Abstract
Despite great advances in tissue engineering, there have been very few reports on the successful clinical use of small-diameter tissue-engineered vascular grafts (TEVGs). Small-diameter (<6 mm internal diameter) is considered as unmet clinical need. This review critically examines the role of stem cells that have been proposed and used in development of TEVGs, and assesses the viability of such graft to clinical pathway. With over 20 years of expertise in development of bypass graft and a number of grafts under clinical trial, this team will offer potential areas for future research that may help improve the current state-of-the-art technology.
Introduction
Cardiovascular diseases (CVDs) are the major cause of death globally claiming over 17 million lives annually (WHO Citation2015). Due to the fact that large numbers of patients are afflicted with CVDs (Liu et al. Citation2007) and peripheral arterial disease (Harris et al. Citation2011), there is a significant demand for small-diameter (<6 mm inner diameter) cardiac and vascular grafts, that are still considered as unmet clinical need. Autologous grafts such as the saphenous vein, which require surgical harvest, may be unavailable due to previous disease and often fail within 10 years due to the inherent differences between arteries and veins (Krawiec and Vorp Citation2012, Liu et al. Citation2007). Various other bypass grafts have been proposed such as synthetic grafts (Kannan et al. Citation2006), cryopreserved allografts (Thakrar et al. Citation2006), and biologically based grafts composed of terminally differentiated cells (Hirai and Matsuda Citation1996, L'Heureux et al. Citation1998, Niklason et al. Citation1999, Weinberg and Bell Citation1986), but none of them succeeded to become clinical practice or commercialized.
Synthetic conduits have shown good mechanical properties, have paved the way for preparation of grafts using an ‘off-the-shelf’ approach, and are convenient alternatives as they minimize the steps before implantation; however, their usefulness in small arteries has not been proven due to development of IH, thrombus formation and calcium deposition. Modification of inner surfaces of the grafts with protein and peptides coatings reduce immediate thrombosis (Solouk et al. Citation2011a, Citation2011b, Citation2015, Yeganeh et al. Citation2008), but do little to improve the long-term arterial graft patency (Cleary et al. Citation2012, Zhao et al. Citation2010). The chronic risk of infection, their lack of capacity to remodel, to integrate with the patient’s native tissue for restoring physiologic function, to respond to vasoactive hormones, and to rebuild after injury, are major disadvantages of currently available synthetic ePTFE and Dacron grafts. These factors result in unacceptable or unproven long-term outcomes (Rocco et al. Citation2014).
In case of using bioabsorbable scaffold, the transition of mechanical strength from the scaffold to neotissue beyond complete polymer degradation is crucial for the function of TEVGs, and this transformation process should be adequately characterized before clinical investigation (Rocco et al. Citation2014). To obtain appropriate burst strength and compliance in grafts reinforced with synthetic materials, the polymer resorption rate needs to be balanced with the TEVG remodeling rate. Besides, it should be noted that remodeling of acellular grafts may be compromised in individuals with cardiovascular disease, leading to incomplete endothelialization (Fernandez et al. Citation2014). The use of allografts may also be limited; in fact, the large variability of mechanical properties of blood vessel tissue among the population raises the need for development of individualized grafts that match tissue properties for specific patients (Kamenskiy et al. Citation2011). In case of biological grafts containing terminally differentiated cells (e.g. ECs or SMCs), the need for an invasive procedure, relatively low proliferative potential, and the difficulty of obtaining a sufficient number of cells from a small biopsy of autologous tissue presents important limitations to clinical availability of mature ECs derived from autologous tissue (Melero-Martin et al. Citation2007). SMCs in the elderly population have limited proliferative capacity and reduced collagen production, which impairs the mechanical strength of engineered vessels. In a recent report, although telomerase (hTERT) gene therapy enabled the culture of cells through extension of the cells life span to produce engineered human blood vessels, such therapy did not apparently alter intrinsic aging-associated changes of vascular SMCs, such as the decline in collagen synthesis (Gong and Niklason Citation2008).
One important goal in production of artificial vascular grafts is to create a living neovessel with structural details and biological response similar to the native vessels (Cleary et al. Citation2012). In order to reach this goal and to overcome the limitations associated with alternative conduits, tissue engineering strategies using stem and progenitor cells have been proposed. Tissue engineers need to consider a number of variables ranging from scaffold materials and their properties such as elasticity, porosity and permeability, to choosing the best source of cells and optimum bioreactor parameters, such as mechanical loading to obtain a functional tissue-engineered construct (Zahedmanesh and Lally Citation2012).
The two major strategies, which have been adopted for tissue engineering of vascular grafts rely on scaffold and scaffold free based approaches. In scaffold-based approach (Hasan et al. Citation2014, Rocco et al. Citation2014), the substrate may be derived from decellularized tissue or either synthetic or natural polymers with the necessary mechanical strength, and may be implanted directly or after the addition of cells to improve patency and vascular reactivity. In scaffold free approach or cell sheet technology, stem cells are grown to high cell densities and, after being subjected to tissue culture over several weeks, the resulting interconnected sheets are wrapped around a mandrel to yield a multi-layered cylindrical tissue.
Despite significant progress towards the development of biomaterials and methods to cultivate vascular constructs, appropriate cell sourcing is a critical issue in tissue engineering of blood vessels (Hashi et al. Citation2007). Using a desirable cell source and both approaches mentioned previously (i.e. using scaffold or not), TEVGs can be created to be immunologically compatible, durable, non-thrombogenic, and capable of cell growth and remodeling under physiological conditions (Liu et al. Citation2007).
The role of stem cells in regenerative medicine is crucial as it leads to the production of cell types of interest and transplantable tissues (Kingham and Oreffo Citation2013). However, each stem cell type presents specific challenges for isolation, expansion, differentiation, and its clinical application.
Herein, we critically review the different strategies that have been used to generate and optimize vascular bypass grafts using either bona fide stem cells, e.g. embryonic stem cells with unlimited self-renewal, and multi-lineage differentiation capacity or progenitor cells, e.g. MSCs with limited self-renewal and differentiation ability (Melero-Martin and Dudley Citation2011). This paper also provides a short review on studies focused on application of terminally differentiated cells for creating TEVGs.
TEVGs using terminally differentiated vascular cells
It was more than 3 decade ago, when Weinberg and Bell first proposed the concept of a vascular tissue equivalent using vascular cell types and collagen as a scaffold (Weinberg and Bell Citation1986). The vascular media was created by casting culture medium, collagen, and bovine SMCs together in an annular mold, which was then gelled at 37 °C and contracted to produce a tubular lattice around the central mandrel. The adventitia was cast around the first lattice with fibroblasts, and an open Dacron mesh sleeve was used to provide additional support. Major differences between the TEVG and normal arteries were insufficient amounts of elastin in the matrix, the longitudinal orientation of the SMCs and collagen fibers (thus, a TEVG that was not supported by a mesh, failed by splitting lengthwise), and the small densities of SMCs and collagen in the model compared to those in normal blood vessels. Later, an opaque, dense, tubular tissue was produced (Hirai and Matsuda Citation1996) using a fragile, transparent and highly hydrated collagen gel containing SMCs, which spontaneously shrank around a glass rod with time. By seeding of ECs on its luminal surface, a hybrid vascular tissue with a hierarchical structure resembling that of natural vessels was prepared. Within 6 months of implantation into a venous system, oriented ECs in the intimal layer, contractile-type SMCs and elastic lamellae in the sub-endothelial layer, and oriented collagen fiber bundles in the deeper layer were observed, resembling tissue organization of natural vessels.
Fibrin and collagen gels are among the most versatile and widely utilized biomaterials in vascular tissue engineering, due to their inherent biocompatibility, short time frame for forming three-dimensional constructs, relative ease of fabricating, and the extent to which cells can attach to and remodel these materials. However, the use of biopolymer gels as scaffolds in transplantable vascular grafts has been limited to venous transplant models due to their relatively poor mechanical strength (Adebayo et al. Citation2013). In an approach utilized in 1999, vascular cells were derived from a biopsy of vascular tissue and grown on polyglycolic acid (a biodegradable polymer matrix). Bovine vessels were then cultured under pulsatile conditions, which had rupture strengths greater than 2000 millimeters of mercury, high suture retention strengths and collagen content (Niklason et al. Citation1999). Another type of TEVGs were generated by the contraction and compaction of collagen type I by porcine aortic SMCs onto a compliant polyester graft scaffold (Baguneid et al. Citation2004, Citation2011). Furthermore, ECs attachment and resistance to shear stress was enhanced by incorporating a period of shear stress preconditioning at 10–20 μN/cm2, as well as by surface modification with fibronectin (FN) and collagen to mimic endogenous ECM (Baguneid et al. Citation2004). Preconditioning, surface modification with FN, or both enhanced ECs resistance to shear stress at 93.2 μN/cm2 compared with control grafts, and TEVGs coated with FN, whether preconditioned or not, demonstrated the best results for EC retention.
Transmission electron microscopy has indicated that natural ECM environment has not yet been properly recreated within TEVGs; collagen in tissue engineered arteries is not adequately surrounded by elastin or other collagen fibrils (Fernandez et al. Citation2014). Because ECM of each tissue is unique in terms of composition and topology, which is generated through interactions between the resident cells and microenvironment, decellularized ECM is one of the best choices for providing cells with the natural microenvironment similar to their parent tissue. Vascular tissue may be subjected to decellularization and solubilization in acidic condition, and then adjusted to physiological pH. The resulting decellularized ECM bioink can encapsulate living stem cells and be used with tissue printing technology followed by gelation at 37 °C (Pati et al. Citation2014) to produce a vascular construct.
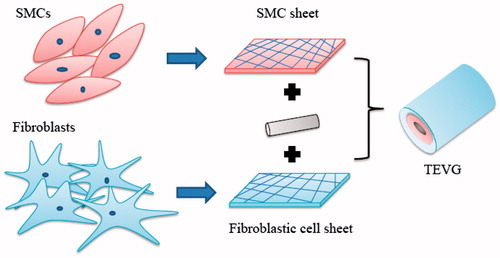
Cellular self-assembly () without exogenous scaffold materials is an attractive alternative approach, which allows for engineered vascular tissue fabrication entirely from cells and CDM. It is a technique developed to produce specific cell orientation relying on the capability of cells to be directed by contact guidance. Although, many cellular self-assembly methods are time consuming and resource intensive, self-assembled constructs have exhibited several potential advantages over scaffold-dependent tissue constructs, including improved strength and biocompatibility, greater cellularity, and increased ECM synthesis (Adebayo et al. Citation2013). The first completely biological TEVG to display burst strength of over 2000 mmHg was created when human SMCs cultured with ascorbic acid produced a cohesive cell sheet which was placed around a tubular support to create the media, and a similar sheet of human fibroblasts was wrapped around the media to provide the adventitia of the vessel (L'Heureux et al. Citation1998). After maturation, the tubular support was removed and ECs were seeded in the lumen. The obtained TEVG featured a well-defined, three-layered organization and ECM composition with numerous extracellular matrix proteins, cell differentiation markers, and cellular functions observed in normal human blood vessels. Short-term grafting experiments in a canine model for 12 weeks demonstrated good handling and suturability characteristics. A decellularized matrix scaffold (dMS) has been produced by self-assembly using human dermal fibroblasts (Bourget et al. Citation2013). The scaffold was seeded with SMCs and rolled into a vascular media. Using this scaffold improved the mechanical and contractile properties, which are two important parameters for a TEVG. Completely biological and nonliving TEVG built from human ECM and allogeneic fibroblasts have also been successful and displayed no signs of degradation or dilation up to 11 months followed by implantation as shunts in hemodialysis patients (Wystrychowski et al. Citation2014).
The relative strength of tissue engineered rings created entirely from self-assembled cells and CDM were compared to cell-populated fibrin or collagen biopolymer gels in a recent study (Adebayo et al. Citation2013). A three-fold increase in tensile strength and stiffness of CDM rings was observed, which were not affected by cell lysis, indicating that ring biomechanics are due to ECM properties. Although the strength of generated CDM tissues (300–650 kPa) was much higher than previously reported values for fibrin and collagen gel constructs, these values were relatively low compared with native arteries (e.g., porcine carotid artery UTS ∼6.6 MPa; rat abdominal aorta, 2.1 MPa).
Since the long times required to synthesize vascular TEVGs are an inherent barrier to their use (Seifu et al. Citation2013), the goal in a recent study was shortening the preparation period, suitable for sub-emergency cases, by in-body tissue architecture technology or “biotube” (Nakayama and Tsujinaka Citation2014). Biotube development is based on tissue capsulation phenomenon, which is one of normal body defense mechanisms induced by weak inflammation reaction against the artificial implant. Using a novel eosin Y-releasing mold, thick walled biotubes primarily colonized by fibroblasts were successfully prepared following a short, 5-day embedding period. Eosin Y stimulation also induced the significant formation of matured capillaries, composed of both ECs and SMCs, which supported the increased thickness of the biotube tissues. Another grafts have also been created, hypothesized to have advantages over completely autologous tissue engineering approaches in terms of reducing the production time (Dahl et al. Citation2011). The TEVGs were generated by culturing human cadaveric donor cells or canine cells on a degradable PGA scaffold to support synthesis of a collagenous extracellular matrix, followed by removing the cellular material to render tissue nonimmunogenic. The grafts retained mechanical properties similar to those of native vessels after 12 months of storage in buffer at 4 °C. TEVGs that were luminally seeded with ECs integrated well with native vasculature and resisted intimal hyperplasia, dilatation and calcification when implanted in canine models of peripheral and coronary bypass.
Controlling vascular cell proliferation and ECM production
IH, where over-proliferation of SMCs occurs in vascular grafts, is due to an imbalance between inhibitory and proliferative mechanisms that control the growth of SMCs. Physiological cyclic strain (less than 15% amplitude) has an anti-proliferative influence on SMCs, and has been shown to upregulate gene expression for synthesis of ECM components such as collagen and elastin.
The mathematical description of tissue growth and resorption as a function of stress and strain have been a complimentary field of research in development of TEVG (Kassab Citation2006). Pathological values of cyclic strain play a conflicting role and increase proliferation of SMCs. Pore fluid velocity within a tissue-engineered construct can significantly improve nutrient delivery and remove waste, which in turn increases the proliferation rate and reduces cell death. Given that it is a challenging task to isolate the dominant cause of IH using in vivo experiments, a mechanobiological model of a TEVG was presented in a study to be used as a predictive and optimization tool for vascular tissue engineering applications, where cyclic strain and pore fluid flow velocity were adopted as the main regulators of SMC growth in TEVGs (Zahedmanesh and Lally Citation2012). It was concluded that to enhance cell growth and reduce culture time, an initial non-pulsatile luminal pressure can be used until cells fully populate the scaffold, following which a pulsatile luminal pressure can enhance ECM synthesis and scaffold remodeling. In an experimental study, porcine aortic SMCs were seeded on non-woven esterified hyaluronic acid (HYAFF 111) sewn tubes and cultured under different mechanical stimulation conditions to analyze their effects on cell proliferation and ECM organization (Arrigoni et al. Citation2008). A bioreactor was used to expose the inner surface of the construct to a low level of pulsatile shear stress (about 1 dyne/cm2), and a simpler rotating vessel with low rotation speed was used to increase mass transfer by mixing culture medium and allowing a more uniform oxygenation of the liquid. The rotating bioreactor allowed obtaining highest ECM production, since oxygen is required as a co-factor by the enzymes involved in the transformation of pro-collagen into functional triple helical collagen. The lower ECM production observed in perfused (and pulsatile) constructs as compared to rotated samples was suggested to be due to metalloproteases-mediated ECM remodeling activated by shear stress.
Besides mechanical stimuli, different growth factors play diverse roles in vascular cell recruitment and proliferation. It has been shown that VEGF can stimulate endothelialization, inhibit excessive proliferation of SMC, and attenuate IH, while PDGF has a high ability to stimulate SMCs proliferation. In a recent study aimed at controlling the rapid delivery of VEGF and prolonged release of PDGF, an electrospun membrane of chitosan hydrogel/poly(ethylene glycol)-b-poly(L-lactide-co-caprolactone) (PELCL) loaded with VEGF was chosen as the inner layer, and an emulsion/PELCL electrospun membrane loaded with PDGF as the outer layer of a tubular construct (Zhang et al. Citation2013). The in vitro cell culture results showed that ECs rapidly adhered and proliferated in the first 6 days, whereas SMCs had a fast growth rate after the 6th day with respect to control samples. After implantation into rabbit carotid artery in vivo for 4 weeks, the vascular grafts permitted ECs adhesion on the lumen, and SMCs grew on the outer layer without thrombosis.
Stem cell-based approaches for development of TEVGs
Pluripotent stem cells
In one of pioneering approaches utilizing hiPS cell lines, the pluripotent cells were first induced into a mesenchymal lineage using a neural crest intermediate stage (Sundaram et al. Citation2014). The derived mesenchymal progenitor cells, which displayed properties similar to marrow stromal cells, were then cultured in a pulsatile bioreactor system over 8 weeks. The cells differentiated into SMCs and organized into a matrix-rich, tubular construct similar to native veins. The resulting TEVGs grown from karyotypically normal iPS derived precursors had burst pressure and suture retention strength approximately half of the saphenous veins. Similarly, a feeder-free strategy was developed for hiPS cell differentiation into a homogeneous population of highly contractile SMC, beginning with epithelial-to-mesenchymal transition and giving rise to an intermediate population of clonogenic MSC (Bajpai et al. Citation2012). The high yield of MSCs and SMCs suggests that this strategy may be used for acquisition of large numbers of cells required for regenerative medicine.
It has been hypothesized that specific combinations of transcription factors and culture conditions could lead to reprogramming from one somatic cell fate to another without an intermediate pluripotent state. Consequently, partially-iPS (PiPS)-SMC differentiation was induced through a DKK3-Wnt signaling pathway, and fibroblasts were reprogrammed into functional SMCs (Karamariti et al. Citation2013). PiPS-SMCs formed vascular structures and differentiated into mature SMC phenotypes when seeded on to decellularized vessel scaffolds in a specially constructed bioreactor. The TEVGs displayed a function in vivo when engrafted into severe combined immunodeficiency mice. Patient-specific integration-free hiPS cells have been developed using episomal vector nucleofection to specifically reprogram non-lymphoid cell subpopulation of peripheral blood mononuclear cells (Hu et al. Citation2015). The iPS cells established by improved reprogramming efficiency were then differentiated into mesoderm-derived cardiovascular progenitor cells.
Multipotent stem cells
Approaches utilizing adult stem cells can address the limitations of using terminally differentiated vascular cells, and the ethical concerns that continue to limit the exploration and use of human embryonic stem cells (Krawiec and Vorp Citation2012).
BM-MNCs
The mononuclear cell fraction in bone marrow consists of progenitor cells, ECs, MSCs, hematopoietic stem cells, monocytes, T cells, B cells, and NK cells, among others. By subjecting the BM-MNCs aspirated from animal models to a differentiation culture period, SMC-like cells expressing α-smooth muscle actin and smooth muscle myosin heavy chain, and EC-like cells expressing von Williebrand factor (vWF) and CD31 can be obtained. A mixed population of these differentiated cells can be seeded on to decellularized arteries or polymeric scaffolds (e.g. poly(glycolic)/poly(l-lactide-co-ɛ-caprolactone)). Alternatively, ECs can be coated onto the luminal surface of a SMC layer seeded onto the scaffold. Such grafts have shown patency for a maximum of 18 weeks with the development of a neo-endothelium and no signs of thrombosis, while unseeded controls have occluded within 2 weeks (Krawiec and Vorp Citation2012).
The preparation time for a TEVG can be reduced by not differentiating stem cells into vascular phenotypes prior to implantation, and this approach may be superior to seeding differentiated vascular cells. Grafts comprising BM-MNCs have shown positive results in animal models with no signs of thrombosis, rupture or calcification for up to 2 years. Following these successful pre-clinical implantations, the TEVGs were used in clinical studies as extra cardiac total cavopulmonary connections, which remained patent for about 5.8 years. The patency of these vascular grafts suggests that implanting BM-MNCs in their undifferentiated state can result in a graft composed of SMCs and ECs, thus it has been theorized that stem cells within the seeded BM-MNC population directly contribute to the cellular composition of neovessel formation. Researchers compared scaffolds seeded with monocytes, the entire population of BM-MNCs, and BM-MNCs without monocytes to provide some insight in to the mechanisms involved. All groups demonstrated patent vessels with endothelium and smooth muscle layers similar to native inferior vena cava, but when explanted at 6 months a significant difference was observed between grafts: grafts seeded with monocytes were significantly larger than those without monocytes. Given the fact that monocytes secrete a variety of angiogenic growth factors and cytokines, it was hypothesized that these cells can contribute to the long-term patency of grafts (Krawiec and Vorp Citation2012). Implanting hBM-MNCs in immunodeficient mice and tracking human cell markers over various time points showed that BM-MNCs remain present in the graft until 1 week, and do not directly differentiate into the mature cells of the neovasculature; In fact, BM-MNCs recruit host cells during this time period in a paracrine fashion by secreting high levels of MCP-1. Given that MCP-1 functions as a potent monocyte chemokine, further investigations into the role of MCP-1 in TEVG development were pursued. biodegradable alginate microparticles encapsulating MCP-1 were embedded into a scaffold to mimic MCP-1 secretion by seeded BM-MNCs. MCP-1 release from embedded microparticles resulted in a vascular graft similar to that produced by hBM-MNC seeded scaffolds, with increased early host monocyte recruitment and improved patency at 10 weeks (Cleary et al. Citation2012).
SMPCs and EPCs
Studies have demonstrated that bone marrow-derived progenitor cells can be used to engineer vasoreactive and implantable TEVG. A highly purified population of ovine BM-SMPCs was obtained that exhibited high proliferation potential, displayed contractile properties and developed a functional mature SMC phenotype (Liu et al. Citation2007). Cells were embedded in fibrin hydrogels, which were polymerized around 4-mm diameter mandrels to engineer cylindrical TEVG. ECs were also isolated from ovine BM-MNCs and seeded in the lumen of TEVG. 5 weeks after implantation of TEVG into the jugular veins of lambs, the grafts demonstrated remarkable ability for matrix remodeling. TEVG prepared from unsorted BM-MNC displayed no contractility, indicating that bone marrow contains only a small fraction of functional SMC, which retain their contractile properties after purification and expansion in vitro.
The mobilization and homing of PB-EPCs has an important role in promoting the endothelialization of TEVG, which prevents thrombosis and IH, thus is important to enhance the patency of TEVG after transplantation. When studying a suitable scaffold for EPCs, it was found that they form tight networks and are more adherent to collagen-chitosan scaffolds compared with collagen scaffolds (Iordache et al. Citation2014). BDNF promotes EC survival and induces neoangiogenesis in ischemic tissues. It was investigated in a study whether BDNF could improve the patency rate of small-diameter tissue-engineered blood vessels through promoting stem cell homing and paracrine activity of EPCs (Zeng et al. Citation2012). The BDNF-modified TEVG grafted to a rat carotid artery, the surface of which was incubated with collagen, formed a complete layer of physiological smooth muscle layer, had significantly less IH compared to the control group, and showed no thrombosis at 2 months after injury.
MSCs
MSCs are adherent adult progenitor cells with the ability to be rapidly expanded, and can be harvested from almost every organ with slight variations in phenotype (Krawiec and Vorp Citation2012). They can differentiate into multiple cell lineages including osteoblasts, adipocytes, chondrocytes, myoblasts, cardiovascular phenotypes and early progenitors of neural cells (Gong and Niklason Citation2008). Cloned hMSCs isolated from a particular source default to give rise to the resident cells of that tissue, suggesting that the tissue environment of origin determines cellular fate (Muraglia et al. Citation2000). When comparing hMSCs isolated from the umbilical cord, bone marrow, and adipose, despite umbilical cord MSCs had the lowest harvest frequency they could be cultured the longest and had the highest proliferation potential (Krawiec and Vorp Citation2012). MSCs need a 3D structure to allow ECM deposition (Nemeno-Guanzon et al. Citation2012). Bioactive factors secreted by MSCs have been found to limit the extent of tissue damage at injured sites and possibly recruit local progenitors (Caplan and Dennis Citation2006).
Guiding MSCs towards a large population of aligned SMCs and ECM
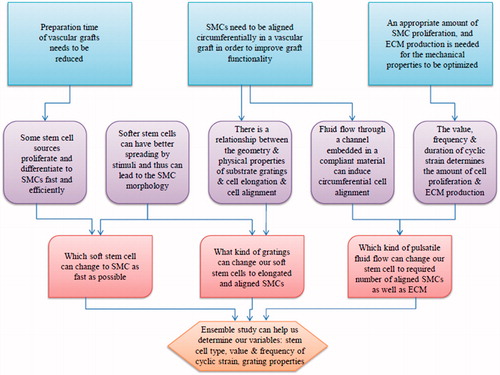
Over the years, many improvements have been made to facilitate SMC culture and to produce vascular media substitutes with higher circumferential mechanical strength. To improve the mechanical resistance and contractility of the vascular media, an alignment of cells and ECM has to be created in the reconstructed tissues (Bourget et al. Citation2013). Cells develop a preferred orientation when they feel a direction-dependent stimulus from their environment. This can be a difference in either the amount of cyclic strain (strain anisotropy), or stiffness value (anisotropy of mechanical resistance) () (Obbink-Huizer et al. Citation2014).
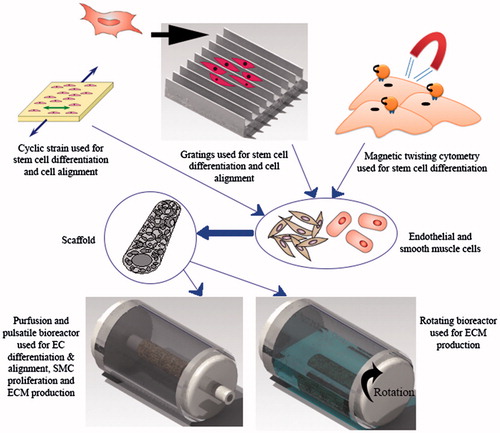
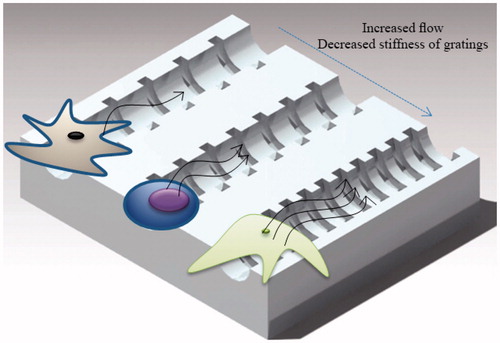
Figure 2. A combination of methods for development of vascular grafts. The frequency of cyclic strain, the geometry of gratings, the amount of magnetic force, the perfusion flow and the number of bioreactor rotations per second need to be quantified and controlled to achieve a vascular graft with required properties.
In tissue engineering, gratings have been used to induce the formation of aligned populations of polarized cells. Stem cell differentiation has been shown to be dependent on substrate rigidity and cell shape; it is therefore possible to use gratings with an optimal geometry to induce cell elongation that corresponds to a desired level of differentiation. A combination of experiments and mathematical modeling has been used to mechanistically understand how anisotropic rigidity directs cell elongation and alignment on gratings, leading to stem cell differentiation (Wong et al. Citation2014). Based on experimental results, it was hypothesized that hMSCs sense the anisotropic rigidity on gratings, which acts as the driving force behind cell elongation. A mathematical model was developed to support this hypothesis, by modeling the individual gratings as beam elements that deflect, tilt or shear when a force is applied to one of the ends. Over the range of grating aspect ratio (0.035–2) and substrate stiffness (0.18–1.43 MPa) investigated, it was shown that both cell elongation and alignment are enhanced on smaller and stiffer gratings. In addition, good correlation was found between cell elongation and the percentage of cells being aligned to the grating axis. Another group of researchers developed a new model of stress fiber formation that allows prediction of cell orientation in complex non-uniform loading conditions, including anisotropic stiffness of the environment, and cyclic tissue deformation (Obbink-Huizer et al. Citation2014). The model qualitatively predicts the following aspects of stress fiber development in response to mechanical stimuli, all observed experimentally: development of more fibers in a direction with higher resistance to mechanical deformation; development of fibers mainly in directions not cyclically strained when the cell resides in a high stiffness environment, with increased strain avoidance for increased strain frequency and strain amplitude; development of more fibers in a cyclically strained direction when the cell resides in a low stiffness environment.
It has been reported that substrate elasticity modulates intracellular rheology and thus, stiffer matrices result in stiffer cells (Chowdhury et al. Citation2009) and larger final spreading area of the cells in a specific time duration (Fan and Li Citation2015). At fixed substrate rigidity, however, intracellular softness can determine cellular biological sensitivity to force. It has been demonstrated that a small local cyclic stress induces time-dependent increase in the spreading and differentiation of the mouse embryonic stem (mES) cell, and that ES cells are much more sensitive to a localized cyclic stress than their differentiated counterparts such as MSCs. The soft mES cells were shown to be more responsive than ES-differentiated cells; when applying the same stress on the same substrate, the greater the cell softness, the stronger the spreading response (Chowdhury et al. Citation2009). Thus, MSCs harvested from different sources may have various spreading capabilities (see for a number of methods of manipulating MSCs). Investigating rheological properties of actin networks can elucidate the mechanics of cells, as it provides insights into the microscopic origin of the viscoelastic behavior of the actin cytoskeleton. By measuring stress in response to applied oscillatory shear strain (the bending and stretching of actin filaments) and thermal fluctuations of individual segments in the polymeric chain, viscoelastic properties of actin-like networks were investigated, and it was shown that the network became increasingly elastic as the prestrain increased (Kim et al. Citation2009).
BMSCs
BMSCs are the most commonly used MSCs typically being harvested from the iliac crest. The MSCs only occupy about 0.01% of the mononuclear cells in bone marrow and this percentage, as well as MSCs differentiation capacity, has been shown to decrease with increased age (Krawiec and Vorp Citation2012). BMSCs can home into sites of vascular injury where they differentiate into vascular cells, and can even be isolated from the same patient or allografted to histocompatible receivers (Liu et al. Citation2007). TEVGs created with BMSCs have been used in vivo and shown positive results. The harvested MSCs from ovine bone marrow were proliferated to sufficient amounts in vitro, induced into ECs-like cells and SMCs-like cells, and seeded on to acellular tubular scaffold produced from ovine carotid artery (Zhao et al. Citation2010). The TEVGs were then implanted into the carotid artery of host ovine and remained patent 5 months after interposition, with no thrombosis or infection observed. Because the labeled MSCs could still be detected after 2 months, it was concluded that the MSCs played a role in the formation of the endothelium and smooth muscle layers. In order to show that the combination of BMSCs with nanofibrous scaffolds made of poly(l-lactic acid) might be a promising approach to fabricate ideal small-diameter vascular grafts, short-term experiments were performed in which platelet adhesion and thrombus formation was induced by nanofibrous scaffolds alone, and was suppressed after BMSC seeding (Hashi et al. Citation2007). It was also showed that nanofibrous TEVGs seeded with BMSCs facilitated efficient vascular cell infiltration and migration, organization of cells into layered structure in vascular wall, and significant ECM synthesis. The gradual loss and removal of BMSCs by the host circulation system, coupled with the organized structure of elastin in intima/media of cellular grafts, suggested that BMSC may have participated in remodeling processes at the early phase post-implantation. To reinforce that it is possible to recapitulate a contractile media layer from MSCs without the need of exogenous scaffolding material, a proportion of BMSCs and umbilical cord blood derived MSCs (UCB-MSCs), which differentiated into SMC-like cells and expressed contraction associated proteins, were induced to assemble into manipulable tissue sheets by being cultured in the presence of ascorbic acid (Bourget et al. Citation2015). BMSC-derived sheets presented a higher mechanical resistance compared to UCB-MSCs, with contractile capability closer to SMC-derived constructs and stronger than dermal fibroblasts derived constructs. BMSCs were, therefore, found to be preferable compared to other cells to reconstruct a media layer.
To evaluate the differential effects of cyclic stretching, cyclic pressure, and shear stress on BMSCs, a set of experiments were performed at confluence to elicit the temporal gene expression response of a selected magnitude of each stimulus, which revealed that EC gene expression can be increased with cyclic pressure and shear stress (Maul et al. Citation2011). In an attempt to design an optimized protocol, whereby hBMSC fate could be controlled in the blood vessel engineering bioreactor, various elements such as growth factors that are elaborated by platelets and vascular cells after vessel injury (PDGF, TGF-β1, and bFGF), extracellular proteins found in native vessel wall, and cyclic mechanical strain were all examined for their effects on hBMSC proliferation and differentiation into SMCs. Under optimized conditions, instead of culturing the vessel walls in the same enhanced DMEM medium for 8 week, the culture period was divided into two phases: 4 weeks of proliferation phase in which PDGF-BB was retained, and another 4 weeks of differentiation phase in which bFGF was removed from the culture medium, PDGF-BB was substituted with 1 ng/ml TGFβ1, and the pulsatile cyclic strain in the bioreactor was initiated. The influence of cyclic strain on hBMSC differentiation was shown to be significantly tied to the presence of ECM as well as PDGF-BB, and among all components of the enhanced DMEM medium, vitamin C, a cofactor for collagen synthesis, stimulated hBMSC proliferation the most. Under the optimized protocol, the researchers observed significantly more collagen deposition and higher expression levels of SMC markers, than those obtained under standard conditions (Gong and Niklason Citation2008).
AFCs, MDSCs and ASCs
Autologous ovine fetal AFCs reveal an immunohistochemical phenotype similar to ovine BMSCs and express stem cell factors described for embryonic stem cells. Isolated ovine amniotic fluid-derived MSCs harvested at different gestational ages successfully differentiated into several mesodermal phenotypes. Myofibroblastic ovine AFC lineages were seeded on to composite scaffolds and exposed to static and dynamic conditioning in a flow bioreactor system to fabricate small- and large-diameter tissue-engineered vascular grafts. While AFC-based tissue-engineered vascular grafts presented high cellularity, glycosaminoglycans and collagen formation, allantoic fluid cell-based controls and all static control constructs showed significantly lower values of ECM components (Weber et al. Citation2016).
The advantages of availability and proliferation during the fabrication process would make the MDSC attractive compared with terminally differentiated cells, even if they would be found to have similar contributions to TEVG patency rate. A novel bilayered scaffold composed of poly(ester urethane)urea, a biodegradable elastomer, has been developed with an inner layer produced by thermally induced phase separation (TIPS) and an outer layer produced by electrospinning (ES) (Nieponice et al. Citation2010). The combination of the two processing techniques to obtain an ES-TIPS scaffold was hypothesized to provide sufficient cell support, tissue ingrowth and mechanical properties. The TIPS layer was used to provide a porous structure for cell infiltration into the graft, and the ES layer was added to give mechanical stability for withstanding arterial conditions. When TIPS and ES-TIPS scaffolds were seeded with MDSCs and then placed in spinner flask culture for 48 h, the newly remodeled tissue consisted of aligned collagen fibers, SMCs, and an endothelial layer, whereas acellular controls showed platelet and fibrin deposition.hASC acquire various endothelial characteristics following culture in EC growth supplement and exposure to fluid shear stress. In vivo evaluation of differentiated ASC towards the EC-phenotype, however, has revealed that they are mildly thrombogenic, possibly due to the lack of endothelial nitric oxide synthase (eNOS) expression. The production of nitric oxide (NO) promotes regulation of systemic blood pressure, modulation of vascular growth, and prevention of coagulation and thrombosis. It was reported in a recent study for the first time, the successful expression of eNOS in ASCs following adenoviral transfection, and the generation of significant concentrations of biologically active NO (McIlhenny et al. Citation2015). The in vitro data suggested that the presence of eNOS, or NO itself, may further improve endothelial differentiation of ASC and subsequent function in vivo. The TEVG lined with eNOS-expressing ASCs that differentiated into an EC-like and non-thrombogenic phenotype, demonstrated a confluent monolayer of cells on their luminal surface after in vivo implantation. Adipose tissue derived MSC have also shown the capability to differentiate into functional ECs using endothelial induced growth factor obtained from human platelet lysates, secreting significantly high quantities of the von willebrand factor (Bertanha et al. Citation2014).
Conclusion and suggestions
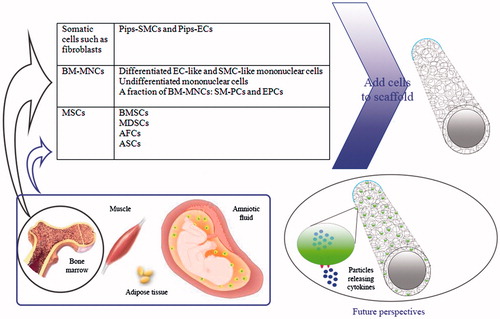
It is known from literature that the ultimate failure of commercial grafts has been mainly due to compliance mismatch and thrombogenicity of inner surface of materials used as vascular bypass graft. Unfortunately, many studies focused on TEVGs lack thorough quantitative data that give us comprehensive information on suturability, collagen and elastin content, mechanical properties of the obtained vessels and the cause of failure after implantation. Non-immunogenic ‘universal donor cells’ could shorten the time required to produce vascular grafts and may be the most appropriate cell sources for vascular tissue engineering. Allogeneic MSCs found to have immunosuppressive effects (Fernandez et al. Citation2014), bone marrow-derived mononuclear cells (Cleary et al. Citation2012), partially iPS cells (Karamariti et al. Citation2013), or AFCs (Weber et al. Citation2016) are also possible candidates (see for characteristics of different stem cell types). Whole bone marrow cell suspensions and the stromal vascular fraction (SVF) of adipose tissue have been used directly with the aim of harnessing the potential of the contained stem cells, both of which represent highly heterogeneous cell populations, thus limiting the translation of MSC-related therapies. Sorting of MSC precursors can address many issues including the purity and potency of progenitors, and the poor regenerative efficiency of SVF (Murray et al. Citation2014, Paredes et al. Citation2011). Pericytes have recently been recognized as distinct cellular entities that have the ability to yield MSCs in culture, which mirrors the broad developmental potential of pericytes (Covas et al. Citation2008, Murray et al. Citation2014). It has been suggested that pericytes are not the only ancestors of MSCs that exclusively present around capillaries and microvessels (Caplan Citation2008), as the adventitia also plays a crucial role in vascular remodeling and the development of vascular diseases (Sartore et al. Citation2001). Pericytes and adventitial cells establish a perivascular compartment as the MSC niche that act to stabilize blood vessels, generating a momentum in the use of adult stem cells for vascular tissue engineering (Murray et al. Citation2014). In a study aimed at finding the origin of coronary arteries, two novel sources of progenitors and also a new program of arteriogenesis (sprouting of the differentiated venous ECs of sinus venous onto the developing heart) were observed (Red-Horse et al. Citation2010), which are promising for future usage in TEVGs. On the other hand, it has been suggested by a group of researchers that the next-generation of TEVGs no longer require seeded cells; modulating the expression of growth factors and cytokines may provide a mechanism to influence the ingrowth of SMCs and ECs in a manner similar to that exerted by seeded cells (Cleary et al. Citation2012). Introducing building blocks that encapsulates certain types of cells with biomolecules (such as inhibitors, growth factors and drugs) and application of untethered micro-robotic coding approach to pattern cell-encapsulating hydrogels, which offers a high level of control over complex tissue architectures, is another direction that future industries in tissue engineering may follow (Tasoglu et al. Citation2014). These works will ideally provide some solutions to the current TEVG limitations by eliminating some of the expense and time required to generate a TEVG (see for a number of current cell sources and future direction).
Table 1. Important features of a number of stem cell types used for TEVG.
Reproducible and solvent-free method of uniaxial thermal stretching has been used to create flexible PCL film with mechanical enhancement and topographical micropatterns comprised of circumferential ridges/grooves (Wang et al. Citation2014). Culturing on such substrate, MSCs self-aligned along the ridges into more elongated morphology and obtained a contractile SMC-like phenotype with ordered cell architecture and potential for tunica media regeneration. To induce mechanical stimuli, a multi-channeled shear flow assay has been reported that allows for simultaneous generation of different pulsatile shear stress levels typical of arterial blood flow in each elastic PDMS microchannel (Song et al. Citation2005). It has been proposed that a combination of existing technologies will help to create progressively more realistic in vitro models of mechanotransduction in living systems. Methods that produce tissue compression might be combined with matrix stretch and chemical gradients, or systems might be produced allowing for simultaneous shear flow and interstitial flow with multiple cell types present (Polacheck et al. Citation2013). Microfluidic systems that incorporate micro- and nano-topographic structures have been developed to investigate synergy between shear stress and ECM topography, and to demonstrate its effect on cytoskeletal and nuclear alignment of SMCs (Yang et al. Citation2011).
Inspired by these works, we propose a device for performing ensemble investigations ( and ), that is, a microfluidic platform made of a flexible material, with several microchannels having circumferential micro-fissures coated with biomolecules or growth factors on their luminal surfaces. A monolayer of stem cells or differentiated SMCs can be coated on the internal surfaces of the micro-channels. The channels within the platform can be fabricated with different-sized gratings to stimulate the cells with various modulus values of substrate, and pulsatile flow can be used to induce different cyclic strains. Some approaches have introduced hydrogel into specific regions of the microfluidic device to culture different types of cells under well-controlled microenvironments (Chung et al. Citation2010). Decellularized ECM gels (Pati et al. Citation2014) from vascular tissue may support and guide the stem cells towards vascular wall resident cells. Gel modulus can always be varied by the introduction of cross-linking, or simply by changing the conditions during polymerization (Chung et al. Citation2010). Then, we may have the capability to regulate the conditions, and also to observe the mechanotransduction response of stem cells at high magnification and in real-time (Polacheck et al. Citation2013). Using optical methods, cell orientation and density as well as ECM density could be quantified and a comparison can be made between the channels into which different mechanical and physical stimuli are introduced. In addition, the results can be scaled up to determine the appropriate bioreactor conditions required for generating TEVGs with similar properties as the native vessels.
Figure 5. A representation of future research derived from the flowchart in figure 4. Circumferentially oriented microscale grooves can be fabricated in different sizes on channel surfaces, providing various physical stimuli and stiffness values of substrate. Multiple types of stem cells can be seeded onto channels and subjected to fluid flow with various properties. The results provide insight into how mechanical stimulation by fluid flow and geometric cues are able to regulate stem cells with desired functions, and have implications for guiding tunica media regeneration with microscale control of cell alignment, shear stress, and pulsatile flow.
| Abbreviations | ||
| AFC(s) | = | amniotic fluid cell(s) |
| (h)ASC(s) | = | (human) adipose derived cell(s) |
| BDNF | = | brain-derived neurotrophic factor |
| (h)BM-MNC(s) | = | (human) bone marrow-derived mononuclear cell(s) |
| (h)BMSC(s) | = | (human) bone marrow-derived MSC(s) |
| BM-SMPC(s) | = | bone marrow-derived SMPC(s) |
| CDM | = | cell-derived matrix |
| EC(s) | = | endothelial cell(s) |
| ECM | = | extracellular matrix |
| EPC(s) | = | endothelial progenitor cells |
| IH | = | intimal hyperplasia |
| (h)ips | = | (human) induced pluripotent stem |
| (h)MSC(s) | = | (human) mesenchymal stem cell(s) |
| MDSC(s) | = | skeletal muscle derived stem cell(s) |
| PB-EPC(s) | = | peripheral blood-derived EPC(s) |
| PDGF | = | platelet derived growth factor |
| SMC(s) | = | smooth muscle cell(s) |
| SMPC(s) | = | smooth muscle progenitor cell(s) |
| TEVG(s) | = | tissue-engineered vascular graft(s) |
| VEGF | = | vascular endothelial growth factor |
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Adebayo O, Hookway TA, Hu JZ, Billiar KL, Rolle MW. 2013. Self-assembled smooth muscle cell tissue rings exhibit greater tensile strength than cell-seeded fibrin or collagen gel rings. J Biomed Mater Res A. 101:428–437.
- Arrigoni C, Chitto A, Mantero S, Remuzzi A. 2008. Rotating versus perfusion bioreactor for the culture of engineered vascular constructs based on hyaluronic acid. Biotechnol Bioeng. 100:988–997.
- Baguneid M, de Mel A, Yildirimer L, Fuller BJ, Hamilton G, Seifalian AM. 2011. In vivo study of a model tissue-engineered small-diameter vascular bypass graft. Biotechnol Appl Biochem. 58:14–24.
- Baguneid M, Murray D, Salacinski HJ, Fuller B, Hamilton G, Walker M, Seifalian AM. 2004. Shear-stress preconditioning and tissue-engineering-based paradigms for generating arterial substitutes. Biotechnol Appl Biochem. 39:151–157.
- Bajpai VK, Mistriotis P, Loh Y-H, Daley GQ, Andreadis ST. 2012. Functional vascular smooth muscle cells derived from human induced pluripotent stem cells via mesenchymal stem cell intermediates. Cardiovasc Res. 96:391–400.
- Bertanha M, Moroz A, Almeida R, Alves FC, Valério MJA, Moura R, et al. 2014. Tissue-engineered blood vessel substitute by reconstruction of endothelium using mesenchymal stem cells induced by platelet growth factors. J Vasc Surg. 59:1677–1685.
- Bourget J-M, Gauvin R, Duchesneau D, Remy M, Auger FA, Germain L. 2015. Potential of newborn and adult stem cells for the production of vascular constructs using the living tissue sheet approach. Biomed Res Int. 2015:168294.
- Bourget J-M, Laterreur V, Guillemette M, Gauvin R, Miville-Godin C, Mounier M, et al. 2013. Recent advances in the development of tissue-engineered vascular media made by self-assembly. Proc Eng. 59:201–205.
- Caplan AI. 2008. All MSCs are pericytes? Cell Stem Cell. 3:229–230.
- Caplan AI, Dennis JE. 2006. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 98:1076–1084.
- Chowdhury F, Na S, Li D, Poh Y-C, Tanaka TS, Wang F, Wang N. 2009. Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nat Mater. 9:82–88.
- Chung S, Sudo R, Vickerman V, Zervantonakis IK, Kamm RD. 2010. Microfluidic platforms for studies of angiogenesis, cell migration, and cell-cell interactions. Sixth International Bio-Fluid Mechanics Symposium and Workshop March 28-30, 2008 Pasadena, California. Ann Biomed Eng. 38:1164–1177.
- Cleary MA, Geiger E, Grady C, Best C, Naito Y, Breuer C. 2012. Vascular tissue engineering: the next generation. Trends Mol Med. 18:394–404.
- Covas DT, Panepucci RA, Fontes AM, Silva WA, Orellana MD, Freitas MC, et al. 2008. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp Hematol. 36:642–654.
- Dahl SL, Kypson AP, Lawson JH, Blum JL, Strader JT, Li Y, et al. 2011. Readily available tissue-engineered vascular grafts. Sci Transl Med. 3:68ra9.
- Fan H, Li S. 2015. Modeling universal dynamics of cell spreading on elastic substrates. Biomech Model Mechanobiol. 14:1265–1280.
- Fernandez CE, Achneck HE, Reichert WM, Truskey GA. 2014. Biological and engineering design considerations for vascular tissue engineered blood vessels (TEBVs). Curr Opin Chem Eng. 3:83–90.
- Gong Z, Niklason LE. 2008. Small-diameter human vessel wall engineered from bone marrow-derived mesenchymal stem cells (hMSCs). FASEB J. 22:1635–1648.
- Harris LJ, Abdollahi H, Zhang P, McIlhenny S, Tulenko TN, DiMuzio PJ. 2011. Differentiation of adult stem cells into smooth muscle for vascular tissue engineering. J Surg Res. 168:306–314.
- Hasan A, Memic A, Annabi N, Hossain M, Paul A, Dokmeci MR, Dehghani F, Khademhosseini A. 2014. Electrospun scaffolds for tissue engineering of vascular grafts. Acta Biomater. 10:11–25.
- Hashi CK, Zhu Y, Yang G-Y, Young WL, Hsiao BS, Wang K, Chu B, Li S. 2007. Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts. Proc Natl Acad Sci USA. 104:11915–11920.
- Hirai J, Matsuda T. 1996. Venous reconstruction using hybrid vascular tissue composed of vascular cells and collagen: tissue regeneration process. Cell Transplant. 5:93–105.
- Hu J, Wang Y, Jiao J, Liu Z, Zhao C, Zhou Z, et al. 2015. Patient-specific cardiovascular progenitor cells derived from integration-free induced pluripotent stem cells for vascular tissue regeneration. Biomaterials. 73:51–59.
- Iordache F, Constantinescu A, Andrei E, Curuţiu C, Grumezescu AM, Voicu G, Maniu H. 2014. In vitro cytocompatibility evaluation of collagen based scaffolds using human endothelial progenitor cells for vascular tissue engineering. Biomaterials. 1:10–16.
- Kamenskiy A, Pipinos II, MacTaggart JN, Kazmi SAJ, Dzenis YA. 2011. Comparative analysis of the biaxial mechanical behavior of carotid wall tissue and biological and synthetic materials used for carotid patch angioplasty. J Biomech Eng. 133:1–10.
- Kannan RY, Salacinski HJ, Edirisinghe MJ, Hamilton G, Seifalian AM. 2006. Polyhedral oligomeric silsequioxane-polyurethane nanocomposite microvessels for an artificial capillary bed. Biomaterials. 27:4618–4626.
- Karamariti E, Margariti A, Winkler B, Wang X, Hong X, Baban D, et al. 2013. Smooth muscle cells differentiated from reprogrammed embryonic lung fibroblasts through DKK3 signaling are potent for tissue engineering of vascular grafts. Circ Res. 112:1433–1443.
- Kassab GS. 2006. Biomechanics of the cardiovascular system: the aorta as an illustratory example. J R Soc Interface. 3:719–740.
- Kim T, Hwang W, Lee H, Kamm RD. 2009. Computational analysis of viscoelastic properties of crosslinked actin networks. PLoS Comput Biol. 5:e1000439.
- Kingham E, Oreffo ROC. 2013. Embryonic and induced pluripotent stem cells: understanding, creating, and exploiting the nano-niche for regenerative medicine. ACS Nano. 7:1867–1881.
- Krawiec JT, Vorp DA. 2012. Adult stem cell-based tissue engineered blood vessels: a review. Biomaterials. 33:3388–3400.
- L'Heureux N, Paquet S, Labbe R, Germain L, Auger FA. 1998. A completely biological tissue-engineered human blood vessel. FASEB J. 12:47–56.
- Liu JY, Swartz DD, Peng HF, Gugino SF, Russell JA, Andreadis ST. 2007. Functional tissue-engineered blood vessels from bone marrow progenitor cells. Cardiovasc Res. 75:618–628.
- Maul TM, Chew DW, Nieponice A, Vorp DA. 2011. Mechanical stimuli differentially control stem cell behavior: morphology, proliferation, and differentiation. Biomech Model Mechanobiol. 10:939–953.
- McIlhenny S, Zhang P, Tulenko T, Comeau J, Fernandez S, Policha A, et al. 2015. eNOS transfection of adipose-derived stem cells yields bioactive nitric oxide production and improved results in vascular tissue engineering. J Tissue Eng Regen Med. 9:1277–1285.
- Melero-Martin JM, Dudley AC. 2011. Concise review: vascular stem cells and tumor angiogenesis. Stem Cells. 29:163–168.
- Melero-Martin JM, Khan ZA, Picard A, Wu X, Paruchuri S, Bischoff J. 2007. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 109:4761–4768.
- Muraglia A, Cancedda R, Quarto R. 2000. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci. 113:1161–1166.
- Murray IR, West CC, Hardy WR, James AW, Park TS, Nguyen A, et al. 2014. Natural history of mesenchymal stem cells, from vessel walls to culture vessels. Cell Mol Life Sci. 71:1353–1374.
- Nakayama Y, Tsujinaka T. 2014. Acceleration of robust “biotube” vascular graft fabrication by in-body tissue architecture technology using a novel eosin Y-releasing mold. J Biomed Mater Res B Appl Biomater. 102:231–238.
- Nemeno-Guanzon JG, Lee S, Berg JR, Jo YH, Yeo JE, Nam BM, Koh Y-G, Lee JI. 2012. Trends in tissue engineering for blood vessels. Biomed Res Int. 2012:956345.
- Nieponice A, Soletti L, Guan J, Hong Y, Gharaibeh B, Maul TM, et al. 2010. In vivo assessment of a tissue-engineered vascular graft combining a biodegradable elastomeric scaffold and muscle-derived stem cells in a rat model. Tissue Eng A. 16:1215–1223.
- Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, Langer R. 1999. Functional arteries grown in vitro. Science. 284:489–493.
- Obbink-Huizer C, Oomens CWJ, Loerakker S, Foolen J, Bouten CVC, Baaijens FPT. 2014. Computational model predicts cell orientation in response to a range of mechanical stimuli. Biomech Model Mechanobiol. 13:227–236.
- Paredes B, Santana A, Arribas MI, Vicente‐Salar N, de Aza PN, Roche E, Such J, Reig JA. 2011. Phenotypic differences during the osteogenic differentiation of single cell‐derived clones isolated from human lipoaspirates. J Tissue Eng Regen Med. 5:589–599.
- Pati F, Jang J, Ha D-H, Kim SW, Rhie J-W, Shim J-H, Kim D-H, Cho D-W. 2014. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun. 5:3935.
- Polacheck WJ, Li R, Uzel SG, Kamm RD. 2013. Microfluidic platforms for mechanobiology. Lab Chip. 13:2252–2267.
- Red-Horse K, Ueno H, Weissman IL, Krasnow MA. 2010. Coronary arteries form by developmental reprogramming of venous cells. Nature. 464:549–553.
- Rocco KA, Maxfield MW, Best CA, Dean EW, Breuer CK. 2014. In vivo applications of electrospun tissue-engineered vascular grafts: a review. Tissue Eng Part B Rev. 20:628–640.
- Sartore S, Chiavegato A, Faggin E, Franch R, Puato M, Ausoni S, Pauletto P. 2001. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: from innocent bystander to active participant. Circ Res. 89:1111–1121.
- Seifu DG, Purnama A, Mequanint K, Mantovani D. 2013. Small-diameter vascular tissue engineering. Nat Rev Cardiol. 10:410–421.
- Solouk A, Cousins BG, Mirahmadi F, Mirzadeh H, Nadoushan MRJ, Shokrgozar MA, Seifalian AM. 2015. Biomimetic modified clinical-grade POSS-PCU nanocomposite polymer for bypass graft applications: a preliminary assessment of endothelial cell adhesion and haemocompatibility. Mater Sci Eng C. 46:400–408.
- Solouk A, Cousins BG, Mirzadeh H, Solati‐Hashtjin M, Najarian S, Seifalian AM. 2011a. Surface modification of POSS‐nanocomposite biomaterials using reactive oxygen plasma treatment for cardiovascular surgical implant applications. Biotechnol Appl Biochem. 58:147–161.
- Solouk A, Solati-Hashjin M, Najarian S, Mirzadeh H, Seifalian AM. 2011b. Optimization of acrylic acid grafting onto POSS-PCU nanocomposite using response surface methodology. Iran Polym J. 20:91–107.
- Song JW, Gu W, Futai N, Warner KA, Nor JE, Takayama S. 2005. Computer-controlled microcirculatory support system for endothelial cell culture and shearing. Anal Chem. 77:3993–3999.
- Sundaram S, One J, Siewert J, Teodosescu S, Zhao L, Dimitrievska S, et al. 2014. Tissue-engineered vascular grafts created from human induced pluripotent stem cells. Stem Cells. Transl Med. 3:1535.
- Tasoglu S, Diller E, Guven S, Sitti M, Demirci U. 2014. Untethered micro-robotic coding of three-dimensional material composition. Nat Commun. 5:3124.
- Thakrar RR, Patel VP, Hamilton G, Fuller BJ, Seifalian AM. 2006. Vitreous cryopreservation maintains the viscoelastic property of human vascular grafts. FASEB J. 20:874–881.
- Wang Z-y, Teoh SH, Johana NB, Chong MSK, Teo EY, Hong M-h, Chan JKY, San Thian E. 2014. Enhancing mesenchymal stem cell response using uniaxially stretched poly (ɛ-caprolactone) film micropatterns for vascular tissue engineering application. J Mater Chem B. 2:5898–5909.
- Weber B, Kehl D, Bleul U, Behr L, Sammut S, Frese L, et al. 2016. In vitro fabrication of autologous living tissue-engineered vascular grafts based on prenatally harvested ovine amniotic fluid-derived stem cells. J Tissue Eng Regen Med. 10:52–71.
- Weinberg CB, Bell E. 1986. A blood vessel model constructed from collagen and cultured vascular cells. Science. 231:397–400.
- WHO [Internet]. 2015. Cardiovascular diseases (CVDs), Fact Sheet N°317. Available from: http://www.who.int/mediacentre/factsheets/fs317/en/
- Wong ST, Teo S-K, Park S, Chiam K-H, Yim EKF. 2014. Anisotropic rigidity sensing on grating topography directs human mesenchymal stem cell elongation. Biomech Model Mechanobiol. 13:27–39.
- Wystrychowski W, McAllister TN, Zagalski K, Dusserre N, Cierpka L, L'Heureux N. 2014. First human use of an allogeneic tissue-engineered vascular graft for hemodialysis access. J Vasc Surg. 60:1353–1357.
- Yang Y, Kulangara K, Sia J, Wang L, Leong KW. 2011. Engineering of a microfluidic cell culture platform embedded with nanoscale features. Lab Chip. 11:1638–1646.
- Yeganeh H, Orang F, Solouk A, Rafienia M. 2008. Synthesis, characterization and preliminary investigation of blood compatibility of novel epoxy-modified polyurethane networks. J Bioact Compat Polym. 23:276–300.
- Zahedmanesh H, Lally C. 2012. A multiscale mechanobiological modelling framework using agent-based models and finite element analysis: application to vascular tissue engineering. Biomech Model Mechanobiol. 11:363–377.
- Zeng W, Wen C, Wu Y, Li L, Zhou Z, Mi J, et al. 2012. The use of BDNF to enhance the patency rate of small-diameter tissue-engineered blood vessels through stem cell homing mechanisms. Biomaterials. 33:473–484.
- Zhang H, Jia X, Han F, Zhao J, Zhao Y, Fan Y, Yuan X. 2013. Dual-delivery of VEGF and PDGF by double-layered electrospun membranes for blood vessel regeneration. Biomaterials. 34:2202–2212.
- Zhao Y, Zhang S, Zhou J, Wang J, Zhen M, Liu Y, Chen J, Qi Z. 2010. The development of a tissue-engineered artery using decellularized scaffold and autologous ovine mesenchymal stem cells. Biomaterials. 31:296–307.