?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
l-Phe-imprinted cryogel cartridge was prepared for the chiral separation of l-Phe. N-Methacryloyl l-phenylalanine (MAPA) was used as a functional monomer for complexing with l-Phe. The selectivity of the membranes was investigated by using d-Phe, l-Trp, and d-Trp as competitor molecules. The PHEMAPA-l-Trp membranes were 6.4, 4.3, and 5.5 times more selective for l-Phe than d-Phe, l-Trp, and d-Trp, respectively. The PHEMAPA-l-Phe cryogel cartridge was incorporated into the fast protein liquid chromatography (FPLC) equipment and was able to separate D,l-Phe racemic mixture efficiently. The PHEMAPA-l-Phe membranes were shown to be reusable many times without significant loss of the adsorption capacity.
Introduction
Amino acids, the main structural components of proteins and enzymes, have attracted significant attention because they are involved in myriad of biological activities, such as protein synthesis and regulation of many metabolic pathways (Song et al. Citation2013). l-Amino acids participate in many physiological processes as the proteins of multicellular organisms are based on l-amino acids (Ilisz et al. Citation2013).
l-Phenylalanine (l-Phe) is an essential amino acid for both human and livestock. It is frequently used for the synthesis of pharmaceutically active chemicals, manufacture of food and drink products. It is also used by itself as a food supplement due to its possible analgesic and antidepressant effects (Mahalakshmi et al. Citation2006). l-Phe has also importance in a case of autosomal recessive genetic disorder, phenylketonuria, which is characterized by phenylalanine hydroxylase enzyme deficiency. This disease results in the accumulation of phenylalanine in the blood and has critical effects on brain development in early infancy (Groselj et al. Citation2015, Jeong et al. Citation2009, Kand’ar and Zakova Citation2009). Therefore, l-Phe level is a direct biomarker for phenylketonuria diagnosis and the analysis of trace levels in blood plays an important role in clinical medicine, as well.
While the enantiomers have the same chemical or physical features, their biological, pharmaceutical, and toxicological activities differ significantly. Therefore, enantioseparation is important and indispensable in pharmaceutical, clinical, and environmental fields (Hernandez et al. Citation2013, Rocco et al. Citation2013). For the separation of amino acid enantiomers, several methods are reported, such as high-performance liquid chromatography (HPLC) (Gheshlagi et al. Citation2008, Ilisz et al. Citation2012, Piccinini et al. Citation2004), capillary electrophoresis (CE) (Kitagawa and Otsuka Citation2011), capillary electrochromatography (CEC) (Aydoğan and Denizli Citation2012, Citation2014, Aydoğan et al. Citation2013), adsorption onto polymeric adsorbents derivatized with different ligands such as β-cyclodextrin (Xiao et al. Citation2007), DNA (Higuchi et al. Citation2005), antibodies (Hofstetter et al. Citation2004), carboxymethyl-β-cyclodextrin (CMCD) (Ghosh et al. Citation2013), crown ether (Yongzhu et al. Citation2006), and chiral extraction (Li et al. Citation2012, Tang et al. Citation2015). The molecular imprinting technique, which allows creation of specific recognition regions compatible with the template molecule in terms of size, geometry, and functionality in a polymeric matrix, has also attracted great interest for the enantiomeric separations due to their high selectivity, ease of preparation, and high stability (Haupt Citation2003). This technique has been successfully used for the imprinting of amino acids and their chiral separation (Aydoğan et al. Citation2012, Gabashvili et al. Citation2007, Hu et al. Citation2011, Huang et al. Citation2008, Kong et al. Citation2011, Li et al. Citation2009, Qin et al. Citation2008, Citation2012).
New generation interconnected supermacroporous cryogels are used as chromatographic support materials and have many advantages due to their large pores, short diffusion path, low-pressure drop, and very short residence time for adsorption and elution (Lozinsky et al. Citation2003). The imprinted cryogels have been successfully used for the separation of various proteins, including albumin, lysozyme, cytochrome c, hemoglobin, and interferon (Andaç et al. Citation2012, Citation2013, Aslıyüce et al. Citation2012, Citation2013, Bereli et al. Citation2008, Citation2011, Derazshamshir et al. Citation2010, Ertürk et al. Citation2013, Tamahkar et al. Citation2011), small molecules such as bilirubin (Baydemir et al. Citation2009a, Citation2009b), cholesterol (Çaktü et al. Citation2014), endocrine disrupters (Koç et al. Citation2011, Le Noir et al. Citation2007), and metal ions (Aslıyüce et al. Citation2010, Ergün et al. Citation2012) for a variety of applications. In this study, l-Phe-imprinted cryogel cartridges were prepared for selective l-Phe recognition and separation. N-Methacryloyl-(l)-phenylalanine (MAPA) and l-Phe was used as functional monomer and template, respectively. After polymerization, the template, l-Phe, was removed to create size, charge, and shape-specific recognition cavities for l-Phe. The surface morphology and bulk properties of membranes were characterized by scanning electron microscopy (SEM), FTIR spectroscopy, and surface area measurements. l-Phe-binding properties, reusability, and selectivity of the cartridges were investigated in aqueous media and reported here.
Experimental
Materials
l-Phenylalanine methylester, methacryloyl chloride, l-phenylalanine (l-Phe), d-phenylalanine (d-Phe), l-tryptophan (l-Trp), and d-tryptophan (d-Trp) were purchased from Sigma-Aldrich (Sigma Chemical Co., St. Louis, MO). Hydroxyethyl methacrylate (HEMA) and N-N′-methylene-bisacrylamide (MBAAm) were obtained from Fluka A.G. (Buchs, Switzerland), distilled under reduced pressure in the presence of hydroquinone inhibitor and stored at 4 °C until use. Ammonium persulfate (APS) and N,N,N′,N′-tetramethylene diamine (TEMED) were also obtained from Fluka. All other chemicals were of reagent grade and purchased from Merck AG (Darmstadt, Germany). All water used in this work was ultrapure grade and purified using a Barnstead (Dubuque, IA) ROpure LP® reverse osmosis unit with a high flow cellulose acetate membrane (Barnstead D2731) followed by a Barnstead D3804 NANOpure® organic/colloid removal and ion-exchange packed-bed system.
Synthesis of functional monomer
2-Methacryloyl-(l)-phenylalanine (MAPA) was chosen as the functional monomer to interact with l-Phe and the experimental procedure reported elsewhere was performed (Öncel et al. Citation2005); briefly l-phenylalanine methylester (5.0 g) and NaNO2 (0.2 g) were dissolved in 30 mL of K2CO3 aqueous solution (5%, w/v). This solution was cooled down to 0 °C. Methacryloyl chloride (4.0 mL) was slowly poured into this solution under nitrogen atmosphere and this solution was then stirred magnetically at room temperature for 2 h. At the end of this chemical reaction period, the pH of the solution was adjusted to 7.0, and subsequently, the solution was extracted with ethyl acetate. The aqueous phase was evaporated in a rotary evaporator. The residue (i.e. MAPA) was crystallized from ether and cyclohexane.
Preparation of l-Phe imprinted cryogel membranes
l-Phe-imprinted poly(2-hydroxyethyl methacrylate-methacryloyl-l-phenylalanine) (PHEMAPA-l-Phe) cryogels were synthesized by using three different ratios of MAPA monomer. The procedure is as follows: 0.273 g of N,N-methylene bisacrylamide (MBAAm) was dissolved in 10 mL of water, then 1.30 mL of HEMA was added and stirred. As summarized in , different amounts of MAPA (51, 102, 153 μL) and 14 mg of l-Phe were mixed in 5 mL of water in order to make functional monomer–template complex and added to the monomer mixture. While the mixture was stirring, 20 mg of APS and 25 μL of H2O2/TEMED were added. The solution was then quickly transferred between two glass plates separated by a 1-mm thick separator and was frozen at −12 °C. The polymerization was carried out for 24 h. After this period, resulting cryogel membrane was removed from the glass surface at room temperature and cut to fit the size of membrane cartridge (diameter: 2 cm). To remove unreacted monomers and other polymeric residuals, cryogels were thoroughly washed with water. The effect of the amount of template molecule in the polymerization recipe was also investigated and it was observed that the resulting cryogels were weak and flimsy in the case of higher amounts of l-Phe.
Table 1. The combination ratios of the template and functional monomer.
Removal of the template molecule from the imprinted polymer was achieved with ethylene glycol/water solution (50%, v/v). Template removal was observed as 91%, 47.2%, and 43.0% from PHEMAPA-l-Phe (A), (B), and (C), respectively. The highest removal ratio and l-Phe rebinding was achieved with the polymer (A) and this imprinted cryogel was used in the rest of the study and named as PHEMAPA-l-Phe in this report. The nonimprinted PHEMAPA cryogel membranes were synthesized using the same polymerization recipe without adding template, l-Phe.
Characterization of the cryogels
The swelling degree (S) of PHEMAPA-l-Phe and the PHEMAPA cryogels were performed in pure water at room temperature. The cryogels were placed in water until constant weight. The mass of wet (mwet gel) and dry (mdry gel) cryogels were recorded and the swelling degree was calculated according to the following equation:
(1)
(1)
The total volume of macropores in the swollen cryogel was roughly estimated by weighing the sample after squeezing (msqueezed) the free water from the swollen gel matrix by hand. These measurements were repeated at least ten times to calculate average and then the porosity was calculated as follows:
(2)
(2)
The morphologies of PHEMAPA and PHEMAPA-l-Phe cryogels were investigated with scanning electron microscope SEM (JEOL, JEM 1200EX, Tokyo, Japan). In order to show the incorporation of MAPA monomer into the polymer structure, FTIR spectra of the PHEMA, PHEMAPA were obtained between 400 and 4000 cm−1 (FTIR 8000 Series, Shimadzu, Japan). The specific surface area of PHEMAPA and PHEMAPA-l-Phe cryogels was measured according to the Brunauer–Emmett–Teller (BET) model using multi point analysis (Flowsorb II 2300, Micromeritics Instrument Corporation, Norcross, GA).
l-Phe adsorption studies
The l-Phe adsorption studies were performed in a continuous membrane cartridge stacked with PHEMAPA and PHEMAPA-l-Phe cryogel membranes. The cartridge was washed with 50 mL deionized water and stabilized with 50 mM working buffer for 15 min. Then, the l-Phe solution was pumped though the cartridge for 2 h with a peristaltic pump. l-Phe adsorption was determined by measuring its absorbance at 258 nm. Effects of pH, flow rate, temperature, ionic strength, and l-Phe concentration on the adsorption capacity were studied. The effect of pH on the adsorption capacity was determined in different buffer systems ranging between 4.0 and 8.0 (acetate buffer for pH 4.0–5.5 and phosphate buffer for pH 6.0–8.0). The effect of flow rate on the adsorption capacity was investigated by changing the flow rate between 0.5 and 3.0 mL/min. The effect of temperature on the adsorption capacity was investigated between 4 °C and 55 °C. The effect of initial concentration on the adsorption capacity was determined by changing l-Phe concentration between 0.1 and 3.0 mg/mL. The ionic strength was varied in the range of 0.001–0.1 M NaCI and the effect of the type of ion was studied with NaCl, Na2SO4, and (NH4)2SO4.
Selectivity experiments
The l-Phe selectivity of PHEMAPA-l-Phe cryogel was investigated by competitive adsorption of l-Phe, d-Phe, l-Trp, and d-Trp in acetate buffer solution (50 mM, pH 5.5). The selectivity of the PHEMAPA-l-Phe cryogel for l-Phe (pI: 5.48) with respect to the competitive amino acids d-Phe, l-Trp, d-Trp (pI:5.89), was evaluated in a continuous system for 2 h. After competitive adsorption, equilibrium was reached, the composition of remaining solution was detected at 258 (Phe) and at 280 nm (Trp). The distribution coefficient (Kd) for l-Trp, d-Trp, and d-Phe with respect to l-Phe were determined according to the following equation:
(3)
(3)
Here, Kd represents the distribution coefficient for the l-Phe (mL g − 1); Ci and Cf are the initial and the final concentration of amino acids (mM), respectively. V is the volume of the solution (mL) and m is the weight of the cryogel used in the column (g). The selectivity coefficient (k) for the binding of l-Phe with respect to competing species (i.e., d-Phe, l-Trp, d-Trp) was determined by the following equation;
(4)
(4)
in which Kd(template) is the distribution coefficient of the template amino acid (l-Phe) and Kd(competing amino acid) is the distribution coefficient of the competing amino acids (d-Phe, l-Trp, d-Trp).
The relative selectivity coefficient (k'), which was used to estimate the effect of imprinting on amino acid selectivity, can be calculated from the following equation:
(5)
(5)
in which kMIP and kNIP is the selectivity coefficients for PHEMAPA- l-Phe and PHEMAPA cryogel, respectively.
Chromatographic separation of amino acid enantiomers
In this experiment, the PHEMAPA-l-Phe membranes were applied to the fast protein liquid chromatography system (FPLC) for enantiomeric separation of l- and d-Phe. FPLC separation was performed by using AKTA–FPLC (Amersham Bioscience, Uppsala, Sweden) system equipped with an UV detector. The system includes M-925 mixer, P-920 pump, UPC-900 monitor, INV-907 injection valve, and Frac920 fraction collector. Separation was carried out with GE Healthcare column (10/10, 19–5001–01) stacked with the PHEMAPA-l-Phe cryogel membranes. FPLC mobile phases A and B were prepared by 10 mM acetate buffer (pH 5.5) and 10 mM acetate buffer (pH 4.0) containing 2 M NaCl, respectively. The chromatographic separation was performed using a linear gradient at 3 mL/min flow rate. After an 8-min starting period with 100% mobile phase A, a linear gradient was applied starting from 0% B to 100% B for 1 min, and continued with 100% eluent B for 6 min and finally finished with 100% buffer A for 1 min. 100 μL of l-Phe and d-Phe (1000 ppm) samples in acetate buffer (pH 5.5) were applied to the column. All buffers and amino acid solutions were filtered before use. Absorbance was monitored at 280 nm. The separation was performed at room temperature. Capacity factor (k, k = (tR−t0)/t0) and separation factor (α, α=k2/k1) were evaluated for the chromatographic performance of the PHEMAPA-l-Phe cartridge. Here, tR defines the retention time of the amino acid and t0 defines the retention time of the void marker (KBr). k2 is the capacity factor for bound l-Phe and k1 is the capacity factor for bound d-Phe. The theoretical plate numbers (N) of the cartridge was calculated using the following equation;
(6)
(6)
Here, w0.5 is the peak width at the corresponding peak height fraction and tR is the retention time of peaks at the baseline.
Desorption and reusability
Desorption of the adsorbed l-Phe from PHEMAPA-l-Phe cryogel was studied in the continuous system. Desorption agent was %50 (v/v) ethyleneglycol/water solution. Desorption time for l-Phe removal from the polymer was determined as two hours. The final l-Phe concentration in desorption solution was determined by UV spectrophotometer at 258 nm. Desorption ratio for l-Phe was calculated based on the ratio of l-Phe released and l-Phe adsorbed. The reusability of the cryogel was shown after 10 adsorption–desorption cycles by using the same cryogel cartridge.
Results and discussion
Characterization of cryogels
N-Methacryloly-l-phenylalanine (MAPA) was used as the comonomer for the hydrophobic interaction of l-Phe, the template molecule, to polymerize with HEMA. The MAPA/ l-Phe complex was used for the imprinting of l-Phe into the molecularly imprinted cryogel structure. The incorporation of MAPA monomer into the polymer structure was confirmed with the FTIR spectrum, which contains strong aromatic C-H stretching band at 1025 cm−1 and aromatic C-C stretching band around 1500 cm−1. The equilibrium swelling degree of the PHEMA, PHEMAPA, and PHEMAPA- l-Phe cryogels were 78.2%, 82.6%, and 88.0% with the macroporosity of 74.0%, 74.0%, and 72.2%, respectively. As can be seen from these values, incorporation of MAPA monomer into the polymer structure results in a decrease in both swelling and macropore ratio due to its hydrophobicity.
Surface area of the cryogels was determined from the nitrogen adsorption/desorption isotherms under the 0.005–1.0 relative pressures (P/P0) at liquid nitrogen temperature. The specific surface area of the PHEMA, PHEMAPA, and the PHEMAPA-l-Phe cryogels were found as 34.0, 36.2, and 37.1 m2/g, respectively. The presence of the hydrophobic l-Phe amino acid in the polymerization media and the presence of specific cavities in the imprinted polymer are responsible for this increase.
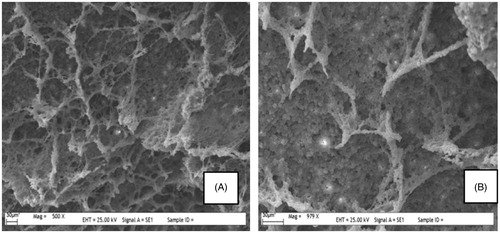
The surface morphology of the imprinted and nonimprinted cryogels was shown in . As can be seen from the figures, both PHEMAPA and PHEMAPA-l-Phe cryogels have characteristic flow channels made up of interconnected macropores (about 150–200 μm size), which allows diffusion of amino acids into the cryogel easily.
Adsorption studies
Effect of pH
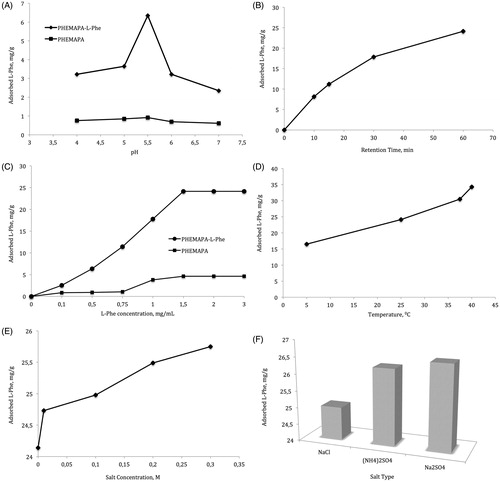
pH is an important separation parameter in the hydrophobic interaction chromatography (HIC). As the pH increases, hydrophobic interactions decrease due to the titration of the charged groups that results in hydrophilicity (Kennedy Citation2000). Inversely pH decrease tends to increase hydrophobic interactions. Generally, optimum binding in hydrophobic interactions occurs near isoelectric point or at slightly above because it minimizes charges and maximizes hydrophobicity (Kennedy Citation2000). shows the adsorption amount of l-Phe at different pH values. As seen in the figure, the maximum adsorption amount of l-Phe was observed at pH 5.5, the isoelectric point of l-Phe, where the net charge on the amino acid is zero. At higher and lower pH values, the adsorption amount was significantly decreased probably due to the charge repulsions.
Figure 2. The effect of adsorption conditions on l-Phe adsorption onto PHEMAPA-l-Phe cryogel membranes. (A) Effect of pH (l-Phe concentration: 0.5 mg/mL; flow rate: 1 mL/min; adsorption time: 2 h; T: 25 °C) (B) Effect of flow rate (l-Phe concentration: 1.0 mg/mL; pH: 5.5; adsorption time: 2 h; T: 25 °C). (C) Effect of l-Phe concentration (pH: 5.5; flow rate: 1.0 mL/min; adsorption time: 2 h; T: 25 °C) (D) Effect of temperature (l-Phe concentration: 1.5 mg/mL; pH: 5.5; adsorption time: 2 h) (E) Effect of ionic strength (l-Phe concentration: 1.5 mg/mL; pH: 5.5; adsorption time: 2 h; T: 25 °C) (F) Effect of salt type (l-Phe concentration: 1.5 mg/mL; salt concentration: 0.1 M; pH: 5.5; adsorption time: 2 h; T: 25 °C).
Effect of flow rate
The effect of retention time on the adsorption of l-Phe with the increase in flow rate is given in . The amount of adsorbed l-Phe decreased significantly from 24.1 to 8.1 mg g−1 as the flow rate increased from 0.5 to 3.0 mL min− 1. This can be explained by the decrease in interaction time, and thus, adsorption capacity due to the decrease in the retention time in the cartridge.
Effect of l-Phe concentration
The effect of l-Phe concentration on l-Phe adsorption onto the PHEMAPA-l-Phe and PHEMAPA cryogels is shown in . As can be seen from the figure increased l-Phe amount in the adsorption media resulted in higher adsorption amount. The l-Phe adsorption was first increased rapidly due to the increase in the concentration difference (ΔC), which is the driving force for the adsorption, and reached a plateau at 1.5 mg/mL l-Phe concentration. The adsorption amount at this concentration was 24.1 mg/g for PHEMAPA-l-Phe and 4.6 mg/g for PHEMAPA cryogels.
Effect of temperature
The hydrophobic interaction is entropy-driven, thus strongly temperature sensitive. The increase in the temperature shifts the equilibrium to the free water rather than the ordered water form and it reduces water exposed hydrophobic surfaces (Kennedy Citation2000). The effect of temperature on l-Phe adsorption onto the PHEMAPA-l-Phe cryogel membranes is given in . As expected, l-Phe adsorption was significantly increased with the increase in temperature. l-Phe adsorption amount was calculated as 16.5 mg/g at 4 °C and 34.3 mg/g at 40 °C. This increase shows the hydrophobic nature of the interaction of PHEMAPA-l-Phe with l-Phe.
Effect of ionic strength
Addition of various salts to the adsorption buffer increases hydrophobic interactions and this increase is directly proportional with the salt concentration until the precipitation point of the species. As given by the Hoffmeister series, in the presence of cosmotropic salts, the salting out effect, and thus, hydrophobic interactions are favored. The effect of salt concentration and type is given in , respectively. The increase in NaCl concentration from 0.001 M to 0.3 M increased the l-Phe adsorption onto PHEMAPA-l-Phe cryogels from 24.2 mg/g to 25.8 mg/g, as expected. The sulfates of sodium and ammonium is shown to be more effective in increasing adsorption amount as can be seen in . l-Phe adsorption was 24.9, 26.3, and 26.5 mg/g for NaCl, Na2(SO)4, and (NH4)2SO4, respectively at 0.1 M salt concentration. This behavior confirms the hydrophobic interaction between the l-Phe and the PHEMAPA-l-Phe cryogel adsorbent.
Adsorption isotherms and kinetics
In order to describe the interaction model between PHEMAPA-l-Phe and l-Phe molecules Langmuir and Freundlich equilibrium isotherm models were applied as linearized in EquationEquations (7)(7)
(7) and Equation(8)
(8)
(8) , respectively.
(7)
(7)
(8)
(8)
With respect to the calculated values, the linear fit confirms that the adsorption process can be defined with the Langmuir model, which means that the binding of l-Phe molecules on the imprinted PHEMAPA-l-Phe cryogels is monolayer and the Qmax value (29.4 mg/g) is consistent with the experimental value (24.14 mg/g). The correlation coefficient (R2) for the Langmuir isotherm was higher (0.97) than that of the Freundlich isotherm (0.93). Additionally, the Freundlich exponent (1/n) is found as 0.709, which represents the homogeneous system rather than the heterogeneous system. The Qf value, roughly the indicator of adsorption capacity, was found as 15.31 mg/g for this system.
Lagergren’s pseudo first-order (9) and pseudo second-order (10) rate equations were applied in their linearized forms in given equations:
(9)
(9)
(10)
(10)
In these equations, k1 (min −1) and k2 (g/mg.min) are rate constants for pseudo first-order and pseudo second-order kinetics, respectively. qeq and qt show the adsorption capacities at equilibrium and at any time in mg/g, respectively.
The analysis of adsorption data indicates that the process was controlled chemically. If follows second-order kinetics and eliminates the diffusion constraints. A comparison of the experimental adsorption capacity with theoretical values is given in . The correlation coefficient for the linear plot for the pseudo first-order equation is lower than the correlation coefficient for the pseudo second-order equation.
Table 2. The pseudo-first-order and pseudo-second-order kinetic constants for l-Phe adsorption.
Selectivity of the PHEMAPA-l-Phe cryogel
In order to show the selectivity of the PHEMAPA-l-Phe cryogel, the adsorption amounts of d-Phe, l-Trp, and d-Trp were compared with the l-Phe adsorption in continuous system. The results of the adsorption studies are given in and Kd and k values are summarized in .
Figure 3. Adsorption of competitor amino acids on PHEMAPA-l-Phe and PHEMAPA cryogels. Amino acid concentration: 1.0 mg/mL; pH: 5.5; adsorption time: 2 h; T: 25 °C.
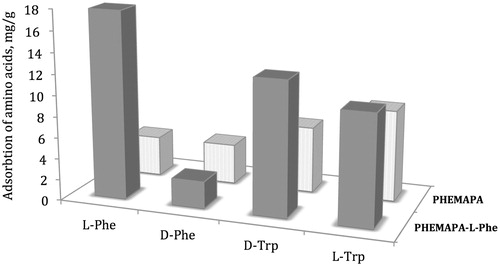
Table 3. Kd and k values for d-Phe, l-Trp, and d-Trp with respect to l-Phe.
These results showed that the Kd value of PHEMAPA-l-Phe cryogel is much more higher for l-Phe than that of the other amino acids. Relative selectivity constant confirms the affinity of the imprinted sites for l-Phe. The imprinted cryogel was 6.36, 4.29, and 5.49 times selective for the l-Phe than the d-Phe and indole side-chain-bearing l-Trp and d-Trp, respectively.
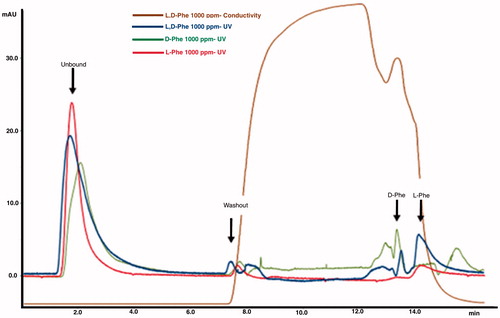
The enantioselective separation of d-Phe and l-Phe was performed by FPLC system with a linear gradient of 10 mM acetate buffer (pH 4.0) containing 2 M NaCl. shows unbound, washout, and bound fractions that were detected by UV absorbance (mAU) at 280 nm as D, l-Phe racemic mixture (blue line), d-Phe (green line), and l-Phe (red line). As seen in the chromatogram, there are two adjacent peaks at washout fraction while only one peak was observed for l-Phe and D-Phe. After unbound and washout fractions were observed, l-Phe was enantioselectively eluted from the PHEMAPA-l-Phe in 14.10 min, while d-Phe came out at 13.50 min. Further investigation was carried out to find the separation capacity of PHEMAPA-l-Phe cartridge toward l-Phe. summarizes the parameters calculated from the FPLC chromatograms. The theoretical plate number (N) and capacity factor (k) of the PHEMAPA-l-Phe cartridge were calculated as 35,290 and 47.62 for l-Phe, respectively. The separation factor was calculated as 1.05. These results confirm that the enantioselective separation of l-Phe from d-Phe was achieved successfully by using PHEMAPA-l-Phe cartridge as chromatographic media.
Table 4. The parameters calculated from the purification chromatograms.
Desorption and reusability of the PHEMAPA-l-Phe
The stability and reusability is a significant parameter for PHEMAPA-l-Phe cartridge. This reduces the total cost of the separation process significantly. Using the same adsorbent in 10 adsorption–desorption cycles showed the reusability of the cryogel membranes. It should be noted that there was no MAPA leakage from the polymeric structure during these cycles. After 10 cycles, there was no remarkable loss in the adsorption capacity of the PHEMAPA-l-Phe membranes.
Conclusion
In this study, l-Phe-imprinted PHEMA-based cartridges were successfully synthesized and enantiomeric separation of racemic D,l-Phe mixture was achieved. The major factors affecting the formation of imprinted regions and template recognition are the hydrophobic interactions and shape memory effect. l-Phe-imprinted PHEMA-based cryogel membranes exhibited high selectivity for the template, l-Phe. Adsorption capacity remained nearly constant after several adsorption– desorption cycles. Performed studies showed that the l-Phe-imprinted cryogels could be an attractive adsorbent for chiral l-Phe separation in chromatographic applications.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Andaç M, Baydemir G, Yavuz H, Denizli A. 2012. Molecularly imprinted composite cryogel for albumin depletion from human serum. J Mol Recognit. 25:555–563.
- Andac M, Galaev IY, Denizli A. 2013. Molecularly imprinted poly(hydroxyethyl methacrylate) based cryogel for albumin depletion from human serum. Colloids Surf B Biointerfaces. 109:259–265.
- Aslıyüce S, Bereli N, Uzun L, Onur MA, Say R, Denizli A. 2010. Ion-imprinted supermacroporous cryogel for in-vitro removal of iron out of human plasma with beta thalassemia. Sep Purif Technol. 73:243–249.
- Aslıyüce S, Uzun L, Rad AY, Ünal S, Say R, Denizli A. 2012. Molecular imprinting based composite cryogel membranes for purification of anti-hepatitis B surface antibody by fast protein liquid chromatography. J Chromatogr B. 95:889–890:95–102.
- Aslıyüce S, Uzun L, Say R, Denizli A. 2013. Immunoglobulin G recognition with Fab fragments imprinted monolithic cryogels: evaluation of the effects of metal-ion assisted-coordination of template molecule. React Funct Polym. 73:813–820.
- Aydoğan C, Andaç M, Bayram E, Say R, Denizli A. 2012. Molecularly imprinted cryogel for L-glutamic acid separation. Biotechnol Progr. 28:459–466.
- Aydoğan C, Denizli A. 2012. Electrochromatographic Enantioseparation of amino acids using polybutylmethacrylate-based chiral monolithic column by capillary electrochromatography. Chirality. 24:606–609.
- Aydoğan C, Denizli A. 2014. Chiral separation-based ligand exchange by open tubular capillary chromatography. Anal Biochem. 447:55–57.
- Aydoğan C, Yılmaz F, Çimen D, Uzun L, Denizli A. 2013. Enantioseparation of aromatic amino acids using CEC monolith with novel chiral selector, N-methacryloyl-L-histidine methyl ester. Electrophoresis 34:1908–1914.
- Baydemir G, Andaç M, Bereli N, Galaev IY, Say R, Denizli A. 2009. Supermacroporous PHEMA based cryogel with embedded bilirubin imprinted particles. React Funct Polym. 69:36–42.
- Baydemir G, Andaç M, Bereli N, Say R, Galaev IY, Denizli A. 2009. Bilirubin recognition via molecularly imprinted supermacroporous cryogels. Colloids Surf B Biointerfaces. 68:33–38.
- Bereli N, Andac M, Baydemir G, Say R, Galaev IY, Denizli A. 2008. Protein recognition via ion coordinated molecularly imprinted supermacroporous cryogels. J Chromatogr A. 1190:18–26.
- Bereli N, Saylan Y, Uzun L, Say R, Denizli A. 2011. L-histidine imprinted supermacroporous cryogels for protein recognition. Sep Purifi Technol. 82:28–35.
- Çaktü K, Baydemir G, Ergün B, Yavuz H. 2014. Cholesterol removal from various samples by cholesterol-imprinted monosize microsphere-embedded cryogels. Artif Cells Nanomed Biotechnol. 42:365–375.
- Derazshamshir A, Baydemir G, Andaç M, Say R, Galaev IY, Denizli A. 2010. Molecularly imprinted PHEMA-based cryogel for depletion of hemoglobin from human blood. Macromol Chem Phys. 211:657.
- Ergün B, Baydemir G, Andaç M, Yavuz H, Denizli A. 2012. Ion imprinted beads embedded cryogels or in-vitro removal of iron from β-thalassemic human plasma. J Appl Polym Sci. 125:254–262.
- Ertürk G, Bereli N, Tümer A, Say R, Denizli A. 2013. Molecularly imprinted cryogels for human interferon purification from human gingival fibroblast culture. J Mol Recognit. 26:633–642.
- Gabashvili A, Medina DD, Gedanken A, Mastai Y. 2007. Templating mesoporous silica with chiral block copolymers and its application for enantioselective separation. J Phys Chem B. 111:11105–11110.
- Gheshlagi R, Scharer JM, Young MM, Douglas PL. 2008. Application of statistical design for the optimization of amino acid separation by reverse-phase HPLC. Anal Biochem. 383:93–102.
- Ghosh S, Fang TH, Uddin MS, Hidajat K. 2013. Enantioselective separation of chiral aromatic amino acids with surface functionalized magnetic nanoparticles. Colloids Surf B Biointerfaces. 105:267–277.
- Groselj U, Murko S, Tansek MZ, Kovac J, Bakija AT, Lampret BR, Battelino T. 2015. Comparison of tandem mass spectrometry and amino acid analyzer for phenylalanine and tyrosine monitoring-Implications for clinical management of patients with hyperphenylalaninemia. Clin Biochem 48:14–18
- Haupt K. 2003. Molecularly imprinted polymers: the next generation. Anal Chem. 9:376A–383A.
- Hernandez LS, Serra NS, Marina ML, Crego AL. 2013. Enantiomeric separation of free L- and D-amino acids in hydrolyzed protein fertilizers by capillary electrophoresis tandem mass spectrometry. J Agric Food Chem. 61:5022–5030.
- Higuchi A, Hayashi A, Kanda N, Sanui K, Kitamura H. 2005. Chiral separation of amino acids in ultrafiltration through DNA-immobilized cellulose membranes. J Mol Struct. 739:145–152.
- Hofstetter O, Lindstrom H, Hofstetter H. 2004. Effect of the mobile phase on antibody-based enantiomer separations of amino acids in high-performance liquid chromatography. J Chromatogr A. 1049:85–95
- Hu YF, Zhang ZH, Zhang HB, Luo LJ, Yao SZ. 2011. Electrochemical determination of L-phenylalanine at polyaniline modified carbon electrode based on b-cyclodextrin incorporated carbon nanotube composite material and imprinted sol-gel film. Talanta. 84:305–313.
- Huang J, Wei Z, Chan J. 2008. Molecular imprinted polypyrrole nanowires for chiral amino acid recognition. Sensor Actuat Chem. 134:573–578.
- Ilisz I, Aranyi A, Pataj Z, Peter A. 2012. Enantiomeric separation of nonproteinogenic amino acids by high-performance liquid chromatography. J Chromatogr A. 1269:94–121.
- Ilisz I, Aranyi A, Peter A. 2013. Chiral derivatizations applied for the separation of unusual amino acid enantiomers by liquid chromatography and related techniques. J Chromatogr A. 1296:119–139.
- Jeong JS, Sim HJ, Lee YM, Yoon HR, Lee DH, Hong SP. 2009. Determination of phenylalanine in blood by high-performance anion-exchange chromatography-pulsed amperometric detection to diagnose phenylketonuria. J Chromatogr A. 1216:5709–5714.
- Kand’ar R, Zakova P. 2009. Determination of phenylalanine and tyrosine in plasma and dried blood samples using HPLC with fluorescence detection. J Chromatogr B. 877:3926–3929.
- Kennedy RM. 2000. Hydrophobic Interaction Chromatography in Current Protocols in Protein Science. New York: John Wiley and Sons.
- Kitagawa F, Otsuka K. 2011. Recent progress in capillary electrophoretic analysis of amino acid enantiomers. J Chromatogr B. 879:3087–3095.
- Koç İ, Baydemir G, Bayram E, Yavuz H, Denizli A. 2011. Selective removal of 17b-estradiol with molecularly imprinted particle-embedded cryogel systems. J Hazard Mater. 192:1819–1826.
- Kong Y, Wei J, Wang W, Chen Z. 2011. Separation of tryptophan enantiomers with polypyrrole electrode column by potential induced technique. Electrochimica Acta. 56:4770–4774.
- Le Noir M, Plieva F, Hey T, Guieyse B, Mattiasson B. 2007. Macroporous molecularly imprinted polymer/cryogel composite systems for the removal of endocrine disrupting trace contaminants. J Chromatogr A. 1154:158–164.
- Li M, Lin X, Xie Z. 2009. Investigation of enantiomer recognition of molecularly imprinted polymeric monoliths in pressurized capillary electrochromatography screening the amino acids and their derivatives. J Chromatogr A. 1216:5320–5326.
- Li Q, Duan J, Wu LJ, Huang Y, Tang G, Min SG. 2012. Sucrose as a chiral selector for determining enantiomeric composition of phenylalanine by UV-vis spectroscopy and chemimetrics. Chin Chem Lett. 23:1055–1058.
- Lozinsky VI, Galaev IY, Plieva FM, Savina IN, Jungvid H, Mattiasson B. 2003. Polymeric cryogels as promising materials. Trends Biotechnol. 21:445–451.
- Mahalakshmi R, Jesuraja SX, Jerome S. 2006. Das Growth and characterization of L-phenylalanine. Cryst Res Technol. 41:780–783.
- Öncel Ş, Uzun L, Garipcan B, Denizli A. 2005. Phenylalanine containing hydrophobic beads for lysozyme adsorption. Eng Chem Res. 44:7049–7056.
- Piccinini AM, Schmid MG, Pajpanova T, Pancheva S, Grueva E, Gübtz G. 2004. Chiral separation of natural and unnatural amino acid derivatives by micro-HPLC on a Ristocetin A stationary phase. J Biochem Biophys Methods 61:11–21.
- Qin L, He XW, Li WY, Zhang YK. 2008. Molecularly imprinted polymer prepared with bonded b-cyclodextrin and acrylamide on functionalized silica gel for selective recognition of tryptophan in aqueous media. J Chromatogr A. 1187:94–102.
- Qiu H, Xi Y, Lu F, Fan L, Luo C. 2012. Determination of L-phenylalanine on-line based on molecularly imprinted polymeric microspheres and flow injection chemiluminescence. Spectrochim Acta A Mol Biomol Spectrosc. 86:456–460.
- Rocco A, Aturki Z, Fanali S. 2013. Chiral separations in food analysis. Trends in Anal Chem. 52:206–225.
- Song Y, Funatsu T, Tsunoda M. 2013. Amino acid analysis using core-shell particle column. J Chromatogr B Analyt Technol Biomed Life Sci. 927:214–217.
- Tamahkar E, Bereli N, Say R, Denizli A. 2011. Molecularly imprinted supermacroporous cryogels for cytochrome c recognition. J Sep Sci. 34:3433–3440.
- Tang K, Luo J, Zhang P, Yi J, Hua J, Yang C. 2015. Kinetic study on reactive extraction of phenylalanine enantiomers with BINAP-copper complexes. Chin J Chem Eng. 23:57–63.
- Xiao Y, Lim HM, Chung TS, Rajagopolan R. 2007. Acetylation of b-cyclodextrin surface-functionalized cellulose dialysis membranes with enhanced chiral separation. Langmuir. 23:12990–12996.
- Yongzhu J, Hirose K, Nakamura T, Nishioka R, Ueshige T, Tobe Y. 2006. Preparation and evaluation of a chiral stationary phase covalently bound with a chiral pseudo-18-crown-6 ether having a phenolic hydroxy group for enantiomer separation of amino compounds. J Chromatogr A. 1129:201–207.