 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The glomerular basement membrane (GBM) is an important tissue structure in kidney function. It is the membrane through which filtrate and solutes must pass to reach the nephron tubules. This review focuses on the spatial location of the main extracellular matrix components of the GBM. It also attempts to explain this organization in terms of their synthesis, transport, and loss. The picture that emerges is that the collagen IV and laminin content of GBM are in a very slow dynamic disequilibrium, leading to GBM thickening with age, and in contrast, some heparan sulfate proteoglycans are in a dynamic equilibrium with a very rapid turnover (i.e. half-life measured in ~hours) and flow direction against the flow of filtrate. The highly rapid heparan sulfate turnover may serve several roles, including an unclogging mechanism for the GBM, compressive stiffness of the GBM fiber network, and/or enabling podocycte-endothelial crosstalk against the flow of filtrate.
1 Introduction
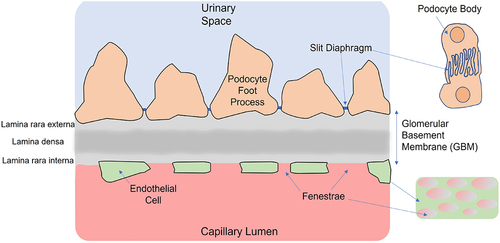
The glomerular basement membrane (or GBM) is a highly specialized basement membrane lying between glomerular capillaries’ endothelial cell layer and podocytes. It is integral to the structural integrity of the capillary walls. The GBM is composed of extracellular matrix (ECM) and, together with the endothelia and podocytes, form the larger main components of the glomerular filtration barrier (GFB) – see . The spatial organization and turnover of the main ECM molecules in the GBM is the focus of this review.
Figure 1. Schematic of cross-section through the glomerular filtration barrier (GFB) showing key components of the barrier, including the fenestrated endothelia, the podocyte foot processes separated by slit diaphragms and the GBM. Not shown is the glycocalyx covering the endothelial cells and podocyte foot processes.Citation1 The small images on the right give a ‘top down’ view of the interdigitating foot processes of adjacent podocytes (top) and the appearance of fenestra in the endothelial cells (bottom).
We begin by describing the histological features of the GBM, and the main ECM components of the GBM. In doing so, we attempt to identify the homeostatic ‘maintenance dynamics,’ including the turnover of ECM molecules in the GBM. This becomes important because GBM maintenance dynamics can help us understand at times, conflicting experimental observations on the distribution and half-life of ECM molecules within the GBM.
This review has two motivations. It is intrinsically interesting to better understand the composition and distribution of components in the GBM. The second motivation relates to the fact that the GBM requires certain characteristics that have to be maintained. For example, it must allow water and small molecules to pass through it and have enough structural integrity to not collapse under a filtrate pressure gradient. This review then provides foundational knowledge for other studies trying to connect GBM component organization to transport and its mechanical properties. An example of where this may be important is estimating the anionic distribution within the GBM.
How the GFB acts as a size and charge filter continues to be the subject of considerable controversy.Citation2 Without wading into this debate, it has been previously proposed that the anionic charge within the GBM contributes to albumin exclusion.Citation3–11 Whether or not this is the case, just to better assess the evidence upon which this assertion is based, would likely be aided by improved estimates of the distribution of all ECM contributions to anionic charge within the GBM. In this review, we will restrict ourselves to the main GBM molecules and leave estimates of the GBM anionic distribution for a future study.
Our focus is on the isolated GBM. We do not include the glycocalyx on the endothelial cells and podocyte foot processes (though glycocalyx is briefly discussed in Section 3.5). The cellular glycocalyx is normally removed together with adjacent cells during processing,Citation12,Citation13 with the remaining ECM barrier known as the ‘isolated GBM’Citation12 or simply the GBM. This means syndecans and glypicans (that facilitate cell–ECM interactionsCitation14) and podocalyxin (a strongly anionic molecule required for normal podocyte foot process development and maintenance)Citation15 that are present in the cell glycocalyx will not be included.
Likewise, the nature and molecular composition of the important structure between podocyte foot processes, known as the slit diaphragms (see ), will not be the focus here. They are only mentioned in that normal operation of the podocyte slit diaphragm is essential for a normal GBM. Podocyte slit diaphragms must allow the filtrate to pass. Their role in albumin filtration has been debated.Citation16 However, and critical to the current study’s focus, they must certainly have a role in preventing large molecular components of the GBM ECM from escaping in significant quantities with the filtrate into the proximal tubule. Many molecules are involved in forming the slit diaphragms and ensuring its pore spacing. Key ones include nephrin, neph1, ZO-1, and podocin.Citation16,Citation17 Loss of these, and others, can lead to proteinuria. Furthermore, claudins are normally associated with epithelial tight junctions. They are present in developing or immature GBM podocytes, but not healthy slit diaphragms. However, claudins may reappear in podocytes (for example, in diabetic nephropathy), leading to a so-called ‘slit diaphragm to tight junction transition’ and proteinuria.Citation18
2 Histological appearance of the GBM
The GBM is one of the thickest basement membranes in the body.Citation19 In the process of normal aging, a progressive increase in the GBM thickness is observed in several mammalian species including human.Citation20 For example, in rats, the GBM increases from 129 nm to 305 nm between 2 months and 18 months.Citation20 In humans, the GBM thickness increases from 145 nm at birthCitation21 to an adult thickness that is typical ~300−350 nm.Citation22
The GBM thickness in adult healthy mice is around 80 nm.Citation23 In human adults, the GBM thickness ~305 nm for women and 330 nm for men.Citation19 Variation in GBM thickness (either thickening or thinning) is also associated with various renal and systemic diseases,Citation24 so, for example, an increase in GBM thickness occurs during the clinically silent period of diabetic nephropathy.Citation22 However, here we only discuss normal kidneys, rather than diseased kidneys.
The fiber network across the GBM appears to be non-uniformly distributed, as can be identified by transmission electron microscopy (TEM) (for example, in [Citation25] or Figure 8a in [Citation26]). Traversing the GBM from the endothelial side to the epithelial side, three distinct regions are usually identified: (i) the lamina rara interna (LRI), (ii) the lamina densa (LD), and (iii) the lamina rara externa (LRE).Citation25 Note that there are several naming conventions for basement membrane that have arisen. We intend to use LRI, LD, and LRE here when referring to the GBM, as this provides a convenient reference to locations within the GBM. Further, lamina densa is sometimes used synonymously with basement membrane (e.g. Mouse Genome Informatics, Gene Ontology BrowserCitation27) or a name given based on optical appearance, rather than location.
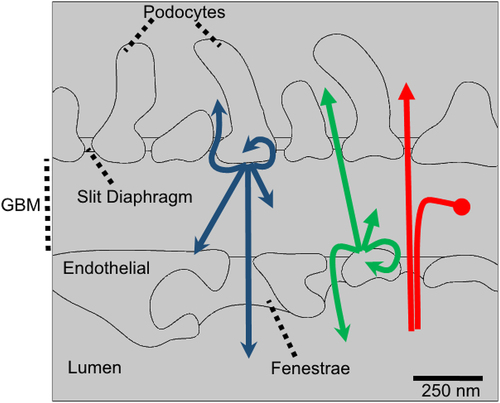
Figure 2. Solutes within the GBM may be from a number of sources and have one of several destinations. The role of GFB as a filter focuses on solutes sourced from the blood plasma (shown by red arrows). These molecules may pass through the GBM, accumulate in the GBM (potentially leading to clogging) or are excluded (e.g. by anion exclusion). Molecules synthesized by either the podocytes (blue arrows) or endothelial cells (green arrows) may potentially leave the GBM via the fenestra or the slit diaphragms. Alternatively, they may spend time in the GBM, contributing to the GBM ECM until they are degraded or transported away. In addition, cross-talk (paracrine signaling) between podocytes-and endothelial cells and autocrine signaling is also possible, though not the focus of the current review.
It is apparent that the lamina rara is not just ‘empty space’ crossed by the few larger ECM fibers or ‘cords’ (see for examples in [Citation25] or Figure 8a in [Citation26]), for when the glomerular tissue is fixed in glutaraldehyde and then freeze substituted, rather than using the conventional tissue preparation methods, a completely different picture emerges – that of a GBM with near uniform electron transmission density, with no discernible lamina rara or lamina densa (see, for example, Figure 8b in [Citation26]).
The preparation-dependent transmission microscope data are puzzling.Citation22 Chen et al. suggest that the difference between standard tissue preparation and one involving freeze substitution preparation is probably due to omission of the standard acetone step. Acetone causes rapid dehydration of the tissue sample.Citation26 This suggests that lamina rara contains molecules that are either removed or condensed using the standard tissue preparation methods. However, it is also evident that removal or condensation of the ECM molecules by organic solvents occurs to a much lesser extent for the lamina densa, presumably because the molecules are either different from those in the lamina rara and bond to one another, or because they are more densely packed together. This suggests that these three regions differ in their ECM.
It appears that there are also real functional differences associated with these three regions too. Large proteins such as ferritin that enter the lamina rara interna face another barrier within the GBM that obstructs large molecular probes from progressing into the lamina densa.Citation3 These transport data support the standard morphological evidence that the lamina densa is a real barrier entity within the GBM.
Based on these images and the transport evidence, we expect to see a non-uniform distribution of ECM molecules across the GBM (though not with ‘step changes’ in ECM density, as suggested by light or TEM images prepared using standard methods). As is discussed later (Section 5), evidence also exists for a non-uniform distribution of ECM proteins across the GBM in high-resolution light microscopy data, but without step changes.Citation28
3 Molecular composition of the GBM
However, some 144 ECM and regulatory proteins have been identified in the ECM of glomeruli, which includes the ECM from both glomerular mesangium and GBM.Citation29 Most proteins occur in very small quantities, and so we only focus on the main ECM protein and proteoglycan components within the GBM. The minor components are much less well studied. Even the definitive roles of the major components are uncertain. Until we know what the major components of the GBM do, it seems even more uncertain what the minor components are doing. However, we can speculate that the minor components may have a role in organizing the structural and functional integration of newly synthesized major ECM components into the existing ECM, stabilizing the pore structure or protecting these major components from degradation (via proteases). Most proteins occur in very small quantities, and so we only identify the main ECM protein and proteoglycan components within the GBM. We proceed to do that here.
3.1 Collagen IV
The fiber network within the GBM is largely made up of collagen IV protomers, which are ~3 nm in diameterCitation30 and ~400 nm long.Citation31 The dominant presence of collagen IV explains the 3 nm diameter fibers reported in the TEM images of the lamina densa (see caption in [Citation25]).
Out of the six distinct alpha (‘α’) chains, labeled one through to six, collagen IV forms from only three distinct heterotrimers, namely, α1α1α2, α3α4α5, and α5α5α6.Citation32 In adult GBM, collagen IV α1α1α2 is primarily produced by endothelial cells, while collagen IV α3α4α5 is produced exclusively by podocytes.Citation33 Note that podocyte collagen synthesis has undergone a switch to collagen α3α4α5 in adult GBM.
While both types of collagen IV are found across the GBM, collagen IV α3α4α5 predominates in the lamina rara externa and lamina densa.Citation28 The collagen IV protomers polymerize, joining at their NC1 domains (8.6 nm in diameterCitation30) to form hexamers (about 11 to 12 nm in diameterCitation30), while cross-linking occurs at the N-terminal 7s domains. The collagen IV network is stabilized by both sulfilimine and allysine-derived cross-links.Citation34 Protomer polymerization provides structural integrity to the collagen IV network within the GBM. Recent research on Drosophila has shown that chloride ion concentration is important in enabling the formation of collagen IV hexamers, and Cummings et al. suggest that low chloride ion concentrations could help ‘reposition’ basement membrane components, by ‘disrupting’ the collagen IV assembly.Citation35
Collagen IV does not form side by side triple helix interactions, as fibril forming collagens do, because
the Gly-X-Y repeat region of collagen IV displays multiple interruptions, imparting flexibility to the collagen IV protomer and to the network that it forms in basement membranes.Citation36
It is likely that collagen IV network in the lamina densa is the primary fiber network obstructing the mobility of albumin (and other proteins) after they enter the GBM. The best evidence for this obstruction is the fact that genetic defects in collagen IV α3α4α5 cause proteinuria, which eventually leads to end-stage renal disease.Citation37
3.2 Laminin 521
As occurs in all basement membranes, laminin molecules polymerize to form a second critically important fiber network in the GBM. One of the laminin’s key roles is to anchor the basement membrane ECM to nearby cells. Laminin alpha5 beta2 gamma1 (i.e. laminin α5β2γ1,Citation38 or laminin 521 for short) is found in the GBM and is produced by both endothelial cells and podocytes. Within the kidney, laminin 521 is only found in GBM and arteriolar basement membranes.Citation39 The C-terminal end of laminin 521 binds to integrins, and the ‘coiled-coil rod’ projects out from the cell membrane into the basement membrane ECM for approximately 77 nm, whereupon it branches into 3 short arms (34 nm in length). The short arms form what is believed to be a hexagonal network (at least in some basement membranes), with the hexagonal network formed by the globular tips of laminin’s ‘three-spoked umbrellas’ binding to one another (see, for example, the depiction shown in Figure 3 of [Citation40]).
The collagen IV fiber network in a typical basement membrane is often depicted distal to the laminin hexagon network (sees for example, Figure 3 in ([Citation41]). In contrast, experimental data for the GBM suggest that the highest concentration of laminin β2 LF domain and laminin α5 LEb/L4b domains are close to the center of the GBM and close to the center of the collagen IV fiber network, that is, in the middle of the lamina densa.Citation28 Furthermore, with the highest concentration in the middle of the lamina densa, laminin beta2 LF and laminin α5 LEb/L4b are reported to form approximately triangular concentration distributions across the width of the GBM, with zero concentration close to the cell membranes. This suggests that laminin is found across the whole GBM, but particularly in the lamina densa.Citation28
3.3 Proteoglycans
It is generally accepted that there are three HS bearing proteoglycans in the GBM: (i) agrin (produced by podocytesCitation42,Citation43), (ii) perlecan (produced by endothelial cells), and (iii) collagen XVIII (produced by podocytes and endothelial cells). Agrin is a long skinny molecule about 95 nm long,Citation44 and is probably the main source of HS in the GBM – agrin is reported to be about six times more abundant than perlecan in rat GBM.Citation45 Agrin also binds to the laminin ‘coiled-coil’ rods and binds to molecules in the cell membrane in a structural role.Citation44
There is a significant quantity of collagen XVIII in the GBM. Based on the location and orientation of collagen XVIII, it is likely that collagen XVIII helps to hold the lamina densa in place (one end of collagen XVIII appears to interact with receptors at the cell membrane, while the other end is buried within the lamina densa).Citation46 We note that the proteoglycan collagen XV may also play some role, but we do not pursue that here.Citation47
There is information on the amount of HS attached to the proteoglycans, though these data are mainly from chicks, with some human data for perlecan. Chick brain agrin core protein is about 220 kDa, while the intact agrin proteoglycan is reported to be in excess of 400 kDa.Citation48 We estimate that about 200 kDa of HS is attached to intact GBM agrin. Human perlecan core protein is reported to be 467 kDa, while intact perlecan has 3 HS side chains and possibly a chondroitin sulfate side chain, with a total weight of up to 750 kDa.Citation49 Intact GBM perlecan has about 200 kDa of HS attached to it.Citation49 We base this estimate on the following. For the collagen XVIII found in the chick embryo, it is reported the collagen XVIII ‘core chain protein’ is 180 kDa, which has 120 kDa of HS attached to it.Citation50 We treble values for the core chain protein of collagen XVIII to approximately estimate a value for the homotrimer of intact collagen XVIII (i.e., we assume 300 kDa of HS is attached to collagen XVIII).
HS is known to carry a strong negative charge. The presence of HSPGs in the GBM has been associated with anionic exclusion by the GBM,Citation51 though the role of HSPG in albumin sieving remains controversial.Citation43,Citation52,Citation53 Less has been said the potential role of HSPG in providing mechanical stiffness of the GBM, especially for compression. Chondroitin sulfate provides articular cartilage with a compressive stiffness.Citation54 It seems highly likely that heparan sulfate inevitably plays a similar role in the GBM to chondroitin sulfate in articular cartilage.
In the GBM, the charge on HS would lead to repulsion between HSPGs (and also with other negatively charged molecules in the GBM). The HS expands against the collagen (generating self-equilibrating forces, with the HS in compression and the collagen IV in tension). This expansive force may (i) play a role in controlling pore size by controlling collagen network tension, and (ii) enable the collagen network pore structure to remain open against the fluid pressure gradient generated by the fluid drag forces that operate throughout the GBM. The concentration of HS in the kidney is substantially lower than CS in cartilage, presumably commensurate with the much lower loads involved.
This does not imply that compressive stiffness and pore opening are the only roles of HS in the GBM. The above does not preclude the role of HS in anionic exclusion, in protein size sieving, or as a contributor to regulating the hydraulic conductivity of the GBM and hence the glomerular filtration rate. In any filter, there is a trade-off between pore size, and thus filter efficacy, and the fluid drag along the pore and compressive stresses on the solid structure from which the filter is made. Potentially a high turnover of HSPGs would allow these compromises to be continuously adjusted.
This HS swelling, not only leads to collagen network tension but, like chondroitin sulfate in articular cartilage, also leads to a transport mechanism for HSPGs (and any free HS) to migrate down their concentration gradient, in addition to transport associated with simple diffusion.Citation55 The expected concentration gradient of HS across the GBM, specifically highest at the podocyte side of the GBM, leads to transport ‘upstream,’ with upstream being used in the sense of being against the flow direction for filtration. Critically, this HSPG and free HS transport may represent a mechanism for unclogging the GBM. It may also enable cross-talk from podocytes to endothelial cells against the filtrate flow, especially if the signaling molecules bind to the HSPG, one example being VEGF.Citation56
3.4 Nidogen
Nidogen 1 and 2 feature prominently in the GBM, binding many different molecules (see Figure 6 in [Citation57]) Nidogen 1 can bind to laminin (in the ‘P1 region’ of lamininCitation58) and collagen IV, while nidogen 2 can also bind to collagens I and IV, endostatin, and collagen XVIII.Citation59 Since nidogen binds both laminin and collagen IV, it has been suggested that nidogens may hold the basement membrane together. Single knockouts of nidogen 1 or nidogen 2 have functional basement membranes, whereas knockouts of both nidogen 1 and 2 result in perinatal lethality.Citation60 Together, we suggest that nidogen is critical but that nidogens 1 and 2 can to some extent substitute for each other, giving basement membrane tissues a level of functional security through redundancy.
Heterozygous nidogen 1 knockdowns result in a wider lamina densa and narrowed lamina rara, while homozygous nidogen 1 knockouts have lamina densa that occupy almost the entire thickness of the GBM, i.e. the lamina rara all but disappear.Citation61 These data suggest that nidogen 1 probably does play a role in holding or binding the lamina densa together.
3.5 Glycocalyx
Having identified the main ECM molecules in the GBM above, we conclude this section with a brief discussion of the glycocalyx. Although glycocalyx is technically part of the cell rather than the GBM, there appears to be crucially important roles played by the molecule podocalyxin for normal GBM function.Citation15 Podocalyxin is a sialomucin with an essential role in the formation and maintenance of podocyte foot processes.Citation15,Citation62 Podocalyxin is a member of the CD34 family of molecules,Citation63 and is a long extensively O-glycosylated and sialylated, type I transmembrane protein (see Figures 1 in [Citation62] and [Citation63]), forming a substantial part of the sialic-acid rich, polyanionic glycocalyx. Attached to the podocyte cell surface, the glycocalyx can be seen to extend some 30 nm to 40 nm in thickness out from the cell membrane (see, for example, Figures 2 and 4 in [Citation64]). This highly anionic glycocalyx covering the podocyte surfacesCitation65 could function as a ‘backstop’ to the GBM, preventing large anionic ECM molecules being transported up to the slit diaphragms and then into Bowman’s space. Otherwise (if they could pass through the slit diaphragms), these large ECM molecules would be immediately lost from the GBM and/or potentially clog the slit diaphragms. Presumably the regions between the foot processes in the GBM, up to the slit diaphragms, are also completely filled with glycocalyx. This barrier must then be traversed before large molecules (e.g. ECM, albumin) can even interact with slit diaphragms.
We surmise the podocyte glycocalyx density and distribution explain how some large particles crossing the GBM are found to have their passage blocked well upstream of the slit diaphragm (see, for example, images Figure 3D and E in [Citation66]). And similarly, ECM molecules that are secreted by podocytes into the GBM cannot access Bowman’s space, rather, the ECM molecules accumulate in the lamina rara externa until they are either degraded, endocytosed,Citation4,Citation67 transported by diffusion laterally along the GBM, or transported across the GBM toward the endothelial cells, along their respective concentration gradients.
4 Turnover of GBM ECM molecules
Having identified the dominant ECM molecules that contribute to fixed charge in the GBM (namely, collagen IV, laminin 521, nidogens, perlecan, agrin, and collagen XVIIICitation39), we wish to understand the turnover of these ECM molecules. Such knowledge will help us interpret the half-life of ECM molecules, as most data represent only snapshots of the GBM. Ultimately, this knowledge will help us understand the processes involved in the maintenance and adaption of the GBM.
The GBM is formed by the fusion of separate endothelial and epithelial basement membranes during the ‘S phase’ of glomerular development.Citation33 Around the time the two basement membranes fuse, they change their laminin expression from laminin 111 expressed in the earliest basement membrane, to laminin 521 in the mature GBM. At the same time, podocytes also change their collagen IV expression (from collagen IV α1α1α2 to collagen IV α3α4α5), while endothelial cells do not change their collagen IV expression but keep on producing collagen IV α1α1α2.Citation33 So, in the mature adult GBM, we might expect to see more collagen α1α1α2 on the endothelial side of the GBM, and more collagen IV α3α4α5 on the epithelial side of the GBM. Allowing for positioning uncertainty, and the difficulties of antibody access to epitope sites on the collagen IV, this expectation is largely confirmed by Suleiman et al. (see Figure 3 in [Citation28]). Podocytes synthesize the majority of the ECM components, with some contribution from endothelial cells.Citation68
It would be very helpful if we understood how the GBM maintains itself. Beyond the broader clinical application of such knowledge, it helps us here to infer the equilibrium distribution of proteins. We can begin to understand the GBM dynamics if we know the half-lives of ECM molecules.
There are some general principles that can be employed to assist in this endeavor. Most importantly, mass balance states that the rate of change of ECM component in the GBM represents the difference between its rate of addition to the GBM and the rate of loss of that component from the GBM (see ). The rate of addition of ECM molecules is controlled by the sum of the separate rates of synthesis by both epithelial and endothelial cells. For example, for some types of molecules, such as laminin 521 and collagen XVIII, there are rates of addition to the GBM from both endothelial and epithelial cells. For other types of molecules, there is only one rate of addition if the molecule is only produced by only one type of cell. For example, agrin is only produced by podocytes, while perlecan is only produced by endothelial cells.
The rate of loss of a number of proteins is governed by the sum of the different rates of degradation of ECM molecules in the GBM via the various degradation pathways. Rates of loss are partly controlled by both the epithelial and endothelial cells. Cellular degradation pathways control endocytosis and the secretion of molecules that regulate rates of ECM degradation in the GBM, such as proteases (e.g., plasmin) and inhibitors of the proteases (e.g., TIMPs), as well as the rate of secretion of heparanase.
The rate of loss of molecules in the GBM is also partly regulated by ‘environmental factors,’ which includes a very large number of molecules in the plasma that can make their way into the GBM during filtration, and thereby interact with and alter the state of the ECM molecules in some way. A list of 144 ECM and regulatory proteins found in the ECM of glomeruli can be found in Lennon et al.Citation29 These molecules include all the different classes of proteases in the plasma, as well as their regulators, which may vary over time with the inflammatory status of the animal.Citation69,Citation70 Environmental factors also include abnormal molecules such as autoantibodies, which may enter the GBM from the plasma and cause glomerulonephritis.Citation71
Finally, there is the rate of loss of ECM molecules to the plasma (via the fenestra in endothelial cells) and the loss to mesangial cells, which are known to be phagocytotic.Citation72,Citation73 Clearly, a model incorporating all the ECM molecule loss pathways is not practical at this stage, but we nevertheless can make some useful observations.
Available evidence suggests that ECM molecules do not have the same turnover rates, and that only some ECM molecules reach steady state. When considering mass balance arguments, we would like to know if an ECM molecule has reached a steady state in the GBM (where the total rate of addition equals the total rate of loss for a molecule). Generally, we expect that the rate of addition equals the rate of loss for a stable GBM ECM. However, depending on the timescale of observation, we might find that some molecules are experiencing a net gain or loss. For example, some ECM molecules may slowly accumulate – indicating they have not reached a steady state. The change in GBM thickness, with age or pathology, is likely evidence of accumulation. For example, the GBM thickness in Wistar rats has been reported to increase from 129 nm at 2 months of age to 305 nm at 18 months,Citation20 while in humans, the GBM is reported to increase from 145 nm (in the range of 246 days post-conception to 1-month old), to 286 nm at 4–12 years old.Citation21 Additional GBM thickening is also known to occur with diabetes.Citation22
GBM thickening is primarily due to thickening of the lamina densa (see in [Citation74] and Table 1 in [Citation21]) Presumably, this thickening corresponds to the slow accumulation of the main proteins comprising the lamina densa, namely, collagen IV α1α1α2, collagen IV α3α4α5, laminin 521 and nidogen 1 and 2. Two of the proteoglycan molecules, namely, collagen XVIII and agrin, probably do accumulate to a small extent as they are also part of the lamina densa, but as we discover below, these molecules are reported to have high turnover rates (see Section 4.3), which suggests both these molecules do reach a steady-state equilibrium.
Finally, we note that there is some recent direct evidence that many ECM components move within the basement membrane, relative to the collagen and laminin networks. For example, a photobleaching studyCitation75 on C. elegans pharynx of some 29 exogenously tagged proteins revealed recovery times along a 5-µm section of the basement membrane occurs within 5–15 mins for smaller ECM molecules. It should be said that transport rates can vary substantially in different tissues and basement membranes under different conditions (e.g. advection induced by muscle contractions plays a role in the photobleaching study mentioned above), so care should be taken in inferring transport rates of these molecules in GBM from these examples. Nevertheless, the photobleaching study clearly shows a surprisingly rapid turnover for some of the small molecules in the C. elegans pharyngeal basement membrane, and the expected very slow turnover for some of the larger molecules.
4.1 Turnover of collagen IV
Perhaps, the best evidence available on the turnover of normal GBM is provided by the rates of incorporation and/or loss of radiolabeled amino acids comprising the GBM.Citation76,Citation77 Using young Lewis rats, Price and Spiro estimated the half-life of proline and glycine (which are the main amino acids in collagen IV) to be greater than 100 days, estimating that leucine in the GBM has a half-life of 65 days, while lysine and phenylalanine are 38 days and 23 days, respectively.Citation76
In a more recent study, Liu et al. performed a so-called stable isotope labeling experiment on mice.Citation77 Commencing 3 weeks after birth, for the next 12 consecutive weeks the mice were fed exclusively on a nitrogen-15 spirulina diet. Twelve weeks after the feed change, 99% of plasma proteins were nitrogen-15 (N-15) labeled and only 1% were nitrogen-14 (N-14) labeled. Liu et al. collected fresh kidney (i.e., the whole renal cortex with a small amount of medulla), and then performed a proteomic analysis using mass spectrometry, to compare the nitrogen-14 and 15 contents of over 4,000 renal proteins they identified in their renal tissue sample. Only about 100 proteins had both nitrogen-14 and 15, and of these proteins, the largest N-14/N-15 ratios were associated with ECM proteins, mainly comprising those proteins found in the basement membranes of glomeruli and tubules.
Based on the spectral intensities of their first mass spectrometer analysis, nitrogen-14 containing collagen α1α1α2 molecules and nitrogen-15 containing collagen α3α4α5 molecules decreased to a fraction of about 55% to 59% of the total number of molecules of each type over the 12 week period (see in [Citation77]), either by nitrogen-14 containing molecules being replaced by nitrogen-15 containing molecules or by the addition of nitrogen-15 containing molecules adding to the total number of molecules. Some molecules of each collagen type contained both N-14 and N-15 nitrogen, but these ‘chimeric’ molecules were in a minority and are excluded from the molecular counts Liu et al. reports,Citation77 presumably for simplicity.
It is important to first note that Liu et al.’s experimental data are for all basement membranes in the renal cortex, including endothelial, tubular, and glomerular, not just the GBM. Nevertheless, if we assume this data also applies to the GBM in about the same way as for the aggregated renal basement membrane data, and that the same rate of increase in GBM thickening reported by Neumann et al. for rats also occurs in the mice (i.e. about a 6% increase per month compounding), then over the 12 week period of the Lui et al. experiment, about 16% (i.e. 1–1/1.19) of the decrease in the proportion of nitrogen-14 containing molecules can be attributed to the 19% addition of nitrogen-15 molecules, and about 25% to 29% of the decrease in nitrogen-14 containing molecules can be attributed to replacing molecules in the GBM. Employing the 25% estimate, this means the half-life of collagen IV in the GBM is approximately 180–200 days. We note that this estimate is consistent with the Price and Spiro data (i.e. half-life of proline and glycine greater than 100 days).
These estimated half-lives for collagen IV are also consistent with data on the time required for the removal of ‘silver salt’ deposits from the GBM.Citation78 Following the initial silver salt labeling of the GBM ECM in Sprague Dawley rats (initial labeling was due to the rats drinking water containing 12 mM silver nitrate for 10 weeks), Walker’s experiment involved periodically observing the silver salt deposit distribution across the GBM.Citation78 Walker reports:
… a major component of GBM is laid down on the epithelial aspect then moves towards the endothelial aspect, and then by way of the lamina rara interna to the mesangial matrix for ingestion by mesangial cells. This process is continuous and slow; the time for complete turnover of the rat glomerular basement membrane is of the order of one year.
We do not see any reason why some fraction of the GBM ECM molecules that Walker suggests are lost (solely) to mesangial cells cannot also be lost via endothelial fenestra, whereupon they would be degraded within the plasma or taken up by cells (e.g. tubular epithelium).Citation79,Citation80 Degraded molecules of different sizes may well have different propensities to be lost from the GBM either through endothelial fenestra (for example, free heparan sulfate may be lost mainly through fenestra), or lost from the GBM by movement toward the mesangial matrix (for examples, significant quantities of silver salt deposits found their way to the mesangial matrix,Citation78 while large molecules such as immune complexes or also found in the mesangial matrix,Citation81 as well as found in mesangial lysosomes).Citation82
4.2 Turnover of laminin and nidogens
To estimate the turnover of laminin and nidogen we again use the data of Liu et al.Citation77 While recalling that the Liu et al. experimental data are an average turnover weighted over all basement membranes in the renal cortex, the assumption that this average weighted turnover is representative of the turnover for collagen IV in the GBM did lead to results broadly consistent with independent data. So, we again assume that this average weighted turnover is representative of the GBM turnover and observe that each of the components of laminin 521 has nitrogen-14 fractions equal to 42% to 50% of the total number of molecules of each type, somewhat lower than that for collagen IV (55% to 59%).Citation77 To minimize the error, we observe that laminin β2 is reported to be the most GBM specific laminin chain comprising laminin 521,Citation39 and this laminin chain has a nitrogen-14 fraction close to 50%,Citation77 i.e. toward the upper end of the range of relevant laminin chains. It is possible that laminin 521 may have accumulated in the lamina densa somewhat more quickly than collagen IV in the GBM, or that it may have been lost from the GBM and replaced somewhat more quickly than collagen IV, or some combination of both.
The highest concentration laminin β2 LF and laminin α5 LEb/L4b is reported to be in the middle of the lamina densa, and that laminin β2 LF and laminin α5 LEb/L4b form triangular concentration distributions across the GBM, with close to zero concentration at the cell membranes.Citation41 This triangular distribution for laminin α5 LEb/L4b is entirely consistent with a labile GBM, in which laminin molecules in the GBM can lose contact with the cell membrane integrins and move deeper into the lamina densa as the lamina densa thickens over time, perhaps to be replaced by new laminins and collagen IV that are secreted continuously into the GBM by the adjacent endothelial and epithelial cells.
After 12 weeks, we note that nidogen 1 and nidogen 2 had N-14 contents of 9% and 31%, respectively,Citation77 suggesting half-lives of around 24 days and 49 days, respectively. These data are consistent with nidogen 2 being produced mainly by podocytes (diffusion across the lamina densa would presumably explain its somewhat longer half-life), and nidogen 1 being produced by endothelial cells.
4.3 Turnover of heparan sulfate
HS enters the GBM attached to secreted proteoglycans, namely, agrin, perlecan, and collagen XVIII. A body of GBM research data, apart from occasionally being mentioned in the GBM literature,Citation22 appears to have been largely overlooked, possibly because its utility is unclear. Not only does these data show that glycoaminoglycans (GAGs) are found within the GBM, particularly HS and hyaluronan,Citation83–88 but most importantly the HS in the GBM turns over at an extraordinarily rapid rate, with a half-life () measured at just a few hours.Citation89,Citation90
Akuffo et al. injected radiolabeled sulfateCitation34 continuously for 5 days into Wistar rats, and then measured how quickly labeled sulfate was lost from different sulfate pools in glomuleri, and more specifically, sulfate was lost from the GBM (e.g. perlecan was immunoprecipitated).Citation90 We note that for the case of immunoprecipitation, Akuffo et al. reports: “there was no evidence for intracellular or cell membrane localization of epitopes.” Based on these data, we calculate the (chase) half-life of HS attached to (immunoprecipitated) perlecan in the GBM could be under one hour, and just a little longer for agrin and other glycoaminoglycan molecules (mainly found in mesangium).Citation90 For example, examining Figure 4D in Akuffo et al., we estimate that a (1.7/0.045 =) 38-fold reduction in perlecan took place over 3.5 hours (i.e. 5.2 half-lives in 3.5 hours).Citation90 This implies that the half-life of (immunoprecipated) perlecan in the rat GBM in vivo to be just 40 minutes. A similar calculation for all HS in the in vivo glomerulus (i.e. this means HS primarily found in the GBM and podocytes (rather than the mesangium, which contains very little or no HS), and includes all ECM perlecan, agrin, collagen XVIII, and HS in the podocyte endothelial glycocalyx, as well as that HS being synthesized within endothelial and podocyte vesicles) (see Figure 4C inCitation90) yields an estimated half-life of just 93 min (22/0.9 = 24.4-fold changes in 7.0 hours i.e. 4.5 half-lives in 7.0 hours).
We observe that the measured half-life for (immunoprecipated) perlecan HS is short relative to the half-life for all HS in the GBM, which is consistent with a comparatively short diffusive transport path length, should perlecan be mainly lost through endothelial fenestra. In contrast to perlecan, agrin HS has to diffuse across the whole thickness of the GBM (requiring transport through the lamina densa to the endothelial fenestra), which presumably explains its longer half-life at around 100 minutes.
Noting that following degradation of all recovered glycoaminoglycans in the glomerulus with chondroitinase ABC and their separation chromatography, remaining sulfate 35 labels only HS. Therefore, the 100 minute half-life estimate is for all HS in the GBM, rather than the half-life of the proteoglycan core proteins themselves, which may have longer half-lives than the labeled HS. Still, it is expected that the core protein half-lives are very much less than collagen, laminin, and nidogen. For example, Lui et al. report that collagen XVIII core protein was not detectable at 12 weeks,Citation77 while agrin core protein had less than 5% remaining at 12 weeks (a more accurate percentage could not be determined by Lui et al.).Citation77 In this context, recall that agrin also has a structural role in stabilizing laminin, by bridging between laminin and molecules in the cell membrane. This may result in a small pool of agrin having a much longer half-life.Citation36 However, the important point here is that the half-life of proteoglycan core proteins can be different from the half-life of HS attached to the core proteins. The above data suggest that the HS chains attached to the core proteins are degraded by heparanase at a faster rate than degradation of the proteoglycan core proteins by proteases.
If our estimated in vivo half-lives for HS are even approximately true, this has very important implications for experimental estimates of the amount of HS in the GBM. HS may well be lost from the GBM in the time required to process renal tissue according to the reported experimental protocols. Processing renal tissue to recover HS (from unfixed tissue) usually involves time to (i) remove the kidney from the animal, (ii) mechanically slice or mince the tissue, and then push slices of the renal cortex through one or more seives, isolating the glomeruli, (iii) followed by decellularization of the glomeruli and (iv) centrifugation to finally obtain the GBMs in a pellet. Sometimes, pellets are aggregated and compressed to form a reconstituted ‘filter,’ which are subsequently analyzed for HS (e.g. [Citation12]) Presumably the decellularization step (using sonication or detergents) would allow the HS within the GBM to escape even more quickly than it can in vivo, as diffusive transport lengths are then decreased substantially.
Indeed, Daniels reports on the behavior of the reconstituted filter:Citation12
Treatment of isolated GBM with heparatinase to remove HS proteoglycan anionic side chains or protamine to effect charge neutralization had no effect on the albumin seiving coefficient … In contrast, when intact glomeruli were treated with either heparantinase or protamine, albumin permeability doubled and protamine treatment of glomeruli resulted in an even greater agumentation of albumin permeability.
Absent tissue fixation, these findings are consistent with most of the HS (and doubtless some of the GBM proteins) being lost from the isolated GBM during preparation. Depending on GBM processing protocols and the time elapsed between cell death and measurement of remaining HS in the GBM, it seems entirely possible that in vivo HS concentrations could be around double (possibly more) than those reported for the processed GBM.
This conjecture, based on Akuffo et al.’s data, is supported by Bevan et al. who report:Citation91
Glomeruli from the kidney which had been perfused with cetylpyridinium chloride [a cationic quaternary ammonium compound] contained approx. 2.6 times more [35S] glycosaminoglycan than those isolated from non-perfused kidneys.
This suggests that the initial perfusion with a cationic compound can act as a ‘fixative,’ at least substantially reducing the rate of loss of heparan sulfate from glomeruli. Indeed, Akuffo et al. state unequivocally that:
cetylpyridinium chloride forms an insoluble complex with proteoglycans and glycosaminoglycans, fixing them in the tissue.Citation90
Further, the contention that anionic charge attributable to heparan sulfate is lost over time from the glomerulus is also supported by data from Comper et al., who report:Citation92
In a non-perfused kidney, the incorporation of 35S-labelled macromolecular material was 0.37 ± 0.09 (n = 3) d.p.m./glomerulus and the residual level in a perfused [ex-situ with Krebs-Henseleit buffer containing 5% BSA with amino acids for 2 hours] kidney was 0.16 ± 0.03 (n = 3) d.p.m./glomerulus … . When the fixed charge concentration is examined in glomeruli isolated from perfused kidneys, it is clear that there is also a substantial decrease (> 50 %) in charge content within the glomerulus (expressed in terms of p-equiv./glomerulus) in accord with a similar proportional loss of 35S-labelled macromolecules.
The fraction of loss (>50%) over 2 hours of perfusion ex-situ kidney (with no fixative used either before, during or after perfusion) demonstrates that any delay (e.g. due to perfusion or tissue processing) reduces the sulfate content of the glomerulus significantly.
We have already learnt that most of the HS in the GBM is produced by podocytes in the form of agrin.Citation90 Therefore, it is reassuring that following the intravenous injection of the radioactive label sulfate 35, labeled HS molecules are reported to be normally first detected using autoradiography in the podocyte Golgi apparatus.Citation42 The radiolabels next appear in the podocyte foot processes, and upon reaching the epithelial side of the GBM, they are then detected further across the GBM (see Figure 7C and discussion in [Citation42]). To maintain their extraordinary rate of synthesis and secretion by podocytes, we surmise the proteoglycans accumulate in the lamina rara externa, diffusing along the lamina rara externa, then continuing their journey across the lamina densa by diffusing down their concentration gradient. Finally, these proteoglycans exit the GBM into the plasma, or if sufficiently close to mesangium, diffuse laterally in the lamina rara interna to be taken up within the mesangium.
Smaller amounts of proteoglycans are produced by endothelial cells, so not all sulfate label appears in podocytes Golgi. Endothelial cells mainly produce the HS bearing ECM molecule perlecan.Citation45 Perlecan is secreted into the lamina rara interna, joining the agrin proteoglycans produced by the podocytes moving across the GBM. That is, agrin and perlecan both diffuse down the total HS concentration gradient to empty into either the plasma or mesangium.
As the principal source of proteoglycans, clearly any action that disrupts podocyte production or secretion of HS will disrupt the normal ‘life cycle’ of HS. For example, if some podocyte foot processes disengage from the GBM, then the main source of heparan sulfate to the GBM is removed, and so we would expect the normal HS concentration and its gradient across the GBM would be reduced.
Finally, both endothelial cells and podocytes are reported to produce collagen XVIII, which also bear HS chains.Citation46,Citation93 Kinnunen et al. report that the collagen XVIII is polarized with ‘the endothelial promoter 1-derived short variant of collagen XVIII and the epithelial promoter 2-derived longer variants having their C-terminal endostatin domains embedded in the BM and the N termini at the respective BM-cell interfaces.’Citation46
Interestingly, Liu et al. report that collagen XVIII had an N-14 content of zero, indicating it was completely turned over in less than 12 weeks.Citation77 Based on this data, it seems possible that collagen XVIII could have a half-life as little as one or two weeks, though again we expect that HS will be removed more quickly by heparanase.
5 The spatial distribution of GBM ECM molecules
As most of the GBM extracellular matrix is normally produced by podocytes,Citation68 we might expect to see some ECM density asymmetry across the GBM (e.g. the ECM density increasing in the direction of podocytes). Asymmetry may be expected due to various transport processes (e.g., diffusion) and gradients in regulatory molecules (e.g., proteases). However, a density asymmetry across the GBM is not detectable in the eye based on transmission electron microscope images. Indeed, the lamina densa appears remarkably uniform in many electron microscope images (see, for example, ).Citation25 Nevertheless, using so-named ‘STORM’ technology, which is a high-resolution light microscope technology, Suleiman et al. do report asymmetries in the concentration distributions across the GBM for various ECM molecules. To be accurate, STORM technology detects the density of epitope probes across the GBM, the epitope probes being antibodies conjugated to Alexa 647 (a fluorophore).
We recognize that the STORM data needs to be interpreted with some caution because antibodies may not be able to access epitopes in the ECM molecules, either due to the depth of the epitope from the surface or due to the density of surrounding ECM molecules.Citation28 Clearly, ECM density variations across the GBM may influence access of the probe to the epitope, but we see that there are no observable jumps in epitope probe concentration data,Citation28 for example, at junctions between the lamina rara and the lamina densa (which are visually obvious density transitions in standard TEM images). Indeed, across the GBM most epitope probe concentrations are smooth, suggesting that the corrective measures taken by Suleiman et al. were successful and probe access to epitopes is not likely to be a significant factor in the reported STORM concentration profiles. With these potential shortcomings in mind, we nevertheless recognize STORM technology data as defining the current state-of-the-art information on the spatial distributions of ECM molecules across the GBM.
By fitting the antibody density distribution for the agrin C epitope to two normal probability distributions, and then taking the midpoint between the two probability peaks as their (arbitrary) zero point, Suleiman et al. sets up a ‘spatial reference axis’ across the GBM (the zero point appears to be very close to the mid-point of the GBM, and the positive direction for this axis is assumed to be directed toward the podocyte side of the GBM).Citation28 TEM imaging reveals the mouse GBM to be 200 nm wide for the animals studied. Using STORM technology, the two normal distribution peaks for agrin C antibody concentration were measured to be 133 nm apart (i.e., the peaks are at plus and minus 67.5 nm on the above-mentioned reference axis), indicating that there is about 32.5 nm from each agrin C peak to the endothelial or podocyte cell membranes (Figure 1in [Citation28]). These measured distances are supported by epitope labeling of the C end of laminin 521 (recall the C end of laminin is attached to the integrins at the cell membrane). Specifically, the peaks of these labels are located at about ±100 nm on the above-mentioned reference axis (see Figures 2e and 2f in [Citation28]). Note 2 × 100 nm equals the estimate for GBM thickness made using TEM imaging. Taken together, this consistency in the location data gives some reassurance that the STORM measurements are accurate.
Interestingly, the peak probe density for the laminin region named α5-LEb/L4b is located close to the reference zero point and forms a triangular distribution across the GBM for this labeled epitope. This indicates that this laminin region is distributed across the GBM, rather than in two narrow bands as predicted on the basis of usual laminin models (see, for example, Figure 3 in [Citation41]). As the zero-reference point is 100 nm from the endothelial and epithelial cell membranes, laminin 521 is not long enough to span the shortest distance from the cell membrane to the zero reference point (see previously discussed dimensions of laminin), even if the three laminin short arms are fully extended in the direction of the coiled coil (the laminin region α5-LEb/L4b is not even close to the end of the laminin arms). This suggests that the laminin located at the center of the 200 nm thick GBM, within the lamina densa, may not be bound to cell membranes, but rather some laminin molecules that ‘float’ within the lamina densa, free of a cell membrane attachment. This remains to be confirmed experimentally, but if true, it suggests that laminin attachments to the cell membrane are labile. The notion of labile laminin interactions with the cell membrane appears to be confirmed by data presented in [Citation37], where it is reported that laminin ‘protein therapy’ (i.e. laminin molecules delivered intravenously) enables laminin molecules to enter the GBM through endothelial fenestrae, restoring the functional integrity of the GBM and consequently ‘abrogates the development of nephrotic syndrome.’Citation37
Importantly, nidogen also forms a triangular distribution, with an antibody peak somewhat to the negative side of zero (about −20 nm) (see Figure 3D in [Citation28]) (unfortunately type 1 and 2 nidogens are not distinguished in the data reported by Suleiman et al.).
As might be expected, when collagen IV α1α1α2 is only produced by endothelial cells in the mature GBM, the distribution of the antibody label has an asymmetrical (also triangular) distribution, with the peak density at about −75 nm. The collagen IV α1α1α2 labeling distribution even extends beyond −100 nm, which probably reflects either collagen IV α1α1α2 in the endothelial cells prior to release to the GBM, or it reflects collagen IV α1α1α2 ‘poking’ through the endothelial fenestra as it exits the GBM (see Figure 3C in [Citation28]), or some combination of both.
Collagen IV α3α4α5 NC1 domain epitope labels are also distributed asymmetrically in a trapezoidal distribution (or irregular quadrilateral shape), with a peak concentration at about −50 nm (see Figure 3A in [Citation28]), while collagen α3α4α5 peri-N domain epitope (close to the 7S domain) again has a triangular distribution, with peak at about −25 nm to −50 nm (see Figure 3B in [Citation28]) This suggests some general structure for the way collagen IV is aligned in the GBM, with somewhat more collagen IV toward the endothelial side of the GBM (which is reflected in our proposed GBM protein distribution model). We again observe that there is a significant concentration of collagen IV α3α4α5 extending beyond −100 nm (Figures 3A and 3B in [Citation28]), which may indicate that collagen IV α3α4α5 poking through the endothelial fenestra. Collagen IV α3α4α5 may also extend beyond 100 nm, which is presumably podocyte intracellular collagen or collagen extending into the regions between the podocyte foot processes. These distributions also appear to suggest that collagen IV α3α4α5 dominates the production of collagen IV α1α1α2 (though one needs to keep in mind that the binding strength between antibodies, epitopes and antibody accesses may also differ between the collagen IV types).
6 Conclusions
In reviewing the literature on the organization and turnover of the ECM components of the GBM, we reach the following conclusions:
ECM proteins are mainly produced by the podocytes (with some smaller fraction of production by endothelial cells). These proteins are probably lost from the GBM to both the mesangium (by endocytosis), and to the plasma (by escaping through endothelial fenestra).
GBM proteoglycans are mainly produced by podocytes (with some smaller fraction of total production by endothelial cells). After being degraded, mainly through removal of HS by heparinase and a slower rate of core protein proteolysis, most HS and core proteins probably escape the GBM through endothelial fenestra.
ECM proteins have a variety of half-lives in the GBM. Collagen IV has the longest half-life at greater than 180–200 days (but probably less than 1 year), laminin has a half-life somewhat less than collagen IV, while nidogens 1 and 2 have a half-life of 24 and 49 days, respectively. In contrast to these relatively long-lived ECM proteins, the half-life of heparan sulfate is likely to be measured in less than a few hours, while the half-life of proteoglycan core proteins may be as little as a few days, and at most a few weeks, depending on the proteoglycan.
The rapid turnover of heparan sulfate is likely to confound the interpretation of experimental results where there is any delay between harvest of GBM and measurement (e.g. of HS content or even of proteinuria).
The rapid turnover of HS may facilitate unclogging of the GBM pore network and cross-talk from podocytes-to-endothelia of large molecules.
The ECM proteins are non-uniformly distributed across the GBM. For example, laminin and nidogen have triangular distributions across the GBM.
Grants
Azin Azadi is supported by Murdoch University Strategic Scholarship.
Disclosure statement
We confirm that none of the authors has any conflicts of interest.
Additional information
Funding
References
- Schlöndorff D, Wyatt CM, Campbell KN. Revisiting the determinants of the glomerular filtration barrier: what goes round must come round. Kidney Int. 2017;92(3):222–238. doi:10.1016/j.kint.2017.06.003.
- Naylor RW, Morais M, Lennon R. Complexities of the glomerular basement membrane. Nat Rev Nephrol. 2020;17(2):112–127.
- Kanwar YS, Linker A, Farquhar MG. Increased permeability of the glomerular basement membrane to ferritin after removal of glycosaminoglycans (heparan sulfate) by enzyme digestion. J Cell Biol. 1980;86(2):688–693. doi:10.1083/jcb.86.2.688.
- Rennke HG, Venkatachalam MA. Glomerular permeability: in vivo tracer studies with polyanionic and polycationic ferritins. Kidney Int. 1977;11(1):44–53. doi:10.1038/ki.1977.6.
- Bertolatus JA, Hunsicker LG. Glomerular sieving of anionic and neutral bovine albumins in proteinuric rats. Kidney Int. 1985;28(3):467–476. doi:10.1038/ki.1985.153.
- Ciarimboli G, Schurek HJ, Zeh M, Flohr H, Bokenkamp A, Fels LM, Kilian I, Stolte H. Role of albumin and glomerular capillary wall charge distribution on glomerular permselectivity: studies on the perfused-fixed rat kidney model. Pflugers Arch. 1999;438(6):883–891. doi:10.1007/s004249900120.
- Sverrisson K, Axelsson J, Rippe A, Asgeirsson D, Rippe B. Dynamic, size-selective effects of protamine sulfate and hyaluronidase on the rat glomerular filtration barrier in vivo. Am J Physiol Renal Physiol. 2014;307(10):F1136–43. doi:10.1152/ajprenal.00181.2014.
- Assel E, Neumann KH, Schurek HJ, Sonnenburg C, Stolte H. Glomerular albumin leakage and morphology after neutralization of polyanions. I. Albumin clearance and sieving coefficient in the isolated perfused rat kidney. Ren Physiol. 1984;7:357–364.
- Bertolatus JA, Abuyousef M, Hunsicker LG. Glomerular sieving of high molecular weight proteins in proteinuric rats. Kidney Int. 1987;31(6):1257–1266. doi:10.1038/ki.1987.139.
- Sorensson J, Ohlson M, Lindstrom K, Haraldsson B. Glomerular charge selectivity for horseradish peroxidase and albumin at low and normal ionic strengths. Acta Physiol Scand. 1998;163(1):83–91. doi:10.1046/j.1365-201x.1998.00315.x.
- Haraldsson B, Nystrom J, Deen WM. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev. 2008;88(2):451–487. doi:10.1152/physrev.00055.2006.
- Daniels BS. Increased albumin permeability in vitro following alterations of glomerular charge is mediated by the cells of the filtration barrier. J Lab Clin Med. 1994;124:224–230.
- Mohos SC, Skoza L. Histochemical demonstration and localization of sialoproteins in the glomerulus. Exp Mol Pathol. 1970;12(3):316–323. doi:10.1016/0014-4800(70)90063-8.
- Kasinath BS. The podocyte and the proteoglycan. Am J Physiol Renal Physiol. 2016;311(2):F310–1. doi:10.1152/ajprenal.00295.2016.
- Quaggin SE. Sizing up sialic acid in glomerular disease. J Clin Invest. 2007;117(6):1480–1483. doi:10.1172/JCI32482.
- Kawachi H, Miyauchi N, Suzuki K, Han GD, Orikasa M, Shimizu F. Role of podocyte slit diaphragm as a filtration barrier. Nephrology. 2006;11(4):274–281. doi:10.1111/j.1440-1797.2006.00583.x.
- Grahammer F, Schell C, Huber TB. The podocyte slit diaphragm—from a thin grey line to a complex signalling hub. Nat Rev Nephrol. 2013;9:587–598. doi:10.1038/nrneph.2013.169.
- Gong Y, Sunq A, Roth RA, Hou J. Inducible expression of Claudin-1 in glomerular podocytes generates aberrant tight junctions and proteinuria through slit diaphragm destabilization. J Am Soc Nephrol. 2017;28:106–117. doi:10.1681/ASN.2015121324.
- Akilesh S. Normal kidney function and structure. In: McManus LM, Mitchell RN, editors. Pathobiology of human disease. San Diego, USA: Academic Press; 2014. p. 2716–2733.
- Neumann KH, Kellner C, Kühn K, Stolte H, Schurek H-J. Age-dependent thickening of glomerular basement membrane has no major effect on glomerular hydraulic conductivity. Nephrol Dial Transplant. 2004;19(4):805–811. doi:10.1093/ndt/gfh067.
- McAdams AJ. Glomerular capillary wall basement membrane really does have laminae lucidae: a defense. Pediatr Dev Pathol. 1999;2(3):260–263. doi:10.1007/s100249900121.
- Marshall CB. Rethinking glomerular basement membrane thickening in diabetic nephropathy: adaptive or pathogenic? Am J Physiol. 2016;311:F831–F43.
- Yamada E. The fine structure of the renal glomerulus of the mouse. J Cell Biol. 1955;1(6):551–566. doi:10.1083/jcb.1.6.551.
- Yang H-C, Zuo Y, Fogo AB. Models of chronic kidney disease. Drug Discov Today. 2010;7:13–19.
- Farquhar MG. The glomerular basement membrane: not gone, just forgotten. J Clin Invest. 2006;116(8):2090–2093. doi:10.1172/JCI29488.
- Chan FL, Inoue S. Lamina lucida of basement membrane: an artefact. Microsc Res Tech. 1994;28(1):48–59. doi:10.1002/jemt.1070280106.
- Mouse genome informatics, gene ontology browser: basement membrane. 2022. Available from: http://www.informatics.jax.org/vocab/gene_ontology/GO:0005604. Accessed 29 July 2022.
- Suleiman H, Zhang L, Roth R, Heuser JE, Miner JH, Shaw AS, Dani A. Nanoscale protein architecture of the kidney glomerular basement membrane. Elife. 2013;2:e01149. doi:10.7554/eLife.01149.
- Lennon R, Byron A, Humphries JD, Randles MJ, Carisey A, Murphy S, Knight D, Brenchley PE, Zent R, Humphries MJ, et al. Global analysis reveals the complexity of the human glomerular extracellular matrix. J Am Soc Nephrol. 2014;25(5):939–951. doi:10.1681/ASN.2013030233.
- Timpl R, Wiedemann H, van Delden V, Furthmayr H, Kuhn K. A network model for the organization of type IV collagen molecules in basement membranes. Eur J Biochem. 1981;120(2):203–211. doi:10.1111/j.1432-1033.1981.tb05690.x.
- Eble JA, Ries A, Lichy A, Mann K, Stanton H, Gavrilovic J, Murphy G, Kühn K. The recognition sites of the integrins alpha1beta1 and alpha2beta1 within collagen IV are protected against gelatinase A attack in the native protein. J Biol Chem. 1996;271(48):30964–30970. doi:10.1074/jbc.271.48.30964.
- Khoshnoodi J, Pedchenko V, Hudson BG. Mammalian collagen IV. Microsc Res Tech. 2008;71(5):357–370. doi:10.1002/jemt.20564.
- Abrahamson DR. Role of the podocyte (and glomerular endothelium) in building the GBM. Semin Nephrol. 2012;32(4):342–349. doi:10.1016/j.semnephrol.2012.06.005.
- Sevcnikar B, Schaffner I, Chuang CY, Gamon L, Paumann-Page M, Hofbauer S, Davies MJ, Furtmüller PG, Obinger C. The leucine-rich repeat domain of human peroxidasin 1 promotes binding to laminin in basement membranes. Arch Biochem Biophys. 2020;689:108443. doi:10.1016/j.abb.2020.108443.
- Cummings CF, Pedchenko V, Brown KL, Colon S, Rafi M, Jones-Paris C, Pokydeshava E, Liu M, Pastor-Pareja JC, Stothers C, et al. Extracellular chloride signals collagen IV network assembly during basement membrane formation. J Cell Biol. 2016;213(4):479–494. doi:10.1083/jcb.201510065.
- Suh JH, Miner JH. The glomerular basement membrane as a barrier to albumin. Nat Rev Nephrol. 2013;9(8):470–477. doi:10.1038/nrneph.2013.109.
- Lin MH, Miller JB, Kikkawa Y, Suleiman HY, Tryggvason K, Hodges BL, Miner JH. Laminin-521 protein therapy for glomerular basement membrane and podocyte abnormalities in a model of Pierson syndrome. J Am Soc Nephrol. 2018;29(5):1426–1436. doi:10.1681/ASN.2017060690.
- Hamill KJ, Kligys K, Hopkinson SB, Jones JC. Laminin deposition in the extracellular matrix: a complex picture emerges. J Cell Sci. 2009;122(Pt 24):4409–4417. doi:10.1242/jcs.041095.
- Miner JH. Renal basement membrane components. Kidney Int. 1999;56(6):2016–2024. doi:10.1046/j.1523-1755.1999.00785.x.
- Hohenester E, Adams J. Structural biology of laminins. Essays Biochem. 2019;63(3):285–295. doi:10.1042/EBC20180075.
- Hohenester E, Yurchenco PD. Laminins in basement membrane assembly. Cell Adh Migr. 2013;7(1):56–63. doi:10.4161/cam.21831.
- Lelongt B, Makino H, Kanwar YS. Status of glomerular proteoglycans in aminonucleoside nephrosis. Kidney Int. 1987;31(6):1299–1310. doi:10.1038/ki.1987.143.
- Harvey SJ, Jarad G, Cunningham J, Rops AL, van der Vlag J, Berden JH, Moeller MJ, Holzman LB, Burgess RW, Miner JH, et al. Disruption of glomerular basement membrane charge through podocyte-specific mutation of agrin does not alter glomerular permselectivity. Am J Pathol. 2007;171(1):139–152. doi:10.2353/ajpath.2007.061116.
- Denzer AJ, Schulthess T, Fauser C, Schumacher B, Kammerer RA, Engel J, Ruegg MA. Electron microscopic structure of agrin and mapping of its binding site in laminin-1. EMBO J. 1998;17(2):335–343. doi:10.1093/emboj/17.2.335.
- Groffen AJ, Ruegg MA, Dijkman H, van de Velden TJ, Buskens CA, van den Born J, Assmann KJ, Monnens LA, Veerkamp JH, van den Heuvel LP, et al. Agrin is a major heparan sulfate proteoglycan in the human glomerular basement membrane. J Histochem Cytochem. 1998;46(1):19–27. doi:10.1177/002215549804600104.
- Kinnunen AI, Sormunen R, Elamaa H, Seppinen L, Miller RT, Ninomiya Y, Janmey PA, Pihlajaniemi T. Lack of collagen XVIII long isoforms affects kidney podocytes, whereas the short form is needed in the proximal tubular basement membrane. J Biol Chem. 2011;286(10):7755–7764. doi:10.1074/jbc.M110.166132.
- Noborn F, Gomez Toledo A, Green A, Nasir W, Sihlbom C, Nilsson J, Larson G. Site-specific identification of heparan and chondroitin sulfate glycosaminoglycans in hybrid proteoglycans. Sci Rep. 2016;6:34537. doi:10.1038/srep34537.
- Tsen G, Halfter W, Kroger S, Cole GJ. Agrin is a heparan sulfate proteoglycan. J Biol Chem. 1995;270(7):3392–3399. doi:10.1074/jbc.270.7.3392.
- Martinez JR, Dhawan A, Farach-Carson MC. Modular proteoglycan perlecan/HSPG2: mutations, phenotypes, and functions. Genes. 2018;9(11):556. doi:10.3390/genes9110556.
- Halfter W, Dong S, Schurer B, Cole GJ. Collagen XVIII is a basement membrane heparan sulfate proteoglycan. J Biol Chem. 1998;273(39):25404–25412. doi:10.1074/jbc.273.39.25404.
- Morita H, Yoshimura A, Kimata K. The role of heparan sulfate in the glomerular basement membrane. Kidney Int. 2008;73(3):247–248. doi:10.1038/sj.ki.5002659.
- Comper WD. Resolved: normal glomeruli filter nephrotic levels of albumin. J Am Soc Nephrol. 2008;19(3):427–432. doi:10.1681/ASN.2007090997.
- Miner JH. Glomerular filtration: the charge debate charges ahead. Kidney Int. 2008;74(3):259–261. doi:10.1038/ki.2008.260.
- Smith DW, Gardiner BS, Zhang L, Grodzinsky AJ. Articular cartilage dynamics. Singapore: Springer; 2019.
- Smith DW, Gardiner BS, Davidson J, Grodzinsky AJ, Smith DW. Computational model for the analysis of cartilage and cartilage tissue constructs. J Tissue Eng Regener Med. 2016;19(10):1160–1170. doi:10.1080/10255842.2015.1115022.
- Robinson CJ, Mulloy B, Gallagher JT, Stringer SE. VEGF165-binding sites within heparan sulfate encompass two highly sulfated domains and can be liberated by K5 lyase. J Biol Chem. 2006;281(3):1731–1740. doi:10.1074/jbc.M510760200.
- Aumailley M, Battaglia C, Mayer U, Reinhardt D, Nischt R, Timpl R, Fox JW. Nidogen mediates the formation of ternary complexes of basement membrane components. Kidney Int. 1993;43(1):7–12. doi:10.1038/ki.1993.3.
- Weber M. Basement membrane proteins. Kidney Int. 1992;41(3):620–628. doi:10.1038/ki.1992.95.
- Kohfeldt E, Sasaki T, Gohring W, Timpl R. Nidogen-2: a new basement membrane protein with diverse binding properties. J Mol Biol. 1998;282(1):99–109. doi:10.1006/jmbi.1998.2004.
- Dai J, Estrada B, Jacobs S, Sanchez-Sanchez BJ, Tang J, Ma M, Magadán-Corpas P, Pastor-Pareja JC, Martín-Bermudo MD. Dissection of Nidogen function in Drosophila reveals tissue-specific mechanisms of basement membrane assembly. PLoS Genet. 2018;14(9):e1007483. doi:10.1371/journal.pgen.1007483.
- Lebel SP, Chen Y, Gingras D, Chung AE, Bendayan M. Morphofunctional studies of the glomerular wall in mice lacking entactin-1. J Histochem Cytochem. 2003;51(11):1467–1478. doi:10.1177/002215540305101107.
- Nielsen JS, McNagny KM. The role of podocalyxin in health and disease. J Am Soc Nephrol. 2009;20(8):1669–1676. doi:10.1681/ASN.2008070782.
- Nielsen JS, McNagny KM. Novel functions of the CD34 family. J Cell Sci. 2008;121(Pt 22):3683–3692. doi:10.1242/jcs.037507.
- Caulfield JP, Farquhar MG. Distribution of anionic sites in glomerular basement membranes: their possible role in filtration and attachment. Proc Natl Acad Sci U S A. 1976;73(5):1646–1650. doi:10.1073/pnas.73.5.1646.
- Economou CG, Kitsiou PV, Tzinia AK, Panagopoulou E, Marinos E, Kershaw DB, Kerjaschki D, Tsilibary EC. Enhanced podocalyxin expression alters the structure of podocyte basal surface. J Cell Sci. 2004;117(Pt 15):3281–3294. doi:10.1242/jcs.01163.
- Lawrence MG, Altenburg MK, Sanford R, Willett JD, Bleasdale B, Ballou B, Wilder J, Li F, Miner JH, Berg UB, et al. Permeation of macromolecules into the renal glomerular basement membrane and capture by the tubules. Proc Natl Acad Sci U S A. 2017;114(11):2958–2963. doi:10.1073/pnas.1616457114.
- Schiessl IM, Hammer A, Kattler V, Gess B, Theilig F, Witzgall R, Castrop H. Intravital imaging reveals angiotensin II-induced transcytosis of albumin by podocytes. J Am Soc Nephrol. 2016;27(3):731–744. doi:10.1681/ASN.2014111125.
- Chen S, Wassenhove-McCarthy DJ, Yamaguchi Y, Holzman LB, van Kuppevelt TH, Jenniskens GJ, Wijnhoven TJ, Woods AC, McCarthy KJ. Loss of heparan sulfate glycosaminoglycan assembly in podocytes does not lead to proteinuria. Kidney Int. 2008;74(3):289–299. doi:10.1038/ki.2008.159.
- Altshuler AE, Penn AH, Yang JA, Kim GR, Schmid-Schonbein GW. Protease activity increases in plasma, peritoneal fluid, and vital organs after hemorrhagic shock in rats. Plos One. 2012;7(3):e32672. doi:10.1371/journal.pone.0032672.
- Altara R, Manca M, Hermans KC, Daskalopoulos EP, Brunner-La Rocca HP, Hermans RJ, Struijker-Boudier HA, Blankesteijn MW. Diurnal rhythms of serum and plasma cytokine profiles in healthy elderly individuals assessed using membrane based multiplexed immunoassay. J Transl Med. 2015;13:129. doi:10.1186/s12967-015-0477-1.
- McAdoo SP, Pusey CD. Anti-glomerular basement membrane disease. Clin J Am Soc Nephrol. 2017;12(7):1162–1172. doi:10.2215/CJN.01380217.
- Schreiner GF. The mesangial phagocyte and its regulation of contractile cell biology. J Am Soc Nephrol. 1992;2(10 Suppl):S74–82. doi:10.1681/ASN.V210s74.
- Marek I, Becker R, Fahlbusch FB, Menendez-Castro C, Rascher W, Daniel C, Volkert G, Hartner A. Expression of the alpha8 integrin chain facilitates phagocytosis by renal mesangial cells. Cell Physiol Biochem. 2018;45(6):2161–2173. doi:10.1159/000488160.
- Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, Park J-K, Beck F-X, Müller DN, Derer W, et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med. 2009;15(5):545–552. doi:10.1038/nm.1960.
- Keeley DP, Hastie E, Jayadev R, Kelley LC, Chi Q, Payne SG, Jeger JL, Hoffman BD, Sherwood DR. Comprehensive endogenous tagging of basement membrane components reveals dynamic movement within the matrix scaffolding. Dev Cell. 2020;54(1):60–74.e7. doi:10.1016/j.devcel.2020.05.022.
- Price RG, Spiro RG. Studies on the metabolism of the renal glomerular basement membrane. Turnover measurements in the rat with the use of radiolabeled amino acids. J Biol Chem. 1977;252(23):8597–8602. doi:10.1016/S0021-9258(19)75262-4.
- Liu P, Xie X, Jin J. Isotopic nitrogen-15 labeling of mice identified long-lived proteins of the renal basement membranes. Sci Rep. 2020;10(1):5317. doi:10.1038/s41598-020-62348-6.
- Walker F. The origin, turnover and removal of glomerular basement-membrane. J Pathol. 1973;110(3):233–244. doi:10.1002/path.1711100306.
- Christensen EI, Rennke HG, Carone FA. Renal tubular uptake of protein: effect of molecular charge. Am J Physiol. 1983;244:F436–F41.
- Christensen EI, Birn H. Megalin and cubilin: synergistic endocytic receptors in renal proximal tubule. Am J Physiol. 2001;280:F562–F73.
- Miettinen A, Stow JL, Mentone S, Farquhar MG. Antibodies to basement membrane heparan sulfate proteoglycans bind to the laminae rarae of the glomerular basement membrane (GBM) and induce subepithelial GBM thickening. J Exp Med. 1986;163(5):1064–1084. doi:10.1084/jem.163.5.1064.
- Shinkai Y. Experimental glomerulonephritis induced in rabbits by horseradish peroxidase. Mesangial uptake and processing of immune complexes. Lab Invest. 1982;46:577–583.
- Kanwar YS, Farquhar MG. Isolation of glycosaminoglycans (heparan sulfate) from glomerular basement membranes. Proc Natl Acad Sci U S A. 1979;76(9):4493–4497. doi:10.1073/pnas.76.9.4493.
- Parthasarathy N, Spiro RG. Characterization of the glycosaminoglycan component of the renal glomerular basement membrane and its relationship to the peptide portion. J Biol Chem. 1981;256(1):507–513. doi:10.1016/S0021-9258(19)70167-7.
- Kanwar YS, Rosenzweig LJ, Linker A, Jakubowski ML. Decreased de novo synthesis of glomerular proteoglycans in diabetes: biochemical and autoradiographic evidence. Proc Natl Acad Sci USA. 1983;80(8):2272–2275. doi:10.1073/pnas.80.8.2272.
- Kashihara N, Watanabe Y, Makino H, Wallner EI, Kanwar YS. Selective decreased de novo synthesis of glomerular proteoglycans under the influence of reactive oxygen species. Proc Natl Acad Sci U S A. 1992;89(14):6309–6313. doi:10.1073/pnas.89.14.6309.
- Spiro MJ. Sulfate metabolism in the alloxan-diabetic rat: relationship of altered sulfate pools to proteoglycan sulfation in heart and other tissues. Diabetologia. 1987;30(4):259–267. doi:10.1007/BF00270425.
- Borza DB. Glomerular basement membrane heparan sulfate in health and disease: a regulator of local complement activation. Matrix Biol. 2017;57-58:299–310. doi:10.1016/j.matbio.2016.09.002.
- Beavan LA, Davies M, Couchman JR, Williams MA, Mason RM. In vivo turnover of the basement membrane and other heparan sulfate proteoglycans of rat glomerulus. Arch Biochem Biophys. 1989;269(2):576–585. doi:10.1016/0003-9861(89)90143-4.
- Akuffo EL, Hunt JR, Moss J, Woodrow D, Davies M, Mason RM. A steady-state labelling approach to the measurement of proteoglycan turnover in vivo and its application to glomerular proteoglycans. Biochem J. 1996;320(Pt 1):301–308. doi:10.1042/bj3200301.
- Beavan LA, Davies M, Mason RM. Renal glomerular proteoglycans. An investigation of their synthesis in vivo using a technique for fixation in situ. Biochem J. 1988;251(2):411–418. doi:10.1042/bj2510411.
- Comper WD, Lee AS, Tay M, Adal Y. Anionic charge concentration of rat kidney glomeruli and glomerular basement membrane. Biochem J. 1993;289(Pt 3):647–652. doi:10.1042/bj2890647.
- Dong S, Cole GJ, Halfter W. Expression of collagen XVIII and localization of its glycosaminoglycan attachment sites. J Biol Chem. 2003;278(3):1700–1707. doi:10.1074/jbc.M209276200.
