Abstract
Flavonoid compounds are strong antioxidant and antifungal agents but their applications are limited due to their poor dissolution and bioavailability. The use of nanotechnology in agriculture has received increasing attention, with the development of new formulations containing active compounds. In this study, kaempferol (KAE) was loaded into lecithin/chitosan nanoparticles (LC NPs) to determine antifungal activity compared to pure KAE against the phytopathogenic fungus Fusarium oxysporium to resolve the bioavailability problem. The influence of formulation parameters on the physicochemical properties of KAE loaded lecithin chitosan nanoparticles (KAE-LC NPs) were studied by using the electrostatic self-assembly technique. KAE-LC NPs were characterized in terms of physicochemical properties. KAE has been successfully encapsulated in LC NPs with an efficiency of 93.8 ± 4.28% and KAE-LC NPs showed good physicochemical stability. Moreover, in vitro evaluation of the KAE-LC NP system was made by the release kinetics, antioxidant and antifungal activity in a time-dependent manner against free KAE. Encapsulated KAE exhibited a significantly inhibition efficacy (67%) against Fusarium oxysporium at the end of the 60 day storage period. The results indicated that KAE-LC NP formulation could solve the problems related to the solubility and loss of KAE during use and storage. The new nanoparticle system enables the use of smaller quantities of fungicide and therefore, offers a more environmentally friendly method of controlling fungal pathogens in agriculture.
Introduction
Fungal diseases of plants have been recognized as a continuous and worldwide threat to crops. Synthetic fungicides have been used as an effective method to protect crops from fungal phytopathogens. However, chemical fungicide residues are mostly non-biodegradable and highly toxic to living organisms and affecting the environment in a negative way to all species that occupy it (Hahn Citation2014). Fusarium species are harmful and toxigenic fungi responsible of vascular diseases in a wide range of plants, including: tomato, cucumber, watermelon, cotton, and bean. Many species of this fungus are resistant to common fungicides. The sustainable supply of diverse and ever-evolving of bio-fungicides is beneficial for the environment and managing resistance problems (Satpath et al. Citation2011).
Flavonoid compounds have a significant effect in safe and natural fungicidal applications. Kaempferol (KAE) (3,4′,5,7-tetrahydroxyflavone) is a flavonoid compound present in various natural products such as grapes, red fruits (strawberry, blueberry, cranberry, and cherry), onions and citrus fruits (Wang et al. Citation2009). Although KAE has a wide range of health benefits including anti-inflammatory, anticancer and antioxidant advantages while its biological effects are limited due to low aqueous solubility (Luo et al. Citation2012). Previous studies have reported that limited water solubility and poor dissolution of flavonoids effected their chemical stability and bioavailability (Gupta et al. Citation2016). The use of nanocarriers can emerge to improve the solubility and bioavailability of KAE in a controlled manner through increased absorption and enhanced stability against free radicals during consumption and storage of food compounds (Yadav and Sawant Citation2010).
Polymeric nanoparticles have numerous advantages including improved bioavailability and efficient delivery of poor water soluble compounds (Göktürk et al. Citation2013). Lecithin, a vegetable based phospholipid, is considered a biocompatible substance and is frequently used for the preparation of various nanoparticulate delivery systems. Chitosan is potentially safe, nontoxic, biodegradable, and a biocompatible polycationic polymer (Özcan et al. Citation2013). Studies have concluded that chitosan possesses antifungal activity against plant pathogens via affinity of its cationic amino groups to cellular components (Saharan et al. Citation2013). Chitosan based nanoparticle systems are preferably used as natural antifungal agents against phytopathogens owing to their biodegradability, non-toxicity, broad antifungal activities, and cost effectiveness (Saharan et al. Citation2015). There are several studies on drug loaded lecithin/chitosan nanoparticles (Barbieri et al. Citation2013, Chadha et al. Citation2012, Şenyiğit et al. Citation2010). However, KAE loaded lecithin/chitosan nanoparticle (KAE-LC NP) systems have not yet been reported. Therefore, the aim of the present study is to determine synthesis, physicochemical characterization, and in vitro antifungal mechanisms on hyphal morphology and fungicidal activity of KAE-LC NPs.
Materials and methods
Chemicals
Chitosan (75–85% deacetylated, low molecular weight (LMW)), KAE (99% purity), d-α-Tocopherol polyethylene glycol 1000 succinate (TPGS), phosphate buffer saline (PBS) tablets (pH: 7.4), and dimethylsulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO). Lecithin derived from soybean was obtained from VWR Chemicals (Radnor, PA). Potato dextrose agar (PDA) was supplied by LABM. Other chemicals and solvents were of analytical reagent grade.
Kaempferol loaded lecithin/chitosan nanoparticles preparation
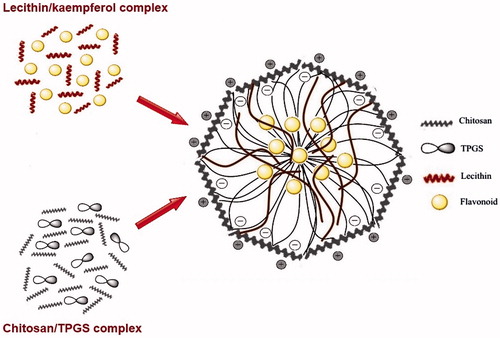
Kaempferol loaded lecithin–chitosan nanoparticles were prepared according to a modified methodology described by Sonvico et al. (Citation2006) based on the self-organizing interaction between lecithin containing KAE and chitosan. Nanoparticles were prepared as described: The LMW chitosan aqueous solution was prepared by dissolving chitosan (0.2%, w/v) in distilled water with 0.1% acetic acid (v/v). Afterwards, TPGS (0.2%, w/v) was dissolved in the chitosan solution. Lecithin (7.5%, w/v) was dissolved in the DMSO (10%, v/v) solution. Kaempferol (10, 20, 40, 60, 80, 100 μg/mL) was then added in the lecithin/DMSO solution under magnetic stirring. Chitosan/TPGS solution (11.5 mL) and lecithin/kaempferol complex were heated at 60 °C and after that, 1 mL of lecithin/KAE solution was injected by dropwise in preheated chitosan aqueous solution at 60 °C under magnetic stirring. After stirring, the pH of the nanoparticles suspension was adjusted to 4.5. The nanoparticles suspension was then, centrifuged (12,000 rpm, 20 min) and washed with water to remove the unencapsulated compounds. The empty lecithin/chitosan NPs (LC NPs) were synthesized with the method described above using lecithin solution without KAE.
Determination of average size, zeta potential, and morphology
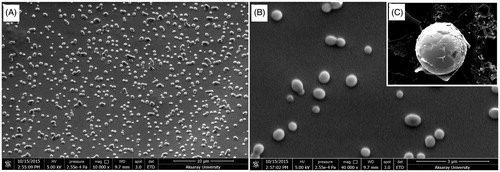
The hydrodynamic diameter (size), polydispersity index (PDI), and zeta potential (ζ-potential) of the prepared KAE-LC NPs were determined by dynamic light scattering (Zetasizer Nano ZS, Malvern Instruments, UK) at 25 °C. The nanoparticle solutions were analyzed in a folded capillary cell. All measurements were carried out in triplicate with three replications. The results were presented as mean ± standard deviation of the obtained values. In addition, Scanning Electron Microscopy (SEM) (Quanta FEG 250; FEI, North America) was used for the surface morphology of the KAE-LC NPs. Before the examination, one drop of diluted NPs suspension was mounted onto aluminum pin-type stubs (12 mm in diameter) with carbon tape. NPs were coated with gold to minimize the effect of heat during high-power magnification after the drying procedure.
Characterization of intermolecular interaction formation
The spectra of Fourier transform infrared spectroscopy (FT-IR) and 1H nuclear magnetic resonance (1H NMR) analyses were used to analyze molecular bonding formation between polymers and KAE. The FT-IR spectra were recorded using Perkin Elmer Nicolet 520 spectrophotometer (Perkin Elmer, Boston, MA). Briefly, the lyophilized samples were ground with spectroscopic grade potassium bromide (KBr) powder and then, pressed into 1 mm pellet for analysis in the range of 450–4000 cm−1 with 4 cm−1 resolution, using 16 scans. In addition, 1H NMR spectra of pure KAE and KAE-LC NPs were obtained from Bruker 2300 NMR system (Bruker, Germany). For analysis, each sample (5 mg) was dissolved into 0.8 mL DMSO-d. All samples were analyzed and recorded in triplicate.
Determination of amorphous transformation
Differential scanning calorimetry (DSC) and X-ray diffractometry (XRD) were used to determine the crystal polymorphism of pure KAE and KAE-LC NPs. The thermal transition properties of samples were recorded using Perkin Elmer DSC Q8000 calorimeter (Perkin Elmer Inc., Boston, MA). The thermal measurements were carried out (scanning rate: 10 °C/min, temperature range: 30–500 °C, under Ar purge 50 mL/min). The crystalline patterns of samples were obtained using the XRD (Panalytical, Empyrean) with Ni-filtered Cu Kα radiation. The measurement was performed at a voltage of 40 kV and 25 mA. The scanned angle was set from 2°≤2θ≤ 50°, and the scan rate was 1° min−1. All determinations were performed in triplicate.
Determination of encapsulation efficiency, loading content, and yield of production
The KAE loading and encapsulation efficiency (%) of the synthesized KAE-LC NPs were measured by UV/Vis spectrophotometry (PG Instruments, T60U UV-Visible, Lutterworth, UK) at wavelength of 373 nm. After NPs were centrifugated for 20 min at 12,000 rpm, non-entrapped KAE concentration in recovered supernatant was determined. Supernatant recovered from nanoparticles without KAE was used as a blank. Possible KAE release was taken into consideration after every washing. Encapsulation efficiency (EE%), loading content (LC%), and yield of production (YP%) were calculated by the following EquationEqs. (1)(1) , Equation(2)
(2) , and Equation(3)
(3) , respectively:
(1)
(2)
(3)
In vitro release studies of KAE-LC NPs
The in vitro release tests of KAE-LC NPs were carried out in PBS (pH: 7.4) and PBS containing DMSO (10%, v/v) release medium at 25 and 37 °C for 24 h. Dialysis bags (pore size 2.5 nm, molecular weight cut off 12,000–14,000 Da, Himedia) containing KAE-LC NPs suspension (10 mg/μL) were suspended in release media (30 mL), incubated at 25 °C and 37 °C and then, stirred at 100 rpm. At pre-determined time intervals, 3 mL of sample was withdrawn and replaced with equal volume of the corresponding fresh medium. KAE concentration was assayed by using UV/Vis spectroscopy at 373 nm.
Determination antioxidant activity
The test ferric reducing antioxidant power (FRAP) was performed to measure antioxidant activities of pure KAE and KAE-LC NPs. Initially, FRAP reagent (290 μL) was mixed with 10 μL of samples and incubated at 37 °C for 15 min. After incubation, the absorbance was determined at 593 nm using UV/Vis spectroscopy. FRAP reagent was freshly prepared by mixing (ratio 10:1:1) acetate buffer (pH 3.6, 300 mM/L), TPTZ (10 mM/L) in HCl (40 mM/L), and FeCl3 (20 mM/L). The results were expressed as micromoles equivalent Fe2+ (Fe2+ μM).
Determination of KAE-LC NPs stability
The prepared KAE-LC NPs were stored at 4 and 25 °C for 60 days in the dark. The stability tests were performed by analysis of the size, PDI, zeta potential, and FRAP reducing power activity. All analyses were carried out in triplicate.
Antifungal activity assay
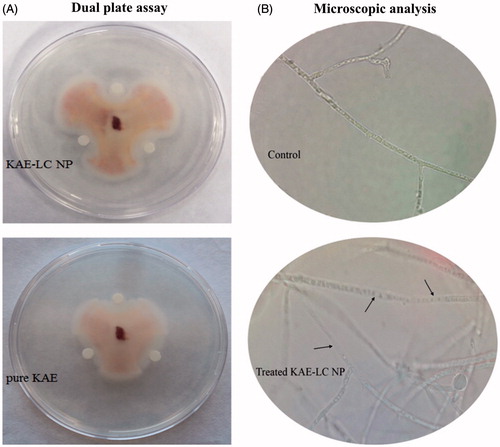
The antifungal activity of the pure KAE and novel KAE-LC NP were estimated by using two methods; hyphal extension-inhibition and mycelium growth inhibition assay against F. oxysporium. The hyphal extension-inhibition assay was evaluated by using the previously described method with some modification (Kopparapu et al. Citation2011). The fresh grown fungal culture (5 days) was inoculated into the center of the petri dishes containing 10 mL PDA. The plates were incubated at 27 ± 1 °C till fungal growth was seen. KAE-LC NPs and pure KAE were spotted on sterile paper discs (6 mm dia.), at a distance of 0.5 cm away from the rim of the mycelia colony. The plates were incubated at 27 ± 1 °C for 72 h until mycelia growth had enveloped discs containing the control and formed zones of inhibition around discs containing samples with antifungal activity. Water and fungal extracts were used as negative controls for antifungal activity. Moreover, the antifungal effectivity of KAE-LC NP against F. oxysporium was observed by a light microscope (Leica, DM500). In order to determine the deformation of fungal hyphae, fungal suspension was treated with nanoparticles (300 ppm) and the untreated nanoparticle culture was used as a control. All experiments were performed in quadruplicate.
The mycelium growth inhibitory effects of the nanocarriers were evaluated by using the poisoned food technique with slight modification (Shahi et al. Citation1999). This method consisted of pouring the 20 mL agar (PDA) in the petri plates (90 mm dia.) containing pure KAE and the KAE-LC nanoparticles (300 ppm) at different storage time points (1, 15, 30, 45, 60 days), so that when the agar solidified, the samples were entrapped in it. Mycelial discs of 5 mm diameter cut out from the periphery of 7 day-old cultures were aseptically inoculated upside-down at the center of each treated PDA petri plate. A separate plate without any samples was used as a control. Inoculated petri plates were incubated at 27 ± 1 °C and the observations were recorded on the seventh day. All experiments were performed in quadruplicate. Once measured, the average value and standard deviation were evaluated. The antifungal index was calculated according to the following formula (Guo et al. Citation2006):
where Da is the diameter of the growth zone on the test plate that contains KAE-LC NPs and Db is the growth zone on the control plate.
Results and discussion
Hydrodynamic diameter of nanoparticles
The KAE-LC NPs were successfully prepared by the injection of lecithin/DMSO solution containing KAE into an aqueous chitosan and TPGS solution which led to the round and stabile formation of nanoparticles due to electrostatic interaction between negatively charged lecithin and the polycationic chitosan. TPGS was used for increasing drug-solubility, drug-absorption, and more stable nanoparticles because of its hydrophobic and hydrophilic molecular structure. In this study, hydrophobic core of nanoparticles might consist of the hydrophobic groups of TPGS and lecithin, which interact with each other and the formation of hydrated shell layer of nanoparticles can be comprised of the hydrophilic chains of TPGS and lecithin as they intertwine with chitosan (Scheme 1).
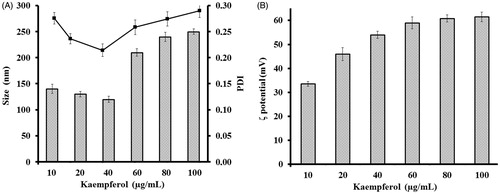
The size of nanoparticles plays an important role in determination of antimicrobial activity as the agents enter the cell walls of microorganisms through channels of protein or ion. Therefore, smaller particle size presents increased uptake of vehicles into microbial cell (Sharma et al. Citation2010). Particle surface charge also plays an important role in the inhibitory effect of nanoparticles because positive charges of improves interaction between nanoparticles and negatively charged surfaces of microbial cell (Wiarachai et al. Citation2012). The chitosan based nanoparticles inhibit microbial growth when they are only positively charged (Zhang et al. Citation2008). We performed a study of average size and PDI of nanoparticles obtained by using KAE at different concentrations (10–100 μg/mL) (). The nanoparticle’s suspension containing higher than 40 μg/mL KAE concentrations were caused aggregation of the nanoparticles and displayed high PDI (≥0.2). We considered that high concentrations of KAE occur to decrease the spacing between nanoparticles and lead to the blockage of the repulsive interaction between the nanoparticles and aggregation. The results are in agreement with the previous studies (Barbieri et al. Citation2013, Pool et al. Citation2012, Souza et al. Citation2014). Based on the results, the formulation of KAE-LC NPs nanoparticles prepared 40 μg/mL KAE concentration was further evaluated. Zeta potential indicated that the surface charge of the nanoparticles affected the stability of nanoparticles because of electrostatic repulsion between particles. shows the effect of the KAE concentration (10–100 μg/mL) on the zeta potential (ζ-potential). Within these concentrations, the obtained colloidal particles had positive surface charge ranged between 33.6 and 61.2 mV. When KAE concentration at 40–80 μg/mL, the surface charge of the NPs ranged from 50 to 60 mV and the size was lower than 300 nm. These values are suitable for stable nanoparticles system due to the absolute value of zeta potential is above +30 mV that is sufficient to prevent aggregation of the nanoparticles. The mean particle size, size distribution with PDI values, and zeta potential of KAE-LC NPs produced in optimum parameters are 270 ± 10 nm, PDI ≤0.2 and net positive surface charge (+56 ± 4 mV) respectively.
Figure 1. Influence of kaempferol concentration (A) on the average size (black squares) and polydispersity index (grey bars) and (B) on the surface charge of KAE-LC NPs.
Surface properties and morphology of the nanoparticles play a crucial role in drug release kinetics. Scanning electron micrographs of nanoparticles were performed to investigate the morphology of nanoparticles. shows that the KAE-LC NPs in the optimal formulation are spherical in shape, uniform, and polydisperse with sizes ranging from 200 to 350 nm with an average size of 273 nm in diameter. In addition, almost no particle aggregation occurred during the drying of the NPs.
Intermolecular interactions between KAE and KAE-LC NPs
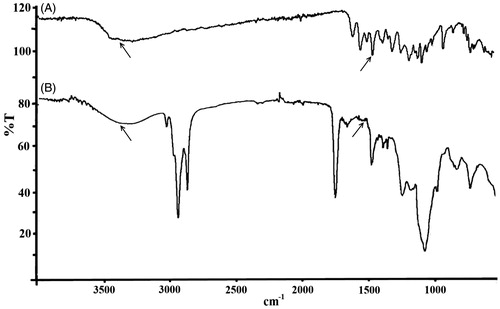
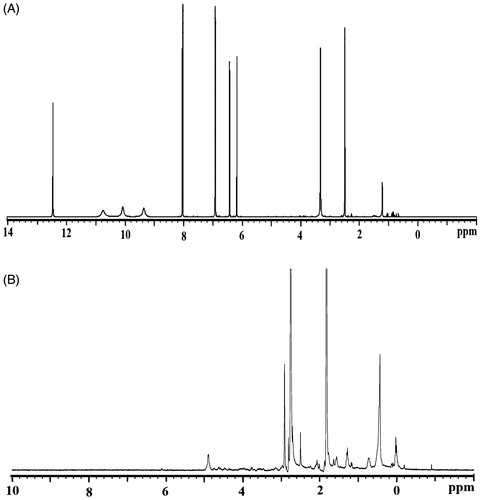
The interactions between the polysaccharides and lipids have been studied to investigate the chemical structure of the KAE-LC NPs by using FTIR. From the FTIR spectrum of the pristine KAE () obtained that bands and typical molecular peaks of its structure: 3323 cm−1 (O–H stretch), 1661 cm−1 (C=O), 1604 cm−1 (C=C), 1378 cm−1 (C–OH), 1257 cm−1 (C–O–C). The KAE-LC NPs spectrum (), by comparison, exhibited the OH stretch of KAE disappeared at 3363 cm−1 and the C=O absorption band (1661 cm−1) of KAE shifted to the 1653 cm−1. Also FTIR results displayed the typical absorption peak of the chitosan at 1644 cm−1 (N–H bands) and 1061 cm−1 (C–O–C) that are indicative for amino groups of glucosamine and saccharide structure of chitosan to appear. Furthermore, in the nanoparticle spectrum, the presence of an absorption band at 1230 cm−1 corresponding to P=O stretching was indicative that ionic interactions between phosphate groups of lipids (lecithin) and amino groups of polysaccharides (chitosan) occurred. From the FTIR spectrum of KAE-LC NPs observed that encapsulation of KAE in the KAE-LC NPs was formed by what appears to be that appear by hydrophobic interactions (Souza et al. Citation2014, Zhang et al. Citation2008). The formation of hydrogen bonding can be also evaluated by the change in chemical shifts in NMR spectra. The 1H NMR data of pure KAE showed that the protons on aromatic groups range from 6 to 8 ppm (H6 and H8) (), the aromatic groups of pristine KAE were clearly shifted by the KAE-LC NPs system (). In the case of KAE-LC NPs, from the obtained results one can determine that the aromatic protons of KAE were clearly shifted and the intramolecular hydrogen bonding disappeared. The 1H NMR spectra results are in agreement with the previous studies (Tzeng et al. Citation2011). These results demonstrated that the aromatic ring of KAE significantly presented intermolecular hydrogen bond with chitosan/lecithin carriers.
The amorphous transformation of KAE by the nanoparticle engineering system
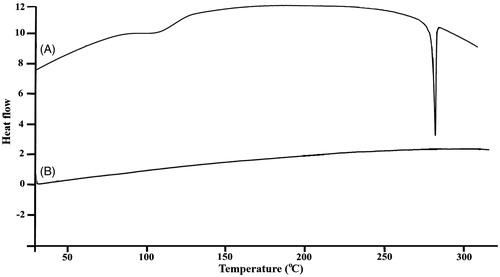
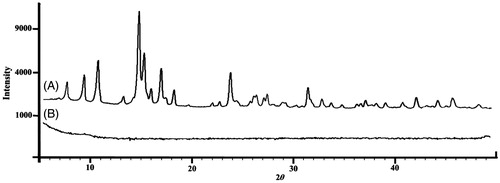
DSC analysis presents information regarding the physical properties (crystalline or amorphous structure) of the samples. DSC was employed to check any variation of crystalline properties of KAE due to the encapsulation into lecithin chitosan nanoparticles. It has been previously reported that when the interactions between a drug and a polymer are suitable, the presence of the drug increases the Tg temperature of the polymer. Thus, when the drug increased and dispersed molecularly in the matrix, there was a positive correlation of Tg with the introduction of asserting drug molecules between polymer chains. The DSC thermograms of pure KAE and KAE loaded lecithin chitosan NPs are presented in . The endothermic peak for KAE was found to be around 285.6 °C as a single Tg peak which indicated that its crystalline structure was formed (). The presence of a single Tg confirms the complete homogeneous dispersion of KAE in the carriers matrix. Still, the peak of endothermic melting for KAE-LC NPs was not observed due to the shifted peak of KAE (). Also, the peak of the chitosan (Patila and Jobanputraa Citation2015) shifted from 190 to 150 °C and the peak of the KAE diminished. It can be assumed, through the proof of inclusion, that the presence of KAE in the lecithin/chitosan nanoparticles caused the melting point disappear due to its thermal profile. The change in the DSC thermogram of pristine KAE and KAE-LC NPs was obtained and correlated to the amorphous nature of KAE after being entrapped in the lecithin–chitosan. The shift in the peak of KAE-LC NPs may attribute to chitosan–lecithin complex formation. XRD studies were carried to investigation of encapsulation properties of nanoparticles by means of change in crystallinity of KAE (). Pristine KAE had a highly crystalline structure (). However, after the encapsulation, the crystalline peaks of pure KAE disappeared and the KAE-LC nanoparticle form had amorphous patterns (). We suggested that the nanoparticle engineering system could form a high-energy amorphous state. The absence of the KAE peak may be due to the formation of KAE molecular dispersion in KAE-LC NPs’ matrix (Tzeng et al. Citation2011). According to DSC and XRD results, KAE was transformed from a crystalline structure into an amorphous structure due to intermolecular interactions with the nanocarriers’ matrix.
Encapsulation efficiency, loading content, yield of production, and dissolution profiles
Encapsulation efficiency has a key role in evaluating drug release from the nanoparticle engineering system (Mora-Huertas et al. Citation2010). The KAE-LC NPs that were produced at optimum parameters had encapsulation efficiency higher than 93.8 ± 4.28% and its yield was 80 ± 3.62% (). The encapsulation efficiency at high values may be related to the core structure of NPs. Both of the core materials (lecithin and KAE) are in a hydrophobic structure, which have an affinity between them. In previous studies, other hydrophobic molecules such as melatonin and progesterone were also successfully loaded in lecithin/chitosan NPs (Hafner et al. Citation2009, Sonvico et al. Citation2006).
Table 1. The correlation between particle size, PDI and encapsulation efficiency, drug loading content, and yield of KAE-LC NPsTable Footnotea.
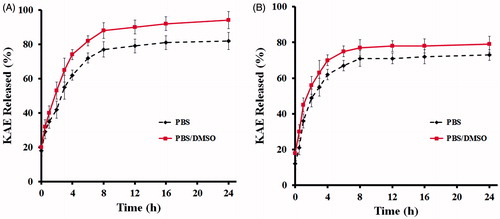
The in vitro KAE release studies were carried out under various conditions to investigate the different temperature and dissolution medium dependency of the KAE-LC NPs. The release profiles of KAE from lecithin/chitosan nanoparticles were recorded under PBS and PBS containing DMSO (PBS + DMSO) at two different temperature (25 °C and 37 °C) conditions (). The dissolution of pure KAE was less than 1% (data not shown). However, a slow and sustained release was observed for KAE loaded LC NPs in PBS + DMSO buffer at 37 °C (). KAE from lecithin/chitosan nanoparticles was released for the first 8 h at 37 °C in both of buffer, no significant drug release was observed for the rest of the remaining time. In this case, the released percentages were limited to 82 and 93% in PBS and PBS/DMSO dissolution media, respectively. In contrast, shows dissolution of KAE from nanoparticles at 25 °C temperature. KAE was dissolved at a greater ratio in terms of released percentage for the first 6 h. Depending on the releasing media (PBS and PBS + DMSO), the final release percentages are 72 and 79%, respectively. When the NPs were exposed to PBS containing DMSO medium at 37 °C, the extended liquid penetration into the systems promoted an increased dissolution of the KAE adsorbed. This meant, KAE was progressively released under the diffusion through the polymer matrix and polymer degradation. In all of the conditional parameters, the release of KAE was slow and the releasing ratios of KAE were high ranging from 72 to 93%. Specifically, the release rate of the KAE from lecithin/chitosan NPs was increased by an average of 78 fold as compared to pure KAE. According to previous studies (Guo et al. Citation2006, Ing et al. Citation2012, Tan et al. Citation2011), this may be related to small nanoparticles with enhanced surface area, resulting in a larger KAE concentration exposed to the releasing media.
Antioxidant activity
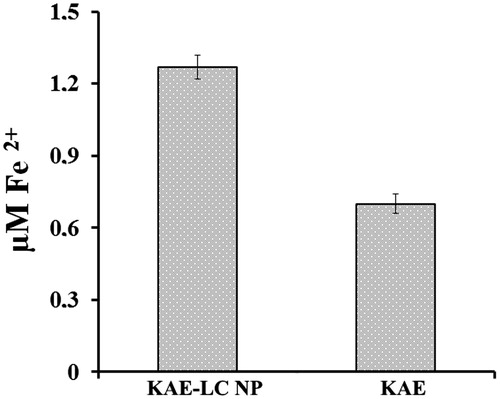
Kaempferol is known to be a potent antioxidant compound and it aids in preventing oxidative damage. However, the utility of such compounds for antimicrobial agents in microorganisms has presented only limited success due to inadequate bioavailability and inefficient systemic delivery. Several factors limit the application of flavonoids. Therefore, novel strategies are required to ensure the sustained release of active compounds for prolonged antioxidant activity to enhance the bioavailability and reduce toxicity of flavonoids. In our study, we examined the antioxidant activity of KAE-LC NPs compared to pure KAE. The reducing power is associated with the presence of reductones that perform antioxidant action by donating a hydrogen atom which breaks the free radical chain. The KAE-LC NPs had more activity in reducing Fe+3 to Fe+2 compared to the free KAE due to a probable improvement in the dissolution properties of KAE in an aqueous medium and the ability to donate protons of the phenolic groups of KAE from KAE-LC NPs (). According to these results, the KAE from the KAE-LC NPs has been improved in its physico-chemical properties and NPs have significantly higher antioxidant potential than pure KAE.
Stability studies
Nanoparticles are regarded as stable when there is no significant change in the hydrodynamic diameter, aggregation and physicochemical characteristics of the particle system over a period of observation. The stability of colloidal biodegradable polymeric nanoparticles depends on their storage conditions. The stability of KAE-LC NPs at storage temperature 4 and 25 °C was assessed during a 60 day observation period by examining the changes in particle size, PDI, zeta potential (ZP) and antioxidant activity (FRAP) (). Insignificant differences were observed in particle size, PDI, ZP, and the antioxidant activity of KAE-LC NPs at 4 °C storage condition. However, KAE-LC NPs stored at room temperature showed linear increase in size, PDI, zeta, and reduction in FRAP consumption at the ninth day of storage. Similar observations have been made previously, where it was noted that the flavonoid loaded lecithin/lipid nanoparticles are stable at 4 °C during 60 days of observation but temperature above this caused the nanoparticles to agglomerate and reduction of antioxidant properties to take place (Tan et al. Citation2011). These results can be explained by the fact that unsaturated phospholipids of the lecithin of NPs are sensitive to spontaneous oxidation by molecular oxygen that dissolved in the suspension. Storage at 4 °C reduced the rate of oxidation of the KAE-LC NPs. It is thus, suitable to store the KAE-LC NPs at 4 °C temperatures, because it did not present any harmful effects. As a result, the antioxidant activity of KAE demonstrated that KAE acts as a good antioxidant but it is also easily oxidized by heat and light. However, the results on the antioxidant activity of KAE-LC NPs results demonstrated that free radical scavenging activity of NPs remained for long time.
Table 2. Changes in KAE-LC NPs characteristics depending on storage time and temperatureTable Footnotea.
Antifungal activity of the KAE-LC NPs
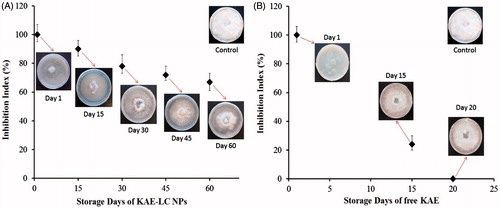
F. oxysporium is a pathogenic fungus, which commonly infects crops, leading to severe crop yield reduction and dramatic economic losses in agriculture. In the present study, the KAE loaded lecithin/chitosan nanoparticles with a sustained controlled release system were developed for the hyphal extension-inhibition activity against F. oxysporium. Hyphal-extension growth of F. oxysporium was strongly inhibited by the KAE-LC nanoparticle system compared to pure KAE (). Moreover, in the present study, a microscopic observation revealed the morphology of hyphae that appeared as distorted and fragmented in the nanoparticles treated culture, whereas the hyphae in the control culture were normal without any distortion (). Most fungal species are composed of thick cell walls in their hyphae, which prevent the cell from antifungal materials. Earlier studies suggested that the hyphae damage is probably associated with cell wall disruption thus causing cellular leakage and loss of cellular integrity. Our results demonstrated that KAE-LC NPs exhibited higher antifungal activity due to their special characteristics such as small and compact formation as well as high surface area to be able to absorb more onto the surface of fungal cell wall. KAE-LC NPs have a higher affinity to binding fungal cells together. This could be explained by the fact that the negatively charged membrane of fungus was targeted from positively charged KAE-LC NPs. Therefore, the polycationic KAE-LC NPs with enhanced surface area interact more effectively with the fungus compared to pristine KAE. Now that chitosan nanoparticles have the ability of cellular uptake into cells (Guerra-Sanchez et al. Citation2009), KAE-LC NPs might be able to diffuse into the cell of fungus and disrupt the synthesis of DNA and RNA (Ing et al. Citation2012). This could explain the damaging of F. oxysporium hyphae by KAE-LC nanoparticle system. Similar results were found by others (Guerra-Sanchez et al. Citation2009, Zhong et al. Citation2007). Our study also demonstrated, for the first time, that the KAE-LC NP system significantly inhibited mycelium growth of F. oxysporium against pure KAE in a time-dependent manner (). Based on the results obtained, KAE-LC NPs presented significantly higher antifungal activity (67%) compared to pure KAE (no inhibition) against F. oxysporium by the end of 60 day storage period due to the sustained release of KAE from the lecithin/chitosan nanocarrier system. While the antifungal activity of KAE-LC NPs on the 15th day of storage presented high inhibition percent value (90%) of F. oxysporium growth (), the antifungal effectivity of pure KAE exhibited inhibition index below 25% (). Overall, the results confirmed that the importance of enhanced encapsulation and effective dissolution of KAE-LC NPs have on the sustainable antioxidant activity of long-term antifungal actions of biofungicide KAE.
Conclusions
Our results highlighted that a newly developed KAE-LC NPs are suitable to be used for the encapsulation of bioactive KAE. Additionally, the present study indicated that a linear relationship between concentration, temperature and ratio of chitosan, lecithin and KAE as well as particle size/PDI/zeta potential was statistically proven. This provided for easy manipulation of physico-chemical properties of KAE-LC NPs suitable for their applications. Results of the present study indicated that the improved antioxidant activity of KAE-LC NPs was correlated with encapsulation efficiency and dissolution properties. Adding KAE into lecithin/chitosan nanoparticles was found to improve its antifungal activity significantly. These findings also illustrate one of the ways in which nanotechnology can be used to develop effective systems for modified release of fungicides, providing greater efficiency in microorganism control. Therefore, KAE-LC NPs have the potential to be a safe and highly effective natural fungicidal agent which can be used in the control of against F. oxysporium for further green environment applications.
Funding information
This work was supported by Hacettepe University Scientific Research Project grant (014 D12 101 005-820) and The Scientific and Technological Research Council of Turkey (TUBITAK) with PhD Scholarship (2211/C).
Acknowledgements
We would like to thank Ayhan Şahenk Foundation for their support for laboratories of Ayhan Şahenk Agricultural Sciences and Technologies Faculty. We are also thankful for Prof. Dr. Feza Korkusuz for his generous help.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Barbieri S, Sonvico F, Como C, Colombo G, Zani F, Buttini F, et al. 2013. Lecithin/chitosan controlled release nanopreparations of tamoxifen citrate: loading, enzyme-trigger release and cell uptake. J Control Release. 167:276–283.
- Chadha R, Bhandari S, Kataria D, Gupta S, Singh Jain D. 2012. Exploring the potential of lecithin/chitosan nanoparticles in enhancement of antihypertensive efficacy of hydrochlorothiazide. J Microencapsul. 29:805–812.
- Göktürk I, Karakoç V, Onur MA, Denizli A. 2013. Characterization and cellular interaction of fluorescent-labeled PHEMA nanoparticles. Artif Cells Nanomed Biotechnol. 41:78–84.
- Guerra-Sanchez MG, Vega-Pérez J, Velázquez-del Valle MG, Hernández-Lauzardo AN. 2009. Antifungal activity and release of compounds on Rhizopus stolonifer (Ehrenb.:Fr.) Vuill. by effect of chitosan with different molecular weights. Pestic Biochem Phys. 93:18–22.
- Guo ZY, Chen R, Xing R, Liu S, Yu H, Wang P, Li C, Li P. 2006. Novel derivatives of chitosan and their antifungal activities in vitro. Carbohydr Res. 341:351–354.
- Gupta A, Kaura CD, Saraf S, Saraf S. 2016. Formulation, characterization, and evaluation of ligand-conjugated biodegradable quercetin nanoparticles for active targeting. Artif Cells Nanomed Biotechnol. 44:960–970.
- Hafner A, Lovric J, Voinovich D, Filipovic-Gr J. 2009. Melatonin-loaded lecithin/chitosan nanoparticles: physicochemical characterisation and permeability through Caco-2 cell monolayers. Int J Pharm. 381:205–213.
- Hahn M. 2014. The rising threat of fungicide resistance in plant pathogenic fungi: Botrytis as a case study. J Chem Res. 7:133–141.
- Ing LY, Zin NM, Sarwar A, Katas H. 2012. Antifungal activity of chitosan nanoparticles and correlation with their physical properties. Int J Biomater. 2012:1–9.
- Kopparapu NK, Liu ZQ, Yan QJ, Jiang ZQ, Zhang SP. 2011. A novel thermostable chitinase (PJC) from pomegranate (Punica granatum) juice. Food Chem. 127:1569–1575.
- Luo H, Jiang B, Li B, Li Z, Jiang B, Chen YC. 2012. Kaempferol nanoparticles achieve strong and selective inhibition of ovarian cancer cell viability. Int J Nanomedicine. 7:3951–3959.
- Mora-Huertas CE, Fessi H, Elaissari A. 2010. Polymer-based nanocapsules for drug delivery. Int J Pharm. 385:113–142.
- Özcan İ, Azizoğlu E, Şenyiğit T, Özyazıcı M, Özer Ö. 2013. Enhanced dermal delivery of diflucortolone valerate using lecithin/chitosan nanoparticles: in-vitro and in-vivo evaluations. Int J Nanomedicine. 8:461–475.
- Patila AG, Jobanputraa AH. 2015. Rutin-chitosan nanoparticles: fabrication, characterization and application in dental disorders. Polym Plast Technol. 54:202–208.
- Pool H, Quintanar D, Figueroa JD, Mano CM, Bechara JEH, Godinez LA, Mendoza S. 2012. Antioxidant effects of quercetin and catechin encapsulated into PLGA nanoparticles. J Nanomater. 2012:1–12.
- Satpath G, Tyagi YK, Gupta RKA. 2011. novel optimized and validated method for analysis of multi residues of pesticides in fruits and vegetables using microwave-reaction system (MRS)-dispersive solid phase extraction(d-SPE)-retention time locked (RTL)-gas chromatography–mass spectrometry with deconvolution reporting software (DRS). Food Chem. 127:1300–1308.
- Şenyiğit T, Sonvico F, Barbieri S, Özer O, Santi P, Colombo P. 2010. Lecithin/chitosan nanoparticles of clobetasol-17-propionate capable of accumulation in pig skin. J Control Release. 142:368–373.
- Shahi SK, Shukla AC, Bajaj AK, Midgely G, Dikshit A. 1999. Board spectrum antimycotic drug for the control of fungal infections in human beings. Curr Sci. 74:836–839.
- Saharan V, Mehrotra A, Khatik R, Rawal P, Sharma SS, Pal A. 2013. Synthesis of chitosan based nanoparticles and their in vitro evaluation against phytopathogenic fungi. Int J Biol Macromol. 62:677–683.
- Saharan V, Sharma G, Yadav M, Choudhary MK, Sharma SS, Pal A, Raliya R, Biswas P. 2015. Synthesis and in vitro antifungal efficacy of Cu-chitosan nanoparticles against pathogenic fungi of tomato. Int J Biol Macromol. 75:346–353.
- Sharma D, Rajput J, Kaith BS, Kaur M, Sharma S. 2010. Synthesis of ZnO nanoparticles and study of their antibacterial and antifungal properties. Thin Solid Films. 519:1224–1229.
- Sonvico F, Cagnani A, Rossi A, Motta S, Di Bari MT, Cavatorta F, et al. 2006. Formation of self-organized nanoparticles by lecithin/chitosan ionic interaction. Int J Pharm. 324:67–73.
- Souza M P, Vaz AFM, Correia MTS, Cerqueira MA, Vicente AA, Carneiro-da-Cunha MG. 2014. Quercetin-loaded lecithin/chitosan nanoparticles for functional food applications. Food Bioprocess Tech. 7:1149–1159.
- Tan Q, Liu W, Guo C, Zhai G. 2011. Preparation and evaluation of quercetin-loaded lecithin–chitosan nanoparticles for topical delivery. Int J Nanomedicine. 6:1621–1630.
- Tzeng CW, Yen FL, Wu TH, Ko HH, Lee CW, Tzeng WS, Lin CC. 2011. Enhancement of dissolution and antioxidant activity of kaempferol using a nanoparticle engineering process. J Agric Food Chem. 59:5073–5080.
- Wang SY, Chen C, Wang CY. 2009. The influence of light and maturity on fruit quality and flavonoid content of red raspberries. Food Chem. 112:676–684.
- Wiarachai O, Thongchul N, Kiatkamjornwong S, Hoven VP. 2012. Surface-quaternized chitosan particles as an alternative and effective organic antibacterial material. Colloids Surf B Biointerfaces. 92:121–129.
- Yadav KS, Sawant KK. 2010. Modified nanoprecipitation method for preparation of cytarabine-loaded PLGA nanoparticles. AAPS PharmSciTech. 11:1456–1465.
- Zhang Y, Yang Y, Tang K, Hu X, Zou G. 2008. Physicochemical characterization and antioxidant activity of quercetin-loaded chitosan nanoparticles. J Appl Polym Sci. 107:891–897.
- Zhong Z, Chen R, Xing R, Chen X, Liu S, Guo Z, et al. 2007. Synthesis and antifungal properties of sulfanilamide derivatives of chitosan. Carbohydr Res. 342:2390–2395.