Abstract
Poor solubility of crocetin has limited its use as a potential chemotherapeutic agent. In this study, a crocetin nanocarrier was formulated based on poly(lactic-co-glycolic acid) (PLGA) polymer. The effect of preparation conditions on the size and entrap efficiency of PLGA nanoparticles (NPs) via single emulsion/solvent evaporation method was evaluated. Cytotoxicity of crocetin and crocetin-encapsulated PLGA NPs was assayed in MCF-7 cell line. In PLGA formulations, the optimal condition was found to be 5% polyvinyl alcohol (PVA), crocetin:polymer ratio 1:20 and dichloromethane as organic solvent. Obtained results demonstrated that the encapsulation of crocetin in PLGA significantly increased the cytotoxicity of this compound.
Keywords:
Introduction
Saffron is the dried stigma which isolated from the flower of Crocus sativus and traditionally cultivated in several Mediterranean countries such as France, Turkey, Italy, Spain, Greece as well as Iran (Giaccio Citation2004, Schmidt and Betti GandHensel Citation2007). It is a non-toxic and not mutagenic natural substance which has been widely used as food additive from ancient times (Schmidt and Betti GandHensel Citation2007). Crocetin is a carotenoid dicarboxylic acid which is one of the major bioactive components of saffron. It showed excellent antioxidant, anti-atherosclerotic, anti-inflammatory, and anti-cancer activities in vitro and in vivo (Bathaie et al. Citation2014, Gutheil et al. Citation2012, Nam et al. Citation2010). As anti-cancer agent, it prevents DNA, RNA, and protein synthesis within the cells and also inhibits growth factor signaling pathways and cell proliferation (Danhier et al. Citation2012, Gutheil et al. Citation2012). Nevertheless, the biological application of crocetin is limited due to its hydrophobic nature and poor aqueous solubility (Giaccio Citation2004).
Encapsulation of crocetin in appropriate nanoparticle (NP) systems could be considered as an ideal solution to overcome aforementioned limitations, enhance water solubility and improve its pharmacokinetics and bioavailability.
Various NPs have been engineered and applied for insoluble bioactive compounds. Poly (lactic-co-glycolic acid) (PLGA) is one of the well-known Food and Drug Administration (FDA)-approved biodegradable and biocompatible polyester which have been widely used as a carrier for gene, vaccine, protein, and peptide delivery (Acharya and Sahoo Citation2011, Danhier et al. Citation2012, Kim et al. Citation2005). A wide variety of hydrophobic drugs including natural phytochemicals like curcumin, coumarin, and many others have been successfully encapsulated in PLGA as nano-formulation for use in therapeutic applications (Bhattacharyya et al. Citation2011, Lu et al. Citation2009, Makadia and Siegel Citation2011).
In this study, corecetin was encapsulated in PLGA NPs in order to improve its solubility and enhance its therapeutic efficiency. Single emulsion/solvent evaporation method was implemented for preparation of corecetin-loaded PLGA NPs. The effect of preparation conditions on the size and entrap efficiency of PLGA NPs was evaluated. Cytotoxicity effect of crocetin and crocetin-loaded PLGA NPs was assayed in MCF-7 cell line by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) test, annexin/PI and PI staining of DNA fragmentation.
Materials and methods
Materials
Poly(d,l-lactic-co-glycolic acid) (PLGA) (Average Mw: 7000–17.000; lactic acid: glycolic acid = 50:50) and MTT was purchased from Sigma. Crocetin was extracted from plant Crocus sativus L. based on the method represented in the Iran patent no. 84459. Polyvinyl alcohol (PVA, 87–89% hydrolyzed, average Mw = 88,000–97,000) and other solvent and chemical reagents were procured from Merck (Darmstadt, Germany) without further purification.
Preparations of PLGA NPs
Crocetin loaded PLGA NPs were prepared by modified single emulsion–solvent evaporation method. Different parameters such as concentration of PVA as stabilizer (2 and 5%), drug:polymer ratios (1:10 and 1:20), organic solutions for PLGA (dichloromethane (DCM) and DCM:acetone (ACE) were considered.
Briefly, 25 mg of PLGA was dissolved in dichloromethane (900 μl). Then, 1.25 mg crocetin was also dissolved in 100 μl of pyridine and mixed with the PLGA solution. In the next step, the resulting solution was dropwise added to 4 ml of PVA aqueous phase and emulsified for 10 min using probe sonicator (Hielscher, UP200Ht, Germany). Then, the emulsion was added to 10 ml of stirring 0.1% PVA at room temperature overnight to evaporate the organic solvent. The PLGA NPs were centrifuged at 15,000 rpm for 15 min and the pellet was washed three times with distilled water (DDW) and finally lyophilized.
Particle size and zeta potential measurement
One milligram lyophilized NPs were dispersed in 1000 μl of deionized water and then after sonication, particle size, and zeta potential were analyzed with Malvern NanoZS instrument and DTS software (Zetasizer Nano Z, Malvern Instruments Ltd, Malvern, Worcestershire, UK). Three independent samples (n = 3) were prepared and the results are reported as mean ± SD.
Determination of encapsulation efficiency (EE) and drug loading
One milligram of lyophilized crocetin-PLGA NPs was dissolved in 1 ml dimethyl sulfoxide (DMSO) and sonicated to release crocetin into DMSO. The concentration of crocetin was determined by a UV spectrophotometer (Shimadzu UV-1700 PharmaSpec, Kyoto, Japan) at 430 nm and using a crocetin standard curve. The EE (%) and loading content (LC%) of PLGA NPs loaded with crocetin were calculated as follows:
In vitro release study
The release of crocetin from PLGA NPs was determined by measuring the cumulative amount of crocetin released from the NPs over predetermined time intervals as described elsewhere (Mukerjee and Vishwanatha Citation2009). Ten milligrams of lyophilized PLGA–crocetin NPs was dispersed in 5 ml phosphate buffer saline (PBS) at physiological pH (7.4) and incubated at 37 °C under constant shaking. At selected time intervals, the solution was centrifuged at 3000 rpm for 10 min to separate the released (pelleted) crocetin from the loaded PLGA. The released crocetin was dissolved in 500 μl DMSO and finally determined using UV spectrophotometer at 430 nm as explained above.
Cell culture
MCF-7 cells (ATCC, Manassas, VA; catalog numbers HTB-22) were cultured in RPMI medium supplemented with 10% fetal bovine serum (FBS), penicillin at 100 U/ml and streptomycin at 100 μg/ml and incubated at 37 °C in a humidified 5% CO2 atmosphere.
Cytotoxicity assay
MCF-7 cells were seeded in 96-well plates at an initial density of 1 × 104 cells per well and incubated for 24 h. Then, the media was removed and 100 μl of different concentrations of crocetin or PLGA–crocetin NPs in RPMI were added to each well. After 24 h incubation, 20 μl of MTT reagent was added to each well and incubated for either 1.5 h at 37 °C. The medium was then removed and the formazan crystals were dissolved in 100 μl DMSO. The absorbance was read at 570/630 nm by an Infinite® 200 PRO multimode microplate reader (Tecan Group Ltd., Männedorf, Switzerland).
PI staining
Apoptotic cells were determined by staining using the propidium iodide (PI) method described elsewhere (Hashemi et al. Citation2015). In brief, MCF-7 cells were seeded at a density of 2 × 104 cells per well in 48-well plates. After 24 h, the cells treated with different concentration of crocetin and PLGA–crocetin NPs for 24 h. Then, floating and adherent cells were harvested, collected, and incubated with 150 μl of hypotonic buffer (50 μg/ml PI in 0.1% sodium citrate, 0.1% triton X-100, and 100 μg/ml RNAase A) in the dark at 4 °C for 2 h. After centrifugation at 5000 x g for 15 min, cells analyzed with flow cytometry (BD FACSCalibur Flow Cytometer with Sorting Option: 4-Color, Cat No. 342976, Franklin Lakes, NJ) to detect the cells that have lost some of their DNA in late stage of apoptosis process (sub-G1 peak).
Cell labeling with annexin V-FITC and PI
The phosphatidyle serine translocation in apoptotic cells was monitored by annexin V-FITC/PI staining using an apoptosis detection kit (BioVision, 100 assays, Cat No. K101-100, Milpitas, CA). MCF-7 cells were seeded at a density of 2 × 105 cells per well in 6-well plates. The cells treated with different concentration of crocetin and PLGA–crocetin NPs for 24 h. Then, cells were collect and resuspend in 100 μl of 1× binding buffer (10 mmol/l HEPES/NaOH, pH 7.4, 2.5 mmol/l CaCl2, 140 mmol/l NaCl). Then 5 μl of annexin V-FITC and 10 μl of PI labeling solutions were added to cell and incubated in the dark for 15 min at room temperature. Then 400 μl of binding buffer was added to solution and analyzed with flow cytometry to identify different types of cell death – either necrosis or apoptosis.
Statistical analysis
Data analysis was performed using student’s t test with InStat software. P values of ≤0.05 were considered significant. Results are reported as the mean ± SD of triplicates.
Results
Encapsulation of crocetin in PLGA NPs
In PLGA–crocetin formulations, the optimal condition was found to be 5% PVA, drug:polymer ratio 1:20, and dichloromethane as organic solvent (). The drug loading efficiency was about 97.20 and the LC was also calculated to be 4.8%. The particle size of the NPs which prepared under aforementioned condition was about 288 nm.
Table 1. Composition and physicochemical parameters of crocetin-encapsulated PLGA nano-formulations.
In vitro drug release
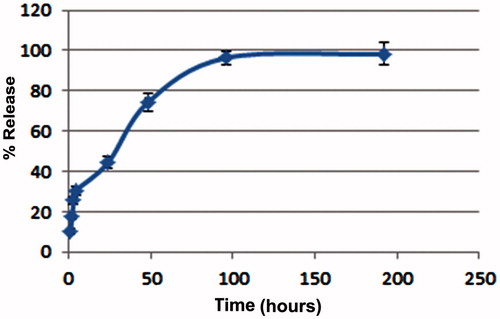
In vitro crocetin release profile from PLGA NPs has been conducted in a PBS solution at pH 7.4. As shown in , our result showed that about 44.49 ± 2.8% of crocetin released after 24 h and drug release gradually increased with overtime hours and maximum released was obtained after 4 d.
Cytotoxicity assay
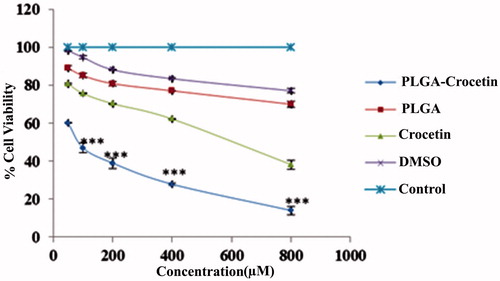
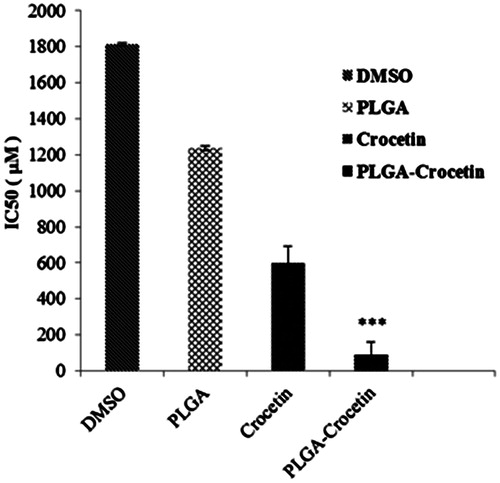
The cytotoxicity of crocetin and crocetin-encapsulated PLGA NPs were evaluated by MTT assay. Our result showed that encapsulation of crocetin in PLGA leads to superior anti-proliferative effect and significantly decreases in the IC50 of crocetin in a dose-dependent manner (). The IC50 of PLGA–crocetin NPs was 84.73 ± 12.14 μM whereas free crocetin showed higher IC50 values (589.65 ± 5.72 μM) on MCF-7 cells ().
Determination of apoptotic cells by PI staining and annexin V-FITC
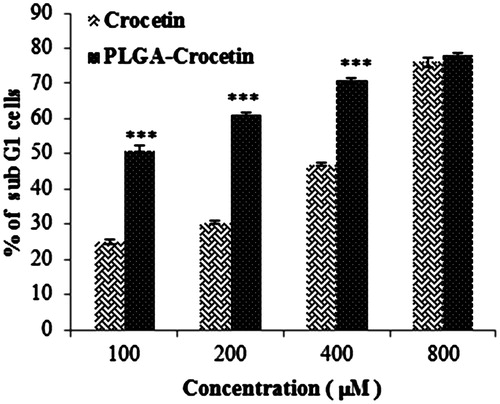
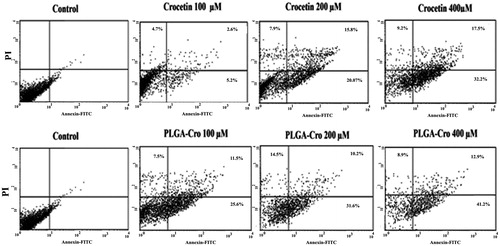
A flow cytometeric study of PI-stained cells was also performed to assess the effect of crocetin on the generation of sub-diploid cells. Treatment of MCF-7 cells with PLGA containing 100, 200, and 400 μM crocetin could significantly increase the induced a sub-G1 population compared with free crocetin (). Furthermore, flow cytometry analysis using annexin V-FITC showed the enhanced percentage of necrosis and apoptosis cells by crocetin–PLGA NPs in comparison with crocetin itself ().
Discussion
Crocetin is one of the most potent carotenoids of saffron which exhibits a wide range of biological activities including anti-tumor activity (Gutheil et al. Citation2012). However, poor aqueous solubility of crocetin has limited the use of this phytochemical as a potential chemotherapeutic agent (Giaccio Citation2004). NP formulation is one of the drug delivery strategies applied for the enhancement of the solubility, safety, pharmacokinetic behavior, and bioavailability of poorly soluble bioactive drugs. For example, to improve the efficiency of curcumin as the principal curcuminoid of turmeric, curcumin has been encapsulated in various types of nanocarriers including liposomes, polymeric NPs and micelles, solid lipid nanoparticle (SLN), nanostructure lipid carrier (NLC), and cyclodextrin (Naksuriya et al. Citation2014, Tsai et al. Citation2011). PLGA, as a biocompatible and biodegradable polymer, has been shown to be one of the attractive systems for the formulation of insoluble compound (Bala et al. Citation2004). In this study, crocetin was encapsulated into PLGA polymers by single emulsion–solvent evaporation method (o/w) to improve its solubility and therapeutic efficiency. The solvent evaporation technique has been used extensively to prepare polymeric NPs containing different agents. Single solvent evaporation is a platform easily adapted with high-entrap efficiency for hydrophobic compounds (Edel Sah and Hongkee Sah Citation2015). However, the preparation conditions should be optimized based on the physicochemical properties of encapsulated drugs. The solubility of the drugs in organic phase is an important parameter when selecting the emulsion phases for a NP preparation process. Crocetin is soluble in DMF, DMSO, and pyridine but when the crocetin solutions in DMF and DMSO were added to DCM or DCM:ACE as organic phase, the solubility of crocetin was decreased. This problem was not observed with pyridine. The effect of other parameters such as organic phase type, PVA concentration, and crocetin:polymer ratio on NPs size and EE% was also evaluated. Previous researches have also studied the effect of various formulation parameters on the properties of PLGA NPs containing different drugs (Narayanan et al. Citation2014, Sharma et al. Citation2016, Wang et al. Citation2013).
In this study, PLGA NPs with a smaller size was produced when DCM was used alone as organic phase. Using acetone with more polarity index along with DCM may cause a decrease in solubility of crocetin. In our work, PVA in 2 and 5% concentration were applied in the formulations. The mean diameter of the crocetin– PLGA NP with PVA 5% was lower than PVA 2% maybe due to more reducing the surface tension of continuous phase. This result is in agreement with other studies (Moayedian et al. Citation2015, Sahoo et al. Citation2002), however, in some researches, it was reported a dramatically increased mean diameter when the PVA concentration was increased (Narayanan et al. Citation2014, Ranjan et al. Citation2012, Wang et al. Citation2013).
It was observed that the particle size decreased with increasing the drug:polymer ratio. Because only a fixed amount of drug can be encapsulated into a certain amount of the polymer, therefore, it seems that further increase in amount of polymer may have resulted in a more encapsulated crocetin and thus reduce viscous dispersed phase resulting in smaller particles. The result of EE% showed an increased from 83.45 to 97.20% when the amount of polymer was increased.
In the optimal condition, the drug loading efficiency was about 97.20 and the LC was also calculated to be 4.8%. The particle size of the NPs which prepared under aforementioned condition was about 288 nm. Obtained results demonstrate that the concentration of surfactant (PVA) and organic solvent were crucial for preparation of nano-sized drug-loaded particles (<300 nm).
In in vitro release study, an initial burst release of crocetin from PLGA NPs was observed during the first 24 h of incubation (44.49 ± 2.8%) and then this value increased gradually from 44.49 to 96.43 ± 3.4% after 4 d. The release profile indicated the burst release was nearly 40% over the first release day, which may relate to crocetin adsorbed on the surface of the PLGA NPs. Other researchers previously reported similar trends for drug release from PLGA formulation (Alebooye Langroodi et al. Citation2016, Sharma et al. Citation2016, Tsai et al. Citation2011, Verderio et al. Citation2013).
Our result showed that encapsulation of crocetin in PLGA NPs lead to superior anti-proliferative effect and significantly decreases in the IC50 of crocetin in a dose-dependent manner. The better efficacy of crocetin loaded into PLGA NPs in comparison to free crocetin maybe due to differential cellular uptake and improve the aqueous solubility of crocetin which further enhances its efficacy to induce apoptosis in cancer cells. This finding is similar to other studies in which drug loaded in PLGA NPs could increase cellular uptake, bioactivity, and bioavailability of compound (Anand et al. Citation2010, Bhattacharyya et al. Citation2011, Nair et al. Citation2012).
Conclusion
The results indicated that the particle size of PLGA–crocetin NPs could be decreased by an increase in drug:polymer ratio, the PVA concentration and using DCM as organic solvent. Obtained results demonstrated that the encapsulation of crocetin into PLGA NPs significantly increased the cytotoxicity and apoptotic properties of this compound in cancer cell line. This study suggested that PLGA–crocetin NPs could be a potential therapeutic tool in breast cancer therapy.
Acknowledgements
The authors are grateful for the financial support received from the Research Council of Mashhad University of Medical Sciences (Grant No. 910744).
Disclosure statement
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Acharya S, Sahoo SK. 2011. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv Drug Deliv Rev. 63:170–183.
- Alebooye Langroodi F, HG Z, Alibolandi M, Ebrahimian M, Hashemi M. 2016. Evaluation of the effect of crocetin on antitumor activity of doxorubicin encapsulated in PLGA nanoparticles. Nanomed J. 3:23–34.
- Anand P, Nair HB, Sung B, Kunnumakkara AB, Yadav VR, Tekmal RR, Aggarwal BB. 2010. Design of curcumin-loaded PLGA nanoparticles formulation with enhanced cellular uptake, and increased bioactivity in vitro and superior bioavailability in vivo. Biochem Pharmacol. 79:330–338.
- Bala I, Hariharan S, Kumar MR. 2004. PLGA nanoparticles in drug delivery: the state of the art. Crit Rev Ther Drug Carrier Syst. 21:387–422.
- Bathaie S, Farajzade A, Hoshyar R. 2014. A review of the chemistry and uses of crocins and crocetin, the carotenoid natural dyes in saffron, with particular emphasis on applications as colorants including their use as biological stains. Biotech Histochem. 89:401–411.
- Bhattacharyya SS, Paul S, De A, Das D, Samadder A, Boujedaini N, Khuda-Bukhsh AR. 2011. Poly (lactide-co-glycolide) acid nanoencapsulation of a synthetic coumarin: cytotoxicity and bio-distribution in mice, in cancer cell line and interaction with calf thymus DNA as target. Toxicol Appl Pharmacol. 253:270–281.
- Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Preat V. 2012. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 161:505–522.
- Edel Sah, Hongkee Sah. 2015. Recent trends in preparation of poly(lactide-co-glycolide) nanoparticles by mixing polymeric organic solution with antisolvent. J Nanomater. 2015:2–22.
- Giaccio M. 2004. Crocetin from saffron: an active component of an ancient spice. Crit Rev Food Sci Nutr. 44:155–172.
- Gutheil WG, Reed G, Ray A, Anant S, Dhar A. 2012. Crocetin: an agent derived from saffron for prevention and therapy for cancer. Curr Pharm Biotechnol. 13:173–179.
- Hashemi M, Parhiz H, Mokhtarzadeh A, Tabatabai SM, Farzad SA, Shirvan HR, Ramezani M. 2015. Preparation of effective and safe gene carriers by grafting alkyl chains to generation 5 polypropyleneimine. AAPS PharmSciTech. 16:1002–1012.
- Kim D, El-Shall H, Dennis D, Morey T. 2005. Interaction of PLGA nanoparticles with human blood constituents. Colloids Surf B Biointerfaces. 40:83–91.
- Lu JM, Wang X, Marin-Muller C, Wang H, Lin PH, Yao Q, Chen C. 2009. Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Rev Mol Diagn. 9:325–341.
- Makadia HK, Siegel SJ. 2011. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers (Basel). 3:1377–1397.
- Moayedian TMF, Khameneh B, Tafaghodi M. 2015. Combined effects of PEGylation and particle size on uptake of PLGA particles by macrophage cells. Nanomed J. 2:299–304.
- Mukerjee A, Vishwanatha JK. 2009. Formulation, characterization and evaluation of curcumin-loaded PLGA nanospheres for cancer therapy. Anticancer Res. 29:3867–3875.
- Nair KL, Thulasidasan AKT, Deepa G, Anto RJ, Kumar GV. 2012. Purely aqueous PLGA nanoparticulate formulations of curcumin exhibit enhanced anticancer activity with dependence on the combination of the carrier. Int J Pharm. 425:44–52.
- Naksuriya O, Okonogi S, Schiffelers RM, Hennink WE. 2014. Curcumin nanoformulations: a review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials. 35:3365–3383.
- Nam KN, Park Y-M, Jung H-J, Lee JY, Min BD, Park S-U, et al. 2010. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur J Pharmacol. 648:110–116.
- Narayanan K, Subrahmanyam VM, Venkata Rao J. 2014. A fractional factorial design to study the effect of process variables on the preparation of hyaluronidase loaded PLGA nanoparticles. Enzyme Res. 2014:162962.
- Ranjan AP, Mukerjee A, Helson L, Vishwanatha JK. 2012. Scale up, optimization and stability analysis of Curcumin C3 complex-loaded nanoparticles for cancer therapy. J Nanobiotechnol. 10:38.
- Sahoo SK, Panyam J, Prabha S, Labhasetwar V. 2002. Residual polyvinyl alcohol associated with poly (D,L-lactide-co-glycolide) nanoparticles affects their physical properties and cellular uptake. J Control Release. 82:105–114.
- Schmidt M, Betti GandHensel A. 2007. Saffron in phytotherapy: pharmacology and clinical uses. Wien Med Wochenschr. 157:315–319.
- Sharma N, Madan P, Lin S. 2016. Effect of process and formulation variables on the preparation of parenteral paclitaxel-loaded biodegradable polymeric nanoparticles: a co-surfactant study. Asian J Pharm Sci. 11:404–416.
- Tsai YM, Chien CF, Lin LC, Tsai TH. 2011. Curcumin and its nano-formulation: the kinetics of tissue distribution and blood–brain barrier penetration. Int J Pharm. 416:331–338.
- Verderio P, Bonetti P, Colombo M, Pandolfi L, Prosperi D. 2013. Intracellular drug release from curcumin-loaded PLGA nanoparticles induces G2/M block in breast cancer cells. Biomacromolecules. 14:672–682.
- Wang H, Jia Y, Hu W, Jiang H, Zhang J, Zhang L. 2013. Effect of preparation conditions on the size and encapsulation properties of mPEG-PLGA nanoparticles simultaneously loaded with vincristine sulfate and curcumin. Pharm Dev Technol. 18:694–700.