Abstract
To take advantages of single-walled carbon nanotubes (SWNTs) for cellular delivery of chemotherapeutic agents (e.g. doxorubicin) in order to decrease general toxicities of doxorubicin (DOX) and to promote the efficacy, we aimed to develop a novel approach to stabilize SWNTs through consequent steps of oxidation and PEG-g-PEI polyionic complexation (PEG-PEI-SWNT). The DOX loading capacity of modified SWNTs was about 900%. Moreover, it showed an enhanced dispersibility in physiologic-stimulated medium. DOX release was prolonged, independent of dilution, and exhibited an acidic pH-stimulated release. Therefore, PEG-PEI-SWNT could be used for cancer chemotherapy in vivo.
Introduction
Although most of the existing anticancer drugs are very potent molecules, their efficacy is constrained not only by their systemic toxicity and narrow therapeutic window but also because of drug resistance and inappropriate pharmacokinetic and cellular entry. That is why the development of efficient delivery systems with the ability to improve bio-distribution and to enhance cellular uptake of existing potent drugs is required (Ali-Boucetta et al. Citation2008). One innovative technological approach to solve this problem is nanotechnology which focuses on the facilitated transfer of nano-sized devices into the target tissues/cells (Klingeler et al. Citation2008). An ideal drug delivery system should exert controlled cargo release (i.e. release triggered by a characteristic feature of the diseased cells) so that minimizes healthy cells injury (Zhang et al. Citation2009). By incorporation of drugs into a suitable carrier, the therapeutic efficacy of the drugs can be improved and general toxicity and overall side effects can be minimized (Liang and Chen Citation2010).
Among the numerous delivery systems currently under investigations, carbon nanotubes (CNTs) have shown great promise as novel delivery systems and theranostics for personalized medicine. Pristine CNTs are made up of carbon atoms arranged in a series of condensed benzene rings and wrapped into a tubular form (Pastorin Citation2009). Single-walled carbon nanotubes (SWNTs), a kind of these novel polyaromatic molecules, have attracted considerable interest as promising carriers for drug delivery. They offer potential advantages, including ultrahigh surface areas, high mechanical strength and structural flexibility, rich electronic properties, and excellent chemical and thermal stability (Liu et al. Citation2009). In the cancer arena, SWNTs have already been investigated as potential delivery vehicles for intracellular transport of pharmacologic agents, diagnostic imaging agents, oligonucleotides, and proteins to detect or treat cancer cells (Gannon et al. Citation2007). SWNTs could traffic in cells through different cellular barriers, and at the same time carry a significant payload of the agent to be delivered (Liang and Chen Citation2010). Also, the intrinsic spectroscopic properties of nanotubes, including Raman and photoluminescence, provide additional advantages for tracking, detecting, and imaging to understand drug delivery efficacy in vivo (Liang and Chen Citation2010). Moreover, they can alter the mechanisms of chemo-resistance in malignant cells (Mahmood et al. Citation2009).
CNTs have highly hydrophobic surfaces, and are not soluble in aqueous solutions. For biomedical applications, surface chemistry or functionalization is required to disperse CNTs and to render biocompatibility (Farvadi et al. Citation2014). Using surfactants to improve nanotube dispersion can be problematic since the surfactant remains in the resulting nanocomposite that could result in allergic reactions. Furthermore, CNTs modified by surfactants are generally unstable in the presence of salt so that aggregate in biological conditions (Moniruzzaman and Winey Citation2006). Polymers such as poly(vinyl alcohol) (Yang et al. Citation2015), poly(vinyl pyrrolidone) (Yang and Nakashima Citation2015), poly(methyl methacrylate) (Mammeri et al. Citation2014), poly(dimethylsiloxane) (Huang et al. Citation2006), poly ethylene glycol (PEG) (Sacchetti et al. Citation2014), and some natural polymers have been used to render CNTs dispersible in water or organic solvents. Most of these methods depend on the physical adsorption of polymers to the surface of the nanotubes (Zhao et al. Citation2004).
Functionalization of CNTs with PEG moieties (also termed as PEGylation) has been widely used to improve the aqueous solubility of CNT conjugates and their stability in biological environments, as well as the biocompatibility of the moieties. In addition to solubilization, PEGylation is one of the most effective methods to prolong the blood-circulation time due to the known “stealth” properties of PEG and to overcome the phagocytic activity of the reticuloendothelial system (Kotagiri and Kim Citation2014). Moreover, PEGylation provides a platform for further modifications such as conjugating specific ligands toward biologic targets.
Polyethyleneimine (PEI) is a cationic water-soluble polymer available in a broad range of molecular weights ranging from 600 to 60 000 Da. Commercial branched PEI contains primary, secondary, and tertiary amino groups in a ratio of approximately 1/4, 1/2, and 1/4, respectively (Kunath et al. Citation2003). The average pKa value of the polymer is between 8.5 and 9, which is influenced by molecular weight and degree of branching (Petersen et al. Citation2002). Despite widespread biological applications of PEI, they demonstrate some safety problems to different extents depending on their molecular weight, charge density, structure, and conformational flexibility (von Harpe et al. Citation2000). Previous studies have shown that PEGylation of PEI could be significant in increasing their dispersion at high concentrations, minimizing aggregation of particulates, reducing interaction of polycation complexes with plasma protein components and erythrocyte aggregation, prolonged blood circulation and reduced systemic toxicity (Najafi et al. Citation2015, Tang et al. Citation2003). Therefore, we aimed to establish a new approach to disperse and stabilize SWNTs for drug delivery application by means of PEG-grafted hyperbranched polyethylenimine (PEG-g-PEI) as a hydrophilic biocompatible block copolymer.
Materials and methods
Materials
SWNTs (purity >90%, mean diameter ∼1–2 nm, length ∼1–3 μm, SSA 380 m2/g) was purchase from Neutrino (Tehran, Iran); methoxy polyethylene glycol 2 kDa (mPEG 2000-COOH) was purchased from Jenkem Technology USA (Allen, TX); hyperbranched polyethyleneimine 25 kDa and doxorubicin hydrochloride were obtained from Sigma-Aldrich (St. Louis, MO) and Pharmacia (Milan, Italy), respectively. Dichloromethane (DCM), dimethylsulfoxide, triethylamine, and potassium bromide (KBr) were obtained from Merck (Darmstadt, Germany).
SWNT surface modification
Surface of SWNTs was modified in two steps: oxidative acid treatment and polyionic complexation with PEG-g-PEI.
Oxidative acid treatment of SWNTs
Shortening, purification, and water dispersion of the SWNTs were carried out via oxidative acid treatment as previously described (Osorio et al. Citation2008) with some modifications. In brief, SWNTs were immersed in a 3:1 (v/v) mixture of H2SO4 (96%) and HNO3 (65%), then put in an ultrasound bath (45 °C, 2 h) and upheld for further 15 h at room temperature. Subsequently, the SWNTs were separated from acid solution by centrifugation and were washed with 5% HCl solution and deionized water for five times until almost neutral pH was reached (Sun et al. Citation2008). The final product (referred to as oxSWNT) was sonicated for 1 h to yield a uniform dispersion. The density of carboxylic acid groups of the oxSWNTs was quantified performing acid-base back-titration as described by Hu et al. (Citation2001) with some modifications. Potentiometery titration showed –COOH content of oxSWNTs was ∼2.5% w/w (∼5 mmol –COOH per 1 g SWNT).
PEG-g-PEI synthesis and characterization
Polyethyleneimine-grafted PEG was synthesized by carbodiimide reaction in the presence of TEA as a proton quencher as reported before (Abolmaali et al. Citation2013). Accordingly, the required volumes of NHS-activated mPEG2000-COOH in methanol reacted with 5% w/v PEI solution in DCM at nominal ethylene oxide weight ratios (fETO) of 0.2. The reaction media were supplemented with 1% triethylamine and the required volumes of methanol to obtain the constant volume ratio of methanol/DCM of 2:1. Then, the vessels were incubated for 3 h while mixing at 25 °C. The products were concentrated using rotational speed-vacuum (Christ RVC 2–18, Germany) until a yellowish white wax was obtained. The reconstituted products in deionized water were dialyzed using Float-A-Lyzer 6–8 kDa (Spectrum, Newport News, VA) according to the manufacturer’s instruction. Then, the product solutions were lyophilized (Christ alpha 1–2 LD, Germany).
To perform gel filtration chromatography (GFC), 100 μl of the polymer solutions were injected into an HPLC system pumped the mobile phase (10 mM EDTA solution in deionized water) at the flow rate of 0.5 ml/min through TSK gel PW5000XL column. Detection was carried out by online refractive index (RI) detector previously equilibrated by the mobile phase. The elution volumes were considered for the products and compared with the pure reactants. Gel filtration chromatography confirmed the successful grafting of mPEG onto the PEI backbone.
1H-NMR (400 MHz, Bruker, Germany) was performed to calculate the degree of PEGyltion and average molecular weight of the copolymer by the proton-integration method. PEG/PEI molar ratio was calculated according to the following equation:
while б (3.5–3.6) and б (2.5–3.1) refer to –O–CH2– units of PEG and –N–CH2–CH2– units of PEI molecules, respectively (Abolmaali et al. Citation2016, Najafi et al. Citation2015).
Preparation of PEG-g-PEI stabilized SWNT aqueous dispersion
About 100 μg.ml−1 of oxSWNT were added drop-wise to the same volume of PEG-g-PEI solution to obtain the final concentration and weight ratios showed in . The mixtures were vortexed for ∼20 s and incubated at room temperature overnight. Free (unbound) PEG-g-PEI was removed by repeated ultracentrifugation. The final product (referred to as PEG-PEI-SWNT) was ultrasonicated for ∼1 min to ensure complete dispersion.
Table 1. Different conditions for preparation of stabilized oxSWNT dispersion with PEG-g-PEI copolymer.
The minimum effective concentration of PEG-g-PEI was defined as the least concentration of the polymer that could sufficiently stabilize oxSWNT against sedimentation in the presence of an increasing salt concentration. The efficiency of PEG-g-PEI on SWNT stabilization was compared with mPEG-NH2 (2 kDa) or PEI (25 kDa) at the same final weigh ratio as previously mentioned for PEG-g-PEI in .
Characterization and analysis PEG-PEI-SWNT
Thermogravimetric analysis
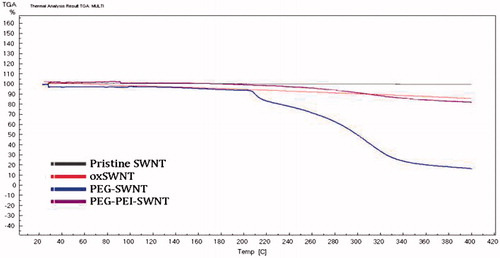
To estimate the degree of surface modification of SWNT, thermogravimetric analysis (TGA) (TGA-50H, SHIMADZU, Japan) was performed for the lyophilized powder of oxSWNT, PEG-SWNT (100:1 PEG:oxSWNT weight ratio), and PEG-PEI-SWNT (10:1 PEI:oxSWNT weight ratio) at a rate of 5 °C.min−1 under nitrogen flow.
FT-IR spectroscopy
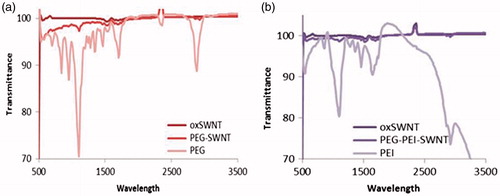
ATR-FTIR spectra were recorded on Bruker (Vertex70, Germany). The experiment was carried out for lyophilized powder of oxSWNT, PEG-SWNT (100:1 PEG:oxSWNT weight ratio), and PEG-PEI-SWNT (10:1 PEI:oxSWNT weight ratio) in comparison with mPEG-NH2 and PEG-g-PEI copolymer. Thirty-two scans were signal averaged with a resolution of 4 cm−1 in the range of 500–4000 cm−1.
Atomic forced microscopy
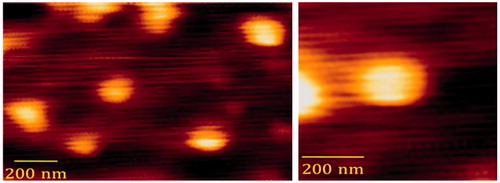
Atomic force microscopy (AFM) was performed to study the shape and size of the PEG-PEI-SWNT. The samples were transferred onto a prewashed glass slide and allowed to immobilize in ambient condition. AFM experiment was performed using Nanowizard II instrument (JPK, Berlin, Germany). Commercial silicon chip containing a pyramidal tip (Micromash, Estonia) attached to rectangular cantilever with a length of 460 μ, width of 50 μ, thickness of 1.5–2.5 μ, resonance frequency of 12 kHz, and a nominal force constant of 0.15 N/m was used. Images were recorded in contact mode at a scan speed of 1 Hz and the resolution of 256 × 256 pixels (Abolmaali et al. Citation2014).
Doxorubicin loading onto the modified nanotubes
Surface-modified SWNT dispersions were incubated with doxorubicin (DOX) hydrochloride at different pH and concentrations at 4 °C overnight while shaking vigorously (). To characterize DOX loading onto SWNT, fluorescence emission spectra of the samples were depicted by fluorimetry (λex = 495) before the removal of free DOX from the samples. Unbound free DOX was removed by centrifugation and the sediment washed thoroughly with deionized water. The amount of DOX loaded onto SWNTs was estimated by measuring the absorbance of the supernatant (free DOX) at the wavelength of maximum absorbance (λmax= 490 nm), and subtracting the corresponding DOX concentration from the initial concentration.
Table 3. Comparison of salt stability of SWNTs modified by various methods.
Loading efficiency and capacity were calculated according to following equations:
Effect of pH on doxorubicin loading parameters
To monitor the effect of pH on DOX loading, the modified SWNTs were redispersed at respective concentration of 50 μg.ml−1 SWNT in 20 mM phosphate buffer solution at different pH values of 5.5, 7.4, and 8.5 by ultrasonication for 1 min. DOX added to each sample at the final concentration of 200 μg.ml−1. The samples were incubated at 4 °C overnight while shaking.
Effect of doxorubicin concentration on loading parameters
The modified SWNTs were redispersed in phosphate buffer solution (pH 8). Various concentrations () of DOX were mixed with the modified SWNTs at a nanotube concentration ∼50 μg.ml−1. The samples were incubated at 4 °C overnight while shaking.
Table 2. Different concentrations of doxorubicin-HCl used for loading onto the modified SWNTs.
Doxorubicin release from the loaded nanotubes
Following removal of free DOX, the sediments (referred to as DOX-SWNT) were resuspended in PBS pH 7.4 (plasma-simulating medium) or pH 5.5 (medium-simulating acidic milieu of tumor tissue and late endosomal micro-environments) and incubated at 37 °C while shaking at 500 rpm. After predetermined time intervals, SWNTs were separated from the buffer by centrifugation. The concentration of released DOX in the supernatant was estimated by UV–Vis–NIR spectroscopy (absorbance in λ = 490 nm).
Since DOX water solubility is 10 mg.ml−1, sink condition was met in the release study; however, it was hypothesized that free DOX in equilibrium may influence the release behavior, therefore, the effect of dilution on release of DOX from SWNT was studied in acidic conditions (pH 5.5) by UV–Vis–NIR spectroscopy of the supernatant following the serial dilution of DOX-SWNT preparations.
Results
Effect of PEG-g-PEI concentration and order of mixing on oxSWNT dispersion
Comparison of the effect of different concentrations of polymer (i.e. PEG-g-PEI or mPEG-NH2) on oxSWNT stabilization showed while 5 mg.ml−1 (respective to weight ratio of 100:1 of PEG:oxSWNT) was the most effective concentration of mPEG-NH2, 0.5 mg.ml−1 PEG-g-PEI (respective to weight ratio of 10:1 PEG-g-PEI: oxSWNT) showed a sufficient dispersion and stability ().
Interestingly the order of mixing was found as an important factor influencing in PEG-g-PEI interaction with SWNTs; SWNT agglomerates were formed upon adding PEG-g-PEI solution to the oxSWNT dispersions, while no aggregation was observable in the case of addition of oxSWNT to PEG-g-PEI solution.
Characterization of PEG-g-PEI stabilized oxSWNT
The TGA analysis () showed the carboxyl content of oxSWNT to be 2.5% w/w (similar to the potentiometric titration result). The polymer content in modified oxSWNTs was estimated to be 20% of the carrier weight for the PEG-g-PEI copolymer (respective to SWNT:PEI 1:0.25 weight ratio) and 90% for the mPEG-NH2 (respective to SWNT:PEG 1:9 weight ratio).
ATR-FTIR spectra are shown in . The comparison of stretching amine bands of PEI and PEG-NH2 prior to and after complexation with oxSWNT shows that the corresponding band shifted to a higher frequency upon complex formation with oxSWNT.
The AFM images of PEG-PEI-SWNTs suggest that polymer modified SWNTs might transform from linear to semispheric posture () with the average diameter of ∼156 nm.
Doxorubicin loading onto the modified nanotubes
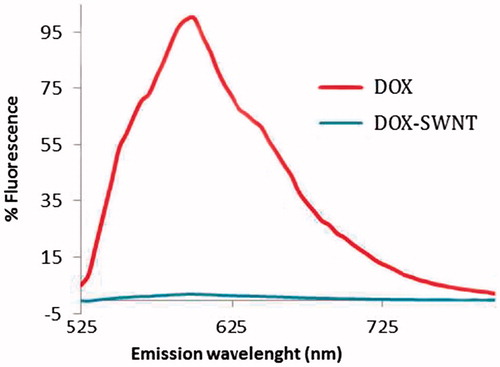
After overnight incubation of the modified SWNTs with DOX, complex formation was proved by fluorescence quenching of loaded DOX compared with free DOX solution at the same concentration ().
Figure 4. Fluorescence quenching of doxorubicin after loading on 50 μg.ml−1 SWNT at pH 8 (doxorubicin concentration is 250 μg.ml−1 in both samples). Significant fluorescence quenching was evident for doxorubicin bound to SWNT.
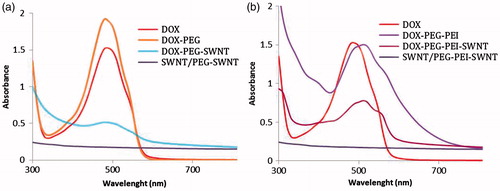
According to , the absorbance peak of bound DOX was quenched to some extent and shifted from 490 to ∼510 nm; the characteristic of complex formation between DOX and PEG-g-PEI not seen in PEG-SWNTs (note that unbound DOX was removed thoroughly to retain only the DOX-SWNT complexes).
Figure 5. UV–Vis–NIR spectra of doxorubicin containing samples in deionized water, SWNT concentration: 50 μg.ml−1, doxorubicin concentration: 250 μg.ml − 1.
The amount of doxorubicin loaded on SWNTs was pH-dependent (). Loading factor decreased from 100 to ∼19.5 to ∼11 as pH was reduced from 8.5 to 7.4 and 5.5, respectively. shows that the loading capacity increased as the DOX concentration increased in a saturable pattern. The loading capacity was much lower for PEG-SWNTs than PEG-PEI-SWNTs.
Figure 6. (a) pH effect on loading factor of doxorubicin on SWNT (error bars represent mean ± SD); (b) loading capacity vs. weight ratio of doxorubicin to the modified oxSWNT. The concentration of oxSWNT was kept constant at 50 μg.ml−1.
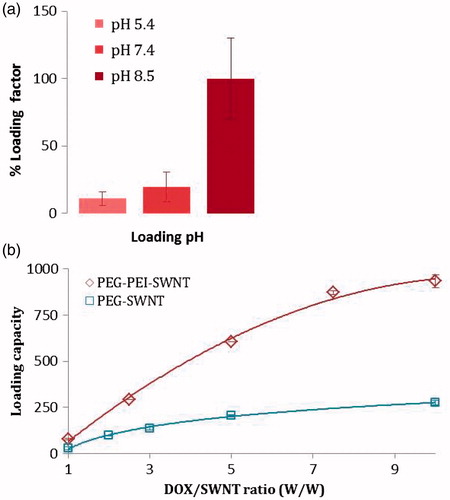
Based on optical absorbance data of DOX and SWNTs, loading efficiency was estimated to be ∼48% and ∼97% and loading capacity to be ∼276% and ∼933% for PEG-SWNT and PEG-PEI-SWNT, respectively (DOX/SWNT weight ratio = 5). summarizes the loading parameters for different modified SWNTs. The optimum DOX/SWNT weight ratio for maximum loading efficiency and capacity to be obtained, estimated to be 5 and 8 for mPEG-NH2- and PEG-g-PEI-modified SWNTs, respectively, to , the proportion of free to total DOX decreased as the DOX:SWNT ratio increased from 1 to 5 that indicating loading increased. A slight increase in fluorescence of the DOX:SWNT ratio 10 (compared to 5) showed the slight increase in free to total DOX fraction, which in turn indicated fulfilling of SWNT payload capacity for DOX loading.
Figure 7. Fluorescence spectra of free doxorubicin- (D1–D10) and doxorubicin-loaded PEG-PEI-SWNT samples (S1–S10) at doxorubicin/SWNT ratio of 1–10 (excitation at 490 nm and emission at 600 nm). Each sample spectrum was normalized by that of free doxorubicin at the corresponding concentration. Note that free DOX was not removed. S1–10: DOX:SWNT weight ratio 1–10, D1–10: equivalent concentration of doxorubicin without SWNT (SWNT concentration is 50 μg.ml−1 in all samples).
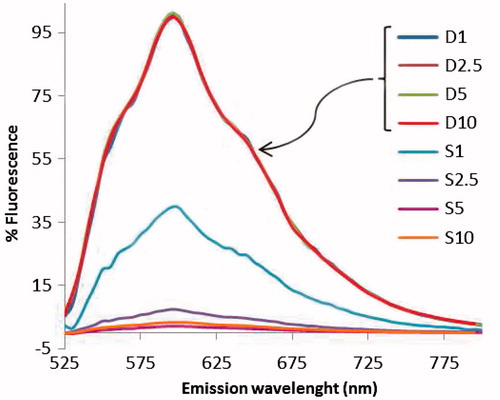
Table 4. DOX-loading parameters for oxSWNT modified by PEG-g-PEI and mPEG-NH2.
Regarding the range of free DOX in all DOX:SWNT ratios that was ∼32–35 μg.ml−1, it was deduced that bound DOX was in equilibrium with unbound drug since even in the lowest ratio (i.e. ratio 1) the amount of unbound DOX was almost the same as higher ratios.
DOX release from loaded nanotubes
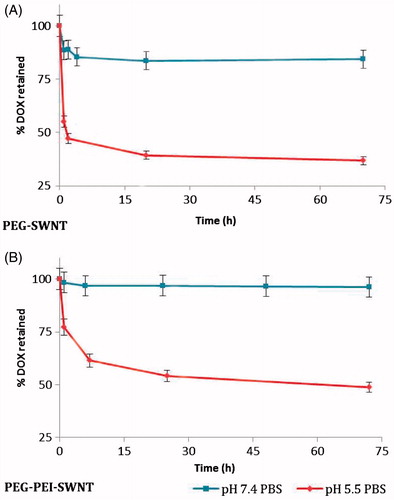
The release of the DOX loaded on the modified SWNTs was pH-stimulated, triggered at acidic conditions (). At pH = 7.4, a burst release was observed for SWNT modified by PEG, but it was absent for the SWNT samples modified with PEG-g-PEI and sustained release pattern was observed. As the systems were put in a relatively acidic condition (pH = 5.5), they replied to the stimuli and released about half of the drug content.
Figure 8. The release profile of doxorubicin loaded on modified SWNTs in isotonic phosphate buffer solutions at 37 °C.
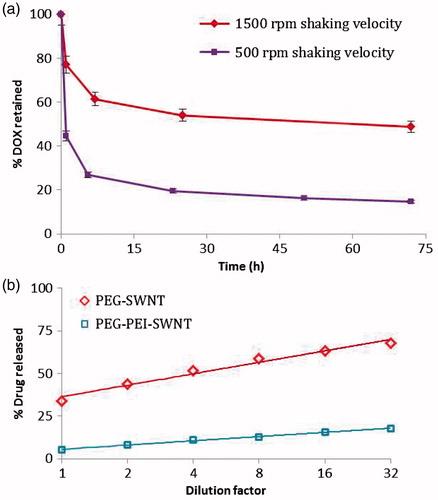
Interestingly, the pattern of DOX loading could alter release profile; the release behavior of DOX loaded onto modified SWNT at 1500 rpm was more sustained than that at 500 rpm although the loading was higher at 1500 rpm (). Moreover, DOX release pattern depended strongly on the volume or dilution factor of release medium. This phenomenon was more pronounced for PEG-stabilized SWNTs while SWNT stabilization via PEG-g-PEI interaction could attenuate this effect to a considerable extent ().
Figure 9. (a) Effect of shaking velocity in the process of doxorubicin loading on the release profile from PEG-PEI-SWNT in saline supplemented phosphate buffer solution pH 5.5 at 37 °C. (b) Dilution effect on doxorubicin release from the modified SWNT in saline supplemented phosphate buffer solution pH 5.5 at 37 °C. The percentages of drug release were plotted vs. dilution factors in a logarithmic scale.
Discussion
Surface modification of oxSWNT with PEG-g-PEI
Oxidative acid treatment results in shortened tube ends and produces abundant carboxylated groups along the nanotubes surface which can provide water dispersion as well as sites for further modifications (Lin et al. Citation2004). Although oxSWNTs were water dispersible, they aggregated in the presence of salts due to charge screening effects as reported previously (Liu et al. Citation2009). Similarly, they are not stable under physiologic conditions because of high salt content of biological media and so may not be directly used for biomedical applications; therefore, it is necessary to stabilize them by hydrophilic shells. Although using polymers such as polyethylene glycol (PEG) has been reported previously (Chattopadhyay et al. Citation2006, Lee et al. Citation2007, Ren et al. Citation2012), but there is no report on using PEI or PEG-g-PEI for SWNT stabilization.
PEG-PEI-SWNTs showed more stability even with lower concentrations of polymer in comparison with PEG-SWNTs, suggesting strong and stable non-covalent adsorption of the PEG-g-PEI molecules on SWNT sidewalls. Interestingly, SWNT weight ratio was found to be key factors in oxSWNT interaction with PEG-g-PEI copolymer. At low concentrations (≤ 200 μg.ml-1 or PEI:SWNT weight ratio ≤4) (or when PEG-g-PEI copolymer solution was added to oxSWNT dispersion), PEG-g-PEI could not sufficiently cover the surface of SWNT and hence cross-linking or “bridging flocculation” may occur, in other words segments of an individual PEG-g-PEI polymer may complex with more than one nanotube so causing SWNTs to agglomerate. PEG-g-PEI-modified SWNTs maintained their dispersion much better in acidic conditions (i.e. pH 5.5 is more than pKa of carboxyl but less than that of the amine group). The pH-dependent re-dispersibility of PEG-g-PEI-modified SWNTs was presumably due to proton absorption of PEI chains forming more quaternary cation to simultaneously interact with SWNTs and solubilize them.
Dilution had no effect on SWNTs modified with either mPEG-NH2 or PEG-g-PEI. Since stabilization of SWNT by mPEG-NH2 or PEG-g-PEI takes place through complex formation, there is almost a strong interaction between polymer and SWNT so that dilution could not influence the intermolecular bond and hence the stabilization effect of the polymer. In contrast, the interaction between pristine SWNT and amphipathic polymers such as PL-PEG takes place trough hydrophobic interaction and could be impacted by micelle concentration and that is why dilution could influence the stabilizing effect of PL-PEG micelle (Liu et al. Citation2009).
Doxorubicin loading and release behavior from the modified SWNTs
Considering pH-dependency of loading (), the mechanism of loading seems to be by deprotonation of the amine group of DOX molecule in alkaline pH (pKa 8.3) that leading to reduce aqueous solubility and settling via hydrophobic interactions onto the SWNT surface which poses polyaromatic structure; Therefore, SWNT can get involved in π–π stacking with DOX molecules. Similarly, regarding the pH-stimulating behavior of the release profile, the release mechanism is probably via formation of positive DOX molecules due to protonation of the amine group and therefore enhanced aqueous solubility and detachment from the carrier surface.
DOX exhibits a unique fluorescence emission spectrum that attenuates upon binding to SWNTs (). Ali-Boucetta et al. (Citation2008) explain the decrease in fluorescence intensity by the static quenching of doxorubicin molecules due to the π–π stacking of its aromatic chromophore groups and the CNT backbone. Interaction with CNT results in changes in its molecular conformation leading to loss in fluorescence signal. This high degree of fluorescence quenching is an evidence of π-stacked DOX (Liu et al. Citation2010). Moreover, this property could be considered for biosensing and reporting applications.
Shaking velocity during the loading process impressed the loading pattern and therefore the release profile of DOX on PEG-PEI-SWNT. It is believed that DOX molecules could bypass the polymeric hyperbranched coat and settle down primarily on SWNT surface at higher shaking velocities while this outer coat avoided DOX to have an easy access to SWNT surface and DOX loading may primarily happen through complexation with PEG-g-PEI coat at lower velocities; hence, DOX could release more readily in acidic environments.
PEG-PEI-SWNTs show more loading capacity of DOX (∼933%) and favored release profile compared with PEG-SWNTs. Improved loading efficiency and capacity seen in PEG-g-PEI copolymer-stabilized SWNTs are presumably due to complex formation between DOX and PEG-g-PEI copolymer as it was confirmed by DOX UV–Vis absorbance shift from 490 to ∼510 nm. In addition, more PEG amounts around SWNTs (∼36 times (w/w) PEI) impede DOX to set down to SWNT surface via π–π stacking leading to low loading capacity for PEG-SWNTs.
Conclusion
A new approach was developed to modify and stabilize SWNTs efficiently without necessity of any catalyst such as EDC, which results in SWNT aggregation or harsh conditions such as long time exposure to high temperature or ultrasonication, which may destroy homing biomolecule ligands attached to nanotubes. Additionally, we obtained an ultra-high loading capacity (∼900%) significantly superior to other drug carriers, including dendrimers, liposomes, and micelles and even to those previously reported for SWNTs (120% (Zhang et al. Citation2009) and 400% (Ali-Boucetta et al. Citation2008, Liu et al. Citation2010). Moreover, the release profile was improved and more sustained in comparison with PEG-modified SWNTs that showed more burst release. Taking into consideration, the above-mentioned properties of the modified SWNT, and the pH-dependency of drug loading and release profile due to protonation and deprotonation of doxorubicin, suggest SWNTs as a successful delivery system that should be studied in vivo.
Funding information
This study was funded by SUMS grant.
Acknowledgements
The authors gratefully acknowledge use of the facilities of the Center for Nanotechnology in Drug Delivery at Shiraz University of Medical Sciences.
Disclosure statement
The authors declare no competing financial interests.
References
- Abolmaali SS, Tamaddon AM, Dinarvand R. 2013. Nano-hydrogels of methoxy polyethylene glycol-grafted branched polyethyleneimine via biodegradable cross-linking of Zn2+-ionomer micelle template. J Nanopart Res. 15:1–21.
- Abolmaali SS, Tamaddon AM, Mohammadi S, Amoozgar Z, Dinarvand R. 2014. Sequential optimization of methotrexate encapsulation in micellar nano-networks of polyethyleneimine ionomer containing redox-sensitive cross-links. Int J Nanomedicine. 9:2833–2848.
- Abolmaali SS, Tamaddon AM, Mohammadi S, Amoozgar Z, Dinarvand R. 2016. Chemically crosslinked nanogels of PEGylated poly ethyleneimine (l-histidine substituted) synthesized via metal ion coordinated self-assembly for delivery of methotrexate: cytocompatibility, cellular delivery and antitumor activity in resistant cells. Mater Sci Eng C Mater Biol Appl. 62:897–907.
- Ali-Boucetta H, Al-Jamal KT, McCarthy D, Prato M, Bianco A, Kostarelos K. 2008. Multiwalled carbon nanotube-doxorubicin supramolecular complexes for cancer therapeutics. Chem Commun. 4:459–461.
- Chattopadhyay J, de Jesus Cortez F, Chakraborty S, Slater NKH, Billups WE. 2006. Synthesis of water-soluble PEGylated single-walled carbon nanotubes. Chem Mater. 18:5864–5868.
- Farvadi F, Tamaddon AM, Abolmaali SS, Sobhani Z, Yousefi GH. 2014. Micellar stabilized single-walled carbon nanotubes for a pH-sensitive delivery of doxorubicin. Res Pharm Sci. 9:1–10.
- Gannon CJ, Cherukuri P, Yakobson BI, Cognet L, Kanzius JS, Kittrell C, et al. 2007. Carbon nanotube-enhanced thermal destruction of cancer cells in a noninvasive radiofrequency field. Cancer. 110:2654–2665.
- Hu H, Bhowmik P, Zhao B, Hamon MA, Itkis ME, Haddon RC. 2001. Determination of the acidic sites of purified single-walled carbon nanotubes by acid–base titration. Chem Phys Lett. 345:25–28.
- Huang YY, Ahir SV, Terentjev EM. 2006. Dispersion rheology of carbon nanotubes in a polymer matrix. Phys Rev B. 73:125422. doi: 10.1103/PhysRevB.73.125422.
- Klingeler R, Hampel S, Buchner B. 2008. Carbon nanotube based biomedical agents for heating, temperature sensoring and drug delivery. Int J Hyperthermia. 24:496–505.
- Kotagiri N, Kim JW. 2014. Stealth nanotubes: strategies of shielding carbon nanotubes to evade opsonization and improve biodistribution. Int J Nanomedicine. 9 Suppl 1:85–105.
- Kunath K, von Harpe A, Fischer D, Petersen H, Bickel U, Voigt K, Kissel T. 2003. Low-molecular-weight polyethylenimine as a non-viral vector for DNA delivery: comparison of physicochemical properties, transfection efficiency and in vivo distribution with high-molecular-weight polyethylenimine. J Control Release. 89:113–125.
- Lee JU, Huh J, Kim KH, Park C, Jo WH. 2007. Aqueous suspension of carbon nanotubes via non-covalent functionalization with oligothiophene-terminated poly(ethylene glycol). Carbon. 45:1051–1057.
- Liang F, Chen B. 2010. A review on biomedical applications of single-walled carbon nanotubes. Curr Med Chem. 17:10–24.
- Lin Y, Taylor S, Li H, Fernando KAS, Qu L, Wang W, et al. 2004. Advances toward bioapplications of carbon nanotubes. J Mater Chem. 14:527–541.
- Liu Z, Tabakman S, Welsher K, Dai H. 2009. Carbon nanotubes in biology and medicine: in vitro and in vivo detection, imaging and drug delivery. Nano Res. 2:85–120.
- Liu Z, Sun X, Nakayama-Ratchford N, Dai H. 2010. Supramolecular chemistry on water-soluble carbon nanotubes for drug loading and delivery. ACS Nano. 4:7726.
- Mahmood M, Karmakar A, Fejleh A, Mocan T, Iancu C, Mocan L, et al. 2009. Synergistic enhancement of cancer therapy using a combination of carbon nanotubes and anti-tumor drug. Nanomedicine (Lond). 4:883–893.
- Mammeri F, Teyssandier J, Darche-Dugaret C, Debacker S, Le Bourhis E, Chehimi MM. 2014. Carbon nanotube–poly(methyl methacrylate) hybrid films: preparation using diazonium salt chemistry and mechanical properties. J Colloid Interface Sci. 433:115–122.
- Moniruzzaman M, Winey KI. 2006. Polymer nanocomposites containing carbon nanotubes. Macromolecules. 39:5194–5205.
- Najafi H, Abolmaali SS, Owrangi B, Ghasemi Y, Tamaddon AM. 2015. Serum resistant and enhanced transfection of plasmid DNA by PEG-stabilized polyplex nanoparticles of L-histidine substituted polyethyleneimine. Macromol Res. 23:618–627.
- Osorio AG, Silveira ICL, Bueno VL, Bergmann CP. 2008. H2SO4/HNO3/HCl-Functionalization and its effect on dispersion of carbon nanotubes in aqueous media. App Surf Sci. 255:2485–2489.
- Pastorin G. 2009. Crucial functionalizations of carbon nanotubes for improved drug delivery: a valuable option? Pharm Res. 26:746–769.
- Petersen H, Fechner PM, Martin AL, Kunath K, Stolnik S, Roberts CJ, et al. 2002. Polyethylenimine-graft-poly(ethylene glycol) copolymers: influence of copolymer block structure on DNA complexation and biological activities as gene delivery system. Bioconjug Chem. 13:845–854.
- Ren J, Shen S, Wang D, Xi Z, Guo L, Pang Z, et al. 2012. The targeted delivery of anticancer drugs to brain glioma by PEGylated oxidized multi-walled carbon nanotubes modified with angiopep-2. Biomaterials. 33:3324–3333.
- Sacchetti C, Liu-Bryan R, Magrini A, Rosato N, Bottini N, Bottini M. 2014. Polyethylene-glycol-modified single-walled carbon nanotubes for intra-articular delivery to chondrocytes. ACS Nano. 8:12280–12291.
- Sun X, Liu Z, Welsher K, Robinson JT, Goodwin A, Zaric S, Dai H. 2008. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 1:203–212.
- Tang GP, Zeng JM, Gao SJ, Ma YX, Shi L, Li Y, Too H-P, Wang S. 2003. Polyethylene glycol modified polyethylenimine for improved CNS gene transfer: effects of PEGylation extent. Biomaterials. 24:2351–2362.
- von Harpe A, Petersen H, Li Y, Kissel T. 2000. Characterization of commercially available and synthesized polyethylenimines for gene delivery. J Control Release. 69:309–322.
- Yang Z, Xu D, Liu J, Liu J, Li L, Zhang L, Lv J. 2015. Fabrication and characterization of poly(vinyl alcohol)/carbon nanotube melt-spinning composites fiber. Prog Nat Sci: Mater Int. 25:437–444.
- Yang Z, Nakashima N. 2015. Poly(vinylpyrrolidone)-wrapped carbon nanotube-based fuel cell electrocatalyst shows high durability and performance under non-humidified operation. J Mater Chem A. 3:23316–23322.
- Zhao B, Hu H, Haddon RC. 2004. Synthesis and properties of a water-soluble single-walled carbon nanotube–poly(m-aminobenzene sulfonic acid) graft copolymer. Adv Funct Mater. 14:71–76.
- Zhang X, Meng L, Lu Q, Fei Z, Dyson PJ. 2009. Targeted delivery and controlled release of doxorubicin to cancer cells using modified single wall carbon nanotubes. Biomaterials. 30:6041–6047.