Abstract
High-resolution structures of yeast ribosomes have improved our understanding of the architecture and organization of eukaryotic rRNA and proteins, as well as eukaryote-specific extensions present in some conserved ribosomal proteins. Despite this progress, assignment of specific functions to individual proteins and/or eukaryote-specific protein extensions remains challenging. It has been suggested that eukaryote-specific extensions of conserved proteins from the small ribosomal subunit may facilitate eukaryote-specific reactions in the initiation phase of protein synthesis. This review summarizes emerging data describing the structural and functional significance of eukaryote-specific extensions of conserved small ribosomal subunit proteins, particularly their possible roles in recruitment and spatial organization of eukaryote-specific initiation factors.
Introduction
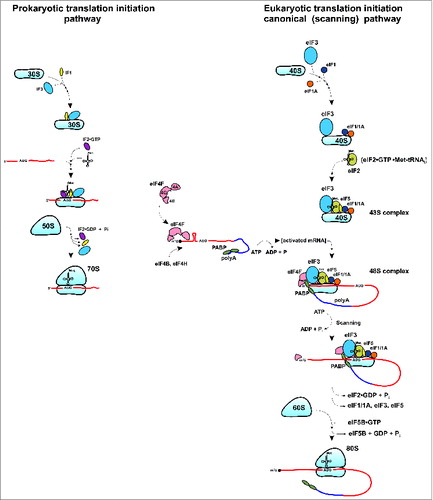
Ribosomes are large ribonucleoprotein (RNP) complexes that decode mRNAs to form proteins.Citation1,2 In both prokaryotes and eukaryotes, ribosomes are composed of 2 subunits with distinct functions, small and large. The small subunit decodes the mRNA within its decoding center (DC).Citation1,2 The large subunit catalyzes peptide bond formation in its peptidyl transferase center (PTC).Citation1,2 Eukaryotic 80S ribosomes (composed of 60S and 40S subunits) have evolved to be much larger in size with more proteins and ribosomal RNA (rRNA) than their archeal, or bacterial counterparts, (70S ribosomes composed of 50S and 30S subunits).Citation3-6 Recent X-ray crystal structures of the yeast 80S ribosome at 3.0 Å resolution permitted detailed analysis of the structural organization of the eukaryotic ribosome.Citation5,6 It became evident that bacterial and eukaryotic ribosomes evolved from a common structural (RNP) core with the majority of changes occurring on the outer shell of the ribosome. These changes include extra rRNA (expansion segments) and additional ribosomal proteins enveloping the core on the solvent side of the ribosome. The 80S yeast ribosome contains 79 proteins, of which 46 are eukaryote-specific (18 in the 40S subunit and 28 in the 60S subunit).Citation5,6 Thirty four proteins (15 in the 40S subunit and 19 in the 60S subunit) are conserved across all kingdoms of life.Citation5 Interestingly, despite general similarity in sequence and structure, many of the conserved ribosomal proteins have evolved eukaryote-specific extensions, the functional significance of which is largely unknown and/or just beginning to emerge.Citation4-11 These polypeptide extensions frequently appear to interact with each other, resulting in complex networks of ribosomal protein interactions on the outer shell of the ribosome in eukaryotes.Citation9,10 It has been hypothesized that eukaryote-specific extensions of the conserved ribosomal proteins evolved to accommodate specific features of the eukaryotic translational apparatus/mechanism such as the increased number of initiation factors (>12 in eukaryotes compared to only 3 in bacteria) ().Citation12,13 This change in particular reflects the appearance of a different (scanning) mode of translation initiation () that is utilized by the majority of eukaryotic mRNAs.Citation12,13 Unlike bacterial systems where the initiation complex (IC; including the small (30S) ribosomal subunit) is positioned directly at the start codon, eukaryotic initiation involves a mechanism whereby the small (40S) ribosomal subunit binds to the 5′-end of mRNA and then scans in search of the initiation AUG codon to form the 48S pre-initiation complex (PIC) (). As the hub for multiple eukaryotic initiation factors (eIFs), the 40S ribosomal subunit plays an important role in the process of translational control of gene expression in eukaryotes.Citation13-15
Table 1. Major eukaryotic translation initiation factors and their functions
Figure 1. Schematic depiction of translation initiation processes (key steps) in prokaryotes and eukaryotes (for eukaryotes, the canonical/scanning pathway is shown). See text for details.
In this review, we discuss emerging data highlighting the structural features and evolutionary significance of eukaryote-specific extensions of conserved proteins from the small ribosomal subunit in ribosome function and, specifically, in the process of translation initiation.
Role of the small ribosomal subunit in translation initiation
Protein synthesis consists of 4 basic steps: initiation, elongation, termination and ribosomal recycling.Citation13,14 While the elongation step is highly similar between prokaryotes and eukaryotes, and termination and ribosome recycling are somewhat similar, the conventional initiation process is strikingly different between bacteria and eukaryotes ().Citation2,13,14 In prokaryotes, start codon selection depends on direct interaction between the 30S ribosomal subunit and the mRNA aided by the 3 single subunit initiation factors IF1, IF2 and IF3. Binding of IF3 to the 30S subunit prevents subunit association and formation of the 70S ribosome.Citation2 IF2 and IF1 bind to the 30S-IF3 complex and promote recruitment of the initiator fMet-tRNAifMet.Citation2,13 The 30S subunit then binds to the mRNA template at a purine-rich (Shine Dalgarno) sequence upstream of the initiation AUG codon. This allows direct positioning of the AUG at the P-site of the 30S subunit and formation of the 30S initiation complex (30S IC). Association of the 30S IC with the 50S ribosomal subunit triggers hydrolysis of IF2-bound GTP and the machinery then proceeds to the elongation phase of translation (, left panel).Citation1,2,13,16,17
In eukaryotes, the small (40S) ribosomal subunit also serves as a central hub for initiation factors (i.e., eIF3, eIF1/1A/5 and the eIF2•GTP•Met-tRNAiMet ternary complex (TC)) as well as mRNA and tRNAs.Citation12-15 After translation termination and ribosome recycling, the 40S and 60S subunits get dissociated, stripped of the mRNA, P-site deacylated tRNA and polypeptide release factor eRF1.Citation18,19 The process of ribosome recycling aided by ATP-binding cassette (ABC) protein ABCE1, eIF3, eIF1 and eIF1A leads to “regeneration” of 40S subunits that become competent for initiation.Citation18,19 Binding of the 40S subunit to multisubunit factor eIF3 prevents association of 40S and 60S subunits, thus enabling formation and engagement of 40S•eIF3•eIF1/1A•TC complex in the next round of initiation. However, in contrast to the situation in prokaryotes, for most eukaryotic mRNAs, the PIC, 43S complex, comprised of 40S, eIF3, eIF1/1A and TC does not land directly on the AUG start codon, binding instead to the 5’ end of the mRNA. The eukaryote-specific initiation factor eIF4G functions as a scaffolding protein that bridges the mRNA and the ribosome.Citation14,20,21 It binds eIF4E (a 5’-end cap-binding protein) and eIF4A (an ATP-dependent RNA helicase that unwinds secondary structure in the mRNA 5′-untranslated region (UTR)) to form the multisubunit factor eIF4F that bridges the mRNA and the ribosome via eIF4G interaction with the 40S-bound initiation factor eIF3.Citation14,20,21 The 40S ribosome (with associated factors) binds to the activated (by eIF4 initiation factors) mRNA and then scans in search of the initiation AUG codon (, right panel). Poly(A)-binding protein (PABP) is believed to enforce a “closed loop” mRNA conformation via interaction with eIF4G. Following recognition of the start codon and eIF5-induced irreversible hydrolysis of eIF2-bound GTP, eIF5B promotes joining of the 40S and 60S subunits and the elongation process begins.Citation14 It should be noted that in yeast, eIFs 1, 3, 5 and TC may form a Multi Factor Complex (MFC) that associates with the 40S subunit as an entire complex to form the 43S PIC.Citation22,23 In addition, evidence from yeast suggests that eIF5 may play a role in bridging the interaction between eIF3 and eIF4G.Citation24,25 Recruitment of the 43S PIC to the mRNA and subsequent scanning is believed to be facilitated by an ‘open’ latch conformation of the 40S subunit maintained by eIF1 and 1A.Citation26,27 However, the exact mechanism by which the mRNA threads through the narrow mRNA channel in the 40S ribosomal subunit remains a mystery. The ‘open’ scanning-competent conformation of the 40S subunit allows sampling of non-AUG triplets during movement of the initiation complex, but does not lead to efficient codon-anticodon base pairing.Citation27,28 During scanning, eIF1 acts as a gatekeeper and blocks the release of Pi from partially hydrolyzed eIF2•GTP into eIF2•GDP•Pi induced by eIF5 and maintains the ‘open’ 40S conformation. Upon recognition of a start AUG codon, factors are rearranged and eIF1 is released. Subsequently, Pi is released from the partially hydrolyzed eIF2•GDP•Pi intermediate and the 40S subunit is converted from an ‘open’ scanning-competent conformation to a ‘closed’ conformation.Citation28,29 This ultimately leads to formation of elongation competent 80S ribosomes (). Thus, in contrast to prokaryotes, where direct binding of the small ribosomal subunit to mRNA mediates proper translation initiation, a complex interplay between the 40S subunit, eIF1, 1A, TC, ribosome movement on the mRNA, eIF5-stimulated GTP hydrolysis and subsequent Pi release is required for efficient start codon recognition in eukaryotes.
Eukaryote-specific extensions in conserved ribosomal proteins of the 40S subunit
The eukaryotic 40S (“small”) ribosomal subunit contains 33 proteins and a single 18S rRNA molecule. Fifteen of the small subunit proteins are highly conserved (). Interestingly, 13 of these highly conserved proteins harbor additional segments () ranging in size and location with respect to the main conserved part of the polypeptide (). Comparison of the available structural data for Escherichia coli and Saccharomyces cerevisiae ribosomes show that 7 out of 13 conserved proteins harbor N-terminal extensions, while only 3 have extensions at the C-terminus (). Two proteins (namely, uS2 and uS12) have extensions on both N- and C-terminal ends and one (uS13) harbors an N-terminal extension and an insertion in the middle region of the protein (). Many of these extensions in yeast are relatively long (e.g., up to ∼100 amino acids (aa) in uS5 and uS15) and comprise 30–70% of the entire ribosomal protein length (e.g., in uS2, uS4, uS5, uS15 and uS19) (). In general, yeast N-terminal extensions appear to be longer (average length ∼46 aa) than C-terminal ones (average length ∼38 aa) (). The extensions can be unstructured and/or form β-sheets or α-helices.Citation5,6,12 X-ray crystal structures of the yeast 80S ribosome indicate that at least 10 eukaryote-specific extensions of conserved proteins in the small subunit are involved in protein-protein interactions specific for the 40S ribosomal subunit.Citation5,6 It should be noted, however, that sequence alignments of proteins (from different organisms) belonging to the same family reveal substantial variability in the lengths of eukaryote-specific extensions (). To illustrate this, we provide comparison of the sequence lengths of uS7 proteins (which were manually annotated) from several species. This shows that many fungi and the fruit fly Drosophila melanogaster have evolved the longest N-terminal extensions (∼65 aa) in comparison with vertebrates (∼40–45 aa) and other eukaryotes ().
Figure 2. Graphical representation of the conservation of small ribosomal subunit protein families between different domains of life (E: Eukarya, A: Archaea, and B: Bacteria; left pie chart). Fifteen protein families are conserved across all domains (EAB) and 13 of these 15 conserved families have evolved eukaryote-specific extensions (right pie chart).
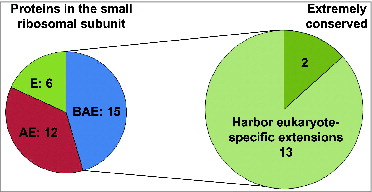
Figure 3. Ribosomal small subunit protein families with eukaryote-specific extensions. (A) Pie chart depicting the distribution of eukaryote-specific extensions (N-terminal, C-terminal, etc.) relative to the conserved part of the protein for the 13 ribosomal small subunit proteins with known extensions (based on the structural information available for the yeast and bacterial ribosomesCitation5,6,12,76,77). (B) Relative lengths of eukaryote-specific extensions in different conserved ribosomal small subunit proteins based on structural alignment of yeast (S. cerevisiae) and bacterial proteins. Blue bars depict N-terminal extensions, red bars depict C-terminal extensions and yellow bars show the length of the entire protein (in the yeast S. cerevisiae for all). (C) Variability in the relative lengths of eukaryote-specific extensions in different conserved ribosomal small subunit proteins based on alignment of S. cerevisiae, Schizosaccharomyces pombe, Mus Musculus, Homo sapiens and Xenopus laevis sequences (relative to corresponding E. coli sequences). Average lengths for the listed eukaryotic species are shown, with standard deviations indicated by error bars. Blue bars depict N-terminal extensions, red bars depict C-terminal extensions and yellow bars show the length of the entire protein. New (universal) nomenclature of ribosomal proteins is used.Citation49
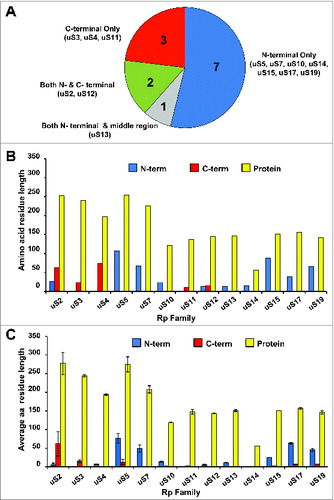
Figure 4. Ribosomal location of eukaryote-specific extension segments of conserved proteins from the small subunit and their known, or potential, functional significance. Only eukaryote-specific segments of conserved protein families are shown. New (universal) nomenclature of ribosomal proteins is used.Citation49 Yeast ribosomal protein names are provided in parentheses. Top – solvent side view. Bottom – interface side view. Extensions are color coded, with the rest of the protein content shown in cyan and rRNA content shown in gray. PDB entries 3U5GCitation6 and 3U5FCitation6 were used to build the models.
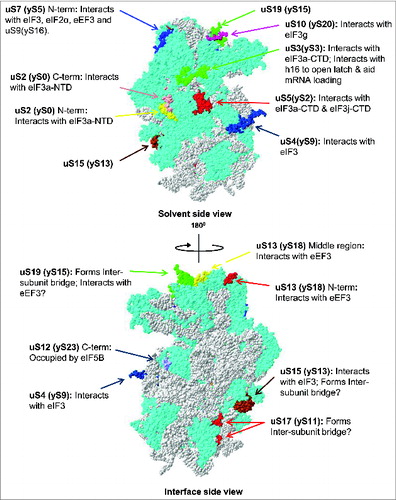
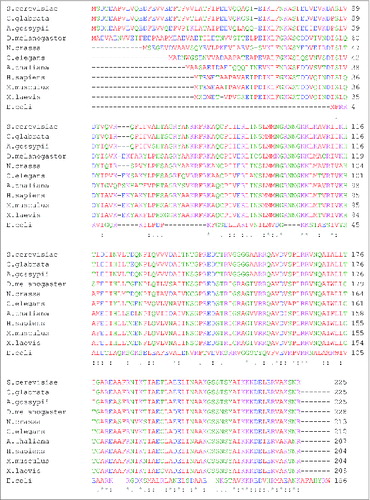
Figure 5. Sequence alignment of uS7 proteins from different organisms showing length variability of eukaryote-specific N-terminal extensions. uS7 protein sequences from fungi (S. cerevisiae, Candida glabrata, Ashbya gossypii, Neurospora crassa), fruit fly (D. melanogaster), worm (Caenorhabditis elegans), plant (Arabidopsis thaliana), vertebrates (Xenopus laevis, Mus musculus, Homo sapiens) and bacteria (E. coli) were aligned using the Clustal W algorithm. S. cerevisiae rpS5 (UniProtKB accession no.: P26783); C. glabrata rpS5 (UniProtKB accession number: Q6FSH6); A. gossypii rpS5 (UniProtKB accession no.: Q75D52); D. melanogaster rpS5 (UniProtKB accession number: Q24186); N. crassa rpS5 (UniProtKB accession no.: Q7RVI1); C. elegans rpS5 (UniProtKB accession number: P49041); A. thaliana rpS5 (UniProtKB accession no.: Q9ZUT9); H. sapiens rpS5 (UniProtKB accession number: P46782); M. musculus rpS5 (UniProtKB accession no.: P97461); X. laevis rpS5 (UniProtKB accession number: Q7SYU3); E. coli rpS7 (UniProtKB accession no.: Q0TCB8) sequences were used.
Below, we discuss emerging structural, genetic and biochemical data suggesting that the extensions present in conserved ribosomal proteins of eukaryotes may be involved in complex networks of interactions with eukaryote-specific translation (initiation) factors as well as other ribosome binding proteins and/or ribosomal proteins themselves.
Potential functional roles of eukaryote-specific extensions in conserved proteins of the 40S ribosomal subunit
While recent studies have yielded novel insights into the mechanism of translation initiation,Citation1,14,28 many details including the exact architecture of the 43S and 48S pre-initiation complexes is lacking or just beginning to emerge.Citation30,31 Information on the placement and orientation of eukaryotic initiation factors on the ribosomal surface has been primarily obtained through genetic and biochemical experiments in yeast,Citation32,33 or more recently, through Cryo-Electron Microscopy (cryo-EM) with yeast and mammalian ribosomes and purified initiation factors.Citation30,31 These data suggested that many eukaryote-specific extensions of conserved ribosomal proteins are involved in recruitment and binding of several key eukaryotic initiation factors (i.e., eIF3, eIF2) as well as other proteins, including fungi-specific eukaryotic elongation factor 3 (eEF3) and eukaryote-specific ribosomal binding protein RACK1 (Asc1)Citation32,33 ().
Table 2. Conserved proteins from the 40S small ribosomal subunit with eukaryote-specific extensions: interacting partners
Apart from their ribosome-associated function(s), some eukaryotic conserved small ribosomal subunit proteins have novel extra-ribosomal functions and, at least in several cases, these can be directly attributed to the eukaryote-specific extensions in the proteins.Citation34 One striking example in this regard is the ribosomal protein uS2, which harbors eukaryote-specific N- and C-terminal extensions. This protein appears to have acquired a second function as a cell surface receptor for laminin (a component of extracellular matrices) specifically in the vertebrate lineage.Citation34 Post-translational modification (i.e., acetylation) of uS2 is a prerequisite for conversion of the monomeric 37 kDa uS2 (known also as laminin receptor precursor (37LRP)) to the mature dimeric 67 kDa laminin receptor (67LR).Citation34 Mature 67LR associates with the plasma membrane, and its C-terminal laminin-binding domain becomes accessible to extracellular ligands.Citation34 The laminin binding capacity was attributed to a specific palindromic sequence LMWWML (found exclusively in vertebrates) in the C-terminal extension of human uS2.Citation34 Phylogenetic analyses have shown that this sequence evolved concomitantly with laminin in vertebrates.Citation34,35 Alterations in uS2 (37LRP) expression are associated with tumor invasion and metastasis.Citation36-38 In addition, this protein was shown to function as a receptor for the pathogenic prion protein PrPScCitation35 and to control the entry of different viruses (such as Sindbis virus) into mammalian cells.Citation35,39
Involvement of eukaryote-specific extensions of conserved ribosomal proteins in eIF3–40S interactions
One of the largest, key and unique factors involved in eukaryotic translation initiation is eIF3.Citation14 eIF3 is a multimeric complex (∼790 kDa) composed of 13 subunits in mammals (referred to by letters a-m) and 5 subunits in yeast (a, b, c, i and g). The five core subunits (a, b, c, i and g) are conserved across all eukaryotes and are essential in yeast.Citation25,40,41 As described above, eIF3 acts as a scaffold for other initiation factors, promotes their binding to the 40S ribosomal subunit and serves as a bridge between the mRNA and the 40S subunit. eIF3 also impedes 40S-60S subunit association.Citation42,43 Studies in a reconstituted yeast system have shown that eIF3 (and eIF5) enhances TC binding to the 43S initiation complex.Citation25,44-46 In addition, genetic and biochemical data have shown that eIF3 can exist as part of a MFC along with eIF1, eIF5 and TC prior to 43S/48S complex formation.Citation22,23 Consistent with this idea, eIF3b mutation (prt1–1) has been shown to compromise mRNA recruitment to the 43S complex in yeast.Citation40
eIF3 architecture and its interaction with the 40S ribosomal subunit is of primary interest and has been the focus of many recent research studies.Citation14,25 eIF3 was originally assumed to cover and interact with a relative large surface of the 40S subunit.Citation25 However, several recent cryo-EM reconstructions using mammalian eIF3 and mammalian ribosomes revealed that “octopus-shaped” eIF3 forms contacts with a relatively limited surface area of the 40S subunit and interacts with the eukaryotic-specific extensions/domains of uS4 and uS15, as well as eukaryotic-specific ribosomal proteins eS27, eS1 and eS26 ().Citation30,47,48 Genetic and biochemical experiments in yeast implicated several other ribosomal proteins (including uS2, uS5, uS3 and uS10) in interaction with eIF3,Citation33 but this was not substantiated by cryo-EM analysis of mammalian eIF3 in complex with mammalian 40S.Citation30,47 This discrepancy may be explained by differences in composition and organization between yeast and mammalian eIF3.Citation25 Assignment of individual eIF3 subunits and/or domains involved in these types of interactions remains challenging, in part due to the relatively low resolution of available cryo-EM structures (∼11.6Å).Citation30 Mutational analysis in yeast suggested that the eIF3a-N-terminal domain eIF3a-(NTD) interacts with the eukaryote-specific C-terminal extension of conserved ribosomal protein uS2Citation49 which is located near the mRNA exit channel of the ribosome ().Citation50 Based on the same type of analysis, yeast eIF3a-C-terminal domain eIF3a-(CTD) was suggested to interact with eukaryote-specific extensions of uS5 and uS3 proteinsCitation51 (). Given that eIF3a is known to modulate GCN4 expression in yeast, it is likely that the above mentioned interactions play a role in regulation of GCN4 translation by controlling reinitiation.Citation52-54 Further, eIF3g was found to interact with uS3 and uS10 located in the 40S subunit beak and head, respectively.Citation55 RpuS10 harbors an eukaryote-specific N-terminal extension that occupies part of the head region where eIF3g is believed to reside.Citation25 The eIF3j subunit has also been shown to interact with uS5 harboring an extremely long (>100 aa) eukaryote-specific N-terminal extension.Citation56 Cryo-EM studies also suggested that the peripheral domain of the eIF3 complex may be located in the vicinity of the eukaryote-specific N-terminal extension of uS7.Citation30 This observation is supported by genetic analyses in yeast showing that truncation of the uS7 N-terminus results in depletion of eIF3 from the initiation complex.Citation57 Very recently, Erzberger and co-workers presented an X-ray structure of the universally conserved core subunits of eIF3 (at ∼3.5 Å) and modeled the eIF3 complex position on the yeast 40S ribosomal subunit by employing cryo-EM and cross-linking of the factor(s) followed by mass spectrometry (MS).Citation58 This reconstruction provided evidence supporting some of the previously reported interactions (between eIF3 and eukaryote-specific 40S regions) observed by biochemical, genetic and structural analyses, but also revealed a number of new/alternative interactions.Citation58
Figure 6. Ribosomal location of eukaryote-specific segments of conserved proteins from the small subunit relative to bound eIF3, ternary complex (TC) and eIF5B. Eukaryote-specific extensions are shown in red, all other protein moieties are in cyan and rRNA is in gray. The location of mammalian eIF3 densitiesCitation30 (shown in orange) and archaeal TCCitation30 (shown in light blue) are traced onto the 40S surface. The putative location of eIF5BCitation15 is shown in pink.
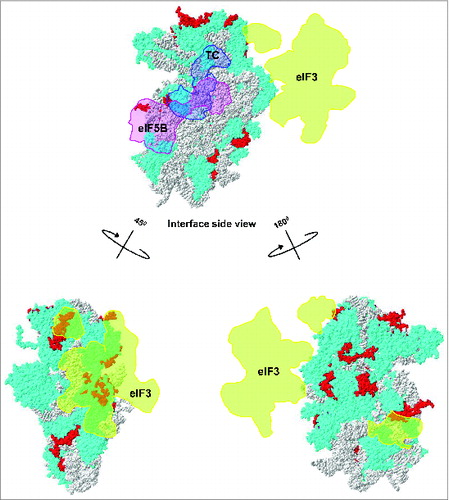
While the exact architecture of the eIF3•40S complex remains to be determined and there are some discrepancies in available data, it is clear that eukaryote-specific extensions in conserved ribosomal proteins have an important functional role in recruiting and retaining eIF3 in the complex.
eIF2, eIF1, eIF1A, eIF5 and eIF5B and t1he complex network of their interactions with the 40S ribosomal subunit
eIF2 is a ∼120 kDa eukaryote-specific heterotrimeric protein composed of α, β and γ subunits.Citation14 eIF2 is the main factor responsible for the GTP-dependent delivery of initiator tRNA to the 40S ribosomal subunit in eukaryotes.Citation14 Initial studies involving directed hydroxyl radical cleavage experiments and mutational analyses showed that eIF2γ's domain III localizes near helix 44 of the 18S rRNA.Citation59 More recent cryo-EM analysis of the 43S complex (∼11.6Å) assembled using archaeal aIF2 and mammalian 40S confirmed this observation and revealed that the TC occupies the 40S subunit interface near the P-site with the eIF2α subunit protruding on the solvent side and the eIF2α-D1 domain making contacts with uS7.Citation30 Crosslinking experiments in rabbit reticulocyte extracts coupled with MS analysis also demonstrated this interaction.Citation60
eIF1 (12.7 kDa) and eIF1A (16.5 kDa) are 2 proteins that work in tandem to ensure correct start codon recognition.Citation27,61 eIF1 suppresses initiation from non-cognate codons or start codons in improper context.Citation61 Directed hydroxyl radical probing and X-ray analyses (∼7.9–9.0 Å) involving mammalian 40S and eIF1 showed that eIF1 binds to helices 44, 24 and 45 of the 18S rRNA near the P-site.Citation31,62 However, eIF1 does not appear to interact with any eukaryote-specific ribosomal protein extensions.
eIF1A (the ortholog of bacterial IF1) cooperates with eIF1 to direct efficient start codon selection.Citation28,63,64 It also plays a role, along with eIF5B, in ensuring proper subunit joining and formation of elongation-competent 80S ribosomes.Citation28,63,64 Hydroxyl radical probing and structural modeling involving eIF1A and Tetrahymena thermophila 40S ribosomal subunits (based on bacterial 30S:IF1 complex information) at ∼3.9Å suggested that eIF1A resides near the A-site and makes contacts with eukaryote-specific proteins eS27 and eS30 as well as conserved protein uS12 which harbors eukaryote-specific extensions.Citation15,65 However, direct involvement of uS12 eukaryote-specific extensions in eIF1A binding has not been proven yet. Compared to IF1 in bacteria, eIF1A has evolved an additional long unstructured N-terminal tail (NTT) and C-terminal tail (CTT). The NTT and CTT have distinct and opposing functions, inhibiting and enhancing scanning, respectively.Citation15 The eIF1A NTT extends toward h30 of the 18S rRNA and is in close proximity to uS19, uS13 and uS10 located on the 40S head.Citation15
eIF5 (49.2 kDa) is another eukaryote-specific initiation factor with 2 structurally and functionally different N- and C-terminal regions.Citation14,15,22-24 The N-terminal region harbors the GTPase-activating protein (GAP) function for eIF2.Citation14,23,24 On the other hand, at least in yeast, the C-terminal region acts as a hub for MFC formation via its interaction with eIF1, eIF3c and eIF2β.Citation61 The exact location of eIF5 on the 40S subunit is not known yet. However, since its CTD is known to interact with other factors as mentioned above, it is believed to occupy regions near the A-site. Given the limited knowledge about its location, it is not yet clear whether the NTD or CTD of eIF5 interacts with any eukaryote-specific proteins or conserved protein extensions.
eIF5B (∼138.8 kDa in mammals) is a ribosome-dependent GTPase protein homologous to bacterial IF2.Citation66,67 The primary function of eIF5B is in subunit joining.Citation66 eIF5B is not essential in yeast; however, its deletion results in a severe slow growth phenotype.Citation67 X-ray analyses of yeast and archaeal eIF5B (∼1.8–3.0Å) in different states (apo-, GDP- and GTP-bound) revealed that the protein has a chalice-like shape with 4 distinct domains (I-IV).Citation68 Cryo-EM reconstruction of yeast eIF5B in complex with 40S ribosomal subunits at 6.6Å showed that it occupies the inter-subunit cleft region.Citation69 Mobility of eIF5B's domains III and IV with respect to domains I and II and the 40S ribosomal subunit is important for efficient subunit joining and GTP hydrolysis.Citation68 Importantly, domain III has been shown to interact with the eukaryote-specific extension of uS12 ().Citation69 It is thus very likely that this interaction might be important for eIF5B function.
Interaction of 40S ribosomal proteins with eEF3
Eukaryotic elongation factor 3 (eEF3) is an essential yeast (fungi) specific ATPase required for protein synthesis.Citation70 eEF3 binds near the E-site and is necessary for clearance of the deacylated tRNA from the ribosome.Citation70,71 Cryo-EM analysis of the eEF3–80S complex (at 9.9Å) showed that the factor binds near the head of the 40S ribosomal subunit and makes contacts with both the 40S and 60S subunits.Citation71 The so-called HEAT domain of eEF3 was mapped to interact with eukaryote-specific extensions of uS13 and 2 protein-rich regions (unassigned due to low resolution) termed rpSX1 and rpSX2.Citation71 However, an X-ray structure of the yeast ribosome subsequently demonstrated that these regions are occupied by eukaryote-specific extensions of the conserved ribosomal proteins uS7, uS10 and uS19, respectively.Citation6 In line with this observation, biochemical analyses showed that deletions/mutations of the uS7 yeast-specific N-terminus affects association of eEF3 with the 80S subunit and results in elongation defects.Citation72 Presence of about 20 extra N-terminal aa (which are negatively charged) in fungi uS7 that are absent in e.g. vertebrates () has been suggested to mediate uS7 interaction with the positively charged C-terminal end of fungi-specific eEF3.Citation72
Role of eukaryote-specific extensions in protein-protein interactions on the surface of the 40S ribosomal subunit
Compared to prokaryotic ribosomes, eukaryotic ribosomes have evolved a much richer protein surface covering the exposed/solvent side of the ribosome.Citation5,6,12 This has resulted in an extensive network of protein-protein interactions.Citation5,6,12 Eukaryotic ribosomes are thought to be stabilized by these interactions, which primarily involve eukaryote-specific ribosomal proteins and/or eukaryote-specific extensions of the conserved ribosomal proteins.Citation9,10 These interactions are more pronounced within the small subunit than within the large subunit and, in particular, involve interacting uS2 - eS17, uS7 – uS9, uS11 - eS26, uS12 - uS17, uS13 - eS25, uS15 - eS27, uS17 - eS8 protein pairs (). As mentioned above, the significance of these interactions remains largely unknown, with only a few having been explored experimentally.
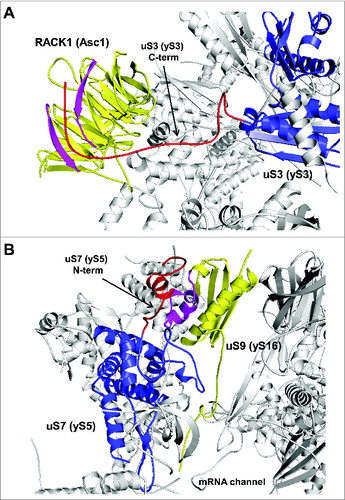
In addition to the interactions among ribosomal proteins and extensions listed above, the C-terminal eukaryote-specific extension of uS3 has been shown to interact in yeast with ribosomal protein Asc1 (). Asc1 is an ortholog of mammalian RACK1 which belongs to the WD-repeat protein family exhibiting a 7 bladed β-propeller structure.Citation6,73 RACK1 is known to play an important role in numerous biological responses/signaling events, in turn involving its interaction with kinases and receptors in a regulated manner.Citation74 Therefore, interaction between uS3 and Asc1 might act as an important link between translational control of gene expression and cellular signaling.Citation74 Whether disruption of uS3-Asc1/RACK1 might abrogate this link remains to be tested.
Figure 7. Ribosomal location of conserved proteins uS3, uS7, uS9. (A) The eukaryote-specific C-terminal extension of uS3 interacts with Asc1/RACK1. The eukaryote specific region of uS3 is shown in red with the rest of the protein shown in blue. Regions of Asc1/RACK1 interacting with the uS3 C-terminus are shown in pink with rest of RACK1 in yellow. (B) Ribosomal locations of uS7 and uS9. The eukaryote-specific N-terminal extension of uS7 is shown in red with rest of the protein in blue. uS9 is shown in yellow, with the region interacting with uS7 N-terminus in pink. PDB entry 3U5G was used.
The eukaryote-specific N-terminal extension of ribosomal uS17 is known to form a eukaryote-specific inter-subunit bridge and might also be important for ribosome assembly and translational control of gene expression in eukaryotes.Citation6,30
An intriguing example of protein-protein interactions being mediated by eukaryote-specific extensions is uS7 - uS9 protein pair. The eukaryote-specific N-terminal extension of uS7 forms contacts with the N-terminal extension of uS9 (). This cross-talk was found to be important for efficient translation initiation in yeast.Citation75 Disruption of the interaction between these 2 eukaryotic-specific segments of uS7 and uS9 impeded productive interaction between the uS9 CTT and Met-tRNAiMet in the P site of 48S PIC, leading to a reduced rate of bulk translation initiation (decreased polysome content), impaired derepression of GCN4 mRNA translation, and accumulation of eIF2 on 40S ribosomal subunits.Citation75 It was hypothesized that interaction between uS7 and uS9 modulates the location of the uS9 CTT to promote correct placement of TC in the P site and eIF5-stimulated GTP-hydrolysis, or Pi release.Citation75 The interaction between uS7 and uS9 was also suggested to influence the placement of eIF1, TC and eIF5 following start codon recognition.Citation75 This study provided the first experimental evidence supporting the functional significance of protein-protein interactions within the ribosome that are absent in prokaryotes but represent a defining feature of eukaryotic ribosomes.
Conclusions and Future Directions
Our understanding of ribosome structure and function has improved significantly in recent years due to the availability of high-resolution structures of eukaryotic ribosomesCitation5,6,9-11 and cryo-EM analysis of eukaryotic ribosomes complexed with translation factors.Citation14,26,30,31,47,69,71 One of the key features differentiating eukaryotic from prokaryotic ribosomes is the extent of protein-protein interactions on the ribosome surface. It is believed that this structural complexity is directly related to the evolution of the translation apparatus, including appearance of new translation factors and multiple sophisticated mechanisms of translation control that are absent in prokaryotic cells.
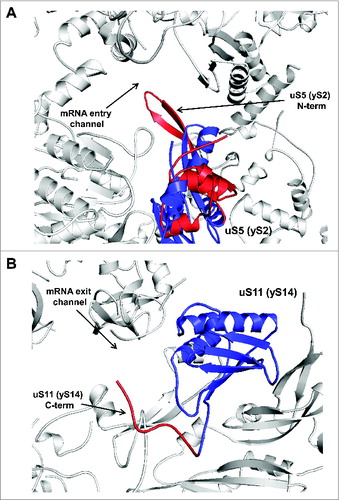
In this review, we focused on the conserved proteins of the small ribosomal subunit that have evolved eukaryote-specific extensions. While much work remains to be done in exploring the functional importance of these extensions, it is becoming clear that in many cases they are involved in the extensive network of interactions on the ribosome surface, forming contacts with ribosomal proteins, other eukaryote-specific extensions of conserved ribosomal proteins, and translation factors. In some cases, available structural information allows readily testable predictions to be made regarding the functional significance of eukaryote-specific extensions. For example, the eukaryote-specific N-terminal extension of uS5, which forms a unique β-hairpin loop structure forming part of the mRNA entry channel (), might have evolved to fulfill an important function during scanning and/or start codon recognition. Another protein, uS11, possesses a eukaryote-specific C-terminal extension that protrudes toward the mRNA exit channel (). It is possible that this extension is also involved in the scanning process, perhaps aiding in inspection of upstream sequences to control start codon selection.
Figure 8. Ribosomal location of conserved proteins uS5 and uS11. (A) uS5 is located in the vicinity of the mRNA entry channel. The eukaryote-specific N-terminal extension forming a β-hairpin loop protruding into the mRNA entry channel is in red and the conserved protein moiety is shown in blue. (B) Ribosomal location of uS11. The eukaryote-specific C-terminal extension of uS11 protruding toward the mRNA exit channel is shown in red and the conserved protein moiety is shown in blue.
Future biochemical and genetic studies combined with structural analyses will help to illuminate the precise structural and functional significance of eukaryote-specific extensions of conserved ribosomal proteins and improve our understanding of the evolution of ribosomes and translational mechanisms in eukaryotes.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Drs Tatyana Pestova, Marat Yusupov and William C. Merrick for many helpful discussions and Patricia Stanhope Baker for help with manuscript preparation and proofreading.
Funding
This work was supported in part by grants from the Human Frontiers Science Program (HFSP) (RGP0024/10; A.A.K.), Cleveland State University Doctoral Dissertation Research Expense Award (A.G.) and funds from the Center for Gene Regulation in Health and Disease (GRHD; A.A.K.) at Cleveland State University.
References
- Ramakrishnan V. Ribosome structure and the mechanism of translation. Cell 2002; 108:557-2; PMID:11909526; http://dx.doi.org/10.1016/S0092-8674(02)00619-0
- Schmeing TM, Ramakrishnan V. What recent ribosome structures have revealed about the mechanism of translation. Nature 2009; 461:1234-1242; PMID:19838167; http://dx.doi.org/10.1038/nature08403
- Dinman JD. The eukaryotic ribosome: current status and challenges. J Biol Chem 2009; 284(18):11761-5; PMID:19117941; http://dx.doi.org/10.1074/jbc.R800074200
- Budkevich TV, El'skaya AV, Nierhaus, KH. Features of 80S mammalian ribosome and its subunits. Nucleic Acids Res 2008; 36(14):4736-4744. PMID:18632761; http://dx.doi.org/10.1093/nar/gkn424
- Ben-Shem A, Jenner L, Yusupova G, Yusupov M. Crystal structure of the eukaryotic ribosome. Science 2010; 330(6008):1203-9; PMID:21109664; http://dx.doi.org/10.1126/science.1194294
- Ben-Shem A, Garreau de Loubresse N, Melnikov S, Jenner L, Yusupova G, Yusupov M. The structure of the eukaryotic ribosome at 3.0 Å resolution. Science 2011; 334(6062):1524-9; PMID:22096102; http://dx.doi.org/10.1126/science.1212642
- Ban N, Nissen P, Hansen J, Moore PB, Steitz TA. The complete atomic structure of the large ribosomal subunit at 2.4 A resolution. Science 2000; 289(5481):905-920; PMID:10937989; http://dx.doi.org/10.1126/science.289.5481.905
- Zhang W, Dunkle JA, Cate JH. Structures of the ribosome in intermediate states of ratcheting. Science 2009; 325(5943):1014-7; PMID:19696352. http://dx.doi.org/ 10.1126/science.1175275
- Klinge S, Voigts-Hoffmann F, Leibundgut M, Ban N. Atomic structures of the eukaryotic ribosome. Trends Biochem Sci 2012; 37(5):189-98; PMID:22436288; http://dx.doi.org/10.1016/j.tibs.2012.02.007
- Ramakrishnan V. Molecular biology. The eukaryotic ribosome. Science 2011; 331(6018):681-2; PMID:21310988; http://dx.doi.org/10.1126/science.1202093
- Anger AM, Armache JP, Berninghausen O, Habeck M, Subklewe M, Wilson DN, Beckmann R. Structures of the human and Drosophila 80S ribosome. Nature 2013; 497(7447):80-5; PMID:23636399; http://dx.doi.org/10.1038/nature12104
- Melnikov S, Ben-Shem A, Garreau de Loubresse N, Jenner L, Yusupova G, Yusupov M. One core, two shells: bacterial and eukaryotic ribosomes. Nat Struct Mol Biol 2012; 19(6):560-7; PMID:22664983; http://dx.doi.org/10.1038/nsmb.2313
- Rodnina MV, Wintermeyer W. Recent mechanistic insights into eukaryotic ribosomes. Curr Opin Cell Biol 2009; 21(3):435-43; PMID:19243929; http://dx.doi.org/10.1016/j.ceb.2009.01.023
- Jackson RJ, Hellen CU, Pestova TV. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat Rev Mol Cell Biol 2010; 11(2):113-27; PMID:20094052; http://dx.doi.org/10.1038/nrm2838
- Voigts-Hoffmann F, Klinge S, Ban N. Structural insights into eukaryotic ribosomes and the initiation of translation. Curr Opin Struct Biol 2012; 22(6):768-77; PMID:22889726; http://dx.doi.org/10.1016/j.sbi.2012.07.010
- Milon P, Konevega AL, Gualerzi CO, Rodnina MV. Kinetic checkpoint at a late step in translation initiation. Mol Cell 2008; 30(6):712-20; PMID:18570874; http://dx.doi.org/10.1016/j.molcel.2008.04.014
- Grigoriadou C, Marzi S, Kirillov S, Gualerzi CO, Cooperman BS. A quantitative kinetic scheme for 70 S translation initiation complex formation. J Mol Biol 2007; 373(3):562-72; PMID:17868692; http://dx.doi.org/10.1016/j.jmb.2007.07.032
- Pisarev AV, Hellen CU, Pestova TV. Recycling of eukaryotic post termination ribosomal complexes. Cell 2007; 131(2):286-99; PMID:17956730; http://dx.doi.org/10.1016/j.cell.2007.08.041
- Pisarev AV, Skabkin MA, Pisareva VP, Skabkina OV, Rakotondrafara AM, Hentze MW, Hellen CU, Pestova TV. The role of ABCE1 in eukaryotic post termination ribosomal recycling. Mol Cell 2010; 37(2):196-210; PMID:20122402; http://dx.doi.org/10.1016/j.molcel.2009.12.034
- Gross JD, Moerke NJ, von der Haar T, Lugovskoy AA, Sachs AB, McCarthy JE, Wagner G. Ribosome loading onto the mRNA cap is driven by conformational coupling between eIF4G and eIF4E. Cell 2003; 115(6):739-50; PMID:14675538; http://dx.doi.org/10.1016/S0092-8674(03)00975-9
- LeFebvre AK, Korneeva NL, Trutschl M, Cvek U, Duzan RD, Bradley CA, Hershey JW, Rhoads RE. Translation initiation factor eIF4G-1 binds to eIF3 through the eIF3e subunit. J Biol Chem 2006; 281(32):22917-32; PMID:16766523; http://dx.doi.org/10.1074/jbc.M605418200
- Asano K, Clayton J, Shalev A, Hinnebusch AG. A multifactor complex of eukaryotic initiation factors, eIF1, eIF2, eIF3, eIF5, and initiator tRNA(Met) is an important translation initiation intermediate in vivo. Genes Dev 2000; 14(19): 2534-46; PMID:11018020; http://dx.doi.org/10.1101/gad.831800
- Asano K, Phan L, Valásek L, Schoenfeld LW, Shalev A, Clayton J, Nielsen K, Donahue TF, Hinnebusch AG. Multifactor complex of eIF1, eIF2, eIF3, eIF5, and tRNA(i)Met promotes initiation complex assembly and couples GTP hydrolysis to AUG recognition. Cold Spring Harb Symp Quant Biol 2001; 66:403-15; PMID:12762043; http://dx.doi.org/10.1101/sqb.2001.66.403
- Asano K, Shalev A, Phan L, Nielsen K, Clayton J, Valásek L, Donahue TF, Hinnebusch AG. Multiple roles for the C-terminal domain of eIF5 in translation initiation complex assembly and GTPase activation. EMBO J 2001; 20(9):2326-37; PMID:11331597; http://dx.doi.org/10.1093/emboj/20.9.2326
- Hinnebusch AG. eIF3: a versatile scaffold for translation initiation complexes. Trends Biochem Sci 2006; 31(10):553-62; PMID:16920360; http://dx.doi.org/10.1016/j.tibs.2006.08.005
- Passmore LA, Schmeing TM, Maag D, Applefield DJ, Acker MG, Algire MA, Lorsch JR, Ramakrishnan V. The eukaryotic translation initiation factors eIF1 and eIF1A induce an open conformation of the 40S ribosome. Mol Cell 2007; 26(1):41-50; PMID:17434125; http://dx.doi.org/10.1016/j.molcel.2007.03.018
- Hinnebusch AG, Lorsch JR. The mechanism of eukaryotic translation initiation: new insights and challenges. Cold Spring Harb Perspect Biol 2012; 4(10): pii: a011544; PMID:22815232; http://dx.doi.org/10.1101/cshperspect.a011544
- Hinnebusch AG. The scanning mechanism of eukaryotic translation initiation. Annu Rev Biochem 2014; 83:779-812; PMID:24499181; http://dx.doi.org/10.1146/annurev-biochem-060713-035802
- Algire MA, Maag D, Lorsch JR. Pi release from eIF2, not GTP hydrolysis, is the step controlled by start-site selection during eukaryotic translation initiation. Mol Cell 2005; 20(2):251-62; PMID:16246727; http://dx.doi.org/10.1016/j.molcel.2005.09.008
- Hashem Y, des Georges A, Dhote V, Langlois R, Liao HY, Grassucci RA, Hellen CU, Pestova TV, Frank J. Structure of the mammalian ribosomal 43S preinitiation complex bound to the scanning factor DHX29. Cell 2013; 153(5):1108-19; PMID:23706745; http://dx.doi.org/10.1016/j.cell.2013.04.036
- Lomakin IB, Steitz TA. The initiation of mammalian protein synthesis and mRNA scanning mechanism. Nature 2013; 500(7462):307-11; PMID:23873042; http://dx.doi.org/10.1038/nature12355
- Dresios J, Panopoulos P, Synetos D. Eukaryotic ribosomal proteins lacking a eubacterial counterpart: important players in ribosomal function. Mol Microbiol 2006; 59(6):1651-63; PMID:16553873; http://dx.doi.org/10.1111/j.1365-2958.2006.05054.x
- Valásek LS. ‘Ribozoomin’-translation initiation from the perspective of the ribosome-bound eukaryotic initiation factors (eIFs). Curr Protein Pept Sci 2012; 13(4):305; PMID:22708493; http://dx.doi.org/10.2174/138920312801619385
- Ardini E, Pesole G, Tagliabue E, Magnifico A, Castronovo V, Sobel ME, Colnaghi MI, Ménard S. The 67-kDa laminin receptor originated from a ribosomal protein that acquired a dual function during evolution. Mol Biol Evol 1998; 15(8):1017-25; PMID:9718729
- Nelson J, McFerran NV, Pivato G, Chambers E, Doherty C, Steele D, Timson DJ. The 67 kDa laminin receptor: structure, function and role in disease. Biosci Rep 2008; 28(1):33-48; PMID:18269348; http://dx.doi.org/10.1042/BSR20070004
- Castronovo V. Laminin receptors and laminin-binding proteins during tumor invasion and metastasis. Invasion Metastasis 1993;13(1):1-30; PMID:8407208
- Martignone S, Ménard S, Bufalino R, Cascinelli N, Pellegrini R, Tagliabue E, Andreola S, Rilke F, Colnaghi MI. Prognostic significance of the 67-kilodalton laminin receptor expression in human breast carcinomas. J Natl Cancer Inst 1993;85(5):398-402; PMID:8433393; http://dx.doi.org/10.1093/jnci/85.5.398
- Berno V, Porrini D, Castiglioni F, Campiglio M, Casalini P, Pupa SM, Balsari A, Ménard S, Tagliabue E. The 67 kDa laminin receptor increases tumor aggressiveness by remodeling laminin-1. Endocr Relat Cancer 2005;12(2):393-406; PMID:15947111; http://dx.doi.org/10.1677/erc.1.00870
- Wang KS, Kuhn RJ, Strauss EG, Ou S, Strauss JH. High-affinity laminin receptor is a receptor for Sindbis virus in mammalian cells. J Virol. 1992; 66(8):4992-5001; PMID:1385835
- Phan L, Zhang X, Asano K, Anderson J, Vornlocher HP, Greenberg JR, Qin J, Hinnebusch AG. Identification of a translation initiation factor 3 (eIF3) core complex, conserved in yeast and mammals, that interacts with eIF5. Mol Cell Biol 1998; 18(8):4935-46; PMID:9671501
- Valásek L, Phan L, Schoenfeld LW, Valásková V, Hinnebusch AG. Related eIF3 subunits TIF32 and HCR1 interact with an RNA recognition motif in PRT1 required for eIF3 integrity and ribosome binding. EMBO J 2001; 20(4):891-904; PMID:11179233; http://dx.doi.org/10.1093/emboj/20.4.891
- Chaudhuri J, Chowdhury D, Maitra U. Distinct functions of eukaryotic translation initiation factors eIF1A and eIF3 in the formation of the 40S ribosomal preinitiation complex. J Biol Chem 1999; 274(25):17975-80; PMID:10364246; http://dx.doi.org/10.1074/jbc.274.25.17975
- Kolupaeva VG, Unbehaun A, Lomakin IB, Hellen CU, Pestova TV. Binding of eukaryotic initiation factor 3 to ribosomal 40S subunits and its role in ribosomal dissociation and anti-association. RNA 2005; 11(4):470-86; PMID:15703437; http://dx.doi.org/10.1261/rna.7215305
- Phan L, Schoenfeld LW, Valásek L, Nielsen KH, Hinnebusch AG. A subcomplex of three eIF3 subunits binds eIF1 and eIF5 and stimulates ribosome binding of mRNA and tRNA(i)Met. EMBO J 2001; 20(11):2954-65; PMID:11387228; http://dx.doi.org/10.1093/emboj/20.11.2954
- Valásek L, Mathew AA, Shin BS, Nielsen KH, Szamecz B, Hinnebusch AG. The yeast eIF3 subunits TIF32/a, NIP1/c, and eIF5 make critical connections with the 40S ribosome in vivo. Genes Dev 2003; 17(6), 786-99; PMID:12651896; http://dx.doi.org/10.1101/gad.1065403
- Valásek L, Nielsen KH, Zhang F, Fekete CA, Hinnebusch AG. Interactions of eukaryotic translation initiation factor 3 (eIF3) subunit NIP1/c with eIF1 and eIF5 promote preinitiation complex assembly and regulate start codon selection. Mol Cell Biol 2004; 24(21):9437-55; PMID:15485912; http://dx.doi.org/10.1128/MCB.24.21.9437-9455.2004
- Siridechadilok B, Fraser CS, Hall RJ, Doudna JA, Nogales E. Structural roles for human translation factor eIF3 in initiation of protein synthesis. Science 2005; 310(5753):1513-5; PMID:16322461; http://dx.doi.org/10.1126/science.1118977
- Sun C, Querol-Audí J, Mortimer SA, Arias-Palomo E, Doudna JA, Nogales E, Cate JH. Two RNA-binding motifs in eIF3 direct HCV IRES-dependent translation. Nucleic Acids Res 2013; 41(15):7512-21; PMID:23766293; http://dx.doi.org/10.1093/nar/gkt510
- Ban N, Beckmann R, Cate JH, Dinman JD, Dragon F, Ellis SR, Lafontaine DL, Lindahl L, Liljas A, Lipton JM, et al. A new system for naming ribosomal proteins. Curr Opin Struct Biol 2014; 24:165-69; PMID:24524803; http://dx.doi.org/10.1016/j.sbi.2014.01.002
- Kouba T, Dányi I, Gunišová S, Munzarová V, Vlčková V, Cuchalová L, Neueder A, Milkereit P, Valásek LS. Small ribosomal protein RPS0 stimulates translation initiation by mediating 40S-binding of eIF3 via its direct contact with the eIF3a/TIF32 subunit. PLoS One 2012; 7(7):e40464; PMID:22792338; http://dx.doi.org/10.1371/journal.pone.0040464
- Chiu WL, Wagner S, Herrmannová A, Burela L, Zhang F, Saini AK, Valásek L, Hinnebusch AG. The C-terminal region of eukaryotic translation initiation factor 3a (eIF3a) promotes mRNA recruitment, scanning, and, together with eIF3j and the eIF3b RNA recognition motif, selection of AUG start codons. Mol Cell Biol 2010; 30(18):4415-34; PMID:20584985; http://dx.doi.org/10.1128/MCB.00280-10
- Nielsen KH, Szamecz B, Valásek L, Jivotovskaya A, Shin BS, Hinnebusch AG. Functions of eIF3 downstream of 48S assembly impact AUG recognition and GCN4 translational control. EMBO J 2004; 23(5):1166-77; PMID:14976554; http://dx.doi.org/10.1038/sj.emboj.7600116
- Munzarová V, Pánek J, Gunišová S, Dányi I, Szamecz B, Valášek LS. Translation reinitiation relies on the interaction between eIF3a/TIF32 and progressively folded cis-acting mRNA elements preceding short uORFs. PLoS Genet 2011; 7(7), e1002137; PMID:21750682; http://dx.doi.org/10.1371/journal.pgen.1002137
- Szamecz B, Rutkai E, Cuchalová L, Munzarová V, Herrmannová A, Nielsen KH, Burela L, Hinnebusch AG, Valásek L. eIF3a cooperates with sequences 5' of uORF1 to promote resumption of scanning by post-termination ribosomes for reinitiation on GCN4 mRNA. Genes Dev 2008; 22(17):2414-25; PMID:18765792; http://dx.doi.org/10.1101/gad.480508
- Cuchalová L, Kouba T, Herrmannová A, Dányi I, Chiu WL, Valásek L. The RNA recognition motif of eukaryotic translation initiation factor 3g (eIF3g) is required for resumption of scanning of posttermination ribosomes for reinitiation on GCN4 and together with eIF3i stimulates linear scanning. Mol Cell Biol 2010; 30(19):4671-86; PMID:20679478; http://dx.doi.org/10.1128/MCB.00430-10
- Elantak L, Wagner S, Herrmannová A, Karásková M, Rutkai E, Lukavsky PJ, Valásek L. The indispensable N-terminal half of eIF3j/HCR1 cooperates with its structurally conserved binding partner eIF3b/PRT1-RRM and with eIF1A in stringent AUG selection. J Mol Biol 2010; 396(4):1097-116; PMID:20060839; http://dx.doi.org/10.1016/j.jmb.2009.12.047
- Lumsden T, Bentley AA, Beutler W, Ghosh A, Galkin O, Komar AA. Yeast strains with N-terminally truncated ribosomal protein S5: implications for the evolution, structure and function of the Rps5/Rps7 proteins. Nucleic Acids Res 2010; 38(4):1261-72; PMID:19969550; http://dx.doi.org/10.1093/nar/gkp1113
- Erzberger JP, Stengel F, Pellarin R, Zhang S, Schaefer T, Aylett CHS, Cimermančič P, Boehringer D, Sali A, Aebersold R, Ban N. Molecular Architecture of the 40S,eIF1,eIF3 Translation Initiation Complex. Cell 2014; 158(5):1123-35; PMID:25171412; http://dx.doi.org/10.1016/j.cell.2014.07.044
- Shin BS, Kim JR, Walker SE, Dong J, Lorsch JR, Dever TE. Initiation factor eIF2γ promotes eIF2-GTP-Met-tRNAi(Met) ternary complex binding to the 40S ribosome. Nat Struct Mol Biol 2011; 18(11):1227-34; PMID:22002225; http://dx.doi.org/10.1038/nsmb.2133
- Sharifulin D, Babaylova E, Kossinova O, Bartuli Y, Graifer D, Karpova G. Ribosomal Protein S5e is Implicated in Translation Initiation through its Interaction with the N-Terminal Domain of Initiation Factor eIF2α. Chembiochem 2013; 14(16):2136-43; PMID:24106102; http://dx.doi.org/10.1002/cbic.201300318
- Hinnebusch AG. Molecular mechanism of scanning and start codon selection in eukaryotes. Microbiol Mol Biol Rev 2011; 75(3):434-67; PMID:21885680; http://dx.doi.org/10.1128/MMBR.00008-11
- Lomakin IB, Kolupaeva VG, Marintchev A, Wagner G, Pestova TV. Position of eukaryotic initiation factor eIF1 on the 40S ribosomal subunit determined by directed hydroxyl radical probing. Genes Dev 2003; 17(22):2786-97; PMID:14600024; http://dx.doi.org/10.1101/gad.1141803
- Fringer JM, Acker MG, Fekete CA, Lorsch JR, Dever TE. Coupled release of eukaryotic translation initiation factors 5B and 1A from 80S ribosomes following subunit joining. Mol Cell Biol 2007; 27(6):2384-97; PMID:17242201; http://dx.doi.org/10.1128/MCB.02254-06
- Nanda JS, Saini AK, Muñoz AM, Hinnebusch AG, Lorsch JR. Coordinated movements of eukaryotic translation initiation factors eIF1, eIF1A, and eIF5 trigger phosphate release from eIF2 in response to start codon recognition by the ribosomal preinitiation complex. J Biol Chem 2013; 288(8):5316-29; PMID:23293029; http://dx.doi.org/10.1074/jbc.M112.440693
- Yu Y, Marintchev A, Kolupaeva VG, Unbehaun A, Veryasova T, Lai SC, Hong P, Wagner G, Hellen CU, Pestova TV. Position of eukaryotic translation initiation factor eIF1A on the 40S ribosomal subunit mapped by directed hydroxyl radical probing. Nucleic Acids Res 2009; 37(15):5167-82; PMID:19561193; http://dx.doi.org/10.1093/nar/gkp519
- Pestova TV, Lomakin IB, Lee JH, Choi SK, Dever TE, Hellen CU. The joining of ribosomal subunits in eukaryotes requires eIF5B. Nature 2000; 403(6767):332-5; PMID:10659855; http://dx.doi.org/10.1038/35002118
- Choi SK, Lee JH, Zoll WL, Merrick WC, Dever TE. Promotion of met-tRNAiMet binding to ribosomes by yIF2, a bacterial IF2 homolog in yeast. Science 1998; 280(5370):1757-60; PMID:9624054; http://dx.doi.org/10.1126/science.280.5370.1757
- Kuhle B, Ficner R. eIF5B employs a novel domain release mechanism to catalyze ribosomal subunit joining. EMBO J 2014; 33(10):1177-1191; PMID:24686316; http://dx.doi.org/10.1002/embj.201387344
- Fernández IS, Bai XC, Hussain T, Kelley AC, Lorsch JR, Ramakrishnan V, Scheres SH. Molecular architecture of a eukaryotic translational initiation complex. Science 2013; 342(6160):1240585; PMID:24200810; http://dx.doi.org/10.1126/science.1240585
- Triana-Alonso FJ, Chakraburtty K, Nierhaus KH. The elongation factor 3 unique in higher fungi and essential for protein biosynthesis is an E site factor. J Biol Chem 1995; 270(35):20473-8; PMID:7657623; http://dx.doi.org/10.1074/jbc.270.35.20473
- Andersen CB, Becker T, Blau M, Anand M, Halic M, Balar B, Mielke T, Boesen T, Pedersen JS, Spahn CM, et al. Structure of eEF3 and the mechanism of transfer RNA release from the E-site. Nature 2006; 443(7112):663-8; PMID:16929303; http://dx.doi.org/10.1038/nature05126
- Galkin O, Bentley AA, Gupta S, Compton BA, Mazumder B, Kinzy TG, Merrick WC, Hatzoglou M, Pestova TV, Hellen CU, et al. Roles of the negatively charged N-terminal extension of Saccharomyces cerevisiae ribosomal protein S5 revealed by characterization of a yeast strain containing human ribosomal protein S5. RNA 2007; 13(12):2116-28; PMID:17901157; http://dx.doi.org/10.1261/rna.688207
- Sengupta J, Nilsson J, Gursky R, Spahn CM, Nissen P, Frank J. Identification of the versatile scaffold protein RACK1 on the eukaryotic ribosome by cryo-EM. Nat Struct Mol Biol 2004; 11(10):957-62; PMID:15334071; http://dx.doi.org/10.1038/nsmb822
- Nilsson J, Sengupta J, Frank J, Nissen P. Regulation of eukaryotic translation by the RACK1 protein: a platform for signalling molecules on the ribosome. EMBO Rep 2004; 5(12):1137-41; PMID:15577927; http://dx.doi.org/10.1038/sj.embor.7400291
- Ghosh A, Jindal S, Bentley AA, Hinnebusch AG, Komar AA. Rps5-Rps16 communication is essential for efficient translation initiation in yeast S. cerevisiae. Nucleic Acids Res 2014; 42(13):8537-55. [epub ahead of print]; PMID:24948608; http://dx.doi.org/10.1093/nar/gku550
- Armache JP, Jarasch A, Anger AM, Villa E, Becker T, Bhushan S, Jossinet F, Habeck M, Dindar G, Franckenberg S, et al. Cryo-EM structure and rRNA model of a translating eukaryotic 80S ribosome at 5.5-A resolution. Proc Natl Acad Sci U S A 2010; 107(46):19748-53; PMID:20980660; http://dx.doi.org/10.1073/pnas.1009999107
- Seidelt B, Innis CA, Wilson DN, Gartmann M, Armache JP, Villa E, Trabuco LG, Becker T, Mielke T, Schulten K, et al. Structural insight into nascent polypeptide chain-mediated translational stalling. Science 2009; 326(5958):1412-5; PMID:19933110; http://dx.doi.org/10.1126/science.1177662
