to prevent cytokine-mediated MLCK1 recruitment to PAMR without interfering with other facilitative mechanisms (e.g., epithelial migration and wound repair).Citation161
6.5 Amino acids and peptides
Some amino acids and peptides also exert protective effects on the GI mucosal barrier. Glutamine (Gln), the most common essential amino acid in the human body, was found to improve GI barrier function and attenuate intestinal permeability, as detected by an increase in TER value in Caco-2 cell monolayers. The depletion of Gln led to the reduction of protein expression levels of CLDN1, occludin, and ZO-1.Citation275–277 Tryptophan (Trp) was also demonstrated to cause a dose-dependent increase in TER in Caco-2 cells.Citation278 Additionally, a casein-derived peptide enhanced the TER value, indicating an increase in GI barrier function, and increased the levels of occludin gene and protein expression in in vitro human intestinal epithelial Caco-2 cells, as well as in ex vivo mouse jejunal and ileal loops.Citation279
6.6 Vitamins and minerals
Other studies of TJ-modulating agents including vitamins and minerals used as food supplements have been performed. Vitamin A is a fat-soluble vitamin applied for maintaining good visual health, antioxidant capacity, and mucosal immune response.Citation280,Citation281 Vitamin A deficiency was reported to worsen diarrhea and GI mucosal damage, which was reversed by vitamin A supplementation.Citation282 Mechanistically, this might occur via the downregulation of TJ-related mRNA expression (e.g., occludin, CLDN1/3/4, and ZO-1).Citation280,Citation283,Citation284 In addition, vitamin A exerted a protective effect on intestinal barrier integrity, as it ameliorated the reduction of TER and restored Clostridium difficile toxin A and LPS-mediated GI mucosal damage in Caco-2 cells and IPEC-J2 cells,Citation285,Citation286 respectively. Vitamin D is essential for bone health and GI barrier integrity. Its deficiency is associated with the risk of GI epithelial defect and IBD.Citation287 In a model of colitis induced by dextran sulfate sodium (DSS), it was demonstrated that vitamin D receptor (VDR)-knockout mice showed more severe diarrhea and ulceration than a control group.Citation287 Consistent with this, upon VDR siRNA knockdown, the downregulation of TJ proteins, including CLDN1, ZO-1/2, and E-cadherin, and the reduction of TER were revealed, which were relieved by 1,25-dihydroxyvitamin D3 [1,25(OH)2D3], suggesting the role of VDR-mediated vitamin D signaling in the regulation of TJs.Citation287 Zinc is an essential mineral found in some foods using as dietary supplements. Zinc also plays a pivotal role in regulating TJ proteins, as zinc supplementation can relieve the GI mucosal damage induced by malnutrition, inflammation, and infection.Citation288,Citation289 GPR39, a zinc-sensing receptor, serves as a sensor for extracellular zinc, and Gpr39 knockout in mice was shown to lead to decreases in ZO-1 and occludin expression and severe symptoms of colitis.Citation290,Citation291 Moreover, in Caco-2 cells, zinc depletion was found to cause a decline in TER and an increase in intestinal epithelial permeability.Citation292 Fortunately, zinc supplementation and GPR39-specific agonist helped reverse the decline in ZO-1 and occludin expression and enabled the reassembly of TJs in Caco-2 cells and T84 cells, at least in part via an AMPK-dependent mechanism.Citation91,Citation293
6.7 Other known drugs
Metformin is known as a drug used in the treatment of type 2 diabetes and cardiovascular complications by attenuating inflammatory responses via the suppression of nuclear factor κB (NF-κB) and the activation of AMPK.Citation294–296 In mice orally administered metformin, the metformin delayed the onset of fructose-induced liver stenosis through reduced fructose function, in which alterations of MMPs and TIMP-1 concentrations in the intestine occurred.Citation297 Apart from being an antidiabetic drug, metformin was found to effectively benefit gut epithelium by suppressing inflammation and improving ileal epithelial barrier function in interleukin-10-deficient mice, probably through AMPK activation.Citation297 AMPK is clearly a target of metformin, which can improve mucosal integrity as a treatment of colitis.Citation205 A recent study showed that metformin can also regulate TJ-related protein expression by reducing the effects of TNF-α on Caco-2 cell permeability, which results in inhibiting the MLCK–MLC signaling pathway.Citation298
7. Tight junction and GI diseases
7.1 Role of the tight junction in the esophagus and related diseases
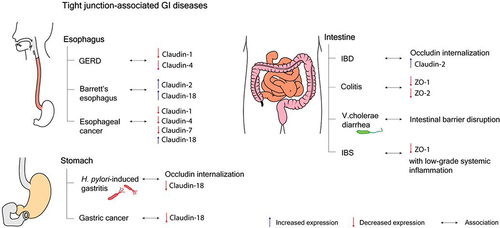
Food travels through the esophagus from the pharynx to the stomach. The barrier functions of esophageal squamous stratified epithelium involve providing mucosal and submucosal defense against noxious intraluminal substances and regulating paracellular ion transport, which is mediated by claudins, the tetra-spanning transmembrane proteins of tight junctions, located at the apical and lateral membranes of esophageal epithelial cells.Citation299–301 Claudins (CLDN)1, 4, and 7 are prominently expressed in the esophagus, which has basal pH of ~5–6.Citation300,Citation302 The esophagus is not normally exposed to a strongly acidic environment. Therefore, a decrease in esophageal pH leads to impaired esophageal barrier functions.Citation303 The changes of claudin expression patterns and the acidic disruption of the esophagus are associated with several esophageal diseases.Citation304
An intra-esophageal pH of <4 caused by acid reflux from the stomach can disrupt the esophageal barrier integrity and can be detected by 24-h ambulatory esophageal impedance pH monitoring.Citation302,Citation305,Citation306 The early morphological lesions caused by unanticipated acidic changes are dilated intercellular spaces related to the expression of tight junctions, which are found in the non-erosive form of gastroesophageal reflux disease (GERD).Citation307 CLDN 1 and 4 were found to be decreased in patients with GERD.Citation308 Consistent with this, the expression of CLDN 4 was downregulated and the esophageal barrier function was attenuated in acid-disrupted esophageal epithelial models.Citation309,Citation310 The damaged esophageal squamous mucosal layers can be replaced with metaplastic columnar epithelium, which is more acid-resistant and contributed by CLDN 18, as found in patients with Barrett’s esophagus.Citation307,Citation311 Moreover, the overexpression of CLDN 2 was found to be related to the reflux of bile acid and the development of BE.Citation312 In eosinophilic esophagitis, CLDN 7 expression was suppressed by the presence of transforming growth factor-β1 (TGF-β1), whereas the upregulation of CLDN 7 relieved TGF-β1-induced esophageal barrier dysfunction.Citation313 Moreover, in esophageal squamous cell carcinoma, reduced expression of CLDN 1, 4, and 7 was identified, which might be candidate prognostic biomarkers for esophageal cancer.Citation314–316 CLDN 18, meanwhile, was found to be expressed in esophageal adenocarcinoma.Citation317
7.2 Role of the tight junction in the stomach and related diseases
In the stomach, the tight junctions formed by adjacent gastric epithelial cells play a pivotal role in regulating the paracellular permeability of ions, nutrients, and other solutes and preventing digestive enzymes and toxins for entering the systemic circulation.Citation318 CLDN 3, 4, 5, 12, 18, and 23 are widely expressed in the stomach.Citation22 The tight gastric epithelium can tolerate local acidity, in contrast to esophageal epithelium. However, the elevation of pH by the urease activity of Helicobacter pylori was found to attenuate TER and interfere with gastric epithelial integrity, relating to occludin internalization and MLC phosphorylation.Citation318–320 H. pylori infection can markedly disrupt both pore and leak pathways of tight junctions. Indeed, the alterations of claudin protein expression can be affected in gastric cancer.Citation318 Notably, the dysregulation of claudins can result in a number of gastric diseases.
The gastric epithelium is considered to have greater tightness, depending on the number and depth of tight junction strands, than the esophageal epithelium.Citation318 Therefore, the gastric mucosal layers have low water and solute permeability and are resistant to stomach-secreted acidic juice.Citation318 The altered expression patterns of CLDNs can give rise to the development of gastric cancers. CLDN 3, 4, and 7 were revealed to be upregulated in gastric cancers, while other CLDNs showed decreased expression.Citation321 In particular, the reduction of stomach-specific CLDN 18 expression was reported in atrophic gastritis and CLDN 18 deficiency was found to be related to the poor prognosis of patients with gastric cancer.Citation322,Citation323 CLDN 18 was also found to help protect gastric epithelium from gastric acid and pepsin and to modulate the paracellular pathway.Citation324 Moreover, CLDN 18 plays a crucial role in regulating cell lineage differentiation in the stomach.Citation322 H. pylori infection is a causal risk factor of chronic gastritis and gastric tumor. In H. pylori-infected mice, the loss of CLDN 18 expression was found to occur first in gastric glands, while subsequently being found in the gastric neck and in the surface epithelial cells. The development of gastric neoplasia was also revealed in gastric tissues from CLDN 18-knockout mice, as the intestinalization of gastric mucosa was addressed.Citation322 Moreover, H. pylori-induced gastritis and gastric cancer were found to be associated with the change of the expression patterns of CLDNs, from gastric-type to intestinal-type CLDNs.Citation324–327 In addition to H. pylori-dependent gastric tumor, the long-term absence of CLDN 18 could initiate the formation of gastric tumors in a chronic gastritis mouse model without H. pylori infection through several signaling cascades related to certain chemokines and Wnt signaling pathways.Citation328
7.3 Role of the tight junction in intestinal epithelium and related diseases
Intestinal epithelium plays important roles in supporting the vectorial transport of nutrients and fluid absorption that requires cell polarity.Citation70,Citation72,Citation75,Citation77 Indeed, epithelial cell polarity in intestinal epithelial cells depends on the integrity of tight junctions.Citation70 Intestinal epithelium also forms physical barriers to protect against noxious substances from the gut lumen, which also relies on the integrity of tight junctions. Therefore, disruption of tight junctions in the intestinal epithelia can disturb homeostasis.
Over the past half-century, several lines of evidence have indicated the association between leaky gut syndrome and IBD.Citation329–332 Indeed, increased gut permeability was found in patients with Crohn’s disease and their relatives.Citation332 Of particular importance is that MLCK activation, claudin-2 overexpression, and occludin internalization have been found in intestinal tissues from both forms of IBD, namely, Crohn’s disease and ulcerative colitis.Citation333–335 In mice with TNFR2 and MLCK knockout specifically in intestinal epithelium, CD4+CD45RBhi adoptive transfer failed to induce gut permeability, MLC phosphorylation, occludin internalization, and claudin-2 expression.Citation84 Furthermore, knockout of intestinal TNFR2 and MLCK also delayed the onset of T-cell transfer-induced colitis.Citation84,Citation116 Consistent with this, the inhibition of MLCK recruitment to PAMR and CK2 inhibitor was found to attenuate immune-mediated leak and pore permeability pathways,Citation21,Citation161 respectively. These findings suggest that the prevention of intestinal tight junction disruption may be a therapeutic option for IBD. A previous study reported that the upregulation of claudin-2 was associated with increased gut permeability.Citation181 In addition, MLCK activation and tight junction disruption were also reported as key etiological factors of T-cell activation-induced diarrhea.Citation85 Furthermore, EPEC infection induced MLCK activation and occludin internalization, resulting in diarrhea.Citation274,Citation336 Clostridium difficile‑induced colitis was also found to be related to loss of ZO-1 and ZO-2.Citation337 Recent studies have additionally identified that enteric infection with V. cholerae El Tor variant caused intestinal barrier dysfunction with an unknown molecular defect and contributed to the pathogenesis of secretory diarrhea.Citation202,Citation338 Notably, enhancement of claudin-2-induced diarrhea in response to Citrobacter rodentium infection-mediated IL-22 upregulation has been considered as a mechanism of innate immunity responsible for bacterial clearance.Citation80 Apart from colitis and diarrhea, intestinal barrier dysfunction was also reported to be found in both adult and pediatric IBS.Citation272 In support of this, MLCK expression was shown to be increased in parallel with a reduction of ZO-1 expression in intestinal tissues from an IBS mouse model.Citation273 Notably, increased intestinal epithelial permeability is IBS could lead to the promotion of low-grade chronic systemic inflammation, resulting in visceral hypersensitivity.Citation273 Indeed, the relationship between increased tight junction-dependent paracellular permeability and gastrointestinal diseases is shown in .
8. Future perspective
In this review, we provide information regarding the physiological roles of intestinal tight junction integrity. We also give examples with experimental evidence supporting the impact of intestinal tight junction dysfunction on the pathogenesis of gastrointestinal diseases. For instance, intestinal tight junction disruption and gut epithelial barrier leakage have been considered as key pathogenic factors in IBD. It has been reported that the upregulation of TNF and IL-13 in intestinal tissues of IBD can promote both leak and pore permeability pathways, as feedforward and positive feedback controls of cytokine-induced intestinal barrier loss and vice versa as a vicious cycle. Increased intestinal barrier permeability was also identified as a factor determining disease severity and progression of IBD. Apart from IBD, TNF-mediated occludin downregulation was also proposed as a drug target for T-cell activation-induced diarrhea. Moreover, tight junction disruption-mediated intestinal barrier loss has been implicated in infectious diarrhea with unknown contributions to the diarrheal pathogenesis. Nevertheless, no FDA-approved therapeutics that can directly restore tight junction integrity are currently available.
At present, accumulating lines of evidence have indicated the existence of many well-recognized drug targets that have been proposed for the recovery of tight junctions, such as MLCK1. Divertin, a small molecule that can specifically bind to IgCAM3 of epithelial long MLCK1, is the first drug candidate for this purpose. Indeed, divertin non-enzymatically suppressed cytokine-induced MLCK1 recruitment to PAMR, which promotes leak pathway-dependent barrier loss. In addition, many AMPK activators, for example, metformin, polyphenols, short-chain fatty acids, oligosaccharides, and some nutrient-sensing GPCR agonists, were also shown to enhance tight junction integrity. Hence, further investigation should focus on the effectiveness and safety profiles of divertin and AMPK activators in human subjects for potential future use.
Claudin-2-mediated paracellular cation permeability is one of the drug targets for IBD. Based on a previous study, although no claudin-2 inhibitor is currently available, the inhibition of CK2 has recently been proposed as an effective strategy for treating experimental colitis. Therefore, CK2 inhibitor may be clinically useful for patients with diseases related to the overactivation of claudin-2, including IBD. However, there are some concerns since claudin-2-mediated intestinal fluid secretion causing diarrhea in response to infection is a component of the innate immunity to promote bacterial clearance. The use of a claudin-2 inhibitor may attenuate diarrhea in the early phase of enteric infection but may also delay the process of bacterial clearance and prolong disease. Furthermore, claudin-2 suppression may result in luminal fecal dehydration, which can promote intestinal obstruction.
Several lines of evidence have also supported the relationship between tight junction disruption and NEC, as well as IBS. In addition to intestinal diseases, tight junction disruption was also reported to be associated with other diseases that arise along the GI tubes, including GERD, Barrett’s esophagus, esophageal adenocarcinoma, and H. pylori-dependent gastric tumor. Despite the pathogenic impacts of tight junction disassembly in these diseases currently being unclear, phenotypic abnormalities of tight junctions may be of interest as diagnostic and prognostic markers for these conditions.
Concluding remarks
In this review, we discuss about the cellular architecture of epithelial tight junction in the gastrointestinal tract. Indeed, based on localizations tight junction at subcellular level, tight junction can be differentially divided into bi- and tri-cellular tight junctions. According to the physical properties of tight junction, there are, at least, pore-forming and barrier-forming tight junctions. In fact, tight junction proteins can be either transmembrane or intracellular scaffolding proteins. In addition, we also furnish the comprehensive views of the relationship between intestinal tight junction disruption and gastrointestinal diseases including Barrett’s esophagus, GERD, H. pylori-induced gastritis, IBD, IBS, cholera, and various types of cancers along the gastrointestinal tract. Nevertheless, there is currently no FDA-approved therapy for tight junction disruption-related diseases. Of particular importance, activation of AMPK and inhibition of MLCK recruitment to PAMR have been markedly proposed as the therapeutic approaches for recovery of intestinal tight junction disruption and related diseases, especially IBD. Furthermore, we also show evidence regarding other possible therapeutic targets, small molecules, and known drugs that have been of interest for the development of new drug for targeting intestinal tight junction recovery.
Author contributions
AM: Writing - original draft, lead; funding acquisition; figure preparation; writing - review and editing
NP: Writing - original draft; writing - review and editing
PRS: Writing - review and editing
PC: Writing - original draft
WS: Writing - original draft, funding acquisition.
PP: Conceptualization, lead; funding acquisition, supporting; lead; writing - original draft, lead; writing - review and editing, lead
Acknowledgments
This project is funded by National Research Council of Thailand (NRCT) (Grant No. N42A650225) (to PP). This work is supported by Chulabhorn Royal Academy (Project code ISFO2-004/2565) (to PP), the Mahidol Medical Scholars Program (to WS), Mahidol University (Basic Research Fund: fiscal year 2021) (to AM), and Office of the Permanent Secretary, Ministry of Higher Education, Science, Research and Innovation (Grant No. RGNS 64-177) (to AM).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol. 1963;17:114–146.
- Machen TE, Erlij D, Wooding FB. Permeable junctional complexes. The movement of lanthanum across rabbit gallbladder and intestine. J Cell Biol. 1972;54:302–312.
- Friend DS, Gilula NB. Variations in tight and gap junctions in mammalian tissues. J Cell Biol. 1972;53:758–776.
- Goodenough DA, Revel JP. A fine structural analysis of intercellular junctions in the mouse liver. J Cell Biol. 1970;45:272–290.
- Staehelin LA. Further observations on the fine structure of freeze-cleaved tight junctions. J Cell Sci. 1973;13:763–786.
- Claude P, Goodenough DA. Fracture faces of zonulae occludentes from ”tight” and ”leaky” epithelia. J Cell Biol. 1973;58:390–400.
- Madara JL, Dharmsathaphorn K. Occluding junction structure-function relationships in a cultured epithelial monolayer. J Cell Biol. 1985;101:2124–2133.
- Meyer HW. Tight junction strands are lipidic cylinders. Naturwissenschaften. 1983;70:251–252.
- Kachar B, Reese TS. Evidence for the lipidic nature of tight junction strands. Nature. 1982;296:464–466.
- Pinto da Silva P, Kachar B. On tight-junction structure. Cell. 1982;28(3):441–450. doi:10.1016/0092-8674(82)90198-2.
- van Meer G, Gumbiner B, Simons K. The tight junction does not allow lipid molecules to diffuse from one epithelial cell to the next. Nature. 1986;322(6080):639–641. doi:10.1038/322639a0.
- Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986;103(3):755–766. doi:10.1083/jcb.103.3.755.
- Citi S, Sabanay H, Jakes R, Geiger B, Kendrick-Jones J. Cingulin, a new peripheral component of tight junctions. Nature. 1988;333(6170):272–276. doi:10.1038/333272a0.
- Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123(6 Pt 2):1777–1788. doi:10.1083/jcb.123.6.1777.
- Raleigh DR, Marchiando AM, Zhang Y, Shen L, Sasaki H, Wang Y, Long M, Turner JR, Omary MB. Tight junction-associated MARVEL proteins marveld3, tricellulin, and occludin have distinct but overlapping functions. Mol Biol Cell. 2010;21(7):1200–1213. doi:10.1091/mbc.e09-08-0734.
- Steed E, Rodrigues NT, Balda MS, Matter K. Identification of MarvelD3 as a tight junction-associated transmembrane protein of the occludin family. BMC Cell Biol. 2009;10:95.
- Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A, et al. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 1998;142:117–127.
- Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and −2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141:1539–1550.
- Sohet F, Lin C, Munji RN, Lee SY, Ruderisch N, Soung A, Arnold TD, Derugin N, Vexler ZS, Yen FT, et al. LSR/angulin-1 is a tricellular tight junction protein involved in blood-brain barrier formation. J Cell Biol. 2015;208:703–711.
- Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S, Tsukita S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol. 2005;171:939–945.
- Raju P, Shashikanth N, Tsai PY, Pongkorpsakol P, Chanez-Paredes S, Steinhagen PR, Kuo WT, Singh G, Tsukita S, Turner JR. Inactivation of paracellular cation-selective claudin-2 channels attenuates immune-mediated experimental colitis in mice. J Clin Invest. 2020;130:5197–5208.
- Gunzel D, Yu AS. Claudins and the modulation of tight junction permeability. Physiol Rev. 2013;93:525–569.
- Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol. 2011;73:283–309.
- Weber C, Fraemohs L, Dejana E. The role of junctional adhesion molecules in vascular inflammation. Nat Rev Immunol. 2007;7:467–477.
- Hartmann C, Schwietzer YA, Otani T, Furuse M, Ebnet K. Physiological functions of junctional adhesion molecules (JAMs) in tight junctions. Biochim Biophys Acta Biomembr. 2020;1862:183299.
- Hirabayashi S, Tajima M, Yao I, Nishimura W, Mori H, Hata Y. JAM4, a junctional cell adhesion molecule interacting with a tight junction protein, MAGI-1. Mol Cell Biol. 2003;23:4267–4282.
- Steinbacher T, Kummer D, Ebnet K. Junctional adhesion molecule-A: functional diversity through molecular promiscuity. Cell Mol Life Sci. 2018;75:1393–1409.
- Otani T, Furuse M. Tight Junction Structure and Function Revisited. Trends Cell Biol. 2020;30:805–817.
- Suzuki H, Tani K, Tamura A, Tsukita S, Fujiyoshi Y. Model for the architecture of claudin-based paracellular ion channels through tight junctions. J Mol Biol. 2015;427:291–297.
- Cummins PM. Occludin: one protein, many forms. Mol Cell Biol. 2012;32:242–250.
- Dorfel MJ, Huber O. Modulation of tight junction structure and function by kinases and phosphatases targeting occludin. J Biomed Biotechnol. 2012;2012:807356.
- Zihni C, Mills C, Matter K, Balda MS. Tight junctions: from simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol. 2016;17:564–580.
- Kuo WT, Zuo L, Odenwald MA, Madha S, Singh G, Gurniak CB, Abraham C, Turner JR. The Tight Junction Protein ZO-1 Is Dispensable for Barrier Function but Critical for Effective Mucosal Repair. Gastroenterology. 2021;161:1924–1939.
- Bosveld F, Wang Z, Bellaiche Y. Tricellular junctions: a hot corner of epithelial biology. Curr Opin Cell Biol. 2018;54:80–88.
- Guillemot L, Paschoud S, Pulimeno P, Foglia A, Citi S. The cytoplasmic plaque of tight junctions: a scaffolding and signalling center. Biochim Biophys Acta. 2008;1778:601–613.
- Utepbergenov DI, Fanning AS, Anderson JM. Dimerization of the scaffolding protein ZO-1 through the second PDZ domain. J Biol Chem. 2006;281:24671–24677.
- Fanning AS, Anderson JM. PDZ domains: fundamental building blocks in the organization of protein complexes at the plasma membrane. J Clin Invest. 1999;103:767–772.
- Herve JC, Derangeon M, Sarrouilhe D, Bourmeyster N. Influence of the scaffolding protein Zonula Occludens (ZOs) on membrane channels. Biochim Biophys Acta. 2014;1838:595–604.
- Gonzalez-Mariscal L, Betanzos A, Avila-Flores A. MAGUK proteins: structure and role in the tight junction. Semin Cell Dev Biol. 2000;11:315–324.
- Nomme J, Antanasijevic A, Caffrey M, Van Itallie CM, Anderson JM, Fanning AS, Lavie A. Structural Basis of a Key Factor Regulating the Affinity between the Zonula Occludens First PDZ Domain and Claudins. J Biol Chem. 2015;290:16595–16606.
- Rouaud F, Sluysmans S, Flinois A, Shah J, Vasileva E, Citi S. Scaffolding proteins of vertebrate apical junctions: structure, functions and biophysics. Biochim Biophys Acta Biomembr. 2020;1862:183399.
- Lye MF, Fanning AS, Su Y, Anderson JM, Lavie A. Insights into regulated ligand binding sites from the structure of ZO-1 Src homology 3-guanylate kinase module. J Biol Chem. 2010;285:13907–13917.
- Beutel O, Maraspini R, Pombo-Garcia K, Martin-Lemaitre C, Honigmann A. Phase Separation of Zonula Occludens Proteins Drives Formation of Tight Junctions. Cell. 2019;179:923–936 e11.
- Odenwald MA, Choi W, Kuo WT, Singh G, Sailer A, Wang Y, Shen L, Fanning AS, Turner JR. The scaffolding protein ZO-1 coordinates actomyosin and epithelial apical specializations in vitro and in vivo. J Biol Chem. 2018;293:17317–17335.
- Otani T, Nguyen TP, Tokuda S, Sugihara K, Sugawara T, Furuse K, Miura T, Ebnet K, Furuse M. Claudins and JAM-A coordinately regulate tight junction formation and epithelial polarity. J Cell Biol. 2019;218:3372–3396.
- Ebnet K, Schulz CU, Meyer Zu Brickwedde MK, Pendl GG, Vestweber D. Junctional adhesion molecule interacts with the PDZ domain-containing proteins AF-6 and ZO-1. J Biol Chem. 2000;275:27979–27988.
- Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273:29745–29753.
- Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, Nakayama M, Matsui T, Tsukita S, Furuse M, Tsukita S. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006;126:741–754.
- Citi S. The mechanobiology of tight junctions. Biophys Rev. 2019;11:783–793.
- Belardi B, Hamkins-Indik T, Harris AR, Kim J, Xu K, Fletcher DA. A Weak Link with Actin Organizes Tight Junctions to Control Epithelial Permeability. Dev Cell. 2020;54:792–804 e7.
- Wu J, Rowart P, Jouret F, Gassaway BM, Rajendran V, Rinehart J, Caplan MJ. Mechanisms involved in AMPK-mediated deposition of tight junction components to the plasma membrane. Am J Physiol Cell Physiol. 2020;318:C486–C501.
- Lynch AM, Grana T, Cox-Paulson E, Couthier A, Cameron M, Chin-Sang I, Pettitt J, Hardin J. A genome-wide functional screen shows MAGI-1 is an L1CAM-dependent stabilizer of apical junctions in C. elegans. Curr Biol. 2012;22:1891–1899.
- Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207–235.
- Michel D, Arsanto JP, Massey-Harroche D, Beclin C, Wijnholds J, Le Bivic A. PATJ connects and stabilizes apical and lateral components of tight junctions in human intestinal cells. J Cell Sci. 2005;118:4049–4057.
- Miyahara M, Nakanishi H, Takahashi K, Satoh-Horikawa K, Tachibana K, Takai Y. Interaction of nectin with afadin is necessary for its clustering at cell-cell contact sites but not for its cis dimerization or trans interaction. J Biol Chem. 2000;275:613–618.
- Huxham J, Tabaries S, Siegel PM. Afadin (AF6) in cancer progression: a multidomain scaffold protein with complex and contradictory roles. Bioessays. 2021;43:e2000221.
- Xu Y, Chang R, Peng Z, Wang Y, Ji W, Guo J, Song L, Dai C, Wei W, Wu Y, et al. Loss of polarity protein AF6 promotes pancreatic cancer metastasis by inducing Snail expression. Nat Commun. 2015;6:7184.
- Sun TT, Wang Y, Cheng H, Xiao HZ, Xiang JJ, Zhang JT, Yu SB, Martin TA, Ye L, Tsang LL, et al. Disrupted interaction between CFTR and AF-6/afadin aggravates malignant phenotypes of colon cancer. Biochim Biophys Acta. 2014;1843:618–628.
- Paschoud S, Yu D, Pulimeno P, Jond L, Turner JR, Citi S. Cingulin and paracingulin show similar dynamic behaviour, but are recruited independently to junctions. Mol Membr Biol. 2011;28:123–135.
- Cordenonsi M, D’Atri F, Hammar E, Parry DA, Kendrick-Jones J, Shore D, Citi S. Cingulin contains globular and coiled-coil domains and interacts with ZO-1, ZO-2, ZO-3, and myosin. J Cell Biol. 1999;147:1569–1582.
- D’Atri F, Citi S. Cingulin interacts with F-actin in vitro. FEBS Lett. 2001;507:21–24.
- Yano T, Torisawa T, Oiwa K, Tsukita S. AMPK-dependent phosphorylation of cingulin reversibly regulates its binding to actin filaments and microtubules. Sci Rep. 2018;8:15550.
- Yano T, Matsui T, Tamura A, Uji M, Tsukita S. The association of microtubules with tight junctions is promoted by cingulin phosphorylation by AMPK. J Cell Biol. 2013;203:605–614.
- Guillemot L, Paschoud S, Jond L, Foglia A, Citi S. Paracingulin regulates the activity of Rac1 and RhoA GTPases by recruiting Tiam1 and GEF-H1 to epithelial junctions. Mol Biol Cell. 2008;19:4442–4453.
- Guillemot L, Citi S. Cingulin regulates claudin-2 expression and cell proliferation through the small GTPase RhoA. Mol Biol Cell. 2006;17:3569–3577.
- Aijaz S, D’Atri F, Citi S, Balda MS, Matter K. Binding of GEF-H1 to the tight junction-associated adaptor cingulin results in inhibition of Rho signaling and G1/S phase transition. Dev Cell. 2005;8:777–786.
- Marchiando AM, Graham WV, Turner JR. Epithelial barriers in homeostasis and disease. Annu Rev Pathol. 2010;5:119–144.
- Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809.
- Zuo L, Kuo WT, Turner JR. Tight Junctions as Targets and Effectors of Mucosal Immune Homeostasis. Cell Mol Gastroenterol Hepatol. 2020;10:327–340.
- Odenwald MA, Turner JR. The intestinal epithelial barrier: a therapeutic target? Nat Rev Gastroenterol Hepatol. 2017;14:9–21.
- Chen L, Tuo B, Dong H. Regulation of Intestinal Glucose Absorption by Ion Channels and Transporters. Nutrients. 2016;8(1).
- Kato A, Romero MF. Regulation of electroneutral NaCl absorption by the small intestine. Annu Rev Physiol. 2011;73:261–281.
- Kellett GL, Brot-Laroche E, Mace OJ, Leturque A. Sugar absorption in the intestine: the role of GLUT2. Annu Rev Nutr. 2008;28:35–54.
- Binder HJ, Rajendran V, Sadasivan V, Geibel JP. Bicarbonate secretion: a neglected aspect of colonic ion transport. J Clin Gastroenterol. 2005;39:S53–S58.
- Barrett KE, Keely SJ. Chloride secretion by the intestinal epithelium: molecular basis and regulatory aspects. Annu Rev Physiol. 2000;62:535–572.
- Pappenheimer JR. On the coupling of membrane digestion with intestinal absorption of sugars and amino acids. Am J Physiol. 1993;265:G409–G417.
- Turnberg LA. Absorption and secretion of salt and water by the small intestine. Digestion. 1973;9:357–381.
- Ong M, Yeruva S, Sailer A, Nilsen SP, Turner JR. Differential regulation of claudin-2 and claudin-15 expression in children and adults with malabsorptive disease. Lab Invest. 2020;100:483–490.
- Nalle SC, Zuo L, Ong M, Singh G, Worthylake AM, Choi W, Manresa MC, Southworth AP, Edelblum KL, Baker GJ, et al. Graft-versus-host disease propagation depends on increased intestinal epithelial tight junction permeability. J Clin Invest. 2019;129:902–914.
- Tsai PY, Zhang B, He WQ, Zha JM, Odenwald MA, Singh G, Tamura A, Shen L, Sailer A, Yeruva S, et al. IL-22 upregulates epithelial claudin-2 to drive diarrhea and enteric pathogen clearance. Cell Host Microbe. 2017;21:671–681 e4.
- Choi W, Yeruva S, Turner JR. Contributions of intestinal epithelial barriers to health and disease. Exp Cell Res. 2017;358:71–77.
- Nalle SC, Kwak HA, Edelblum KL, Joseph NE, Singh G, Khramtsova GF, Mortenson ED, Savage PA, Turner JR. Recipient NK cell inactivation and intestinal barrier loss are required for MHC-matched graft-versus-host disease. Sci Transl Med. 2014;6:243ra87.
- Odenwald MA, Turner JR. Intestinal permeability defects: is it time to treat? Clin Gastroenterol Hepatol. 2013;11:1075–1083.
- Su L, Nalle SC, Shen L, Turner ES, Singh G, Breskin LA, Khramtsova EA, Khramtsova G, Tsai PY, Fu YX, et al. TNFR2 activates MLCK-dependent tight junction dysregulation to cause apoptosis-mediated barrier loss and experimental colitis. Gastroenterology. 2013;145:407–415.
- Clayburgh DR, Barrett TA, Tang Y, Meddings JB, Van Eldik LJ, Watterson DM, Clarke LL, Mrsny RJ, Turner JR. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. J Clin Invest. 2005;115:2702–2715.
- Amasheh S, Meiri N, Gitter AH, Schoneberg T, Mankertz J, Schulzke JD, Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci. 2002;115:4969–4976.
- Van Itallie CM, Tietgens AJ, Anderson JM. Visualizing the dynamic coupling of claudin strands to the actin cytoskeleton through ZO-1. Mol Biol Cell. 2017;28:524–534.
- Van Itallie CM, Fanning AS, Bridges A, Anderson JM. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol Biol Cell. 2009;20:3930–3940.
- Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci. 2013;70:631–659.
- Sasaki H, Matsui C, Furuse K, Mimori-Kiyosue Y, Furuse M, Tsukita S. Dynamic behavior of paired claudin strands within apposing plasma membranes. Proc Natl Acad Sci U S A. 2003;100:3971–3976.
- Pongkorpsakol P, Buasakdi C, Chantivas T, Chatsudthipong V, Muanprasat C. An agonist of a zinc-sensing receptor GPR39 enhances tight junction assembly in intestinal epithelial cells via an AMPK-dependent mechanism. Eur J Pharmacol. 2019;842:306–313.
- Pearce SC, Al-Jawadi A, Kishida K, Yu S, Hu M, Fritzky LF, Edelblum KL, Gao N, Ferraris RP. Marked differences in tight junction composition and macromolecular permeability among different intestinal cell types. BMC Biol. 2018;16:19.
- Lechuga S, Ivanov AI. Disruption of the epithelial barrier during intestinal inflammation: Quest for new molecules and mechanisms. Biochim Biophys Acta Mol Cell Res. 2017;1864:1183–1194.
- Krug SM, Amasheh S, Richter JF, Milatz S, Gunzel D, Westphal JK, Huber O, Schulzke JD, Fromm M. Tricellulin forms a barrier to macromolecules in tricellular tight junctions without affecting ion permeability. Mol Biol Cell. 2009;20:3713–3724.
- Capaldo CT, Nusrat A. Claudin switching: physiological plasticity of the Tight Junction. Semin Cell Dev Biol. 2015;42:22–29.
- Buckley A, Turner JR. Cell biology of tight junction barrier regulation and mucosal disease. Cold Spring Harb Perspect Biol. 2018;10(1).
- Marchiando AM;, Shen L, Graham WV, Weber CR, Schwarz BT, Austin JR 2nd, Raleigh DR, Guan Y, Watson AJ, Montrose MH, et al. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J Cell Biol. 2010;189:111–126.
- Su L, Shen L, Clayburgh DR, Nalle SC, Sullivan EA, Meddings JB, Abraham C, Turner JR. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology. 2009;136:551–563.
- Shen L, Black ED, Witkowski ED, Lencer WI, Guerriero V, Schneeberger EE, Turner JR. Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J Cell Sci. 2006;119:2095–2106.
- Turner JR, Rill BK, Carlson SL, Carnes D, Kerner R, Mrsny RJ, Madara JL. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am J Physiol. 1997;273(4):C1378–85. doi:10.1152/ajpcell.1997.273.4.C1378.
- Atisook K, Carlson S, Madara JL. Effects of phlorizin and sodium on glucose-elicited alterations of cell junctions in intestinal epithelia. Am J Physiol. 1990;258(1 Pt 1):C77–85. doi:10.1152/ajpcell.1990.258.1.C77.
- Buschmann MM, Shen L, Rajapakse H, Raleigh DR, Wang Y, Wang Y, Lingaraju A, Zha J, Abbott E, McAuley EM, et al. Occludin OCEL-domain interactions are required for maintenance and regulation of the tight junction barrier to macromolecular flux. Mol Biol Cell. 2013;24(19):3056–3068. doi:10.1091/mbc.e12-09-0688.
- Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol. 2009;1(2):a002584. doi:10.1101/cshperspect.a002584.
- Van Itallie CM, Holmes J, Bridges A, Gookin JL, Coccaro MR, Proctor W, Colegio OR, Anderson JM. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. J Cell Sci. 2008;121(Pt 3):298–305. doi:10.1242/jcs.021485.
- Watson CJ, Hoare CJ, Garrod DR, Carlson GL, Warhurst G. Interferon-gamma selectively increases epithelial permeability to large molecules by activating different populations of paracellular pores. J Cell Sci. 2005;118(Pt 22):5221–5230. doi:10.1242/jcs.02630.
- Fihn BM, Sjoqvist A, Jodal M. Permeability of the rat small intestinal epithelium along the villus-crypt axis: effects of glucose transport. Gastroenterology. 2000;119(4):1029–1036. doi:10.1053/gast.2000.18148.
- Naftalin RJ, Tripathi S. Passive water flows driven across the isolated rabbit ileum by osmotic, hydrostatic and electrical gradients. J Physiol. 1985;360:27–50. doi:10.1113/jphysiol.1985.sp015602.
- Marcial MA, Carlson SL, Madara JL. Partitioning of paracellular conductance along the ileal crypt-villus axis: a hypothesis based on structural analysis with detailed consideration of tight junction structure-function relationships. J Membr Biol. 1984;80(1):59–70. doi:10.1007/BF01868690.
- Kiesler P, Fuss IJ, Strober W. Experimental Models of Inflammatory Bowel Diseases. Cell Mol Gastroenterol Hepatol. 2015;1(2):154–170. doi:10.1016/j.jcmgh.2015.01.006.
- Nalle SC, Turner JR. Intestinal barrier loss as a critical pathogenic link between inflammatory bowel disease and graft-versus-host disease. Mucosal Immunol. 2015;8(4):720–730. doi:10.1038/mi.2015.40.
- Pongkorpsakol P, Satianrapapong W, Wongkrasant P, Steinhagen PR, Tuangkijkul N, Pathomthongtaweechai N, Muanprasat C. Establishment of Intestinal Epithelial Cell Monolayers and Their Use in Calcium Switch Assay for Assessment of Intestinal Tight Junction Assembly. Methods Mol Biol. 2021;2367:273–290.
- Pongkorpsakol P, Turner JR, Zuo L. Culture of Intestinal Epithelial Cell Monolayers and Their Use in Multiplex Macromolecular Permeability Assays for In Vitro Analysis of Tight Junction Size Selectivity. Curr Protoc Immunol. 2020;131(1):e112. doi:10.1002/cpim.112.
- Chanez-Paredes SD, Abtahi S, Kuo WT, Turner JR. Differentiating Between Tight Junction-Dependent and Tight Junction-Independent Intestinal Barrier Loss In Vivo. Methods Mol Biol. 2021;2367:249–271.
- Colegio OR, Van Itallie CM, McCrea HJ, Rahner C, Anderson JM. Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am J Physiol Cell Physiol. 2002;283(1):C142–7. doi:10.1152/ajpcell.00038.2002.
- Van Itallie CM, Anderson JM. Measuring size-dependent permeability of the tight junction using PEG profiling. Methods Mol Biol. 2011;762:1–11.
- Weber CR, Raleigh DR, Su L, Shen L, Sullivan EA, Wang Y, Turner JR. Epithelial myosin light chain kinase activation induces mucosal interleukin-13 expression to alter tight junction ion selectivity. J Biol Chem. 2010;285(16):12037–12046. doi:10.1074/jbc.M109.064808.
- Rosenthal R, Gunzel D, Theune D, Czichos C, Schulzke JD, Fromm M. Water channels and barriers formed by claudins. Ann N Y Acad Sci. 2017;1397(1):100–109. doi:10.1111/nyas.13383.
- Tamura A, Hayashi H, Imasato M, Yamazaki Y, Hagiwara A, Wada M, Noda T, Watanabe M, Suzuki Y, Tsukita S. Loss of claudin-15, but not claudin-2, causes Na+ deficiency and glucose malabsorption in mouse small intestine. Gastroenterology. 2011;140(3):913–923. doi:10.1053/j.gastro.2010.08.006.
- Rosenthal R, Milatz S, Krug SM, Oelrich B, Schulzke JD, Amasheh S, Gunzel D, Fromm M. Claudin-2, a component of the tight junction, forms a paracellular water channel. J Cell Sci. 2010;123(Pt 11):1913–1921. doi:10.1242/jcs.060665.
- Fujita H, Chiba H, Yokozaki H, Sakai N, Sugimoto K, Wada T, Kojima T, Yamashita T, Sawada N. Differential expression and subcellular localization of claudin-7, −8, −12, −13, and −15 along the mouse intestine. J Histochem Cytochem. 2006;54(8):933–944. doi:10.1369/jhc.6A6944.2006.
- Holmes JL, Van Itallie CM, Rasmussen JE, Anderson JM. Claudin profiling in the mouse during postnatal intestinal development and along the gastrointestinal tract reveals complex expression patterns. Gene Expr Patterns. 2006;6(6):581–588. doi:10.1016/j.modgep.2005.12.001.
- Barmeyer C, Fromm M, Schulzke JD. Active and passive involvement of claudins in the pathophysiology of intestinal inflammatory diseases. Pflugers Arch. 2017;469(1):15–26. doi:10.1007/s00424-016-1914-6.
- Weber CR, Nalle SC, Tretiakova M, Rubin DT, Turner JR. Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation. Lab Invest. 2008;88(10):1110–1120. doi:10.1038/labinvest.2008.78.
- Yu AS, Cheng MH, Angelow S, Gunzel D, Kanzawa SA, Schneeberger EE, Fromm M, Coalson RD. Molecular basis for cation selectivity in claudin-2-based paracellular pores: identification of an electrostatic interaction site. J Gen Physiol. 2009;133(1):111–127. doi:10.1085/jgp.200810154.
- Weber CR, Liang GH, Wang Y, Das S, Shen L, Yu AS, Nelson DJ, Turner JR. Claudin-2-dependent paracellular channels are dynamically gated. Elife. 2015;4:e09906. doi:10.7554/eLife.09906.
- Madara JL. Tight junction dynamics: is paracellular transport regulated? Cell. 1988;53(4):497–498. doi:10.1016/0092-8674(88)90562-4.
- Turner JR, Buschmann MM, Romero-Calvo I, Sailer A, Shen L. The role of molecular remodeling in differential regulation of tight junction permeability. Semin Cell Dev Biol. 2014;36:204–212. doi:10.1016/j.semcdb.2014.09.022.
- Yu D, Marchiando AM, Weber CR, Raleigh DR, Wang Y, Shen L, Turner JR. MLCK-dependent exchange and actin binding region-dependent anchoring of ZO-1 regulate tight junction barrier function. Proc Natl Acad Sci U S A. 2010;107(18):8237–8241. doi:10.1073/pnas.0908869107.
- Shen L, Weber CR, Turner JR. The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. J Cell Biol. 2008;181(4):683–695. doi:10.1083/jcb.200711165.
- Fanning AS, Little BP, Rahner C, Utepbergenov D, Walther Z, Anderson JM. The unique-5 and −6 motifs of ZO-1 regulate tight junction strand localization and scaffolding properties. Mol Biol Cell. 2007;18(3):721–731. doi:10.1091/mbc.e06-08-0764.
- Li Y, Fanning AS, Anderson JM, Lavie A. Structure of the conserved cytoplasmic C-terminal domain of occludin: identification of the ZO-1 binding surface. J Mol Biol. 2005;352(1):151–164. doi:10.1016/j.jmb.2005.07.017.
- Muller SL, Portwich M, Schmidt A, Utepbergenov DI, Huber O, Blasig IE, Krause G. The tight junction protein occludin and the adherens junction protein alpha-catenin share a common interaction mechanism with ZO-1. J Biol Chem. 2005;280(5):3747–3756. doi:10.1074/jbc.M411365200.
- Schmidt A, Utepbergenov DI, Mueller SL, Beyermann M, Schneider-Mergener J, Krause G, Blasig IE. Occludin binds to the SH3-hinge-GuK unit of zonula occludens protein 1: potential mechanism of tight junction regulation. Cell Mol Life Sci. 2004;61(11):1354–1365. doi:10.1007/s00018-004-4010-6.
- Raleigh DR, Boe DM, Yu D, Weber CR, Marchiando AM, Bradford EM, Wang Y, Wu L, Schneeberger EE, Shen L, et al. Occludin S408 phosphorylation regulates tight junction protein interactions and barrier function. J Cell Biol. 2011;193(3):565–582. doi:10.1083/jcb.201010065.
- Cording J, Berg J, Kading N, Bellmann C, Tscheik C, Westphal JK, Milatz S, Gunzel D, Wolburg H, Piontek J, et al. In tight junctions, claudins regulate the interactions between occludin, tricellulin and marvelD3, which, inversely, modulate claudin oligomerization. J Cell Sci. 2013;126(Pt 2):554–564. doi:10.1242/jcs.114306.
- Ikenouchi J, Sasaki H, Tsukita S, Furuse M, Tsukita S, Mostov KE. Loss of occludin affects tricellular localization of tricellulin. Mol Biol Cell. 2008;19(11):4687–4693. doi:10.1091/mbc.e08-05-0530.
- Saito AC, Higashi T, Fukazawa Y, Otani T, Tauchi M, Higashi AY, Furuse M, Chiba H. Occludin and tricellulin facilitate formation of anastomosing tight-junction strand network to improve barrier function. Mol Biol Cell. 2021;32(8):722–738. doi:10.1091/mbc.E20-07-0464.
- Inai T. The coculture method to examine interactions between claudin isoforms in tight junction-free HEK293 cells and tight junction-bearing MDCK II cells. Methods Mol Biol. 2011;762:101–114.
- Kachar B, Pinto da Silva P. Rapid massive assembly of tight junction strands. Science. 1981;213(4507):541–544. doi:10.1126/science.7244652.
- Zhao J, Krystofiak ES, Ballesteros A, Cui R, Van Itallie CM, Anderson JM, Fenollar-Ferrer C, Kachar B. Multiple claudin-claudin cis interfaces are required for tight junction strand formation and inherent flexibility. Commun Biol. 2018;1:50. doi:10.1038/s42003-018-0051-5.
- Piontek A, Rossa J, Protze J, Wolburg H, Hempel C, Gunzel D, Krause G, Piontek J. Polar and charged extracellular residues conserved among barrier-forming claudins contribute to tight junction strand formation. Ann N Y Acad Sci. 2017;1397(1):143–156. doi:10.1111/nyas.13341.
- Daugherty BL, Ward C, Smith T, Ritzenthaler JD, Koval M. Regulation of heterotypic claudin compatibility. J Biol Chem. 2007;282(41):30005–30013. doi:10.1074/jbc.M703547200.
- Milatz S, Piontek J, Hempel C, Meoli L, Grohe C, Fromm A, Lee IM, El-Athman R, Gunzel D. Tight junction strand formation by claudin-10 isoforms and claudin-10a/-10b chimeras. Ann N Y Acad Sci. 2017;1405(1):102–115. doi:10.1111/nyas.13393.
- Koval M. Claudins–key pieces in the tight junction puzzle. Cell Commun Adhes. 2006;13(3):127–138. doi:10.1080/15419060600726209.
- Furuse M, Tsukita S. Claudins in occluding junctions of humans and flies. Trends Cell Biol. 2006;16(4):181–188. doi:10.1016/j.tcb.2006.02.006.
- Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol. 2006;68:403–429. doi:10.1146/annurev.physiol.68.040104.131404.
- Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286(6):C1213–28. doi:10.1152/ajpcell.00558.2003.
- Furuse M, Sasaki H, Tsukita S. Manner of interaction of heterogeneous claudin species within and between tight junction strands. J Cell Biol. 1999;147(4):891–903. doi:10.1083/jcb.147.4.891.
- Piontek J, Winkler L, Wolburg H, Muller SL, Zuleger N, Piehl C, Wiesner B, Krause G, Blasig IE. Formation of tight junction: determinants of homophilic interaction between classic claudins. FASEB J. 2008;22(1):146–158. doi:10.1096/fj.07-8319com.
- Colegio OR, Van Itallie C, Rahner C, Anderson JM. Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am J Physiol Cell Physiol. 2003;284(6):C1346–54. doi:10.1152/ajpcell.00547.2002.
- Blue EK, Goeckeler ZM, Jin Y, Hou L, Dixon SA, Herring BP, Wysolmerski RB, Gallagher PJ. 220- and 130-kDa MLCKs have distinct tissue distributions and intracellular localization patterns. Am J Physiol Cell Physiol. 2002;282:C451–60.
- Cao F, Zhu L, Zhang J, Pongkorpsakol P, Kuo WT, Turner JR, Zhou Q, Wang Y, Chen F, Liu Y, et al. Myosin light chain kinase is a potential target for hypopharyngeal cancer treatment. Biomed Pharmacother. 2020;131:110665.
- Zhi G, Ryder JW, Huang J, Ding P, Chen Y, Zhao Y, Kamm KE, Stull JT. Myosin light chain kinase and myosin phosphorylation effect frequency-dependent potentiation of skeletal muscle contraction. Proc Natl Acad Sci U S A. 2005;102:17519–17524.
- Kamm KE, Stull JT. Dedicated myosin light chain kinases with diverse cellular functions. J Biol Chem. 2001;276:4527–4530.
- Clayburgh DR, Rosen S, Witkowski ED, Wang F, Blair S, Dudek S, Garcia JG, Alverdy JC, Turner JR. A differentiation-dependent splice variant of myosin light chain kinase, MLCK1, regulates epithelial tight junction permeability. J Biol Chem. 2004;279:55506–55513.
- Cunningham KE, Turner JR. Myosin light chain kinase: pulling the strings of epithelial tight junction function. Ann N Y Acad Sci. 2012;1258:34–42.
- Madara JL, Stafford J, Barenberg D, Carlson S. Functional coupling of tight junctions and microfilaments in T84 monolayers. Am J Physiol. 1988;254:G416–23.
- Madara JL, Moore R, Carlson S. Alteration of intestinal tight junction structure and permeability by cytoskeletal contraction. Am J Physiol. 1987;253:C854–61.
- Madara JL, Pappenheimer JR. Structural basis for physiological regulation of paracellular pathways in intestinal epithelia. J Membr Biol. 1987;100:149–164.
- Burgess DR. Reactivation of intestinal epithelial cell brush border motility: ATP-dependent contraction via a terminal web contractile ring. J Cell Biol. 1982;95:853–863.
- Graham WV, He W, Marchiando AM, Zha J, Singh G, Li HS, Biswas A, Ong M, Jiang ZH, Choi W, et al. Intracellular MLCK1 diversion reverses barrier loss to restore mucosal homeostasis. Nat Med. 2019;25:690–700.
- Wang F, Schwarz BT, Graham WV, Wang Y, Su L, Clayburgh DR, Abraham C, Turner JR. IFN-gamma-induced TNFR2 expression is required for TNF-dependent intestinal epithelial barrier dysfunction. Gastroenterology. 2006;131:1153–1163.
- Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol. 2005;166:409–419.
- Van Itallie CM, Balda MS, Anderson JM. Epidermal growth factor induces tyrosine phosphorylation and reorganization of the tight junction protein ZO-1 in A431 cells. J Cell Sci. 1995;108:1735–1742.
- Kurihara H, Anderson JM, Farquhar MG. Increased Tyr phosphorylation of ZO-1 during modification of tight junctions between glomerular foot processes. Am J Physiol. 1995;268:F514–24.
- Stevenson BR, Anderson JM, Braun ID, Mooseker MS. Phosphorylation of the tight-junction protein ZO-1 in two strains of Madin-Darby canine kidney cells which differ in transepithelial resistance. Biochem J. 1989;263:597–599.
- Schofield AV, Bernard O. Rho-associated coiled-coil kinase (ROCK) signaling and disease. Crit Rev Biochem Mol Biol. 2013;48:301–316.
- Saadeldin IM, Tukur HA, Aljumaah RS, Sindi RA. Rocking the Boat: The Decisive Roles of Rho Kinases During Oocyte, Blastocyst, and Stem Cell Development. Front Cell Dev Biol. 2020;8:616762.
- Hinkel R, Trenkwalder T, Petersen B, Husada W, Gesenhues F, Lee S, Hannappel E, Bock-Marquette I, Theisen D, Leitner L, et al. MRTF-A controls vessel growth and maturation by increasing the expression of CCN1 and CCN2. Nat Commun. 2014;5:3970.
- Shimada H, Rajagopalan LE. Rho-kinase mediates lysophosphatidic acid-induced IL-8 and MCP-1 production via p38 and JNK pathways in human endothelial cells. FEBS Lett. 2010;584:2827–2832.
- Mavria G, Vercoulen Y, Yeo M, Paterson H, Karasarides M, Marais R, Bird D, Marshall CJ. ERK-MAPK signaling opposes Rho-kinase to promote endothelial cell survival and sprouting during angiogenesis. Cancer Cell. 2006;9:33–44.
- Totsukawa G, Yamakita Y, Yamashiro S, Hartshorne DJ, Sasaki Y, Matsumura F. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J Cell Biol. 2000;150:797–806.
- Acharya BR, Nestor-Bergmann A, Liang X, Gupta S, Duszyc K, Gauquelin E, Gomez GA, Budnar S, Marcq P, Jensen OE, et al. A Mechanosensitive RhoA Pathway that Protects Epithelia against Acute Tensile Stress. Dev Cell. 2018;47:439–452 e6.
- Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446–456.
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science. 1996;273:245–248.
- Wu J, Li J, Hu H, Liu P, Fang Y, Wu D. Rho-kinase inhibitor, fasudil, prevents neuronal apoptosis via the Akt activation and PTEN inactivation in the ischemic penumbra of rat brain. Cell Mol Neurobiol. 2012;32:1187–1197.
- Holvoet T, Devriese S, Castermans K, Boland S, Leysen D, Vandewynckel YP, Devisscher L, Van den Bossche L, Van Welden S, Dullaers M, et al. Treatment of Intestinal Fibrosis in Experimental Inflammatory Bowel Disease by the Pleiotropic Actions of a Local Rho Kinase Inhibitor. Gastroenterology. 2017;153:1054–1067.
- Soliman M, Cho EH, Park JG, Kim JY, Alfajaro MM, Baek YB, Kim DS, Kang MI, Park SI, Cho KO. Rotavirus-Induced Early Activation of the RhoA/ROCK/MLC Signaling Pathway Mediates the Disruption of Tight Junctions in Polarized MDCK Cells. Sci Rep. 2018;8:13931.
- Wang Y, Wang X, Yang W, Zhao X, Zhang R. Effect of Simvastatin on the Intestinal Rho/ROCK Signaling Pathway in Rats With Sepsis. J Surg Res. 2018;232:531–538.
- Grothaus JS, Ares G, Yuan C, Wood DR, Hunter CJ. Rho kinase inhibition maintains intestinal and vascular barrier function by upregulation of occludin in experimental necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2018;315:G514–G528.
- Ares G, Buonpane C, Sincavage J, Yuan C, Wood DR, Hunter CJ. Caveolin 1 is Associated with Upregulated Claudin 2 in Necrotizing Enterocolitis. Sci Rep. 2019;9:4982.
- Carracedo A, Pandolfi PP. The PTEN-PI3K pathway: of feedbacks and cross-talks. Oncogene. 2008;27:5527–5541.
- He S, Guo Y, Zhao J, Xu X, Wang N, Liu Q. Ferulic Acid Ameliorates Lipopolysaccharide-Induced Barrier Dysfunction via MicroRNA-200c-3p-Mediated Activation of PI3K/AKT Pathway in Caco-2 Cells. Front Pharmacol. 2020;11:376.
- Mitchell J, Kim SJ, Koukos G, Seelmann A, Veit B, Shepard B, Blumer-Schuette S, Winter HS, Iliopoulos D, Pothoulakis C, et al. Colonic Inhibition of Phosphatase and Tensin Homolog Increases Colitogenic Bacteria, Causing Development of Colitis in Il10-/- Mice. Inflamm Bowel Dis. 2018;24:1718–1732.
- Meloche S, Pouyssegur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene. 2007;26:3227–3239.
- Jiang J, Huang K, Xu S, Garcia JGN, Wang C, Cai H. Targeting NOX4 alleviates sepsis-induced acute lung injury via attenuation of redox-sensitive activation of CaMKII/ERK1/2/MLCK and endothelial cell barrier dysfunction. Redox Biol. 2020;36:101638.
- Moonwiriyakit A, Koval M, Muanprasat C. Pharmacological stimulation of G-protein coupled receptor 40 alleviates cytokine-induced epithelial barrier disruption in airway epithelial Calu-3 cells. Int Immunopharmacol. 2019;73:353–361.
- Xu T, Dong Z, Wang X, Qi S, Li X, Cheng R, Liu X, Zhang Y, Gao MQ. IL-1beta induces increased tight junction permeability in bovine mammary epithelial cells via the IL-1beta-ERK1/2-MLCK axis upon blood-milk barrier damage. J Cell Biochem. 2018;119:9028–9041.
- Ryu WI, Lee H, Bae HC, Jeon J, Ryu HJ, Kim J, Kim JH, Son JW, Kim J, Imai Y, et al. IL-33 down-regulates CLDN1 expression through the ERK/STAT3 pathway in keratinocytes. J Dermatol Sci. 2018;90:313–322.
- Al-Sadi R, Guo S, Ye D, Ma TY. TNF-alpha modulation of intestinal epithelial tight junction barrier is regulated by ERK1/2 activation of Elk-1. Am J Pathol. 2013;183:1871–1884.
- Iglesias DE, Cremonini E, Fraga CG, Oteiza PI. Ellagic acid protects Caco-2 cell monolayers against inflammation-induced permeabilization. Free Radic Biol Med. 2020;152:776–786.
- Al-Sadi R, Ye D, Said HM, Ma TY. Cellular and molecular mechanism of interleukin-1 beta modulation of Caco-2 intestinal epithelial tight junction barrier. J Cell Mol Med. 2011;15:970–982.
- Kim CY, Kim KH. Curcumin prevents leptin-induced tight junction dysfunction in intestinal Caco-2 BBe cells. J Nutr Biochem. 2014;25:26–35.
- Wang Z, Litterio MC, Muller M, Vauzour D, Oteiza PI. (-)-Epicatechin and NADPH oxidase inhibitors prevent bile acid-induced Caco-2 monolayer permeabilization through ERK1/2 modulation. Redox Biol. 2020;28:101360.
- Chen Y, Zhang L, Zhang Y, Bai T, Song J, Qian W, Hou X. EphrinA1/EphA2 promotes epithelial hyperpermeability involving in Lipopolysaccharide-induced intestinal barrier dysfunction. J Neurogastroenterol Motil. 2020;26:397–409.
- Samak G, Aggarwal S, Rao RK. ERK is involved in EGF-mediated protection of tight junctions, but not adherens junctions, in acetaldehyde-treated Caco-2 cell monolayers. Am J Physiol Gastrointest Liver Physiol. 2011;301:G50–9.
- Suzuki T, Yoshinaga N, Tanabe S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J Biol Chem. 2011;286:31263–31271.
- Inai T, Kitagawa N, Hatakeyama Y, Ikebe T, Iida H, Fujita M. Inhibition of extracellular signal-regulated kinase downregulates claudin-2 expression and alters paracellular permeability in mouse rectum CMT93-II cells. Tissue Cell. 2013;45:175–182.
- Ikari A, Sato T, Watanabe R, Yamazaki Y, Sugatani J. Increase in claudin-2 expression by an EGFR/MEK/ERK/c-Fos pathway in lung adenocarcinoma A549 cells. Biochim Biophys Acta. 2012;1823:1110–1118.
- Kim B, Breton S. The MAPK/ERK-Signaling Pathway Regulates the Expression and Distribution of Tight Junction Proteins in the Mouse Proximal Epididymis. Biol Reprod. 2016;94:22.
- Muanprasat C, Wongkrasant P, Satitsri S, Moonwiriyakit A, Pongkorpsakol P, Mattaveewong T, Pichyangkura R, Chatsudthipong V. Activation of AMPK by chitosan oligosaccharide in intestinal epithelial cells: mechanism of action and potential applications in intestinal disorders. Biochem Pharmacol. 2015;96:225–236.
- Pongkorpsakol P, Satitsri S, Wongkrasant P, Chittavanich P, Kittayaruksakul S, Srimanote P, Chatsudthipong V, Muanprasat C. Flufenamic acid protects against intestinal fluid secretion and barrier leakage in a mouse model of Vibrio cholerae infection through NF-kappaB inhibition and AMPK activation. Eur J Pharmacol. 2017;798:94–104.
- Moonwiriyakit A, Wattanaphichet P, Chatsudthipong V, Muanprasat C. GPR40 receptor activation promotes tight junction assembly in airway epithelial cells via AMPK-dependent mechanisms. Tissue Barriers. 2018;6:1–12.
- Zhang L, Li J, Young LH, Caplan MJ. AMP-activated protein kinase regulates the assembly of epithelial tight junctions. Proc Natl Acad Sci U S A. 2006;103:17272–17277.
- Chen L, Wang J, You Q, He S, Meng Q, Gao J, Wu X, Shen Y, Sun Y, Wu X, et al. Activating AMPK to Restore Tight Junction Assembly in Intestinal Epithelium and to Attenuate Experimental Colitis by Metformin. Front Pharmacol. 2018;9:761.
- Muanprasat C, Wongkrasant P, Satitsri S, Moonwiriyakit A, Pongkorpsakol P, Mattaveewong T, Pichyangkura R, Chatsudthipong V. Activation of AMPK by chitosan oligosaccharide in intestinal epithelial cells: Mechanism of action and potential applications in intestinal disorders. Biochem Pharmacol. 2015;96:225–236.
- Glucagon control of glucose, energy and lipid metabolism. Nat Rev Endocrinol. 2013;9(3):131–131.
- Shiomi R, Shigetomi K, Inai T, Sakai M, Ikenouchi J. CaMKII regulates the strength of the epithelial barrier. Sci Rep. 2015;5:13262.
- Xiang RL, Mei M, Cong X, Li J, Zhang Y, Ding C, Wu LL, Yu GY. Claudin-4 is required for AMPK-modulated paracellular permeability in submandibular gland cells. J Mol Cell Biol. 2014;6:486–497.
- Gopalakrishnan S, Hallett MA, Atkinson SJ, Marrs JA. aPKC-PAR complex dysfunction and tight junction disassembly in renal epithelial cells during ATP depletion. Am J Physiol Cell Physiol. 2007;292:C1094–102.
- Ghosh P. The stress polarity pathway: AMPK ‘GIV’-es protection against metabolic insults. Aging (Albany NY). 2017;9:303–314.
- Assemat E, Bazellieres E, Pallesi-Pocachard E, Le Bivic A, Massey-Harroche D. Polarity complex proteins. Biochim Biophys Acta. 2008;1778:614–630.
- Qiu RG, Abo A, Steven Martin G. A human homolog of the C. elegans polarity determinant Par-6 links Rac and Cdc42 to PKCzeta signaling and cell transformation. Curr Biol. 2000;10:697–707.
- Joberty G, Petersen C, Gao L, Macara IG. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat Cell Biol. 2000;2:531–539.
- Suzuki A, Yamanaka T, Hirose T, Manabe N, Mizuno K, Shimizu M, Akimoto K, Izumi Y, Ohnishi T, Ohno S. Atypical protein kinase C is involved in the evolutionarily conserved par protein complex and plays a critical role in establishing epithelia-specific junctional structures. J Cell Biol. 2001;152:1183–1196.
- Rosse C, Linch M, Kermorgant S, Cameron AJ, Boeckeler K, Parker PJ. PKC and the control of localized signal dynamics. Nat Rev Mol Cell Biol. 2010;11:103–112.
- Jain S, Suzuki T, Seth A, Samak G, Rao R. Protein kinase Czeta phosphorylates occludin and promotes assembly of epithelial tight junctions. Biochem J. 2011;437:289–299.
- Seth A, Sheth P, Elias BC, Rao R. Protein phosphatases 2A and 1 interact with occludin and negatively regulate the assembly of tight junctions in the CACO-2 cell monolayer. J Biol Chem. 2007;282:11487–11498.
- Palazzo AF, Lee ES. Non-coding RNA: what is functional and what is junk? Front Genet. 2015;6:2.
- Teng CL, Yang VC. A facile colorimetric protamine titration method. J Lab Clin Med. 1989;113:498–504.
- O’Brien J, Hayder H, Zayed Y, Peng C. Overview of MicroRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol (Lausanne). 2018;9:402.
- Breving K, Esquela-Kerscher A. The complexities of microRNA regulation: mirandering around the rules. Int J Biochem Cell Biol. 2010;42:1316–1329.
- Zhang X, Wang W, Zhu W, Dong J, Cheng Y, Yin Z, Shen F. Mechanisms and Functions of Long Non-Coding RNAs at Multiple Regulatory Levels. Int J Mol Sci. 2019;20:22.
- Yao RW, Wang Y, Chen LL. Cellular functions of long noncoding RNAs. Nat Cell Biol. 2019;21:542–551.
- Lashgarian HE, Karkhane M, Marzban A, Yazdi M, Shahzamani K. Emerging Involvement of long non-coding RNAs in gastrointestinal associated inflammatory disorders. Comp Immunol Microbiol Infect Dis. 2020;69:101428.
- Hossian A, Mackenzie GG, MattheolabakisG. miRNAs in gastrointestinal diseases: can we effectively deliver RNA-based therapeutics orally?. Nanomed (Lond). 2019;14(21):2873–2889.
- Zhang L, Cheng J, Fan XM. MicroRNAs: new therapeutic targets for intestinal barrier dysfunction. World J Gastroenterol. 2014;20:5818–5825.
- Veltman K, Hummel S, Cichon C, Sonnenborn U, Schmidt MA. Identification of specific miRNAs targeting proteins of the apical junctional complex that simulate the probiotic effect of E. coli Nissle 1917 on T84 epithelial cells. Int J Biochem Cell Biol. 2012;44:341–349.
- Zhao X, Zeng H, Lei L, Tong X, Yang L, Yang Y, Li S, Zhou Y, Luo L, Huang J, et al. Tight junctions and their regulation by non-coding RNAs. Int J Biol Sci. 2021;17:712–727.
- Yang Y, Ma Y, Shi C, Chen H, Zhang H, Chen N, Zhang P, Wang F, Yang J, Yang J, et al. Overexpression of miR-21 in patients with ulcerative colitis impairs intestinal epithelial barrier function through targeting the Rho GTPase RhoB. Biochem Biophys Res Commun. 2013;434:746–752.
- Zhang L, Zhang F, He DK, Fan XM, Shen J. MicroRNA-21 is upregulated during intestinal barrier dysfunction induced by ischemia reperfusion. Kaohsiung J Med Sci. 2018;34:556–563.
- Nakata K, Sugi Y, Narabayashi H, Kobayakawa T, Nakanishi Y, Tsuda M, Hosono A, Kaminogawa S, Hanazawa S, Takahashi K. Commensal microbiota-induced microRNA modulates intestinal epithelial permeability through the small GTPase ARF4. J Biol Chem. 2017;292:15426–15433.
- Wang H, Chao K, Ng SC, Bai AH, Yu Q, Yu J, Li M, Cui Y, Chen M, Hu JF, et al. Pro-inflammatory miR-223 mediates the cross-talk between the IL23 pathway and the intestinal barrier in inflammatory bowel disease. Genome Biol. 2016;17:58.
- Ye D, Guo S, Al-Sadi R, Ma TY. MicroRNA regulation of intestinal epithelial tight junction permeability. Gastroenterology. 2011;141:1323–1333.
- Zhu Y, Wang W, Yuan T, Fu L, Zhou L, Lin G, Zhao S, Zhou H, Wu G, Wang J. MicroRNA-29a mediates the impairment of intestinal epithelial integrity induced by intrauterine growth restriction in pig. Am J Physiol Gastrointest Liver Physiol. 2017;312:G434–G442.
- Martinez C, Rodino-Janeiro BK, Lobo B, Stanifer ML, Klaus B, Granzow M, Gonzalez-Castro AM, Salvo-Romero E, Alonso-Cotoner C, Pigrau M, et al. miR-16 and miR-125b are involved in barrier function dysregulation through the modulation of claudin-2 and cingulin expression in the jejunum in IBS with diarrhoea. Gut. 2017;66:1537–1538.
- Statello L, Guo CJ, Chen LL, Huarte M. Author Correction: Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol. 2021;22:159.
- Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146:353–358.
- Wang JY, Cui YH, Xiao L, Chung HK, Zhang Y, Rao JN, Gorospe M, Wang JY. Regulation of Intestinal Epithelial Barrier Function by Long Noncoding RNA uc.173 through Interaction with MicroRNA 29b. Mol Cell Biol. 2018;38:13.
- Ma D, Cao Y, Wang Z, He J, Chen H, Xiong H, Ren L, Shen C, Zhang X, Yan Y, et al. CCAT1 lncRNA Promotes Inflammatory Bowel Disease Malignancy by Destroying Intestinal Barrier via Downregulating miR-185-3p. Inflamm Bowel Dis. 2019;25:862–874.
- Chen SW, Wang PY, Liu YC, Sun L, Zhu J, Zuo S, Ma J, Li TY, Zhang JL, Chen GW, et al. Effect of Long Noncoding RNA H19 Overexpression on Intestinal Barrier Function and Its Potential Role in the Pathogenesis of Ulcerative Colitis. Inflamm Bowel Dis. 2016;22:2582–2592.
- Zou T, Jaladanki SK, Liu L, Xiao L, Chung HK, Wang JY, Xu Y, Gorospe M, Wang JY. H19 Long Noncoding RNA Regulates Intestinal Epithelial Barrier Function via MicroRNA 675 by Interacting with RNA-Binding Protein HuR. Mol Cell Biol. 2016;36:1332–1341.
- Chen T, Xue H, Lin R, Huang Z. MiR-34c and PlncRNA1 mediated the function of intestinal epithelial barrier by regulating tight junction proteins in inflammatory bowel disease. Biochem Biophys Res Commun. 2017;486:6–13.
- Xiao L, Rao JN, Cao S, Liu L, Chung HK, Zhang Y, Zhang J, Liu Y, Gorospe M, Wang JY. Long noncoding RNA SPRY4-IT1 regulates intestinal epithelial barrier function by modulating the expression levels of tight junction proteins. Mol Biol Cell. 2016;27:617–626.
- Cosme P, Rodriguez AB, Espino J, Garrido M. Plant Phenolics: Bioavailability as a Key Determinant of Their Potential Health-Promoting Applications. Antioxidants (Basel). 2020;9(12).
- Yang G, Bibi S, Du M, Suzuki T, Zhu MJ. Regulation of the intestinal tight junction by natural polyphenols: A mechanistic perspective. Crit Rev Food Sci Nutr. 2017;57:3830–3839.
- Gupta SC, Tyagi AK, Deshmukh-Taskar P, Hinojosa M, Prasad S, Aggarwal BB. Downregulation of tumor necrosis factor and other proinflammatory biomarkers by polyphenols. Arch Biochem Biophys. 2014;559:91–99.
- Carrasco-Pozo C, Morales P, Gotteland M. Polyphenols protect the epithelial barrier function of Caco-2 cells exposed to indomethacin through the modulation of occludin and zonula occludens-1 expression. J Agric Food Chem. 2013;61:5291–5297.
- Suzuki T, Tanabe S, Hara H. Kaempferol enhances intestinal barrier function through the cytoskeletal association and expression of tight junction proteins in Caco-2 cells. J Nutr. 2011;141:87–94.
- Suzuki T, Hara H. Quercetin enhances intestinal barrier function through the assembly of zonula [corrected] occludens-2, occludin, and claudin-1 and the expression of claudin-4 in Caco-2 cells. J Nutr. 2009;139:965–974.
- Amasheh M, Schlichter S, Amasheh S, Mankertz J, Zeitz M, Fromm M, Schulzke JD. Quercetin enhances epithelial barrier function and increases claudin-4 expression in Caco-2 cells. J Nutr. 2008;138:1067–1073.
- Li Q, Liang X, Guo N, Hu L, E MP, Wu Y, Xue X, Wu L, Wang K. Protective effects of Bee pollen extract on the Caco-2 intestinal barrier dysfunctions induced by dextran sulfate sodium. Biomed Pharmacother. 2019;117:109200.
- Wang K, Jin X, Chen Y, Song Z, Jiang X, Hu F, Conlon MA, Topping DL. Polyphenol-rich propolis extracts strengthen intestinal barrier function by activating AMPK and ERK signaling. Nutrients. 2016;8(5).
- Nallathambi R, Poulev A, Zuk JB, Raskin I. Proanthocyanidin-Rich Grape Seed Extract Reduces Inflammation and Oxidative Stress and Restores Tight Junction Barrier Function in Caco-2 Colon Cells. Nutrients. 2020;12:6.
- Bianchi MG, Chiu M, Taurino G, Brighenti F, Del Rio D, Mena P, Bussolati O. Catechin and procyanidin B2 modulate the expression of tight junction proteins but do not protect from inflammation-induced changes in permeability in human intestinal cell monolayers. Nutrients. 2019;11(10).
- Cai TT, Ye XL, Li RR, Chen H, Wang YY, Yong HJ, Pan ML, Lu W, Tang Y, Miao H, et al. Resveratrol modulates the gut microbiota and inflammation to protect against diabetic nephropathy in mice. Front Pharmacol. 2020;11:1249.
- Muanprasat C, Chatsudthipong V. Chitosan oligosaccharide: biological activities and potential therapeutic applications. Pharmacol Ther. 2017;170:80–97.
- Grenha A. Chitosan nanoparticles: a survey of preparation methods. J Drug Target. 2012;20:291–300.
- Pathomthongtaweechai N, Muanprasat C. Potential Applications of Chitosan-Based Nanomaterials to Surpass the Gastrointestinal Physiological Obstacles and Enhance the Intestinal Drug Absorption. Pharmaceutics. 2021;13:6.
- Wang J, Zhang C, Guo C, Li X. Chitosan Ameliorates DSS-Induced Ulcerative Colitis Mice by Enhancing Intestinal Barrier Function and Improving Microflora. Int J Mol Sci. 2019;20:22.
- Wang Y, Wen R, Liu D, Zhang C, Wang ZA, Du Y. Exploring Effects of Chitosan Oligosaccharides on the DSS-Induced Intestinal Barrier Impairment In Vitro and In Vivo. Molecules. 2021;26(8).
- Carvalho PLA, Andrade MER, Trindade LM, Leocadio PCL, Alvarez-Leite JI, Dos Reis DC, Cassali GD, Souza EMELS, Dos Santos Martins F, Fernandes SOA, et al. Prophylactic and therapeutic supplementation using fructo-oligosaccharide improves the intestinal homeostasis after mucositis induced by 5- fluorouracil. Biomed Pharmacother. 2021;133:111012.
- Wongkrasant P, Pongkorpsakol P, Ariyadamrongkwan J, Meesomboon R, Satitsri S, Pichyangkura R, Barrett KE, Muanprasat C. A prebiotic fructo-oligosaccharide promotes tight junction assembly in intestinal epithelial cells via an AMPK-dependent pathway. Biomed Pharmacother. 2020;129:110415.
- Nopvichai C, Pongkorpsakol P, Wongkrasant P, Wangpaiboon K, Charoenwongpaiboon T, Ito K, Muanprasat C, Pichyangkura R. Galactomannan Pentasaccharide Produced from Copra Meal Enhances Tight Junction Integration of Epithelial Tissue through Activation of AMPK. Biomedicines. 2019;7:4.
- Miyoshi M, Usami M, Ohata A. Short-chain fatty acids and trichostatin A alter tight junction permeability in human umbilical vein endothelial cells. Nutrition. 2008;24:1189–1198.
- Ohata A, Usami M, Miyoshi M. Short-chain fatty acids alter tight junction permeability in intestinal monolayer cells via lipoxygenase activation. Nutrition. 2005;21:838–847.
- Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104–119.
- Wang HB, Wang PY, Wang X, Wan YL, Liu YC. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig Dis Sci. 2012;57:3126–3135.
- Van Deun K, Pasmans F, Van Immerseel F, Ducatelle R, Haesebrouck F. Butyrate protects Caco-2 cells from Campylobacter jejuni invasion and translocation. Br J Nutr. 2008;100:480–484.
- Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. 2009;139:1619–1625.
- Perez-Reytor D, Puebla C, Karahanian E, Garcia K. Use of Short-Chain Fatty Acids for the Recovery of the Intestinal Epithelial Barrier Affected by Bacterial Toxins. Front Physiol. 2021;12:650313.
- Hanning N, Edwinson AL, Ceuleers H, Peters SA, De Man JG, Hassett LC, De Winter BY, Grover M. Intestinal barrier dysfunction in irritable bowel syndrome: a systematic review. Therap Adv Gastroenterol. 2021;14:1756284821993586.
- Long Y, Du L, Kim JJ, Chen B, Zhu Y, Zhang Y, Yao S, He H, Zheng X, Huang Z, et al. MLCK-mediated intestinal permeability promotes immune activation and visceral hypersensitivity in PI-IBS mice. Neurogastroenterol Motil. 2018;30:e13348.
- Zolotarevsky Y, Hecht G, Koutsouris A, Gonzalez DE, Quan C, Tom J, Mrsny RJ, Turner JR. A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. Gastroenterology. 2002;123:163–172.
- Kim MH, Kim H. The Roles of Glutamine in the Intestine and Its Implication in Intestinal Diseases. Int J Mol Sci. 2017;18(5).
- Li N, Lewis P, Samuelson D, Liboni K, Neu J. Glutamine regulates Caco-2 cell tight junction proteins. Am J Physiol Gastrointest Liver Physiol. 2004;287:G726–33.
- Li N, DeMarco VG, West CM, Neu J. Glutamine supports recovery from loss of transepithelial resistance and increase of permeability induced by media change in Caco-2 cells. J Nutr Biochem. 2003;14:401–408.
- Filipovic T. Changes in the interpupillary distance (IPD) with ages and its effect on the near convergence/distance (NC/D) ratio. Coll Antropol. 2003;27:723–727.
- Yasumatsu H, Tanabe S. The casein peptide Asn-Pro-Trp-Asp-Gln enforces the intestinal tight junction partly by increasing occludin expression in Caco-2 cells. Br J Nutr. 2010;104:951–956.
- Liang D, Yang Q, Tan B, Dong X, Chi S, Liu H, Zhang S. Dietary vitamin A deficiency reduces growth performance, immune function of intestine, and alters tight junction proteins of intestine for juvenile hybrid grouper (Epinephelus fuscoguttatus female symbol x Epinephelus lanceolatus male symbol). Fish Shellfish Immunol. 2020;107:346–356.
- Li Y, Gao Y, Cui T, Yang T, Liu L, Li T, Chen J. Retinoic Acid Facilitates Toll-Like Receptor 4 Expression to Improve Intestinal Barrier Function through Retinoic Acid Receptor Beta. Cell Physiol Biochem. 2017;42:1390–1406.
- Xiao L, Cui T, Liu S, Chen B, Wang Y, Yang T, Li T, Chen J. Vitamin A supplementation improves the intestinal mucosal barrier and facilitates the expression of tight junction proteins in rats with diarrhea. Nutrition. 2019;57:97–108.
- Retana C, Sanchez E, Perez-Lopez A, Cruz A, Lagunas J, Cruz C, Vital S, Reyes JL. Alterations of intercellular junctions in peritoneal mesothelial cells from patients undergoing dialysis: effect of retinoic Acid. Perit Dial Int. 2015;35:275–287.
- Osanai M, Nishikiori N, Murata M, Chiba H, Kojima T, Sawada N. Cellular retinoic acid bioavailability determines epithelial integrity: role of retinoic acid receptor alpha agonists in colitis. Mol Pharmacol. 2007;71:250–258.
- He C, Hu X, Xiao D, Wu J, Zhou S, Deng J, Xu S, Huang Y, Peng M, Yang X. Vitamin A prevents lipopolysaccharide-induced injury on tight junctions in mice. Food Sci Nutr. 2020;8:1942–1948.
- Maciel AA, Oria RB, Braga-Neto MB, Braga AB, Carvalho EB, Lucena HB, Brito GA, Guerrant RL, Lima AA. Role of retinol in protecting epithelial cell damage induced by Clostridium difficile toxin A. Toxicon. 2007;50:1027–1040.
- Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, Bissonnette M, Li YC. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol. 2008;294:G208–16.
- Suzuki T. Regulation of the intestinal barrier by nutrients: The role of tight junctions. Anim Sci J. 2020;91:e13357.
- Sturniolo GC, Fries W, Mazzon E, Di Leo V, Barollo M, D’Inca R. Effect of zinc supplementation on intestinal permeability in experimental colitis. J Lab Clin Med. 2002;139:311–315.
- Sunuwar L, Medini M, Cohen L, Sekler I, Hershfinkel M. The zinc sensing receptor, ZnR/GPR39, triggers metabotropic calcium signalling in colonocytes and regulates occludin recovery in experimental colitis. Philos Trans R Soc Lond B Biol Sci. 2016;371(1700).
- Cohen L, Sekler I, Hershfinkel M. The zinc sensing receptor, ZnR/GPR39, controls proliferation and differentiation of colonocytes and thereby tight junction formation in the colon. Cell Death Dis. 2014;5:e1307.
- Finamore A, Massimi M, Conti Devirgiliis L, Mengheri E. Zinc deficiency induces membrane barrier damage and increases neutrophil transmigration in Caco-2 cells. J Nutr. 2008;138:1664–1670.
- Shao YX, Lei Z, Wolf PG, Gao Y, Guo YM, Zhang BK. Zinc supplementation, via GPR39, upregulates PKCzeta to Protect intestinal barrier integrity in Caco-2 cells challenged by salmonella enterica serovar typhimurium. J Nutr. 2017;147:1282–1289.
- Forouzandeh F, Salazar G, Patrushev N, Xiong S, Hilenski L, Fei B, Alexander RW. Metformin beyond diabetes: pleiotropic benefits of metformin in attenuation of atherosclerosis. J Am Heart Assoc. 2014;3:e001202.
- Hattori Y, Suzuki K, Hattori S, Kasai K. Metformin inhibits cytokine-induced nuclear factor kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension. 2006;47:1183–1188.
- Isoda K, Young JL, Zirlik A, MacFarlane LA, Tsuboi N, Gerdes N, Schonbeck U, Libby P. Metformin inhibits proinflammatory responses and nuclear factor-kappaB in human vascular wall cells. Arterioscler Thromb Vasc Biol. 2006;26:611–617.
- Xue Y, Zhang H, Sun X, Zhu MJ. Metformin Improves Ileal Epithelial Barrier Function in Interleukin-10 Deficient Mice. PLoS One. 2016;11:e0168670.
- Zhou HY, Zhu H, Yao XM, Qian JP, Yang J, Pan XD, Chen XD. Metformin regulates tight junction of intestinal epithelial cells via MLCK-MLC signaling pathway. Eur Rev Med Pharmacol Sci. 2017;21:5239–5246.
- Blevins CH, Iyer PG, Vela MF, Katzka DA. The Esophageal Epithelial Barrier in Health and Disease. Clin Gastroenterol Hepatol. 2018;16:608–617.
- Oshima T, Miwa H. Gastrointestinal mucosal barrier function and diseases. J Gastroenterol. 2016;51:768–778.
- Oshima T, Koseki J, Chen X, Matsumoto T, Miwa H. Acid modulates the squamous epithelial barrier function by modulating the localization of claudins in the superficial layers. Lab Invest. 2012;92:22–31.
- Sifrim D, Castell D, Dent J, Kahrilas PJ. Gastro-oesophageal reflux monitoring: review and consensus report on detection and definitions of acid, non-acid, and gas reflux. Gut. 2004;53:1024–1031.
- Farre R, van Malenstein H, De Vos R, Geboes K, Depoortere I, Vanden Berghe P, Fornari F, Blondeau K, Mertens V, Tack J, et al. Short exposure of oesophageal mucosa to bile acids, both in acidic and weakly acidic conditions, can impair mucosal integrity and provoke dilated intercellular spaces. Gut. 2008;57:1366–1374.
- Gyawali CP, Sonu I, Becker L, Sarosiek J. The esophageal mucosal barrier in health and disease: mucosal pathophysiology and protective mechanisms. Ann N Y Acad Sci. 2020;1482:49–60.
- Kessing BF, Bredenoord AJ, Weijenborg PW, Hemmink GJ, Loots CM, Smout AJ. Esophageal acid exposure decreases intraluminal baseline impedance levels. Am J Gastroenterol. 2011;106:2093–2097.
- Farre R, Blondeau K, Clement D, Vicario M, Cardozo L, Vieth M, Mertens V, Pauwels A, Silny J, Jimenez M, et al. Evaluation of oesophageal mucosa integrity by the intraluminal impedance technique. Gut. 2011;60:885–892.
- Jovov B, Van Itallie CM, Shaheen NJ, Carson JL, Gambling TM, Anderson JM, Orlando RC. Claudin-18: a dominant tight junction protein in Barrett’s esophagus and likely contributor to its acid resistance. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1106–13.
- Bjorkman EV, Edebo A, Oltean M, Casselbrant A. Esophageal barrier function and tight junction expression in healthy subjects and patients with gastroesophageal reflux disease: functionality of esophageal mucosa exposed to bile salt and trypsin in vitro. Scand J Gastroenterol. 2013;48:1118–1126.
- Chen H, Hu Y, Fang Y, Djukic Z, Yamamoto M, Shaheen NJ, Orlando RC, Chen X. Nrf2 deficiency impairs the barrier function of mouse oesophageal epithelium. Gut. 2014;63:711–719.
- Chen X, Oshima T, Shan J, Fukui H, Watari J, Miwa H. Bile salts disrupt human esophageal squamous epithelial barrier function by modulating tight junction proteins. Am J Physiol Gastrointest Liver Physiol. 2012;303:G199–208.
- Que J, Garman KS, Souza RF, Spechler SJ. Pathogenesis and cells of origin of Barrett’s esophagus. Gastroenterology. 2019;157:349–364 e1.
- Abu-Farsakh S, Wu T, Lalonde A, Sun J, Zhou Z. High expression of Claudin-2 in esophageal carcinoma and precancerous lesions is significantly associated with the bile salt receptors VDR and TGR5. BMC Gastroenterol. 2017;17:33.
- Nguyen N, Fernando SD, Biette KA, Hammer JA, Capocelli KE, Kitzenberg DA, Glover LE, Colgan SP, Furuta GT, Masterson JC. TGF-beta1 alters esophageal epithelial barrier function by attenuation of claudin-7 in eosinophilic esophagitis. Mucosal Immunol. 2018;11:415–426.
- Sung CO, Han SY, Kim SH. Low expression of claudin-4 is associated with poor prognosis in esophageal squamous cell carcinoma. Ann Surg Oncol. 2011;18:273–281.
- Miyamoto K, Kusumi T, Sato F, Kawasaki H, Shibata S, Ohashi M, Hakamada K, Sasaki M, Kijima H. Decreased expression of claudin-1 is correlated with recurrence status in esophageal squamous cell carcinoma. Biomed Res. 2008;29:71–76.
- Usami Y, Chiba H, Nakayama F, Ueda J, Matsuda Y, Sawada N, Komori T, Ito A, Yokozaki H. Reduced expression of claudin-7 correlates with invasion and metastasis in squamous cell carcinoma of the esophagus. Hum Pathol. 2006;37:569–577.
- Coati I, Lotz G, Fanelli GN, Brignola S, Lanza C, Cappellesso R, Pellino A, Pucciarelli S, Spolverato G, Guzzardo V, et al. Claudin-18 expression in oesophagogastric adenocarcinomas: a tissue microarray study of 523 molecularly profiled cases. Br J Cancer. 2019;121:257–263.
- Caron TJ, Scott KE, Fox JG, Hagen SJ. Tight junction disruption: Helicobacter pylori and dysregulation of the gastric mucosal barrier. World J Gastroenterol. 2015;21:11411–11427.
- Marcus EA, Vagin O, Tokhtaeva E, Sachs G, Scott DR. Helicobacter pylori impedes acid-induced tightening of gastric epithelial junctions. Am J Physiol Gastrointest Liver Physiol. 2013;305:G731–9.
- Wroblewski LE, Shen L, Ogden S, Romero-Gallo J, Lapierre LA, Israel DA, Turner JR, Peek RM Jr. Helicobacter pylori dysregulation of gastric epithelial tight junctions by urease-mediated myosin II activation. Gastroenterology. 2009;136:236–246.
- Okugawa T, Oshima T, Chen X, Hori K, Tomita T, Fukui H, Watari J, Matsumoto T, Miwa H. Down-regulation of claudin-3 is associated with proliferative potential in early gastric cancers. Dig Dis Sci. 2012;57:1562–1567.
- Hagen SJ, Ang LH, Zheng Y, Karahan SN, Wu J, Wang YE, Caron TJ, Gad AP, Muthupalani S, Fox JG. Loss of Tight Junction Protein Claudin 18 Promotes Progressive Neoplasia Development in Mouse Stomach. Gastroenterology. 2018;155:1852–1867.
- Sanada Y, Oue N, Mitani Y, Yoshida K, Nakayama H, Yasui W. Down-regulation of the claudin-18 gene, identified through serial analysis of gene expression data analysis, in gastric cancer with an intestinal phenotype. J Pathol. 2006;208:633–642.
- Hayashi D, Tamura A, Tanaka H, Yamazaki Y, Watanabe S, Suzuki K, Suzuki K, Sentani K, Yasui W, Rakugi H, et al. Deficiency of claudin-18 causes paracellular H+ leakage, up-regulation of interleukin-1beta, and atrophic gastritis in mice. Gastroenterology. 2012;142:292–304.
- Lu B, Li M. Helicobacter pylori eradication for preventing gastric cancer. World J Gastroenterol. 2014;20:5660–5665.
- Wroblewski LE, Peek RM Jr., Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010;23:713–739.
- Necchi V, Candusso ME, Tava F, Luinetti O, Ventura U, Fiocca R, Ricci V, Solcia E. Intracellular, intercellular, and stromal invasion of gastric mucosa, preneoplastic lesions, and cancer by Helicobacter pylori. Gastroenterology. 2007;132:1009–1023.
- Suzuki K, Sentani K, Tanaka H, Yano T, Suzuki K, Oshima M, Yasui W, Tamura A, Tsukita S. Deficiency of Stomach-Type Claudin-18 in Mice Induces Gastric Tumor Formation Independent of H pylori Infection. Cell Mol Gastroenterol Hepatol. 2019;8:119–142.
- Michielan A, D’Inca R. Intestinal Permeability in Inflammatory Bowel Disease: Pathogenesis, Clinical Evaluation, and Therapy of Leaky Gut. Mediators Inflamm. 2015;2015:628157.
- Hollander D. Intestinal permeability, leaky gut, and intestinal disorders. Curr Gastroenterol Rep. 1999;1:410–416.
- Hollander D. Crohn’s disease–a permeability disorder of the tight junction? Gut. 1988;29:1621–1624.
- Hollander D, Vadheim CM, Brettholz E, Petersen GM, Delahunty T, Rotter JI. Increased intestinal permeability in patients with Crohn’s disease and their relatives. A possible etiologic factor. Ann Intern Med. 1986;105:883–885.
- Zeissig S, Burgel N, Gunzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007;56:61–72.
- Blair SA, Kane SV, Clayburgh DR, Turner JR. Epithelial myosin light chain kinase expression and activity are upregulated in inflammatory bowel disease. Lab Invest. 2006;86:191–201.
- Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Burgel N, Fromm M, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–564.
- McNamara BP, Koutsouris A, O’Connell CB, Nougayrede JP, Donnenberg MS, Hecht G. Translocated EspF protein from enteropathogenic Escherichia coli disrupts host intestinal barrier function. J Clin Invest. 2001;107:621–629.
- Nusrat A, von Eichel-Streiber C, Turner JR, Verkade P, Madara JL, Parkos CA. Clostridium difficile toxins disrupt epithelial barrier function by altering membrane microdomain localization of tight junction proteins. Infect Immun. 2001;69:1329–1336.
- Satitsri S, Pongkorpsakol P, Srimanote P, Chatsudthipong V, Muanprasat C. Pathophysiological mechanisms of diarrhea caused by the Vibrio cholerae O1 El Tor variant: an in vivo study in mice. Virulence. 2016;7:789–805.