?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The aim of the present study was to prepare and evaluate microparticle formulation encapsulated with glycyrrhetinic acid (GA) based on bovine serum albumin (BSA). The drug-loaded nanoparticles were firstly formed by a simple desolvation method, and were further assembled into microparticles using zinc chloride and glutaraldehyde as crosslinkers. The obtained microparticles contained approximately 30% (w/w) drug and showed as spherical particles with a size of about 2 μm. Differential scanning calorimetry (DSC) and X-ray powder diffraction (XRPD) analysis indicated that GA lost its crystallinity during the nano/microencapsulation process. In vitro dissolution study demonstrated a typical sustained-release pattern for 24 h with a burst of 28.1% at the first 30 min, which fitted well by Higuchi model. After intravenous administration into mice, the microparticle formulation remained a higher drug level than the solution formulation in blood and liver for more than 18 h. These results suggested the potential benefit of using the prepared albumin microparticles as a promising vector for enhanced liver delivery of poorly water-soluble drug.
Introduction
Over the last few decades, the nano/microparticles have gained special interests in delivering various therapeutic agents as depots (D'Mello et al. Citation2014, Patino et al. Citation2015). Currently, numerous natural and synthetic materials are typically used during the production of drug-loaded nano/microparticles (Ci et al. Citation2015, D'Mello et al. Citation2014). Among them, bovine serum albumin (BSA) is an excellent natural building material to fabricate nano/microparticles due to its abundance, low cost, and biocompatibility. BSA has been proven to be safe and clinically applied (Viúdez et al. Citation2014). BSA-based particulate drug delivery systems demonstrate several specific advantages such as good stability, high binding capacity for drugs with different physicochemical properties, and potentials for surface modification (Xing et al. Citation2015, Zhang et al. Citation2012). In addition, these nano/microparticles can be utilized to provide prolonged therapeutic effect by controlling drug release, and/or reduced systematic toxicity by targeted drug delivery (Wilson et al. Citation2012). Previous reports have suggested that proper size control of nano/microparticles could provide enhanced drug distribution in specific tissues. For instance, particles over 7 μm will be mechanically intercepted by pulmonary vascular after intravenous administration, and those in size range of 0.1–7 μm will be uptaken by macrophages of liver and spleen (Birnbaum et al. Citation2000, Minamimura et al. Citation2000). There are rich varieties of techniques for the preparation of BSA nano/microparticles, including the desolvation (or coacervation), emulsification and spray drying methods (Elzoghby et al. Citation2012). During the desolvation process, the addition of coacervating agents (usually ethanol) and crosslink agents (glutaraldehyde) usually tends to form colloidal systems with nanoscale size (Kouchakzadeh et al. Citation2014, Weber et al. Citation2000). To obtain BSA-based microparticles, the emulsification-solvent evaporation method or the spray drying method is commonly acquired (Elzoghby et al. Citation2012). Here, we aimed to obtain drug-loaded BSA nanoparticles by a simple desolvation method, and then to assemble these nanoparticles into microparticles with size range using cross-linking agents.
Glycyrrhetinic acid (GA) was chosen as the model drug in the present study. GA is the hydrolysis product of glycyrrhizic acid, which is one of the major active flavonoids extracted from the roots of Glycyrrhiza uralensis Fisch. (Chinese licorice or Gan-Cao in Chinese) (Zhao et al. Citation2012). GA has been proven by a lot of researchers on its potential antiviral, anti-inflammatory, antioxidant, antitumor and antimutagenic activities, as well as hepatoprotective effects (Lu et al. Citation2008, Zhao et al. Citation2012). It has been clinically used for many years in the treatment of various liver diseases. However, GA is poorly soluble in water and its short biological half-time presents obstacles for its clinical efficacy (Lu et al. Citation2008). Theoretically, some solvents and surfactants (glycol, Tween 80 etc.) could be adopted into the formulation for parenteral administration to improve its solubility, but the injection may cause serious adverse effects. In order to overcome the above pharmaceutical obstructions, various drug delivery systems have been studied, including chitosan composite hydrogel beads (Zhong et al. Citation2012), liposome (Guo et al. Citation2010), emulsions (Han et al. Citation2012). However, these preparations were usually at a low level of drug loading and drug delivery into the liver, which could not satisfy the goal of low injection dose and preferable therapeutic results.
To construct the multifunctional microparticles with hepatic-passive targeting behavior and high drug loading, the desolvation method was adopted in the present study to prepare GA–BSA nanoparticles followed by cross-linking into microparticles. The related features of these microparticles were investigated by transmission electron microscopy (TEM), X-ray powder diffraction (XRPD), and differential scanning calorimetry (DSC). The in vitro drug release, in vivo pharmacokinetics, and tissue distribution of GA-loaded microparticles were also assessed.
Materials and methods
Materials
Glycyrrhetinic acid (GA, purity ≥98%) was purchased from Jingzhu Biological Technology Co., Ltd. (Nanjing, China). Bovine serum albumin (BSA, purity ≥98%) was obtained from Sigma-Aldrich Co. (St. Louis, MO). Dioxane was provided by Fisher Co. Glutaraldehyde 50%, Methyl tert-butyl ether (MTBE) and Tween 20 were supplied by Damao Pharmaceutical Co., Ltd. (Tianjin, China). Zinc chloride (ZnCl2) was procured from Aladdin Industrial Corporation (Shanghai, China). Cremophor EL was received from Yunhong Pharmaceutical Excipients & Technology Co., Ltd. (Shanghai, China). Double-distilled water (Milli-Q Plus, Millipore, Merck, Germany) prepared in our laboratory was used throughout the experiments. All other reagents were of analytical grade and used as received.
Preparation of GA nanoparticles and GA–BSA microparticles
BSA microparticles encapsulated with GA were prepared according to the following protocol: Firstly, GA solution in dioxane and BSA solution in water were prepared at a concentration of 10% and 0.75% (w/v), respectively. The GA–BSA nanoparticles were obtained by quickly injecting 1 mL GA solution into 20 mL BSA solution and treating for 5 min by an ultrasonic processor (JY99-IIDN, Ningbo Scientz Biotechnology Co. Ltd., China) under an ice-water bath. The input of ultrasonic power was 800 W, and the period of ultrasound burst was set to 90 s with a pause of 1 s between two ultrasound bursts. Then, 600 μL 25% ZnCl2 and 240 μL 0.1% glutaraldehyde were sequentially added into 8 mL of the obtained suspension under vortex to form the microparticles. The suspension was kept stirring at 600 rpm for 1 h, and then centrifuged at 4000 rpm for 8 min to remove free protein molecules and unreacted chemicals (Langer et al. Citation2003). The precipitate was rinsed for two times using 2 mL water and ethanol, respectively, and then freeze-dried by an FD-1–50 freeze-dryer (Beijing Boyikang Laboratory Instrument Co. LTD., China) under vacuum (pressure <10 Pa) for 24 h.
Characterization of GA–BSA microspheres
Morphological observation
The appearances of BSA solution, the suspensions of GA–BSA nano/microparticles were observed before and after centrifugation at 4000 rpm for 8 min, and then recorded by a digital camera (Fujinon 3× Optical Zoom, China).
The morphology of GA–BSA nano/microparticles was observed under TEM ( H-7650, Hitachi, Japan). One droplet of GA nanoparticles or resuspended microparticles was placed on the carbon-coated copper grid, the excess solution was removed with a filter paper. After air dried, the films were viewed and photographed.
Particle size and size distribution
The suspensions of GA–BSA nano/mircoparticles were diluted by water, and the mean size and size distribution were then analyzed by a NICOMPTM 380 Submicron particle analyzer (PSS Nicomp, Santa Barbara, CA).
Differential scanning calorimetry
DSC thermograms were recorded on a SETSYS-1750CS Evolution thermogravimetry analyzer (Setaram, France). Accurately weighed samples were sealed into aluminum pans and heated at a heating rate of 10 °C/min over a temperature of 25 to 450 °C under dry nitrogen atmosphere (Pu et al. Citation2013).
X-ray powder diffraction
The crystalline state of GA in different samples was examined using a D/MARX2200/PC XRPD (Rigaku Co., Tokoyo, Japan) with a CuKα radiation detector (40 kV, 30 mA) at a scan speed of 0.02°/min over a 2θ range of 3–85°.
Drug loading and encapsulation efficiency
The dry powder of microparticles was accurately weighed, and 10 mL of 0.05 mol/L NaOH solution was then added and sonicated for 5 min to dissolve the sample (Liu and Zhang Citation2005). The obtained solution was immediately filtered through a 0.22 μm syringe filter and drug content was determined by a HPLC (Model-L2000, Hitachi, Japan) method. The analytical column was a PntulipsTM-C18 (5 μm, 4.6 × 250 mm). The mobile phase was composed of methanol and water (95:5, v/v; pH = 3) at a flow rate of 1.0 mL/min, and the detection was performed at 254 nm by a UV-Vis detector (L7420, Hitachi, Japan). The drug loading (DL) and encapsulation efficiency (EE) were calculated according to the following equations:
(1)
(1)
(2)
(2)
In vitro drug release test
The drug release profiles of nano/microparticles were analyzed. Briefly, 5 mg dry powders were dispersed in 90 mL phosphate buffer saline containing 0.1% Tween 20 (PBST, pH 7.4) and incubated at 37 °C/100 rpm in a THZ-100B shaking air bath (Shanghai, China). At regular time intervals, 8 mL of aliquots were withdrawn after centrifuged at 4000 rpm for 3 min and replaced with an equal volume of fresh medium. The samples were filtered through 0.22 μm syringe filters and analyzed by the above-mentioned HPLC method.
Pharmacokinetic and tissue distribution studies
Kunming mice, body weight between 20 and 25 g (provided by the Experimental Animals Center, Ningxia Medical University) were randomly divided into two groups, GA–BSA microparticle group and GA solution group (control). GA solution of 0.5 mg/mL was prepared by dissolving the accurately weighted drug in normal saline with 5% Cremophor EL as solubilizer. Mice were administered with the formulations at the dose of 10 mg/kg by tail-vein injection. At predetermined time (5, 15, 30 min and 1, 2, 4, 8, 12, 18, 24 h), blood was collected via postorbital vein from six animals at each time point for each group and centrifuged to obtain the serum samples. The animals were then sacrificed by cervical dislocation. Major organs (heart, liver, spleen, lung, kidney and brain) were removed and washed with normal saline, then were dried with tissue paper and weighed accurately.
Serum and tissue sample analysis
Tissue samples were homogenized with normal saline at a volume of 4 mL/g tissue by a homogenizer. Tissue homogenates of 300 μL (or 100 μL serum sample) and 900 μL MTBE (300 μL for serum sample) were added to a centrifuge tube, followed by vortex mixing for 1 min. The mixture was centrifuged at 12,000 rpm for 10 min, and 600 μL of the supernatant (200 μL for serum sample) was removed to another tube and volatilized under nitrogen gas flow. Subsequently, 100 μL methanol was added to redissolve the residue and centrifuged at 12,000 rpm for 10 min, 20 μL supernatant was used for HPLC analysis (Model L1220, Agilent, USA). The chromatographic conditions were similar to the HPLC analysis mentioned above. The mixture of methanol and water at a volumetric ratio of 90:10 was used as the mobile phase during the analysis.
Data analysis
Pharmacokinetic parameters of GA in different experimental groups were analyzed by a DAS 3.0 software package (Bojia Corp., Shanghai, China). Comparisons between two groups were carried out by F-test and unpaired Student’s t-test using SPSS Statistics 17.0 software (IBM Corporation, Armonk, NY). Differences were considered statistically significant when p < 0.05.
Results
Preparation of GA–BSA microspheres
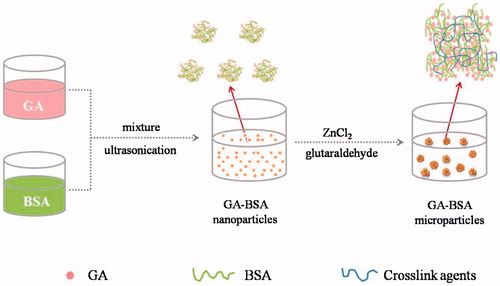
As shown in , the key procedures for preparation of GA–BSA microparticles including the formation of GA–BSA nanoparticles and the assembly of these nanoparticles into larger particles. GA nanoparticles were prepared by the anti-solvent precipitation-ultrasonication method previously described by Liu et al with some modifications (Liu et al. Citation2012). Dioxane was selected as the appropriate solvent because GA showed excellent solubility in dioxane (> 100 mg/mL). BSA was functioned as the encapsulating material and the stabilizer, simultaneously. The nanoprecipitation process was facilitated with ultrasonication and provided a milky white suspension without any visible particles. These results indicated that nano-sized GA–BSA nanoparticles were formed immediately after the mixing of two miscible phases. This process is most likely to the formation of drug nanosuspensions by nanoprecipitation (Chorny et al. Citation2002), however, the difference lies in that BSA and GA were simultaneously precipated out and BSA itself can serve as the stabilizer to prevent close contact and aggregation of particles.
The obtained suspension of nanoparticles was then added with ZnCl2 solution (ionic crosslinker) to form protein precipitate, and the proteins were further cross-linked by the addition of glutaraldehyde (covalent cross-linker). The BSA precipitation process induced the aggregation of GA-loaded nanoparticles, and finally the composite microparticles were collected after centrifugation. The microparticles were directly freeze-dried to yield a white powder, without the addition of any other lyoprotectants.
According to previous literatures, the ionic interactions between protein molecules and divalent metal ions (such as Zn2+) could create intermolecular function zones and result in the instant formation of BSA-Zn precipitate, which can be used to enhance the stability and duration of protein action to prepare a sustained-release formulation (Prabhu et al. Citation2001, Kwon et al. Citation2004). In addition, the –CHO groups of glutaraldehyde reacted with the –OH groups of proteins to form an acetal linkage, leading to the strengthened BSA microparticles (Bera et al. Citation2015, Das et al. Citation2011).
The obtained GA–BSA microparticles displayed a reasonable EE of 75.0 ± 2.36% and DL of 30.2 ± 1.18%, which was due to the formation of crosslinked protein precipitate, thereby preventing drug leakage from the matrix. A drug loss of nearly 25% might be due to drug leakage into the external aqueous medium during preparation.
Morphology and particle size observation
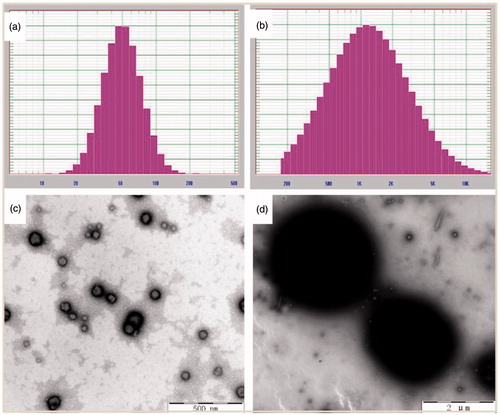
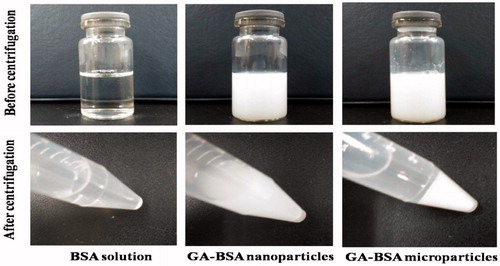
Milky white GA–BSA nanoparticles were obtained by injecting GA solution into the clear BSA solution under ultrasonication (). The GA–BSA microparticles were formed via adding ZnCl2 solution and glutaraldehyde to the suspension under vortex. After centrifugation at 4000 rpm for 8 min, the nanoparticle system was still homogeneous and showed a good stability, but the microparticle system was sedimented completely (), which suggested a big size jump after the cross-linking process.
Figure 2. Images of BSA solution, the suspensions of GA–BSA nano/microparticles before and after centrifugation.
The morphology and particle size distribution of GA–BSA nanoparticles before and after cross-linking were analyzed by TEM and DLS, respectively. As shown in , the nanoparticles appeared as spherical in shape with their particle size ranging from 50 nm to 100 nm, approximately (). The nanoparticles were formed sphere-like particles with an approximate size of 2 μm () after addition of cross-linkers. The average particle size was 58.6 nm (PDI = 0.158) for GA nanoparticles and 2035.3 nm (PDI = 0.828) for the final microparticles, respectively (), which was in accordance with the TEM images.
Differential scanning calorimetry
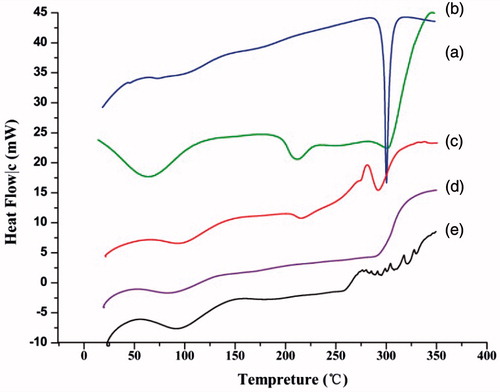
The DSC thermograms of GA, BSA, physical mixture, and microparticles are shown in . The DSC curve of BSA is in good agreement with the previous reports (Norde and Giacomelli Citation2000). A denaturation peak at about 57 °C was observed (), and it was shifted to a slightly higher temperature either in the physical mixture or in the microparticles samples, which may be due to the influence of drug. GA showed an endothermic peak at about 300 °C corresponding to its melting point, indicating the crystalline nature of the drug (). The endothermic peak of GA was still observable in thermogram of the physical mixture, but it was absent in that of the BSA–GA microparticles (), indicating the formation of amorphous state or molecular dispersion of drug in the matrix.
X-ray powder diffraction
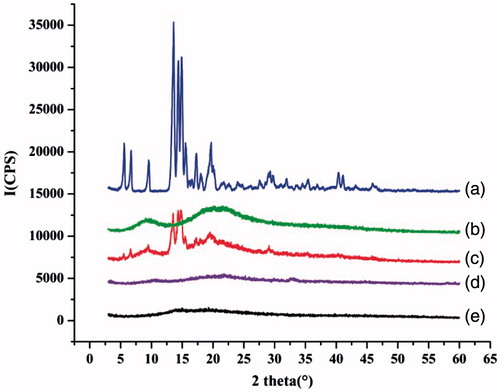
To further confirm the solid state transformation of GA, XRPD patterns of GA, BSA, physical mixtures and the microparticles with or without drug were analyzed (). A slight shift above the baseline without any dominant peaks was detected for BSA powder and BSA microparticles (blank microparticles), which indicated a basically amorphous state of the protein. The diffractogram of GA depicted characteristic peaks at 6°, 7°, 9°, 13° and 20° (2θ) that were intense and sharp, indicating its crystalline nature. The characteristic peaks of the drug appeared at the same 2θ values for the physical mixture of drug and protein at a ratio of 3:7 (w/w). However, the peaks of the drug were absent in the drug-loaded microparticles, which suggested that the process of drug encapsulation into microparticles resulted in the loss of drug crystallinity. These results were in accordance with those of DSC.
In vitro drug release test
shows the in vitro release profile of drug-loaded nano/microparticles. The nanoparticles showed a fast-release pattern with approximately 85% of drug released at the first 3 min. However, the cumulative drug released from the microparticle formulation was 50% at 2 h and reached over 70% after 8 h. The typical sustained release behavior of microparticle formulation was possibly due to reduced free volume spaces of the matrix treated with glutaradehyde and ZnCl2, which could restrict drug diffusion across the protein network. We also noticed a burst release of less than 10% drug at 5 min, which probably suggested drug adsorption on the surface of the microparticles and/or a relatively loose structure of the obtained microparticles.
Figure 6. The in vitro drug release profile of GA–BSA nanoparticles and GA–BSA microparticles (mean ± S.D, n = 3).
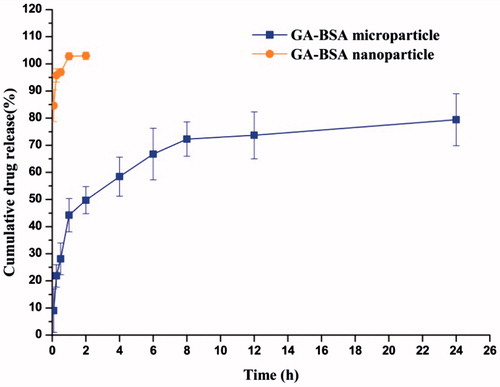
The release data of GA–BSA microparticles were fitted into different models to determine the release kinetics. As shown in , Higuchi equation was the best among the fitted model equations, revealing the release mechanism of GA from BSA microparticles primarily dominated by diffusion. Since GA was highly dispersed inside the microparticles, drug molecules were ready for diffusion out of the microparticles when water was penetrated into the matrix and dissolved the GA.
Table 1. Correlation coefficient values from different model simulation of in vitro GA–BSA microparticles release data (mean ± S.D, n = 3).
Pharmacokinetic and tissue distribution studies
and show the drug concentration–time profiles and pharmacokinetic parameters of GA solution and microparticles after intravenous injection, respectively. There was no significant difference in Cmax and T1/2 values between GA–BSA microparticles and GA solution, which may be due to the burst of GA from GA–BSA microparticles at the first 30 min. However, for the solution group, GA was quickly removed from blood circulation and the drug level reduced to the minimum after 12 h. The microparticle group showed a delayed elimination, and the drug level was still detectable in serum even after injection for 24 h. The MRT0–24 of GA–BSA microparticle was 2.35-fold higher than that of GA solution, indicating that the microparticle formulation prolonged the average residence time of GA (). Besides, the AUC0 − 24 value of microparticle group was about 2.8 times than that of solution group, which suggested a more preferable relative bioavailability for microparticle than for solution (Wang et al. Citation2015). Compared with the solution formulation, BSA-based microparticles may circulate for a longer time in vivo and/or partly accumulate in some specific tissues and then slowly release drug. Therefore, tissue distribution of GA after intraveneously administrated microparticles or solution was further tested and compared.
Figure 7. Plasma concentration–time curves for GA solution and GA–BSA microparticles in mice after intravenous injection (mean ± S.D, n = 6).
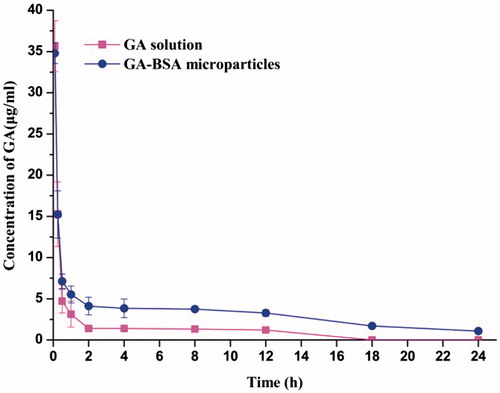
Table 2. Pharmacokinetic parameters following oral administration of GA–BSA microparticles and GA solution (mean ± S.D, n = 6).
GA concentrations in tissue samples are shown in and the related parameters are listed in . Generally, drug level for the microparticle group was lower than that for the solution group in the heart, lung, kidney, and brain. But in the liver and spleen, drug concentrations are significantly higher and more sustained for GA–BSA microparticles than for GA solution. These data indicated that the microparticles were taken up by the mononuclear phagocytic system (MPS) after administrated intraveneously, which was also reflected by the decreased volume of distribution.
Figure 8. Tissue distribution of GA in mice after i.v. administration of GA solution and GA–BSA microparticles ((a) heart, (b) liver, (c) spleen,(d) lung, (e) kidney and (f) brain) (mean ± S.D, n = 6).
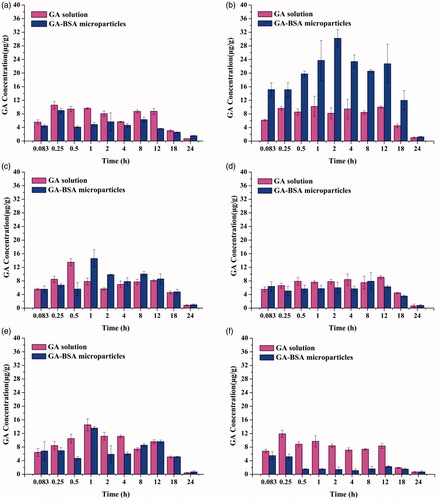
Table 3. AUC of GA solution and GA–BSA microparticles of GA and Re values (mean ± S.D, n = 6).
As deduced from , the AUC for liver tissue after administrated with microparticles formulation was about 2.5 times more than that with solution formulation, suggesting the hepatic tropism of the microencapsulated drug. Due to the possible liver-targeted delivery route of GA–BSA microparticles, much amount of GA would be retained in the liver as compared to GA solution. The previous reports by other authors observed similar results as well (Guo et al. Citation2012). And, the liver-specific delivery characteristic of the prepared GA–BSA microparticles may further improve the therapeutic effect of GA for various liver diseases, and also avoid the toxicity or side effects on other major organs.
Conclusion
The present study showed that the poorly water-soluble drug GA could be encapsulated into BSA-based microparticles by a simple desolvation and coacervation/cross-linking technique. GA was highly dispersed into the albumin matrix at a preferable loading capacity of about 30%. In vitro drug release profile of GA–BSA microparticles showed an initial burst effect and then sustained release phase. Biodistribution test in mice indicated that the GA–BSA microparticles provided higher serum drug concentration for a prolonged period and enhanced liver uptake in comparison with GA solution, suggesting its potential application as liver-targeted drug delivery vector.
Funding information
This work was supported by the National Natural Science Foundation of China (81360644).
Disclosure statement
The authors declare no conflicts of interest.
References
- Bera H, Boddupalli S, Nayak AK. 2015. Mucoadhesive-floating zinc-pectinate-sterculia gum interpenetrating polymer network beads encapsulating ziprasidone HCl. Carbohydr Polym. 131:108–118.
- Birnbaum DT, Kosmala JD, Henthorn DB, Brannon-Peppas L. 2000. Controlled release of beta-estradiol from PLAGA microparticles: the effect of organic phase solvent on encapsulation and release. J Control Release. 65:375–387.
- Chorny M, Fishbein I, Danenberg HD, Golomb G. 2002. Lipophilic drug loaded nanospheres prepared by nanoprecipitation: effect of formulation variables on size, drug recovery and release kinetics. J Control Release. 83:389–400.
- Ci Y, Wang L, Guo Y, Sun R, Wang X, Li J. 2015. Study on encapsulation of chlorine dioxide in gelatin microsphere for reducing release rate. Int J Clin Exp Med. 8:12404–12410.
- Das S, Ng KY, Ho PC. 2011. Design of pectin-based microparticle formulation using zinc ions as the cross-linking agent and glutaraldehyde as the hardening agent for colonic-specific delivery of resveratrol: in vitro and in vivo evaluations. J Drug Target. 19:446–457.
- D'Mello SR, Yoo J, Bowden NB, Salem AK. 2014. Microparticles prepared from sulfenamide-based polymers. J Microencapsul. 31:137–146.
- Elzoghby AO, Samy WM, Elgindy NA. 2012. Albumin-based nanoparticles as potential controlled release drug delivery systems. J Control Release. 157:168–182.
- Guo BH, Cheng Y, Lin LP. 2010. Preparation and pharmaceutical characterization of glycyrrhetinic acid liposomes. Chin Trad Herb Drug. 41:380–383.
- Guo BH, Cheng Y, Wu W, Lin LP, Lin DH. 2012. HPLC assay and pharmacokinetics and tissue distribution study of glycyrrhetinic acid liposomes modified with galactosylated lipid. J Liposome Res. 22:120–127.
- Han TF, Yan JH, Dou JJ, Hui X, Xu K, Chen G, et al. 2012. Study on the preparation technique of glycyrrhetinic acid ethosomes. Northwest Pharmaceut J. 27:64–66.
- Kouchakzadeh H, Shojaosadati SA, Shokri F. 2014. Efficient loading and entrapment of tamoxifen in human serum albumin based nanoparticulate delivery system by a modified desolvation technique. Chem Eng Res Des. 92:1681–1692.
- Kwon JH, Lee BH, Lee JJ, Kim CW. 2004. Insulin microcrystal suspension as a long-acting formulation for pulmonary delivery. Eur J Pharm Sci. 22:107–116.
- Langer K, Balthasar S, Vogel V, Dinauer N, von Briesen H, Schubert D. 2003. Optimization of the preparation process for human serum albumin (HSA) nanoparticles. Int J Pharm. 257:169–180.
- Liu D, Xu H, Tian B, Yuan K, Pan H, Ma S, et al. 2012. Fabrication of carvedilol nanosuspensions through the anti-solvent precipitation-ultrasonication method for the improvement of dissolution rate and oral bioavailability. AAPS PharmSciTech. 13:295–304.
- Liu HQ, Zhang XJ. 2005. Study on the preparation and properties in vitro of fluorouracil albumin sustained release microsphere. Guangdong Chem Ind. 8:37–39.
- Lu Y, Li J, Wang G. 2008. In vitro and in vivo evaluation of mPEG-PLA modified liposomes loaded glycyrrhetinic acid. Int J Pharm. 356:274–281.
- Minamimura T, Sato H, Kasaoka S, Saito T, Ishizawa S, Takemori S, et al. 2000. Tumor regression by inductive hyperthermia combined with hepatic embolization using dextran magnetite-incorporated microspheres in rats. Int J Oncol. 16:1153–1158.
- Norde W, Giacomelli CE. 2000. BSA structural changes during homomolecular exchange between the adsorbed and the dissolved states. J Biotechnol. 79:259–268.
- Patino T, Soriano J, Barrios L, Ibanez E, Nogues C. 2015. Surface modification of microparticles causes differential uptake responses in normal and tumoral human breast epithelial cells. Sci Rep. 5:1–12.
- Prabhu S, Jacknowitz AI, Stout PJ. 2001. A study of factors controlling dissolution kinetics of zinc complexed protein suspensions in various ionic species. Int J Pharm. 217:71–78.
- Pu XH, Sun J, Han JH, Lian H, Zhang P, Yan ZT, et al. 2013. Nanosuspensions of 10-hydroxycamptothecin that can maintain high and extended supersaturation to enhance oral absorption: preparation, characterization and in vitro/in vivo evaluation. J Nanopart Res. 15:1–13.
- Viúdez A, Ramírez N, Hernández-García I, Carvalho FL, Vera R, Hidalgo M. 2014. Nab-paclitaxel: a flattering facelift. Crit Rev Oncol Hematol. 92:166–180.
- Wilson B, Ambika TV, Patel RD, Jenita JL, Priyadarshini SR. 2012. Nanoparticles based on albumin: preparation, characterization and the use for 5-flurouracil delivery. Int J Biol Macromol. 51:874–878.
- Wang Z, Li Z, Zhang D, Miao L, Huang G. 2015. Development of etoposide-loaded bovine serum albumin nanosuspensions for parenteral delivery. Drug Deliv. 22:79–85.
- Weber C, Kreuter J, Langer K. 2000. Desolvation process and surface characteristics of HSA nanoparticles. Int J Pharm. 196:197–200.
- Xing X, Zhang B, Wang X, Liu F, Shi D, Cheng Y. 2015. An “imaging-biopsy” strategy for colorectal tumor reconfirmation by multipurpose paramagnetic quantum dots. Biomaterials. 48:16–25.
- Zhang B, Wang X, Liu F, Cheng Y, Shi D. 2012. Effective reduction of nonspecific binding by surface engineering of quantum dots with bovine serum albumin for cell-targeted imaging. Langmuir. 28:16605–16613.
- Zhao K, Ding M, Cao H, Cao ZX. 2012. In-vitro metabolism of glycyrrhetinic acid by human and rat liver microsomes and its interactions with six CYP substrates. J Pharm Pharmacol. 64:1445–1451.
- Zhao X, Liu J, Hu Y, Fan Y, Wang D, Yuan J, et al. 2012. Optimization on condition of glycyrrhetinic acid liposome by RSM and the research of its immunological activity. Int J Biol Macromol. 51:299–304.
- Zhong DG, Liu ZH, Huang YC, Shi YF, Zhang Y, Xue W. 2012. Preparation and in vitro release of alginate-carboxymethyl chitosan composite hydrogel beads for 18β-glycyrrhetinic acid delivery. Mater Rev B. 26:77–80.