Abstract
There has been huge interest in applications of nanomaterials in biomedical science, including diagnosis, drug delivery, and development of human organs. Number of these nanomaterials has been already studied in human or at pre-clinical trial. There is a growing concern on potential toxicity and adverse effects of nanomaterials on human health, including lack of standard method of assessment of toxicology of these materials. Our investigation indicated that the bare and small nanoparticle have higher toxicity than modified and bulk materials, respectively. In addition, spherical nanoparticles have less toxicity than rod nanoparticles due to immune response of body.
Introduction
Nanotechnology is an emerging field involving manipulation of the matter at nanometer scale, which results in a novel class of materials with improved properties for a wide range of applications. Nanomaterials are defined as substances with one or more dimensions in the size range of 1–100 nanometers (Bleeker et al. Citation2012). Their use in diverse areas has been vastly explored in recent years, offering great advantages over conventional materials. In particular, the engineered nanomaterials have been widely investigated for the biomedical applications, including development of new diagnostic tools such as nanobiosensors and precise imaging modalities, novel therapeutics based on targeted drug delivery systems, and scaffolds for tissue engineering (Chang Citation2014, Karimi et al. Citation2015, Ketabchi et al. Citation2016, Naghibzadeh and Adabi Citation2014). Due to the increasing usage of nanomaterials in various fields of science and technology, the concerns have been emerged about their safety, biocompatibility, and toxicity.
Nanotoxicology as a branch of toxicology has been attracted researchers attention to specifically investigate potential toxic effects of nanomaterials. Nanotoxicology is an interdisciplinary field dealing with different aspects of the potential toxicity of nanomaterials. While there is a growing interest in the use of nanomaterials in different fields, the safety concerns about their use is also on the rise. Hence, there is an urgent need to address these concerns and expand our knowledge on the safety, biocompatibility, and toxicity of nanomaterials. In biomedical applications, in particular, there are serious concerns on the safety and biocompatibility of nanomaterials, considering the possibility of greater interactions between nanomaterials and biological system.
According to a consensus conference of the European Society for biomaterials in 1986, the biocompatibility is defined as the ability of a substance to present an appropriate host response in a particular application (Duncan and Izzo Citation2005, Williams Citation1989). It is worth noting that the interactions between a material and a host are influenced by several factors including the host factors and the properties of the material and the site and duration of the exposure. To understand the type and scale of these interactions, nanomaterials should be tested for potential toxicity in a variety of in vitro and in vivo settings. However, there is no harmonized standards for evaluating toxicity and biocompatibility of nanomaterials in biological systems and the rules are still being investigated (Dobrovolskaia and McNeil Citation2007). The aim of this research was to critically review the biocompatibility and toxicology of nanomaterials.
Several nanostructured materials have been explored for the biomedical applications. The most commonly studied materials are based on carbon, silica, and metals in different shapes (i.e., spheres, tubes, and rods) (Adabi et al. Citation2011, Ketabchi et al. Citation2016, Shakoori et al. Citation2015, Tavakol et al. Citation2014). The toxicity and biocompatibility of these materials depends on several factors such as the size, surface area, functional groups, concentration, and dosage (Foldvari and Bagonluri Citation2008). In general, the toxicity responses induced by ultrafine particles is higher in comparison with larger sizes of similar composition (Donaldson et al. Citation2001, Kang et al. Citation2008, Oberdürster Citation2000). Not only the size but also the surface plays an important role in the toxicity of nanomaterials. Each parameter could affect the toxicity and biocompatibility of a nanomaterial independently or in association with other parameters.
Clinical applications of nanostructures
Nanomaterials with its large surface area and nano size has huge applications for drug delivery (Kamali et al. Citation2015). As nanomaterials can cross the blood brain barrier, they have become the top research theme in delivery of drugs to brain. Nanomaterials with capability to be used in medical field, e.g., tissue engineering, drug delivery, and diagnosis, have potential toxicity and harmful effects on human health. To more clarify about importance of nanoparticles safety, clinical applications of nanomaterials with a focus in regenerative medicine and tissue engineering and blood brain barrier are mentioned in the following.
Regenerative medicine and tissue engineering
Nanotechnology provides the basic scientific foundation for the development of regenerative medicine along with tissue engineering. Applications of nanomaterials in biomedical sciences include molecules delivery (drugs, growth factors, and DNA), imaging and tracking iPSC, surface modifications of implantable materials or nanodevices (biosensor), and nanofibers for tissue scaffolds (Engel et al. Citation2008, Ramalingam and Rana Citation2015).
Nanoparticles research within regenerative medicine has been addressed mainly towards the development of entrapment and delivery systems. Delivery systems can enhance the success of therapeutic agents in nanoparticles for continuous release in a controlled manner which will boost the success rate of regeneration (Martin et al. Citation2004, Reddy et al. Citation2006). Nanoparticles are also useful for the delivery of molecules to stem cells, since stem cells undergoing lineage commitment require a specific spatio-temporal presentation of factors. Efforts have been made to incorporate these nanoparticles into biomaterials for controlled release rates (La Francesca Citation2012). Solid surface-modified nanoparticles might also be used for regenerative purposes. Although polymers are the most used nanoparticles in the delivery area, the use of ceramics has also been investigated. Hydroxyapatite nanoparticles conjugated with biomolecules could enhance osteoblast adhesion and bone regeneration (Liu and Webster Citation2007).
In addition, nanofibers are used for preparing tissue scaffolds and for modifying the surface of implantable materials and nanodevices such as biosensors (Adabi et al. Citation2015a, Citation2015b, Yang and Leong Citation2010). Depending upon the cells that need to be targeted, functionalization of scaffold is done accordingly with a variety of biological molecules. Ceramic nanoparticles and nanofibers have been reported by Sarvestani et al. (Citation2007) to be suitable in the elaboration of bio-inspired nanocomposites for bone tissue engineering applications, acting as the reinforcing phase of a polymer matrix, and improving scaffold bioactivity. The tantalum blocks were also found to provide even better bone fusion rates than structural bone grafts in several different clinical applications (Levine et al. Citation2006, Wigfield et al. Citation2003), indicating the importance of nanoparticles in tissue and surface engineering. Despite the fact that nanoparticles utilization in tissue engineering and regenerative medicine is increasing, their toxicity has not been fully addressed. Therefore, comprehensive studies are required about their toxicity before the use.
Blood brain barrier
Blood brain barrier is composed of different cell types including endothelial cells, astrocytes, pericytes, and microglial cells (Begley Citation2004). The transfer of almost all drugs is limited by the highly restrictive tight junctions and only small lipophilic molecules with molecular weight less than 500 Da could cross blood brain barrier (Pardridge Citation1998, Reese and Karnovsky Citation1967, Wolburg and Lippoldt Citation2002, Wu and Pardridge Citation1998). Likewise, these molecules may be transported out of blood brain barrier by active efflux mechanisms specially P-glycoprotein even after successful endothelial cell absorption (Begley Citation1996, Cordon-Cardo et al. Citation1989).
The size of particles can influence entrance of the particles into the cells. For example, particles less than 12 nm are able to cross the blood brain barrier (Sarin et al.Citation2008). Besides, the size may affect the mechanism of endocytotic uptake. Totally, clathrin-mediated endocytosis was proposed as the predominant pathway for the uptake of particles less 200 nm, whereas the uptake of particles 200–500 nm seems to be caveolae-mediated (Hillaireau and Couvreur Citation2009).
Other properties such as surface charge and hydrophobicity also affect transcytosis rate due to the effect on proteins adsorbed from plasma (Gessner et al. Citation2002). After intravenous injection, bare nanoparticles are immediately adsorbed via plasma and cleared from the blood stream by the macrophages of RES within 5 min (Pardridge Citation1992). However, by modifying nanoparticle surface, their blood circulation time can enhanced and their distribution in the body may alter and increase their uptake in the brain (Tiwari and Amiji Citation2006, Tröster et al. Citation1990). For example, 30 min, 2 h, and 4 h after intravenous injection of doxorubicin solution containing 1% of polysorbate 80, doxorubicin-loaded poly(butyl cyanoacrylate) nanoparticles with and without polysorbate 80-coating into the rats, their brains were removed. Considerable concentrations of doxorubicin in the were only observed after injection of the polysorbate 80-coated nanoparticles, proving the successful crossing of the blood brain barrier (Triguero et al. Citation1990). After administration of drug solution alone, doxorubicin was not taken up into the brain. Moreover, toxicological investigation of doxorubicin bound to poly(butyl cyanoacrylate) demonstrated that the doxorubicin toxicity significantly decrease after intravenous injection of the polysorbate 80-coated nanoparticles in comparison with the doxorubicin solutions. A considerably reduced cardio- and hepatotoxicity occur in comparison with free drug. The lower toxicity of the nanoparticulate formulations of doxorubicin can be attributed to the altered biodistribution of the doxorubicin loaded nanoparticles. As the tumor therapy with doxorubicin is limited due to cardiotoxicity, the reduced cardiotoxicity with doxorubicin loaded nanoparticles can play great importance. Likewise, the binding doxorubicin to nanoparticles alter distribution of the drug, resulting in lower accessible to hepatocytes and consequently less toxicity (Gelperina et al. Citation2002, Gulyaev et al. Citation1999, Pereverzeva et al. Citation2007).
The effects of nanomaterials properties on toxicity in clinical applications
Many factors could potentially influence material’s biocompatibility and toxicity including their surface chemistry (Chou et al. Citation1999, Tsai et al. Citation2001), roughness (Campoccia et al. Citation2003), surface energy (hyrophobicity/hydrophilicity) (Cai et al. Citation2002), the level of degradation products and release of by-products (Sun et al. Citation2007), concentration (Sun et al. Citation2011), particle size (Singh et al. Citation2007), oxidative stress functions (Reddy et al. Citation2010, Yang et al. Citation2009), crystallinity (Braydich-Stolle et al. Citation2009), coating (Harris et al. Citation2010, Lin et al. Citation2012), and the longevity of particles (Ai et al. Citation2011). Although, it is difficult to determine the specific role of any individual factor in toxicity and biocompatibility of nanomaterials, but some of the most important characteristics were summarized in the following.
The size
For the application of nanomaterials in biomedicine, all of effective characteristics on toxicity and biocompatibility should be considered. One of the most effective parameters on toxicity of nanomaterials is the size of the materials. Nanoparticles could pass through cell membranes and go into the blood and organs (Gatti and Rivasi Citation2002). Hence, the areas of biological systems which are normally inaccessible for larger particles may be accessible for nanoparticles (Dhawan and Sharma Citation2010). Besides, when the size of a bulk material decreases below a specific critical threshold, it results in an increase in surface area (Ai et al. Citation2011). Thus, the number of chemical molecules bounded to the surface increases and consequently the reactivity enhances. This can be a reason behind the potential toxic effects of nanoparticles which could be more significant compared to the larger particles (Ai et al. Citation2011, Linkov et al. Citation2008, Suh et al. Citation2009). For instance, studies demonstrated that the size-dependent cytotoxicity of gold nanoparticle (with diameter 1.4 nm) capped with triphenyl phosphine monosulfonate (TPPMS) resulted in cell death via induction of oxidative stress and mitochondrial damage (Kunzmann et al. Citation2011, Pan et al. Citation2009) whereas gold nanoparticles (with size 3.7 nm) modified with poly(ethylene glycol) (PEG) had no toxic effects despite their entrance into the nucleus of cells (Gu et al. Citation2009, Kunzmann et al. Citation2011). Likewise, titanium dioxide (TiO2) with the size of 20 nm induced 43-fold more inflammation than Tio2 with the size of 250 nm in short-term experiments of pulmonary toxicity in rats (Hallock et al. Citation2009). Park et al. (Citation2011) demonstrated that silver nanoparticles with the size of 20 nm are more toxic than the larger ones and Shi et al. (Citation2013) in a study revealed that there was negative correlation between the particle size and the toxicity effects; it means, small silver nanoparticles (5–10 nm) had higher toxicity effects than large ones (15–25 nm) in a model organism of Tetrahymena pyriformis. Other investigations also indicated that smaller particles had more pathological effects on the lungs in comparison with the larger particles of the same material (Oberdörster et al. Citation1994, Singh et al. Citation2007).
In general, nanomaterials in comparison with the bulk materials have higher surface energy and catalytic activities. For example, a number of nanoparticles such as metal oxide nanoparticles, fullerenes and silica particles can cause reactive oxygen species (ROS) generation in cell-free systems (Cristina Yeber et al. Citation2000, Fubini and Hubbard Citation2003, Isakovic et al. Citation2006, Kim et al. Citation2004). ROS production via nanoparticles with the size of 2–4 nm was 100–1000 times faster in comparison with 100 nm nanoparticles (Hoffman et al. Citation1994). Therefore, the enhanced catalytic activity might be a size-dependent phenomenon. However, the effect of other parameters on potential toxicity of nanoparticles in correlation with the size such as shape and charges could be considered.
The shape
Studies have demonstrated that the shape could influence the biocompatibility and toxicity of a nanoparticle. It has been illustrated with altering material’s shape from an equiaxed to acicular one, the toxic response was enhanced. Wang et al. reported that gold nanorods are highly toxic to the presence of hexadecyl cetyl trimethyl ammonium bromide (CTAB) as coating material for human skin cells whereas spherical gold nanoparticles are not inherently toxic. They explained that it is difficult to understand the cytotoxicity of gold nanomaterials individually, because CTAB was used for synthesis of gold nanorods and this surfactant alone show cytotoxicity whereas CTAB is not in gold nanoparticles (Wang et al. Citation2008). Likewise, Hsiao et al. reported that the nanorod zinc oxide (ZnO) particles are more toxic than the spherical ones on human lung epithelial cell (A549) at a fixed size (Hsiao and Huang Citation2011). It could be due to the interaction forces of lengthwise-oriented nanomaterials which enhance proportionally with their lengths (Brown et al. Citation2007). Therefore, the van der Waals forces of rod-shaped nanomaterials are greater in comparison with spherical ones.
The shape of nanomaterials could also influence the cellular internalization rate. Spherical particles are more easily internalized into the cell membrane in comparison with the large length-to-radius ratio (elongated) particles laying parallel to the cell membrane (Decuzzi et al. Citation2009). For example, the uptake of spherical-shaped gold nanoparticles is more than rod-shaped counterparts (Chithrani et al. Citation2006). Therefore, nanomaterials can be designed in an appropriate shape in order to enter the cells more easily for therapeutic purposes such as cancer therapy.
Surface charge
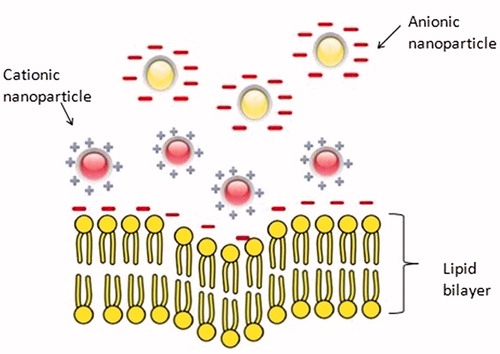
Surface charge of the nanoparticles is another important factor which can affect biocompatibility. The zeta potential is commonly used for characterizing the surface charge of nanoparticles. Zeta potential of nanoparticles in solutions in the ranges above (±) 30 mV leads to stability and prevents aggregation of the particles (Mohanraj and Chen Citation2007). However, surface charge affects the behavior of particles with biological moieties like cell–membrane interactions, penetration, protein adsorption, and stability in biological fluid (Maffre et al. Citation2011). Neutral particles show slower opsonization rates than the charged particles (Owens Iii and Peppas Citation2006, Roser et al. Citation1998) and nanoparticles with slight negative charges tend to accumulate in tumor tissues more efficiently (He et al. Citation2010, Patil et al. Citation2007). Positive charged particles could be more easily uptaken by the cells than the other nanoparticles due to the attractive interaction between positively charged nanoparticles and the negative cell membranes (Chen et al. Citation2011, Zhu et al. Citation2010) as seen in schematic . On the other hand, cationic nanoparticles are much more potent in activation of immune response than neutral or anionic nanoparticles (Kedmi et al. Citation2010, Zolnik et al. Citation2010).
Other factors
The aforementioned properties of nanoparticles as well as hydrophilicity, hydrophobicity, and surface topography, roughness, and tension may affect the protein adsorption, platelet activation, cellular growth, and consequently biocompatibility (Hsu and Lin Citation2004). Toxicity of nanoparticles can be decreased or eliminated using various surface modification techniques. For instance, it has been shown that coating of superparamagnetic iron oxide nanoparticles with pullulan significantly reduced toxicity of these nanoparticles (Ai et al. Citation2011). Uncoated magnetic nanoparticles (MNP) were associated with increased acidity of cell media to a cytotoxic level, leading to greater cytotoxicity in comparison with MNP coated with polyethyleneglycol-co-fumarate or polyvinyl-alcohol (Arsianti et al. Citation2010, Citation2011, Cedervall et al. Citation2007b, Nel et al. Citation2009). The hydrophobicity of nanoparticles determines the type and the amount of adsorbed biological components, mainly proteins (opsonins). Hydrophobicity influences both the amount of opsonization and the features of nanomaterial-binding proteins (Cedervall et al. Citation2007a, Goppert and Muller Citation2005). Opsonin proteins bound to hydrophobic nanoparticles facilitate macrophage-mediated phagocytosis of nanoparticles. In general, it seems that hydrophilic nanoparticles are safer and less toxic than the hydrophobic ones in biological systems (Sadaf et al. Citation2012, Yan et al. Citation2006, Zhu et al. Citation2010). In order to increase the blood circulation half-life of nanoparticles, one solution is to increase hydrophilicity of the surface. The most preferred method is modification of the surface by physical adsorption or chemical grafting of the poly(ethylene glycol) (PEG) to the surface of nanoparticles (Aggarwal et al. Citation2009, Jokerst et al. Citation2011, Moghimi and Szebeni Citation2003, Owens Iii and Peppas Citation2006). This could increase the circulation half-life of the nanoparticle by avoiding phagocytosis and rapid clearance from the blood. Likewise, it has been shown that the presence of poly-(vinyl pyrrolidone (PVP), a hydrophilic block polymer, on the surface of polyethersulfone (PES) membranes, which is used as hemodialysis membranes, could improve the biocompatibility (Ran et al. Citation2011). For some applications, however, hydrophobic surface could be preferred. For instance, surface modification of nanoparticles by hydrophobic polymers showed more adsorption in intestinal mucus (Ai et al. Citation2011). Surface modification of the polymeric membrane with amphiphilic triblock co-polymer, poly(vinyl pyrrolidone)–b-poly(methyl methacrylate)–b-poly(vinyl pyrrolidone), revealed a superior biocompatibility (Ran et al. Citation2011). The blood-compatibility of these modified membranes enhanced in comparison with PES membrane without modification (Ran et al. Citation2011). Different surface modification methods have been explored to enhance biocompatibility of various nanomaterials. However, the optimum modification technique with the best effect on biocompatibility for a particular nanomaterial is yet to be found.
Nanomaterials and immune system
The immune system protects the body from foreign invasions. Antigen-presenting dendritic cells, macrophages and the other phagocytic cells recognize foreign bodies and trigger an appropriate immunological response to the foreign materials. The recognition of nano-scaled particles as foreign stimuli may promote different levels of immune responses. Therefore, minimized or diminished immunogenicity of nanomaterials is favored in biomedical application of nanomaterials and nanoparticles as novel diagnostic or therapeutic modalities.
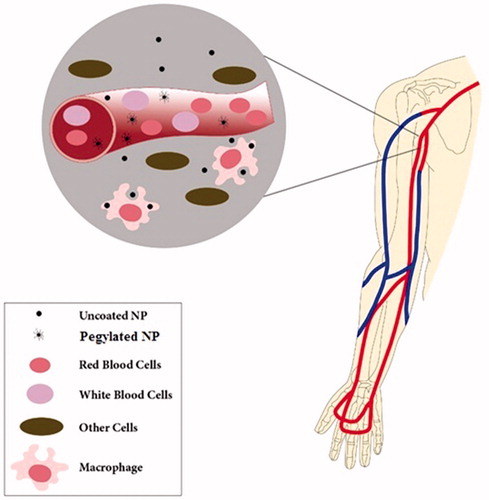
Most materials are coated by a layer of proteins, as exposed to the biological system after entrance to the body (Cedervall et al. Citation2007b, Lynch and Dawson Citation2008, Sahoo et al. Citation2007). The proteins corona on nanoparticles, depending on the amounts and the type of the adsorbed proteins, determines the subsequent interactions between nanoparticles and the immune cells and plays an effective role on biodistribution and uptake of nanomaterials by the reticuloendothelial system (RES) (Aggarwal et al. Citation2009, Chonn et al. Citation1992, Kiwada et al. Citation1987, Patel Citation1992, Tyrrell et al. Citation1977). It is now widely known that nanoparticles remarkable properties (e.g., size, surface charge and coating, hydrophobicity and hydrophilicity, etc) could be effective on their biocompatibility (Aggarwal et al. Citation2009, Dobrovolskaia and McNeil Citation2007, Dobrovolskaia et al. Citation2008). According to the reports, unmodified nanoparticles are taken up from the bloodstream within seconds by phagocytic cells, i.e., macrophages via opsonization (a process conducted by opsonins, a constituent of plasma proteins that makes nanomaterials more susceptible to ingestion) (Gref et al. Citation1994). Modifying the nanoparticles surface may considerably reduce their immunotoxicity and enhance their biocompatibility (Aggarwal et al. Citation2009, Dobrovolskaia and McNeil Citation2007, Dobrovolskaia et al. Citation2008, Gref et al. Citation1994). For example, the surface characteristics of nanoparticles such as hydrophobicity and hydrophilicity affect opsonization potential, as hydrophilic materials are opsonized slower than the hydrophobic ones, most likely due to reduced absorbability of hydrophilic surfaces (Carrstensen et al.Citation1992, Müller et al. Citation1992, Norman et al. Citation1992, Owens Iii and Peppas Citation2006). Therefore, surface modification of hydrophobic nanoparticles by coating with poly(ethylene glycol) (PEG), called “PEGylation”, or other kinds of hydrophilic polymers leads to a hydrophilic surface and act as an effective strategy for shielding of nanoparticles from plasma proteins, thereby hindering them from immune recognition and resulting in a prolong blood circulation half-life () (Gref et al. Citation1994, Citation2000, Kim et al. Citation2007, Lemarchand et al. Citation2006, Moghimi Citation2002, Owens Iii and Peppas Citation2006, Paciotti et al. Citation2004, Peracchia et al. Citation1999a, Citation1999b). It has been also reported that opsonization rate of neutrally charged particles is slower in comparison with the charged particles, representing a direct relationship between protein binding and the surface charge of particles (Owens Iii and Peppas Citation2006, Roser et al. Citation1998). Gessner et al. (Citation2002) reported that adsorption of plasma proteins enhanced as the surface charge density increased no significant differences in the type of adsorbed proteins.
Toxicity and biocompatibility studies: in vivo
Pre-clinical evaluation of nanoparticles in appropriate animal models is a crucial step of characterization to prove their safety and efficacy. The type of the nanomaterial and its particular application mainly determines the choice of animal model, route of administration, dosage, study end points, and other parameters. The interactions between nanomaterial as a foreign body and the animal as a host are mainly influenced by physicochemical properties of the nanomaterial. Totally, in most of the cases, there is no enough evidence to prove the safety or toxicity of nanomaterials. Besides, there is no a unique protocol for in vivo biocompatibility studies and several different procedures can be adapted to investigate the safety and efficacy of a specific nanomaterial in vivo.
However, before this can become a clinical reality, toxicity, and biocompatibility of the nanoparticles has to be carefully evaluated, with emphasis on an understanding of the physio-chemical properties that account for the adverse biological responses (Fadeel and Garcia-Bennett Citation2010).
One of the most widely accepted defining characteristic of nanoparticle-based medicine is particle size and distribution, because size can significantly impact pharmaco-kinetics, biodistribution, and safety (Moghimi et al. Citation2001). The pharmacokinetics and distribution of nanoparticles in the body depends on their surface physicochemical characteristics, shape as well as size (Hoet et al. Citation2004). Distribution can be either monodisperse or polydisperse, whereas the former with narrow distribution are desirable for consistency. It has been proposed that nanoparticles population of mixed sizes (polydisperse) is intentionally introduced for different rates of drug release to sustain delivery over time. To show that adsorption and distribution of nanoparticles are size dependent, Hillyer and Albrecht administered metallic colloidal gold nanoparticles with different sizes orally to mice. They noticed that distribution of nanoparticles increases with a decrease in size of nanoparticles to several organs (Hillyer and Albrecht Citation2001), and concluded that bigger particles reside in the gastrointestinal tract. In addition, nanoparticles with 10 nm in size were found in blood, liver, spleen, kidney, testis, thymus, heart, lung, and brain but larger particles were only in spleen, liver, and blood (De Jong et al. Citation2008). Chen et al. investigated the effect of colloidal gold nanoparticles in different sizes (3–100 nm) on physical and behavioral status of mice model. Intraperitoneal administration of 3–5 nm gold nanoparticles did not induce sickness but larger gold nanoparticles induced loss of appetite, fatigue, change of fur’s color, and weight loss and most of them died within 21 days (Chen et al. Citation2009).
Route of administration of nanoparticles include oral, pulmonary and dermal delivery. After the absorption process of nanoparticles by the various ports of entrance, the systemic circulation can distribute them towards all organs and tissues in the body. Several studies have shown distribution of particles to several organs including liver, spleen, heart, and brain (Hillyer and Albrecht Citation2001, Ji et al. Citation2006, Nemmar et al. Citation2002, Oberdörster Citation2002). Nanoparticles distribution to these organs is often mediated by their combination with human serum albumin (HSA), coagulant factors, RBC, WBC, and platelets available in systemic circulation. Therefore, their interaction with serum component (like Apo-A1) in vitro result in cytotoxic effect reduction (Barrett et al. Citation1999). Cartiera et al. (Citation2009) reported that the intracellular distribution of particles within cells is also time and dose-dependent. PLGA nanoparticles within renal tubule cells appeared to co-localize with early endosomes 2 h after exposure whereas they were also found in other compartments after 4–24 h. This is in agreement with finding of Panyam and Labhasetwar (Citation2003) who reported endo-lysosomal escape of nanoparticles. Cellular uptake of nanoparticles did not involve endocytosis (Geiser et al. Citation2005) since erythrocytes have no phagocytotic receptors (Rothen-Rutishauser et al. Citation2006). This finally suggests that nanoparticles are able to cross the cell membrane using processes other than phagocytosis and endocytosis.
The clearance of nanoparticles is also size and surface characteristics dependent. Small nanoparticles with size smaller than 20–30 nm are rapidly cleared by renal excretion, while 200 nm particles or those greater in size are more efficiently taken up by the Kupffer cells and mononuclear phagocytic system (reticuloendothelial system) located in the liver, spleen, and bone marrow (Moghimi et al. Citation2001). Previous reports have shown that nanoparticles of 150–300 nm are localize mainly in the liver and spleen (Gaumet et al. Citation2008), and colloids of sizes 200 to 400 nm undergo rapid hepatic clearance. The nanoparticle clearance is facilitated by the opsonization of blood components and complement proteins on the particle surface (Moghimi Citation2003). The inhibition of opsonization and evasion of detection by macrophages with approaches such as pegylation prolong the circulation of nanoparticles in the case of liposomal doxorubicin (Doxil) (Cattel et al. Citation2002).
In addition to cellular uptake of nanomaterials via different pathways of body, biodegradation, size- and dose-dependent cytotoxicities of nanomaterials, and the interaction between organs cells and nanoparticles are important issues that should be considered. The studies have been shown that various nanoparticles, e.g., micelles, liposomes, polymeric, and inorganic nanoparticles, interact with plasma proteins via different mechanisms (Karmali and Simberg Citation2011).
Dose is other determinant factor in the toxicity of nanomaterials in vivo. In a study in mice model, the toxicity of 13.5 nm citrate coated gold nanoparticles proved adverse effects of higher concentration of the nanoparticles such as weight loss, decreased red blood cells count (Zhang et al. Citation2010). In another study, different groups of rats received 4, 10, 20, and 40 mg/kg silver nanoparticles, intravenously. Results indicated that low doses (4 and 10 mg/kg) did not affect the hematological parameters of the animals, while in higher concentrations, 20 and 40 mg/kg, there was a significant change in the level of ROS, liver function enzymes such as ALT, AST, ALP, and bilirubin. DNA damage was also observed in the high dose groups, showing genotoxicity of these nanoparticles in high concentrations (Tiwari et al. Citation2011).
Properties related to the surface of nanoparticles determine the type and extent of interactions between nanoparticles and different plasma proteins such as immunoglobulin, lipoproteins, coagulation, and complement factors. These proteins can be adsorbed to the surface of nanoparticles and affect the metabolism, clearance, and long-term fate of nanoparticles. Manipulating the surface of nanoparticles via altering the surface charge and coating with different materials is an effective strategy to make the particles more soluble and biocompatible. Different coating material has been used to modulate surface properties of nanoparticles and control the biological response to those particles in living organisms. Surface modification can also affect the biodistribution of nanomaterials in different organs. Thi Ha Lien et al. (Citation2012) showed that PEG and BSA coated gold nanoparticles are accumulated in liver Kupfer cells and no gold nanoparticles were found in other cell types and other organs like kidney. PEG is one of the most widely used polymers for in vivo application of nanoparticles owing to its good solubility and biocompatibility. Nanoparticles with PEG coating have shown more blood circulation time due to the ability to escape from RES system (Gref et al. Citation1994, Peracchia et al. Citation1999a).
Conclusion
There are still several hindrances in the use of nanomaterials in various fields in general, and in biomedical field in particular, which should be resolved. One of the main restricting factors in the application of the nanomaterials is their safety, which remains a real concern. The concern is more serious when these materials are supposed to enter into human body, either intentionally or accidentally. One might be very optimistic about the potential benefits that nanomaterials offer, underscoring the risks associated with their use. However, studies conducted on testing toxicity and biocompatibility of nanomaterials reveals various ranges of toxicity and biocompatibility, which reflects the distinct response of biological system toward different nanostructures and nanomaterials. To date, our knowledge on the interactions of nanomaterials with biological systems is limited and harmonized standards do not exist for evaluating toxicity of nanomaterials on biological systems. There is also a huge controversy on the influence of different parameters related to nanomaterials properties on their toxicity and safety. Therefore, despite rapid development in the field of nanotechnology, there are still important challenges that necessitate further studies to provide more accurate data on the potential risks and hazards of biomedical applications of nanomaterials.
Funding information
This study was supported by Tehran University of Medical Sciences.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Adabi M, Saber R, Adabi M, Sarkar S. 2011. Examination of incubation time of bare gold electrode inside cysteamine solution for immobilization of multi-walled carbon nanotubes on a gold electrode modified with cysteamine. Microchim Acta. 172:83–88.
- Adabi M, Saber R, Faridi-Majidi R, Faridbod F. 2015a. Performance of electrodes synthesized with polyacrylonitrile-based carbon nanofibers for application in electrochemical sensors and biosensors. Mater Sci Eng C. 48:673–678.
- Adabi M, Saber R, Naghibzadeh M, Faridbod F, Faridi-Majidi R. 2015b. Parameters affecting carbon nanofiber electrodes for measurement of cathodic current in electrochemical sensors: an investigation using artificial neural network. RSC Adv. 5:81243–81252.
- Aggarwal P, Hall JB, McLeland CB, Dobrovolskaia MA, McNeil SE. 2009. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv Drug Deliv Rev. 61:428–437.
- Ai J, Biazar E, Jafarpour M, Montazeri M, Majdi A, Aminifard S, et al. 2011. Nanotoxicology and nanoparticle safety in biomedical designs. Int J Nanomedicine. 6:1117–1127.
- Arsianti M, Lim M, Lou SN, Goon IY, Marquis CP, Amal R. 2011. Bi-functional gold-coated magnetite composites with improved biocompatibility. J Colloid Interface Sci. 354:536–545.
- Arsianti M, Lim M, Marquis CP, Amal R. 2010. Polyethylenimine based magnetic iron-oxide vector: the effect of vector component assembly on cellular entry mechanism, intracellular localization, and cellular viability. Biomacromolecules. 11:2521–2531.
- Barrett EG, Johnston C, Oberdörster G, Finkelstein JN. 1999. Silica binds serum proteins resulting in a shift of the dose-response for silica-induced chemokine expression in an alveolar type II cell line. Toxicol Appl Pharmacol. 161:111–122.
- Begley DJ. 1996. The blood-brain barrier: principles for targeting peptides and drugs to the central nervous system. J Pharm Pharmacol. 48:136–146.
- Begley DJ. 2004. Delivery of therapeutic agents to the central nervous system: the problems and the possibilities. Pharmacol Ther. 104:29–45.
- Bleeker EA, de Jong WH, Geertsma RE, Groenewold M, Heugens EH, Koers-Jacquemijns M, et al. 2012. Considerations on the EU definition of a nanomaterial: science to support policy making. Regul Toxicol Pharmacol. 65:119–125.
- Braydich-Stolle LK, Schaeublin NM, Murdock RC, Jiang J, Biswas P, Schlager JJ, Hussain SM. 2009. Crystal structure mediates mode of cell death in TiO2 nanotoxicity. J Nanopart Res. 11:1361–1374.
- Brown SC, Kamal M, Nasreen N, Baumuratov A, Sharma P, Antony VB, Moudgil BM. 2007. Influence of shape, adhesion and simulated lung mechanics on amorphous silica nanoparticle toxicity. Adv Powder Technol. 18:69–80.
- Cai K, Yao K, Cui Y, Yang Z, Li X, Xie H, Qing T, Gao L. 2002. Influence of different surface modification treatments on poly(D,L-lactic acid) with silk fibroin and their effects on the culture of osteoblast in vitro. Biomaterials. 23:1603–1611.
- Campoccia D, Arciola CR, Cervellati M, Maltarello MC, Montanaro L. 2003. In vitro behaviour of bone marrow-derived mesenchymal cells cultured on fluorohydroxyapatite-coated substrata with different roughness. Biomaterials. 24:587–596.
- Carrstensen H, Müller R, Müller B. 1992. Particle size, surface hydrophobicity and interaction with serum of parenteral fat emulsions and model drug carriers as parameters related to RES uptake. Clin Nutr. 11:289–297.
- Cartiera MS, Johnson KM, Rajendran V, Caplan MJ, Saltzman WM. 2009. The uptake and intracellular fate of PLGA nanoparticles in epithelial cells. Biomaterials. 30:2790–2798.
- Cattel L, Ceruti M, Dosio F. 2002. From conventional to stealth liposomes: a new frontier in cancer chemotherapy. Tumori. 89:237–249.
- Cedervall T, Lynch I, Foy M, Berggard T, Donnelly SC, Cagney G, Linse S, Dawson KA. 2007a. Detailed identification of plasma proteins adsorbed on copolymer nanoparticles. Angew Chem Int Ed Engl. 46:5754–5756.
- Cedervall T, Lynch I, Lindman S, Berggård T, Thulin E, Nilsson H, Dawson KA, Linse S. 2007b. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc Natl Acad Sci USA. 104:2050–2055.
- Chang TMS. 2014. Artificial cells: the beginning of nanomedicine. Select Top Nanomedicine. 3:1–44.
- Chen L, McCrate JM, Lee JC, Li H. 2011. The role of surface charge on the uptake and biocompatibility of hydroxyapatite nanoparticles with osteoblast cells. Nanotechnology. 22:105708. doi(2011 Feb 2):10.1088/0957-4484/22/10/105708.
- Chen Y-S, Hung Y-C, Liau I, Huang GS. 2009. Assessment of the in vivo toxicity of gold nanoparticles. Nanoscale Res Lett. 4:858–864.
- Chithrani BD, Ghazani AA, Chan WC. 2006. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 6:662–668.
- Chonn A, Semple S, Cullis P. 1992. Association of blood proteins with large unilamellar liposomes in vivo. Relation to circulation lifetimes. J Biol Chem. 267:18759–18765.
- Chou L, Marek B, Wagner W. 1999. Effects of hydroxylapatite coating crystallinity on biosolubility, cell attachment efficiency and proliferation in vitro. Biomaterials. 20:977–985.
- Cordon-Cardo C, O'Brien JP, Casals D, Rittman-Grauer L, Biedler JL, Melamed MR, Bertino JR. 1989. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci USA. 86:695–698.
- Cristina Yeber M, Rodrı´guez J, Freer J, Durán N, Mansilla DH. 2000. Photocatalytic degradation of cellulose bleaching effluent by supported TiO2 and ZnO. Chemosphere. 41:1193–1197.
- De Jong WH, Hagens WI, Krystek P, Burger MC, Sips AJ, Geertsma RE. 2008. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials. 29:1912–1919.
- Decuzzi P, Pasqualini R, Arap W, Ferrari M. 2009. Intravascular delivery of particulate systems: does geometry really matter? Pharm Res. 26:235–243.
- Dhawan A, Sharma V. 2010. Toxicity assessment of nanomaterials: methods and challenges. Anal Bioanal Chem. 398:589–605.
- Dobrovolskaia MA, Aggarwal P, Hall JB, McNeil SE. 2008. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol Pharmaceut. 5:487–495.
- Dobrovolskaia MA, McNeil SE. 2007. Immunological properties of engineered nanomaterials. Nat Nanotechnol. 2:469–478.
- Donaldson K, Stone V, Clouter A, Renwick L, MacNee W. 2001. Ultrafine particles. Occup Environ Med. 58:211–216.
- Duncan R, Izzo L. 2005. Dendrimer biocompatibility and toxicity. Adv Drug Deliv Rev. 57:2215–2237.
- Engel E, Michiardi A, Navarro M, Lacroix D, Planell JA. 2008. Nanotechnology in regenerative medicine: the materials side. Trends Biotechnol. 26:39–47.
- Fadeel B, Garcia-Bennett AE. 2010. Better safe than sorry: understanding the toxicological properties of inorganic nanoparticles manufactured for biomedical applications. Adv Drug Deliv Rev. 62:362–374.
- Foldvari M, Bagonluri M. 2008. Carbon nanotubes as functional excipients for nanomedicines: II. Drug delivery and biocompatibility issues. Nanomed Nanotechnol. 4:183–200.
- Fubini B, Hubbard A. 2003. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosis. Free Radic Biol Med. 34:1507–1516.
- Gatti AM, Rivasi F. 2002. Biocompatibility of micro- and nanoparticles. Part I: in liver and kidney. Biomaterials. 23:2381–2387.
- Gaumet M, Vargas A, Gurny R, Delie F. 2008. Nanoparticles for drug delivery: the need for precision in reporting particle size parameters. Eur J Pharm Biopharm. 69:1–9.
- Geiser M, Rothen-Rutishauser B, Kapp N, Schürch S, Kreyling W, Schulz H, et al. 2005. Ultrafine particles cross cellular membranes by nonphagocytic mechanisms in lungs and in cultured cells. Environ Health Perspect. 113:1555–1560.
- Gelperina SE, Khalansky AS, Skidan IN, Smirnova ZS, Bobruskin AI, Severin SE, et al. 2002. Toxicological studies of doxorubicin bound to polysorbate 80-coated poly(butyl cyanoacrylate) nanoparticles in healthy rats and rats with intracranial glioblastoma. Toxicol Lett. 126:131–141.
- Gessner A, Lieske A, Paulke BR, Müller RH. 2002. Influence of surface charge density on protein adsorption on polymeric nanoparticles: analysis by two-dimensional electrophoresis. Eur J Pharm Biopharm. 54:165–170.
- Goppert TM, Muller RH. 2005. Protein adsorption patterns on poloxamer- and poloxamine-stabilized solid lipid nanoparticles (SLN). Eur J Pharm Biopharm. 60:361–372.
- Gref R, Lück M, Quellec P, Marchand M, Dellacherie E, Harnisch S, Blunk T, Müller R. 2000. ‘Stealth’ corona-core nanoparticles surface modified by polyethylene glycol (PEG): influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloid Surface B. 18:301–313.
- Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. 1994. Biodegradable long-circulating polymeric nanospheres. Science. 263:1600–1603.
- Gu Y-J, Cheng J, Lin C-C, Lam YW, Cheng SH, Wong W-T. 2009. Nuclear penetration of surface functionalized gold nanoparticles. Toxicol Appl Pharmacol. 237:196–204.
- Gulyaev AE, Gelperina SE, Skidan IN, Antropov AS, Kivman GY, Kreuter J. 1999. Significant transport of doxorubicin into the brain with polysorbate 80-coated nanoparticles. Pharm Res. 16:1564–1569.
- Hallock MF, Greenley P, DiBerardinis L, Kallin D. 2009. Potential risks of nanomaterials and how to safely handle materials of uncertain toxicity. JCHAS. 16:16–23.
- Harris TJ, Green JJ, Fung PW, Langer R, Anderson DG, Bhatia SN. 2010. Tissue-specific gene delivery via nanoparticle coating. Biomaterials. 31:998–1006.
- He C, Hu Y, Yin L, Tang C, Yin C. 2010. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials. 31:3657–3666.
- Hillaireau H, Couvreur P. 2009. Nanocarriers' entry into the cell: relevance to drug delivery. Cell Mol Life Sci.66:2873–2896.
- Hillyer JF, Albrecht RM. 2001. Gastrointestinal persorption and tissue distribution of differently sized colloidal gold nanoparticles. J Pharm Sci. 90:1927–1936.
- Hoet PHM, Brüske-Hohlfeld I, Salata OV. 2004. Nanoparticles – known and unknown health risks. J Nanobiotechnol. 2:12–27.
- Hoffman AJ, Carraway ER, Hoffmann MR. 1994. Photocatalytic production of H2O2 and organic peroxides on quantum-sized semiconductor colloids. Environ Sci Technol. 28:776–785.
- Hsiao I-L, Huang Y-J. 2011. Effects of various physicochemical characteristics on the toxicities of ZnO and TiO nanoparticles toward human lung epithelial cells. Sci Total Environ. 409:1219–1228.
- Hsu S-h, Lin Z-C. 2004. Biocompatibility and biostability of a series of poly(carbonate)urethanes. Colloids Surf B Biointerfaces. 36:1–12.
- Isakovic A, Markovic Z, Todorovic-Markovic B, Nikolic N, Vranjes-Djuric S, Mirkovic M, et al. 2006. Distinct cytotoxic mechanisms of pristine versus hydroxylated fullerene. Toxicol Sci. 91:173–183.
- Ji ZQ, Sun H, Wang H, Xie Q, Liu Y, Wang Z. 2006. Biodistribution and tumor uptake of C60 (OH) x in mice. J Nanopart Res. 8:53–63.
- Jokerst JV, Lobovkina T, Zare RN, Gambhir SS. 2011. Nanoparticle PEGylation for imaging and therapy. Nanomedicine (Lond). 6:715–728.
- Kamali M, Dinarvand R, Maleki H, Arzani H, Mahdaviani P, Nekounam H, Adabi M, Khosravani M. 2015. Preparation of imatinib base loaded human serum albumin for application in the treatment of glioblastoma. RSC Adv. 5:62214–62219.
- Kang S, Herzberg M, Rodrigues DF, Elimelech M. 2008. Antibacterial effects of carbon nanotubes: size does matter!. Langmuir. 24:6409–6413.
- Karimi MA, Pourhakkak P, Adabi M, Firoozi S, Adabi M, Naghibzadeh M. 2015. Using an artificial neural network for the evaluation of the parameters controlling PVA/chitosan electrospun nanofibers diameter. e-Polymers. 15:127–138.
- Karmali PP, Simberg D. 2011. Interactions of nanoparticles with plasma proteins: implication on clearance and toxicity of drug delivery systems. Expert Opin Drug Deliv. 8:343–357.
- Kedmi R, Ben-Arie N, Peer D. 2010The systemic toxicity of positively charged lipid nanoparticles and the role of Toll-like receptor 4 in immune activation. Biomaterials. 31:6867–6875.
- Ketabchi N, Naghibzadeh M, Adabi M, Esnaashari SS, Faridi-Majidi R. 2016. Preparation and optimization of chitosan/polyethylene oxide nanofiber diameter using artificial neural networks. Neural Comput Appl. [Epub ahead of print]. DOI: 10.1007/s00521-016-2212-0.
- Kim HR, Andrieux K, Delomenie C, Chacun H, Appel M, Desmaële D, et al. 2007. Analysis of plasma protein adsorption onto PEGylated nanoparticles by complementary methods: 2-DE, CE and Protein Lab-on-chip system. Electrophoresis. 28:2252–2261.
- Kim SY, Nishioka M, Taya M. 2004. Promoted proliferation of an SOD-deficient mutant of Escherichia coli under oxidative stress induced by photoexcited TiO2. FEMS Microbiol Lett. 236:109–114.
- Kiwada H, Miyajima T, Kato Y. 1987. Studies on the uptake mechanism of liposomes by perfused rat liver. II. An indispensable factor for liver uptake in serum. Chem Pharm Bull. 35:1189–1195.
- Kunzmann A, Andersson B, Thurnherr T, Krug H, Scheynius A, Fadeel B. 2011. Toxicology of engineered nanomaterials: focus on biocompatibility, biodistribution and biodegradation. Biochim Biophys Acta. 1810:361–373.
- La Francesca S. 2012. Nanotechnology and stem cell therapy for cardiovascular diseases: potential applications. Methodist DeBakey Cardiovasc J. 8:28–35.
- Lemarchand C, Gref R, Passirani C, Garcion E, Petri B, Müller R, Costantini D, Couvreur P. 2006. Influence of polysaccharide coating on the interactions of nanoparticles with biological systems. Biomaterials. 27:108–118.
- Levine BR, Sporer S, Poggie RA, Della Valle CJ, Jacobs JJ. 2006. Experimental and clinical performance of porous tantalum in orthopedic surgery. Biomaterials. 27:4671–4681.
- Lin I, Liang M, Liu T-Y, Jia Z, Monteiro MJ, Toth I. 2012. Effect of polymer grafting density on silica nanoparticle toxicity. Bioorgan Med Chem. 20:6862–6869.
- Linkov I, Satterstrom FK, Corey LM. 2008. Nanotoxicology and nanomedicine: making hard decisions. Nanomedicine. 4:167–171.
- Liu H, Webster TJ. 2007. Nanomedicine for implants: a review of studies and necessary experimental tools. Biomaterials. 28:354–369.
- Lynch I, Dawson KA. 2008. Protein-nanoparticle interactions. Nano Today. 3:40–47.
- Maffre P, Nienhaus K, Amin F, Parak WJ, Nienhaus GU. 2011. Characterization of protein adsorption onto FePt nanoparticles using dual-focus fluorescence correlation spectroscopy. Beilstein J Nanotechnol. 2:374–383.
- Martin I, Wendt D, Heberer M. 2004. The role of bioreactors in tissue engineering. Trends Biotechnol. 22:80–86.
- Moghimi S. 2002. Chemical camouflage of nanospheres with a poorly reactive surface: towards development of stealth and target-specific nanocarriers. Biochim Biophys Acta. 1590:131–139.
- Moghimi SM. 2003. Modulation of lymphatic distribution of subcutaneously injected poloxamer 407-coated nanospheres: the effect of the ethylene oxide chain configuration. FEBS Lett. 540:241–244.
- Moghimi SM, Hunter AC, Murray JC. 2001. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev. 53:283–318.
- Moghimi SM, Szebeni J. 2003. Stealth liposomes and long circulating nanoparticles: critical issues in pharmacokinetics, opsonization and protein-binding properties. Prog Lipid Res. 42:463–478.
- Mohanraj V, Chen Y. 2007. Nanoparticles – a review. Trop J Pharm Res. 5:561–573.
- Müller R, Wallis K, Tröster S, Kreuter J. 1992. In vitro characterization of poly (methyl-methaerylate) nanoparticles and correlation to their in vivo fate. J Control Release. 20:237–246.
- Naghibzadeh M, Adabi M. 2014. Evaluation of effective electrospinning parameters controlling gelatin nanofibers diameter via modelling artificial neural networks. Fibers Polym. 15:767–777.
- Nel AE, Mädler L, Velegol D, Xia T, Hoek EM, Somasundaran P, et al. 2009. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 8:543–557.
- Nemmar A, Hoylaerts MF, Hoet PHM, Dinsdale D, Smith T, Xu H, Vermylen J, Nemery B. 2002. Ultrafine particles affect experimental thrombosis in an in vivo hamster model. Am J Respir Crit Care Med. 166:998–1004.
- Norman M, Williams P, Illum L. 1992. Human serum albumin as a probe for surface conditioning (opsonization) of block copolymer-coated microspheres. Biomaterials. 13:841–849.
- Oberdörster G. 2002. Toxicokinetics and effects of fibrous and nonfibrous particles. Inhal Toxicol. 14:29–56.
- Oberdörster G, Ferin J, Lehnert BE. 1994. Correlation between particle size, in vivo particle persistence, and lung injury. Environ Health Persp. 102:173–179.
- Oberdürster G. 2000. Toxicology of ultrafine particles: in vivo studies. Philos T Roy Soc A. 358:2719–2740.
- Owens Iii DE, Peppas NA. 2006. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 307:93–102.
- Paciotti GF, Myer L, Weinreich D, Goia D, Pavel N, McLaughlin RE, Tamarkin L. 2004. Colloidal gold: a novel nanoparticle vector for tumor directed drug delivery. Drug Deliv. 11:169–183.
- Pan Y, Leifert A, Ruau D, Neuss S, Bornemann J, Schmid G, et al. 2009. Gold nanoparticles of diameter 1.4 nm trigger necrosis by oxidative stress and mitochondrial damage. Small. 5:2067–2076.
- Panyam J, Labhasetwar V. 2003. Dynamics of endocytosis and exocytosis of poly(D,L-lactide-co-glycolide) nanoparticles in vascular smooth muscle cells. Pharm Res. 20:212–220.
- Pardridge WM. 1992. Recent developments in peptide drug delivery to the brain. Pharmacol. Toxicol. 71:3–10.
- Pardridge WM. 1998. CNS drug design based on principles of blood-brain barrier transport . J. Neurochem. 70:1781–1792.
- Park MV, Neigh AM, Vermeulen JP, de la Fonteyne LJ, Verharen HW, Briedé JJ, van Loveren H, de Jong WH. 2011. The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. Biomaterials. 32:9810–9817.
- Patel H. 1992. Serum opsonins and liposomes: their interaction and opsonophagocytosis. Crit Rev Ther Drug Carrier Syst. 9:39–90
- Patil S, Sandberg A, Heckert E, Self W, Seal S. 2007. Protein adsorption and cellular uptake of cerium oxide nanoparticles as a function of zeta potential. Biomaterials. 28:4600–4607.
- Peracchia M, Fattal E, Desmaele D, Besnard M, Noel J, Gomis J, et al. 1999a. Stealth PEGylated polycyanoacrylate nanoparticles for intravenous administration and splenic targeting. J Control Release. 60:121–128.
- Peracchia M, Harnisch S, Pinto-Alphandary H, Gulik A, Dedieu J, Desmaele D, et al. 1999b. Visualization of in vitro protein-rejecting properties of PEGylated stealth polycyanoacrylate nanoparticles. Biomaterials. 20:1269–1275.
- Pereverzeva E, Treschalin I, Bodyagin D, Maksimenko O, Langer K, Dreis S, et al. 2007. Influence of the formulation on the tolerance profile of nanoparticle-bound doxorubicin in healthy rats: focus on cardio- and testicular toxicity . Int J Pharm. 337:346–356.
- Ramalingam M, Rana D. 2015. Impact of nanotechnology in induced pluripotent stem cells-driven tissue engineering and regenerative medicine. J Bionanosci. 9:13–21.
- Ran F, Nie S, Zhao W, Li J, Su B, Sun S, Zhao C. 2011. Biocompatibility of modified polyethersulfone membranes by blending an amphiphilic triblock co-polymer of poly(vinyl pyrrolidone)-b-poly(methyl methacrylate)-b-poly(vinyl pyrrolidone). Acta Biomater. 7:3370–3381.
- Reddy ARN, Reddy YN, Krishna DR, Himabindu V. 2010. Multi wall carbon nanotubes induce oxidative stress and cytotoxicity in human embryonic kidney (HEK293) cells. Toxicology. 272:11–16.
- Reddy ST, Rehor A, Schmoekel HG, Hubbell JA, Swartz MA. 2006. In vivo targeting of dendritic cells in lymph nodes with poly (propylene sulfide) nanoparticles. J Control Release. 112:26–34.
- Reese TS, Karnovsky MJ. 1967. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol. 34:207–217.
- Roser M, Fischer D, Kissel T. 1998. Surface-modified biodegradable albumin nano- and microspheres. II: effect of surface charges on in vitro phagocytosis and biodistribution in rats. Eur J Pharm Biopharm. 46:255–263.
- Rothen-Rutishauser BM, Schürch S, Haenni B, Kapp N, Gehr P. 2006. Interaction of fine particles and nanoparticles with red blood cells visualized with advanced microscopic techniques. Environ Sci Technol. 40:4353–4359.
- Sadaf A, Zeshan B, Wang Z, Zhang R, Xu S, Wang C, Cui Y. 2012. Toxicity evaluation of hydrophilic CdTe quantum dots and CdTe@SiO2 nanoparticles in mice. J Nanosci Nanotechnol. 12:8287–8292.
- Sahoo B, Goswami M, Nag S, Maiti S. 2007. Spontaneous formation of a protein corona prevents the loss of quantum dot fluorescence in physiological buffers. Chem Phys Lett. 445:217–220.
- Sarin H, Kanevsky AS, Wu H, Brimacombe KR, Fung SH, Sousa AA, et al. 2008. Effective transvascular delivery of nanoparticles across the blood-brain tumor barrier into malignant glioma cells. J Transl Med. 6:80–95.
- Sarvestani AS, He X, Jabbari E. 2007. Effect of osteonectin-derived peptide on the viscoelasticity of hydrogel/apatite nanocomposite scaffolds. Biopolymers. 85:370–378.
- Shakoori Z, Salimian S, Kharrazi S, Adabi M, Saber R. 2015. Electrochemical DNA biosensor based on gold nanorods for detecting hepatitis B virus. Analyt Bioanalyt Chem. 407:455–461.
- Shi J, Xu B, Sun X, Ma C, Yu C, Zhang H. 2013. Light induced toxicity reduction of silver nanoparticles to Tetrahymena Pyriformis: effect of particle size. Aquat Toxicol. 132–133:53–60.
- Singh S, Shi T, Duffin R, Albrecht C, van Berlo D, Höhr D, et al. 2007. Endocytosis, oxidative stress and IL-8 expression in human lung epithelial cells upon treatment with fine and ultrafine TiO2: role of the specific surface area and of surface methylation of the particles. Toxicol Appl Pharm. 222:141–151.
- Suh WH, Suslick KS, Stucky GD, Suh Y-H. 2009. Nanotechnology, nanotoxicology, and neuroscience. Prog Neurobiol. 87:133–170.
- Sun J, Dai Z, Zhao Y, Chen G-Q. 2007. In vitro effect of oligo-hydroxyalkanoates on the growth of mouse fibroblast cell line L929. Biomaterials. 28:3896–3903.
- Sun L, Li Y, Liu X, Jin M, Zhang L, Du Z, et al. 2011. Cytotoxicity and mitochondrial damage caused by silica nanoparticles. Toxicol In Vitro. 25:1619–1629.
- Tavakol S, Nikpour MR, Hoveizi E, Tavakol B, Rezayat SM, Adabi M, Abokheili SS, Jahanshahi M. 2014. Investigating the effects of particle size and chemical structure on cytotoxicity and bacteriostatic potential of nano hydroxyapatite/chitosan/silica and nano hydroxyapatite/chitosan/silver; as antibacterial bone substitutes. J Nanopart Res. 16:2622–2635.
- Thi Ha Lien N, Thi Tuyen N, Emmanuel F, Thanh Phuong N, Thi My Nhung H, Thi Quy N, Hong Nhung T. 2012. Capping and in vivo toxicity studies of gold nanoparticles. Adv Nat Sci Nanosci Nanotechnol. 3:015002–015007.
- Tiwari DK, Jin T, Behari J. 2011. Dose-dependent in vivo toxicity assessment of silver nanoparticle in Wistar rats. Toxicol Mech Methods. 21:13–24.
- Tiwari SB, Amiji MM. 2006. A review of nanocarrier-based CNS delivery systems. Curr Drug Deliv. 3:219–232.
- Triguero D, Buciak J, Pardridge WM. 1990. Capillary depletion method for quantification of blood-brain barrier transport of circulating peptides and plasma proteins. J Neurochem. 54:1882–1888.
- Tröster SD, Müller U, Kreuter J. 1990. Modification of the body distribution of poly (methyl methacrylate) nanoparticles in rats by coating with surfactants. Int J Pharm. 61:85–100.
- Tsai C-C, Chang Y, Sung H-W, Hsu J-C, Chen C-N. 2001. Effects of heparin immobilization on the surface characteristics of a biological tissue fixed with a naturally occurring crosslinking agent (genipin): an in vitro study. Biomaterials. 22:523–533.
- Tyrrell DA, Richardson VJ, Ryman BE. 1977. The effect of serum protein fractions on liposome-cell interactions in cultured cells and the perfused rat liver. Biochim Biophys Acta. 497:469–480.
- Wang S, Lu W, Tovmachenko O, Rai US, Yu H, Ray PC. 2008. Challenge in understanding size and shape dependent toxicity of gold nanomaterials in human skin keratinocytes. Chem Phys Lett. 463:145–149.
- Wigfield C, Robertson J, Gill S, Nelson R. 2003. Clinical experience with porous tantalum cervical interbody implants in a prospective randomized controlled trial. Br J Neurosurg. 17:418–425.
- Williams D. 1989. A model for biocompatibility and its evaluation. J Biomed Eng. 11:185–191.
- Wolburg H, Lippoldt A. 2002. Tight junctions of the blood-brain barrier: development, composition and regulation. Vasc Pharmacol. 38:323–337.
- Wu D, Pardridge WM. 1998. Pharmacokinetics and blood-brain barrier transport of an anti-transferrin receptor monoclonal antibody (OX26) in rats after chronic treatment with the antibody. Drug Metab Dispos. 26:937–939.
- Yan A, Lau BW, Weissman BS, Külaots I, Yang NYC, Kane AB, Hurt RH. 2006. Biocompatible, hydrophilic, supramolecular carbon nanoparticles for cell delivery. Adv Mater. 18:2373–2378.
- Yang H, Liu C, Yang D, Zhang H, Xi Z. 2009. Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: the role of particle size, shape and composition. J Appl Toxicol. 29:69–78.
- Yang Y, Leong KW. 2010. Nanoscale surfacing for regenerative medicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2:478–495.
- Zhang X-D, Wu H-Y, Wu D, Wang Y-Y, Chang J-H, Zhai Z-B, et al. 2010. Toxicologic effects of gold nanoparticles in vivo by different administration routes. Int J Nanomedicine. 5:771–781.
- Zhu Z-J, Carboni R, Quercio MJ, Yan B, Miranda OR, Anderton DL, et al. 2010. Surface properties dictate uptake, distribution, excretion, and toxicity of nanoparticles in fish. Small. 6:2261–2265.
- Zolnik BS, González-Fernández Á, Sadrieh N, Dobrovolskaia MA. 2010. Minireview: nanoparticles and the immune system. Endocrinology. 151:458–465.