Abstract
The aim of the present study is to develop embelin-loaded enteric-coated microspheres and investigate their pharmacological potential in acetic acid induced ulcerative colitis. The optimized formulation of embelin-loaded microspheres has shown significant sustained release of embelin. Further this formulation significantly reduced the ulcer activity score and oxidative stress, and attenuated the inflammatory changes. Thus it may be concluded that embelin-loaded enteric-coated microspheres have shown delayed release capacity than plain embelin and exerts colon ulcer protective effect in rats.
Introduction
The therapeutic strategy for treating inflammatory bowel disease is now focused on the use of anti-inflammatory agents (Xu et al. Citation2004). Glucocorticoids and aminosalicylate have been used for the treatment of inflammatory bowel disease, but their side effects remain a major clinical problem. Plant remedies play an important role in the therapy of many inflammatory disease conditions including inflammatory bowel disease (Mahgoub Citation2003, Medhi et al. Citation2008). A growing body of literature suggests that inflammatory bowel disease results from a deregulated immune response to normal bacterial antigens. This uncontrolled immune system activation results in the sustained overproduction of reactive metabolites of oxygen and nitrogen. Some of the intestinal and/or colonic injury and dysfunction observed in inflammatory bowel disease is due to elaboration of these reactive species. In many studies, it has been reported that antioxidants showed beneficial effects on experimental colitis. Quinone derivatives from plants are known to possess protective effects against colitis induced by acetic acid (Thippeswamy et al. Citation2011). Targeted drug delivery to colon via oral route is highly desirable for ameliorative localization and controlled release of drug, for the selective treatment of inflammatory bowel diseases such as ulcerative colitis (UC), Crohn’s disease, colon cancer, amoebiasis, etc. (Glavas-Dodov Citation2013).
The commonly used drugs for UC are sulfasalazine, mesalamine, olsalazide, balsalazide, budesonide, etc. But these drugs show various side effects such as diarrhea, nausea, vomiting, which result in maximum removal of drug from body. Therefore, it necessitates using drugs from herbal origin, which hardly shows any side effects. Recently, embelin is extensively documented to exert its pleotropic protective effects. It is commonly known as false black pepper or vidanga and is known for its anti-inflammatory (Chitra et al. Citation1994) and antioxidant activities (Joshi et al. Citation2007). Embelin has also been reported to inhibit NF-κB activation by blocking its signaling pathways, which further inhibits pro-inflammatory gene expression in inflamed mucosa (Ahn et al. Citation2007). Furthermore, Kumar et al. (Citation2011) proved that embelin ameliorates dextran sodium sulfate-induced colitis by suppressing the levels of pro-inflammatory cytokines, such as TNF-α, IL-1β, IL-6 in the colonic tissues.
Embelin (2,5-dihydroxy-3-undecyl-1,4-benzoquinone), a major constituent of Embelia ribes Burm., is a naturally occurring alkyl substituted hydroxyl benzoquinone. The plant is indicated in traditional medicine for the treatment of various diseases (Thippeswamy et al. Citation2011). The fruit is bitter in taste, used to treat fever, inflammatory diseases and a variety of gastrointestinal ailments for thousands of years (Gupta et al. Citation1976). Embelin is reported to possess anti-inflammatory, analgesic (Chitra et al. Citation1994), antioxidant (Joshi et al. Citation2007) and wound healing (Swamy et al. Citation2007) activities. It is also reported to impair the inflammatory signaling through inhibition of nuclear factor kappa B (NF-kappa B) activity (Ahn et al. Citation2007). It is reported to possess potent antioxidant (Joshi et al. Citation2007) and anti-inflammatory (Chitra et al. Citation1994) activities. Various animal models of experimental colitis to screen drugs effective against inflammatory bowel disease have been established and acetic acid-induced colitis is an animal model that mimics some of the acute inflammatory responses seen in UC (Gonzalez et al. Citation1999). Boreddy et al., has already reported the protective effect of embelin against acetic acid induced UC in rats. It has also been reported that embelin treatment significantly decreased clinical activity score, gross lesion score, percent affected area, and wet colon weight when compared to acetic induced colitis (Thippeswamy et al. Citation2011). Among these approaches, polymer-based microspheres are found to be a promising approach to increase drug stability by either encapsulating or dispersing drug in polymeric matrix. The drug released from microspheres by different mechanism depends on composition of polymer and its preparative techniques (Faisant et al. Citation2002). Eudragit S 100, a co-polymer of methacrylic acid and methyl methacrylate ester is a commonly used pH sensitive polymer for targeting drugs to intestine (Ibekwe et al. Citation2006). Although, it protects drug to dissolve in upper intestinal tract, due to its pH sensitive properties, it starts dissolving at pH >6. This lack of site specificity needs another hydrophobic polymer for sustained drug release (Davis et al. Citation1986). Ethyl cellulose is one of the best encapsulating hydrophobic delayed release polymers, which controls the release behavior of drug. Thus, by combining both pH-dependent and time-controlled polymer, site-specific drug delivery to colon can be easily achieved. It has also been reported that optimal particle size of carrier for improved localization in colonic region is the utmost importance and it should be in the range of 4–15 μm, in order to achieve excessive accumulation in inflamed region by internalization of macrophages (Glavas-Dodov Citation2013). It is possible by using multiparticulate drug delivery system in which blend of combined pH-dependent, i.e., Eudragit S 100 and delayed release, i.e., ethyl cellulose polymers has been used.
The aim of present study is to develop pH-dependent sustained-release embelin-loaded microspheres prepared by using combination of crosslinking technique and w/o emulsion solvent evaporation technique employing combined pH-dependent, i.e., Eudragit S 100 and delayed release, i.e., ethyl cellulose hydrophobic polymers for the treatment of UC.
Materials and methods
Materials
Embelin was purchased from Indo Fine Chemicals, Hillsborough Township, NJ. Eudragit S 100 was procured as free gift sample from Rohm Pharma, Darmstadt, Germany. Span 80 was purchased from Himedia Laboratories (Mumbai, India). Glutaraldehyde was purchased from CDH. Petroleum ether, ethyl cellulose, methanol, acetone, diethyl ether and liquid light paraffin were purchased from Loba Chemie, Mumbai, India. Dianisidine was purchased from Sigma (St. Louis, MO). Mesalamine was purchased from Sun Pharma (Sikkim, India). All other chemicals were of analytical grade.
Methods
Preparation of embelin containing microparticles
As per the studies of Thippeswamy et al. (Citation2011), we have taken 50 mg/kg of embelin. It was loaded in microspheres and prepared by using combined techniques of solvent evaporation and crosslinking method. In this method, polymer solution was prepared by dissolving ethyl cellulose in 5 ml methanol and Eudragit S 100 in 5 ml acetone. Embelin was also dissolved in methanolic solution using bath sonicator (Steryl Medi Equip System, 40050, Chennai). This polymeric methanolic solution was mixed with above polymeric acetone solution under magnetic stirring (Tarsons Product Pvt. Ltd., Kolkata, India). This drug polymer solution was taken in syringe and dropped into 20 ml of liquid light paraffin containing Span 80 (2% w/v) as an emulsifier. After complete dispersion of polymer–drug solution into continuous oil phase, stirring was continued for 15 min using mechanical stirrer at 1500 rpm and thereafter, 0.2 ml of glutaraldehyde was added into continuous phase. Simultaneously, 0.2 ml of NaOH (0.1 M) was added into the mixture. Stirring proceeded for 2 h until complete removal of solvent. Microspheres get hardened and were washed successively with petroleum ether to remove adhering oil. For the removal of crosslinking agent, microspheres were washed with buffer solution of pH 1.2. Microspheres were allowed to dry at room temperature and kept in desiccator until further use (Shahi et al. Citation2014). To assess the impact of varying manufacturing parameters on particle size, entrapment efficiency, and drug release, the basic protocol was modulated step-by-step.
Surface morphology and particle size analyses
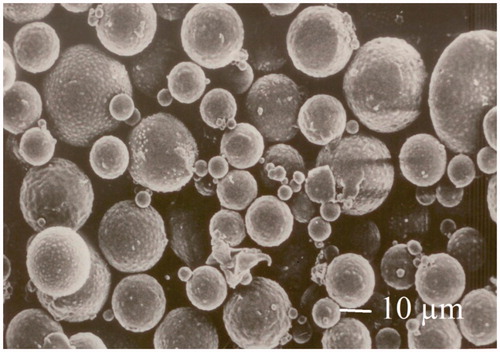
The particle size and surface morphology were analyzed by using motic and scanning electron microscope. The microspheres were dried and coated with gold palladium and placed under the atmosphere of argon for at least 150 s for getting thin 20 nm film. These coated microspheres were examined in scanning electron microscope (JEOL JSM-840, Tokyo, Japan) for surface morphology and particle size analyses.
Drug entrapment efficiency
The amount of embelin entrapped in microspheres was determined by centrifugating 12 ml of microparticle solution at 6000 rpm for 30 min in ultracentrifuge (Sigma Laborzentrifugen, SV Instruments Pvt. Ltd., Gurgaon, India). After 30 min, supernatant was collected and analyzed using UV-spectrophotometry (Perkin Elmer, 1900). Entrapment efficiency was calculated by using the following formula:
The entrapment efficiency of embelin was determined in triplicate for each formulation. The results were expressed as mean ± standard deviation.
In vitro drug release study
In vitro release study of microspheres was carried out using dialysis bag method. For this study, microspheres equivalent to 5 mg of embelin were measured and added to dialysis membrane-70 (Himedia, LA393-10MT). This dialysis membrane was taken into 50 ml of simulated gastric fluid of pH 1.2 in a conical flask for 2 h. After 2 h, this buffer was replaced with a fresh simulated intestinal buffer of pH 6.8 for small intestine. Thereafter, it was replaced with a buffer of pH 7.4 for colon. This flask was taken in an incubator shaker and the speed of shaker was maintained at 60 rpm at 37 ± 0.5 °C. At specific time intervals, samples (5 ml) were withdrawn and filtered. Same volume (5 ml) of the phosphate buffer pH 7.4 was replaced after each sampling. The drug content in the sample was determined spectrophotometrically at 292.37 nm (Perkin Elmer, Waltham, MA) (Rai et al. Citation2016) ().
In vitro release kinetics
Different release kinetic models were applied on release data in order to determine the drug release mechanism. The R2 values determine the mechanism of drug release. Greater the R2 values, more is the probability of determining release kinetics. Release kinetics was determined by using BCP software.
In vivo study
In vivo study was carried out as per the approved protocol by Institutional Animal Ethical Committee (IAEC) formed as per the norms of Committee for Prevention, Control and Supervision of Experiments on Animals (CPCSEA). Healthy wistar rats (180–200 g) were subjected to standard laboratory conditions (i.e., room temperature, 25 ± 2 °C; relative humidity, 55 ± 5%; 12/12 h light/dark cycles) with free access to commercial rodent diet and water.
Induction of ulcerative colitis
A solution of 1.5 ml of acetic acid (3%, v/v) in 0.9% saline was instilled into the lumen of the colon to a distance of 8 cm via rectal route with help of a polypropylene tube of 2 mm diameter and maintained in a supine trendelenburg position for 30 s to prevent the leakage of the intracolonic instillate. After 72 h of single dose administration of acetic acid (8th day), stool consistency and change in body weight were measured and the animals were anaesthetized with ether and sacrificed by cervical dislocation. The colon was dissected out. It was flushed gently with saline and weighed. It is used for macroscopic scoring, histopathological and biochemical estimations (Thippeswamy et al. Citation2011).
Evaluation of ulcerative colitis
Clinical activity score
The ulcer activity score was reported by Thippeswamy et al. (Citation2011). Colitis was quantified with a clinical score assessing weight loss, stool consistency and bleeding of the colon (measured by guaiac reaction, hemoccult) as described previously. No weight loss was counted as 0 point, weight loss of 1–5% as 1 point, 5–10% as 2 points, 10–20% as 3 points, and 20% as 4 points. For stool consistency, the stool was collected in a thick paper and observed, 0 points were given for well-formed pellets, 2 points for pasty and semiformed stools that did not stick to the anus, and 4 points for liquid stools that did stick to the anus. Bleeding was scored 0 points for no blood in hemoccult, 2 points for positive hemoccult, and 4 points for gross bleeding. These scores were added and divided by 3, forming a total clinical score that ranged from 0.0 (healthy) to 4.0 (maximal activity of colitis).
Macroscopic characters
The severity of colitis was evaluated by an independent observer who was blinded to the treatment. For each animal, the distal 10 cm portion of the colon was removed and cut longitudinally, slightly cleaned in physiological saline to remove fecal residues and weighed. Macroscopic inflammation scores were assigned based on clinical features of the colon using the following scoring pattern. No visible change was counted as 0 point, hyperemia at sites as 1 point, lesions having diameter l mmor or less counted as 2 points, lesions having diameter 2 mmor or less (number b5, 5–10 and N10) as 3, 4, and 5 points, respectively and lesions having diameter more than 2 mm (number b5, 5–10, N10) counted as 6, 7, and 8 points, respectively. Weight of rats was taken on first and last day, and then change in weight was calculated for all the groups according to the given formula.
Biochemical studies
Colon was homogenized in 10% (w/v) of ice-cold potassium phosphate buffer (pH 7.4) using Elvenjan homogenizer (Remi Motors Ltd., Mumbai, India) and the homogenate was used for the measurement of myeloperoxidase activity (MPO), lipid peroxidation, and reduced glutathione (GSH).
Colonic MPO activity
Colonic MPO activity was reported by Thippeswamy et al. (Citation2011). It has been calculated by using the following formula:
Colonic lipid peroxides concentration (LPO)
Lipid peroxide level was estimated according to the standard protocol reported by Ohkawa et al. Citation1979. The amount of lipid peroxide was determined by using the following formula:
Colonic glutathione level (GSH)
Glutathione level was estimated according to the standard protocol reported by Moron et al. (Citation1979). The amount of GSH was calculated by the following formula:
Histopathological study
Histopathological studies were done by the standard protocol of Thippeswamy et al. (Citation2011).
Experimental groups
Animals were divided into five groups. Each group consists of six animals. Group I normal control, Group II UC control rats (1.5 ml of acetic acid (3%, v/v) in 0.9% saline), Group III UC rats + embelin (50 mg/kg., p.o), Group IV UC rats + embelin microspheres (50 mg/kg., p.o), Group V UC rats + mesalamine (100 mg/kg., p.o). Acetic acid was administered to Groups II, III, IV, and V for induction of UC. On the 4th day of the treatment, the animals were fasted overnight with access to water and libitum. On the 5th day after 1 h of the aforementioned treatments, the animals (Groups II, III, IV, and V) were sacrificed and assessment of UC was done.
Statistical analyses
The values were expressed as mean ± SD. The statistical analysis was carried out by one-way ANOVA followed by comparison test of Bonferroni. P values <0.05 were considered as significant (Thippeswamy et al. Citation2011).
Results
Particle size analyses
Enteric-coated microsphere was prepared by using combined technique of solvent evaporation and crosslinking method. The microspheres were found to have spherical surface as shown in . The effect of formulation variables on entrapment efficiency, particle size, and drug release of embelin-loaded microspheres is shown in .
Table 1. Effect of formulation parameters on particle size and entrapment efficiency.
Effect of ethyl cellulose and Eudragit S 100 on particle size and entrapment efficiency
Polymer concentration has substantial impact on average diameter of prepared microspheres. With increase in polymer concentration, entrapment efficiency goes on decreasing. This is due to the fact that with increase in polymer concentration, viscosity also increases which leads to large solvent droplet, making it difficult for drug to diffuse inside droplet, which ultimately decreases entrapment efficiency.
Effect of amount of crosslinking agent on particle size and entrapment efficiency
Microspheres prepared by using different amounts of crosslinking agent were found to be fragile. Crosslinking of polymers with glutaraldehyde is very necessary for controlled drug delivery to colon. In the present study, with varying amounts of crosslinking agent from 0.1 to 0.3 ml, particle size of microspheres was also increased from 17 μm to 24.3 μm and entrapment efficiency also increases from 73.6 ± 4.4 to 81.2 ± 3.4% as given in . This is due to the formation of rigid network, which prevents the drug from leaching out during crosslinking of microspheres.
Effect of Span 80 on particle size analysis
On increasing Span 80 concentration from 0.5 to 2%, particle size of microspheres goes on decreasing, but to some extent. After this, it again goes on increasing. Optimized microsphere F11 formulation has shown size and entrapment efficiency 12.6 μm ± 2.1 size and 79.5 ± 2.3%, respectively, by using 2% span, 1:1:0.3 EC:ES 100 drug ratio and 0.2 ml crosslinking agent. This is due to the fact that low concentration of surfactant is unable to cover the entire organic droplet. Therefore on increasing Span 80 concentration, it covers the entire droplet surface and forms a surface coat over entire droplets, which help in the miscibility of continuous phase with dispersed phase and form a stable emulsion with relatively larger droplets.
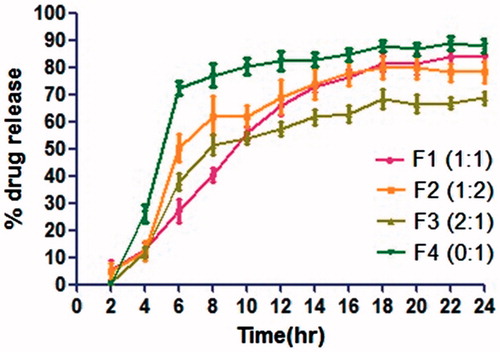
In vitro drug release
In vitro release study of microspheres was carried out using dialysis bag method. As shown in , it can be concluded that with increase in the concentration of hydrophobic polymer, % drug release was decreased after 24 h. F1 (EC:ES 100, 1:1 w/w) exhibited nearly 84.1% drug release within 24 h of incubation in dissolution medium compared to only 66.5% drug release in F2 (EC:ES 100, 2:1 w/w). There is a great impact of concentration of both the polymers in drug release. F3 also shows nearly same drug release as that of F1 within 24 h of incubation in dissolution medium. But during first 5 h, F3 shows nearly 49.4% of drug release as compared to F1 which shows only 19% of drug release. This is due to the fact that with increase in concentration of ethylcellulose, i.e., hydrophobic polymer, viscosity goes on increasing, which leads to large particle size and less surface area for drug to expose in release medium, which decreases drug release in the first 5 h. Thus, showed delayed release. Another reason might be decreasing the wettability of microspheres in medium due to increased concentration of hydrophobic polymer, i.e., ethyl cellulose around the drug. Thus, dissolution rate of drug decreases. While, with increase in concentration of Eudragit S 100, it creates pores around drug at pH 6.8 during first 5 h. Thus, on exposing it in release medium, medium diffuses inside particles and drug starts releasing faster from that pores. But due to the presence of ethyl cellulose, only 50% of drug was released during the first 5 h, and 82% of drug was released after 24 h. F4 showed 72.8% of drug release during the first 5 h in which no ethyl cellulose was incorporated. This can be explained by the fact that at pH 6.8, eudragit starts dissolving and whole drug was released from matrix due to absence of any delayed release polymer, i.e., ethyl cellulose. Thus, 90% of drug was released during 24 h. This type of formulation is suitable for targeting in small intestine.
In vitro release kinetics
The kinetics of embelin release from the prepared microspheres was studied by fitting the release data to zero order, first order, Peppas model, and Higuchi model. shows different drug release kinetic models.
Table 2. Drug release kinetics of different formulations.
It was found that F1 (1:1) shows both zero-order and first-order kinetics, which indicates that R2 value of F1 in case of zero order was found to be 0.9588 and in case of first order is 0.9961, which shows that it follows diffusion as well as dissolution mechanism. Eudragit S 100 polymer was dissolved at pH 6.8, where it shows first-order release and after dissolution, drug starts diffusing out of ethyl cellulose and follows the zero-order kinetics and provides sustained drug release.
In vivo study
Effect of embelin on ulcer activity scores
Acetic acid produces significant inflammation followed by ulcers in the colon assessed in terms of increase in scores for % weight change, stool consistency, lesion score, and macroscopic scores. Embelin-loaded microspheres (F11) has shown % weight change, stool consistency, lesion score, and macroscopic scores, 2 ± 0.03, 1 ± 0.05, 2 ± 0.05, and 2 ± 0.03, respectively, which are comparable with results obtained with marketed formulation ( and ). It can be concluded from above studies that embelin-loaded microspheres (F11) has significantly (P < 0.05) reduced the ulcer activities.
Table 3. Macroscopic scoring for evaluation of disease in different treatment groups.
Table 4: Scoring chart for the evaluation of disease.
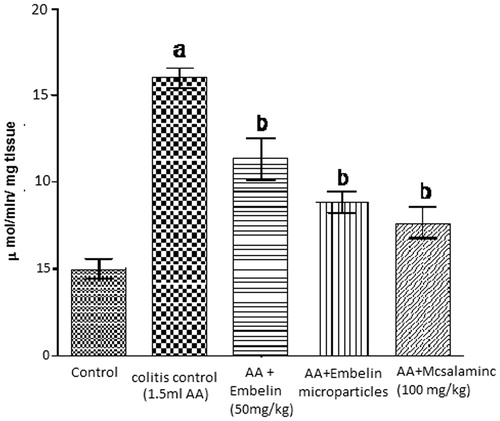
Effect of embelin on myeloperoxidase level in acetic acid induced colitis in rats
Intrarectal administration of acetic acid showed significant increase in the concentration of MPO, i.e., 16 μmol/min/mg tissue, while embelin-loaded microparticle (F11) shows significant decrease in the concentration of MPO, i.e., 8.8 μmol/min/mg as compared to disease control. Plain embelin (50 mg/kg) significantly reduced biochemical parameter (11.3 μmol/min/mg) as compared to disease control but less than embelin-loaded microspheres ().
Effect of embelin on lipid peroxidation in acetic acid induced colitis in rats
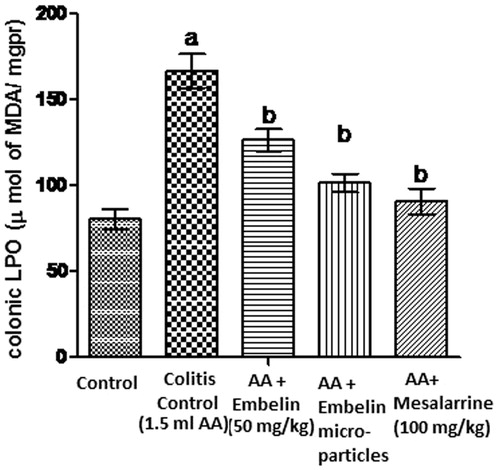
Lipid peroxidase is an enzyme that occurs in colonic tissue. Increased lipid peroxide generates more and more reactive free radicals, which exhausts cellular antioxidants and ultimately favors the consequent development of further inflammation and ulceration. It is assumed that embelin-loaded microsphere treatment improves colonic oxidative balance in colitis induced animals, because microspheres were able to reduce the level of malondialdehyde, a good indicator of lipid peroxidation.
Intrarectal administration of acetic acid showed significant increase in the concentration of LPO, i.e., 166.3 μmol of MDA/mg pr, while embelin-loaded microspheres show significantly decreased concentration of LPO, i.e., 101.33 μmol of MDA/mg pr as compared to disease control. Plain embelin (50 mg/kg) significantly reduced biochemical parameter (126.3 μmol of MDA/mg pr) as compared to disease control but less than embelin-loaded microparticles ().
Effect of embelin on reduced glutathione level in acetic acid induced colitis in rats
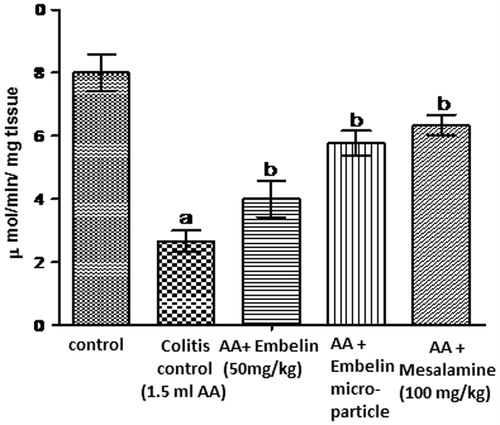
Pre-treatment of embelin-loaded microspheres reversed the depletion of GSH and restored the level towards normal. Intrarectal administration of acetic acid showed significant decrease in the concentration of GSH, i.e., 2.66 μmol of GSH/mg pr, while embelin-loaded microparticle shows significant increase in the concentration of GSH, i.e., 5.7 μmol of MDA/mg pr as compared to disease control. Plain embelin (50 mg/kg) significantly increased GSH (4 μ mol of GSH/mg pr) as compared to disease control but not as much potent as embelin-loaded microparticles. Standard drug mesalamine significantly increases GSH (6.3 μmol of GSH/mg pr) as compared to colitis control. shows the effect of embelin on GSH level in acetic acid induced colitis in rats.
Histopathological study
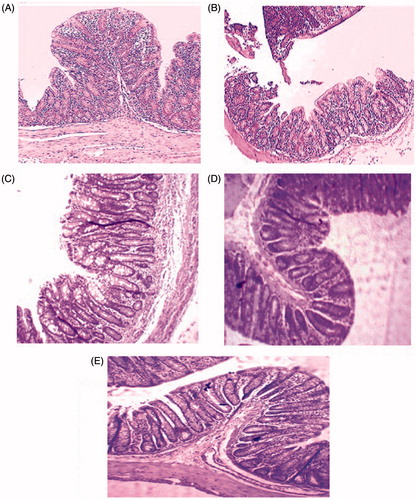
Embelin-loaded microspheres showed minimal damage of mucosa with slight submucosal edema and mild inflammatory cell infiltration. Embelin-loaded microspheres and standard drug, i.e., mesalamine showed remarkable recovery of colonic mucosa from acetic acid induced colitis damage. shows the histological image of colonic tissue of normal control group received CMC as vehicle. Normal control group shows papillary mucosa having a lining of columnar epithelium. The nucleus was placed in the basal portion and the cytoplasm fills the majority of the cell. The submucosa showed an infiltrate of lymphocytes while the deeper zone showed muscular layer. The case of acetic acid induced colitis group showed the massive destruction of epithelium, submucosal edema and inflammatory cell infiltration in submucosa and muscularis. Plain embelin at 50 mg/kg b.w. showed minimum damage of mucosa with submucosal edema and mild inflammatory cell infiltration. But embelin-loaded microspheres showed almost complete recovery of colonic mucosa from acetic acid induced colitis damage as compared to other groups. Thus, it has been concluded that embelin-loaded microspheres showed comparable results with standard drug, i.e., mesalamine, which also showed a healthy colonic epithelium as shown in .
Discussion
Acetic acid induced colitis is a model wherein inflammatory mediators such as reactive oxygen species, vasoactive amines, and eicosanoids play a prominent role (Carty et al. Citation2000). The underlying pathophysiological mechanisms involved include colon structure and mucosa barrier destruction by chemical stimulation, enhanced vessel permeability, increased inflammatory mediators, promotion of fibrin hydrolysis, and disturbance of cruor process.
Myeloperoxidase is an enzyme present in neutrophils and in a much lower concentration in monocytes and macrophages. The level of MPO activity is directly proportional to the neutrophil concentration in the inflamed tissue. Therefore, a measurement of MPO activity has been considered a quantitative and sensitive assay for acute intestinal inflammation. In addition, increased MPO activity has been reported to be an index of neutrophil infiltration and inflammation (Choudhary et al. Citation2001). Embelin at both the doses exhibited a significant decrease in the MPO levels when compared to acetic acid induced colitis control. Increased lipid peroxides that occur in colonic tissue can initiate a vicious cycle that generates more and more reactive metabolites, which exhausts cellular antioxidants, vitamins C and E, and ultimately favors the consequent development of further inflammation and ulceration. Embelin at both the doses significantly inhibited the increase in the lipid peroxides activity in the colonic tissue. It is therefore reasonable to assume that the embelin treatment improves colonic oxidative balance in colitis induced animals, because embelin was able to reduce the level of malondialdehyde, a good indicator of lipid peroxidation (Ohkawa et al. Citation1979). GSH is involved in the synthesis and repair of DNA, assists the recycling of vitamins C and E, blocks free radical damage, enhances the antioxidant activity of vitamin C, facilitates the transport of amino acids and plays a critical role in detoxification (Chavan et al. Citation2005). Pre-treatment of embelin reversed the depletion of GSH and restored the levels towards the normal. LDH, a cytosolic enzyme is involved in the biochemical regulation reaction of the body tissues and fluids. An elevation of LDH in serum indicates a shift towards anaerobiosis resulting in the enhanced production of lactic acid (Manna et al. Citation2004). In the present study, the embelin pre-treatment altered the serum LDH level induced by acetic acid towards the normal. The clinical, macroscopic, and biochemical evidence for the protective effect of embelin on acetic acid induced colitis in rats was well correlated by the histopathological studies. The histological science of inflammation such as leukocyte infiltration, edema and tissue injury was found to be low following the pre-treatment with embelin. The results obtained from embelin-treated acetic acid induced colitis in the present study are in well correlation with earlier results of its ability to inhibit carrageen, an induced paw edema in rats, inhibition of NF-kappa B activation, which makes it a potentially effective suppressor of tumor cell survival, proliferation, invasion, angiogenesis, and inflammation (Ahn et al. Citation2007, Chitra et al. Citation1994, Gupta et al. Citation1976). In addition, embelin is known to suppress the NF-kappa B activation induced by TNF-α and various other inflammatory and carcinogenic agents (Ahn et al. Citation2007). The results from the present study are also in consistent with the earlier study of its ability to scavenge physiologically relevant oxidizing radicals (Joshi et al. Citation2007). In the present study, combined pH-dependent and delayed release embelin-loaded microspheres were examined in vitro and in vivo to elucidate their gastrointestinal behavior. In order to improve the local targeting, it is essential to use a suitable polymeric carrier system, which is able to deliver the drugs specifically to inflamed region and avoid the gastrointestinal absorption as far as possible (Tozaki et al. Citation2002). With the advancement in novel formulation technology, the complete knowledge and understanding of cellular and molecular pathogenesis of UC along with microparticulate system could overcome the drawbacks of conventional therapy and provide efficacious delivery of newly developed drugs. At present, various anti-inflammatory drugs are available in market such as sulfasalazine, mesalamine, balsalazide, but their side effects always remain a major clinical problem. Therefore, present study emphasis on the use of embelin (2,5-dihydroxy-3-undecyl-1,4-benzoquinone) from herbal origin, which is reported to possess anti-inflammatory and antioxidant activities. Thus, embelin-loaded microspheres were developed by combining the concept of pH dependent and delayed release for targeting drugs specifically to inflamed local site of colon. In addition, particle size ranging 4–15 μm is optimum, in order to achieve large surface area and prolonged residence time in colon. Embelin-loaded microspheres (F1) showed average particle size of 13.5 μm ± 1.2, which was considered to be suitable for macrophages to accumulate particles in the inflamed region. Other important parameters were also optimized based on particle size and entrapment efficiency such as Span 80 and crosslinking agent. The surfactant as well as crosslinking agent play a major role in the particle size, entrapment efficiency, and release study. With increase in Span 80 concentration, particle size, and entrapment efficiency goes on decreasing. This was due to the reason that high concentration of surfactant forms a coating over entire organic droplet and gets miscible with continuous phase and forms stable emulsion, which decreases the particle size. Thus, because of small particle size, more micelle formation containing drug takes place and these micelles easily miscible with continuous phase causing increased drug diffusion, which ultimately reduces entrapment efficiency. But, due to the presence of ethyl cellulose, viscosity of medium increases, which increases particle size. The data indicated that increasing ethyl cellulose concentration resulted in decrease of entrapment efficiency. This was due to the reason that at high polymer concentration, larger droplets were formed resulting in the inability of drug to diffuse inside the particles and consequently, entrapment efficiency decreases. In addition, with increase in crosslinking agent, particle size as well as entrapment efficiency increases. This was due to the formation of rigid network in the medium, which prevents the drug from leaching out of polymer. In vitro study also concluded that F1 formulation showed both zero- and first-order releases. Very less amount of drug was released up to 2 h in the presence of simulated gastric fluid using pepsin (pH 1.2). This is due to the presence of pH-sensitive and time-controlled polymer, which prevents the drug from releasing into a gastric environment, but with increase in time, drug started releasing at pH 6 and followed first-order release but with further increase in time, it started showing zero-order release. Thus, controlled drug delivery was achieved, which is considered to be a promising approach for UC. The in vivo study concluded that pre-treatment of embelin prevents acetic acid-induced colitis in rats, and this effect is due to its antioxidant and anti-inflammatory actions.
Conclusion
This study has demonstrated that embelin-loaded microspheres (F1) formulation consisted of 1:1:0.3 w/w/w EC:ES100:embelin ratio using 2% Span 80 for stabilization could deliver drug specifically to colon. The drug release is pH- and time dependent. This approach produces time-dependent and pH-dependent sustained release as compared to other conventional dosage forms. The study examined the application of microparticulate system in enhancing stability and controlled release with brightening results concerning the therapeutic efficacy using blend of both pH-dependent and time controlled polymers.
Acknowledgements
The authors are thankful to Mr. Praveen Garg, Chairman, ISF College of Pharmacy, Moga, Punjab, for his continuous support and encouragement.
Disclosure statement
The authors have declared no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ahn KS, Sethi G, Aggarwal BB. 2007. Embelin, an inhibitor of X chromosome-linked inhibitor-of-apoptosis protein, blocks nuclear factor-κB (NF-κB) signaling pathway leading to suppression of NF-κB-regulated antiapoptotic and metastatic gene products. Mol Pharmacol. 71:209–219.
- Carty E, De Brabander M, Feakins R, Rampton D. 2000. Measurement of in vivo rectal mucosal cytokine and eicosanoid production in ulcerative colitis using filter paper. Gut. 46:487–492.
- Chavan S, Sava L, Saxena V, Pillai S, Sontakke A, Ingole D. 2005. Reduced glutathione: importance of specimen collection. Indian J Clin Biochem. 20:150–152.
- Chitra M, Sukumar E, Suja V, Devi CS. 1994. Antitumor, anti-inflammatory and analgesic property of embelin, a plant product. Chemotherapy. 40:109–113.
- Choudhary S, Keshavarzian A, Yong S, Wade M, Bocckino S, Day B, Banan A. 2001. Novel antioxidants zolimid and AEOL11201 ameliorate colitis in rats. Digest Dis Sci. 46:2222–2230.
- Davis S, Hardy J, Fara J. 1986. Transit of pharmaceutical dosage forms through the small intestine. Gut. 27:886–892.
- Faisant N, Siepmann J, Benoit J. 2002. PLGA-based microparticles: elucidation of mechanisms and a new, simple mathematical model quantifying drug release. Eur J Pharm Sci. 15:355–366.
- Glavas-Dodov M. 2013. Particulate carriers for local colon drug delivery. J Bioequiv Availab. 5:e25.
- Gonzalez R, Rodriguez S, Romay C, González A, Armesto J, Remirez D, Merino N. 1999. Anti-inflammatory activity of phycocyanin extract in acetic acid-induced colitis in rats. Pharmacol Res. 39:55–59.
- Gupta O, Ali MM, Ray GB, Atal C. 1976. Some pharmacological investigations of embelin and its semisynthetic derivatives. Indian J Physiol Pharmacol. 21:31–39.
- Ibekwe VC, Fadda HM, Parsons GE, Basit AW. 2006. A comparative in vitro assessment of the drug release performance of pH-responsive polymers for ileo-colonic delivery. Int J Pharm. 308:52–60.
- Joshi R, Kamat J, Mukherjee T. 2007. Free radical scavenging reactions and antioxidant activity of embelin: biochemical and pulse radiolytic studies. Chem-Biol Interact. 167:125–134.
- Kumar GK, Dhamotharan R, Kulkarni NM, Srinivasa H, Murugesan S. 2011. Embelin ameliorates dextran sodium sulfate-induced colitis in mice. Int Immunopharmacol. 11:724–731.
- Mahgoub AA. 2003. Thymoquinone protects against experimental colitis in rats. Toxicol Lett. 143:133–143.
- Manna S, Bhattacharyya D, Basak D, Mandal T. 2004. Single oral dose toxicity study of a-cypermethrin in rats. Indian J Pharmacol. 36:25.
- Medhi B, Prakash A, Avti P, Saikia U, Pandhi P, Khanduja K. 2008. Effect of Manuka honey and sulfasalazine in combination to promote antioxidant defense system in experimentally induced ulcerative colitis model in rats. Indian J Exp Biol. 46:583.
- Moron MS, Depierre JW, Mannervik B. 1979. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta (BBA) – Gen Subj. 582:67–78.
- Ohkawa H, Ohishi N, Yagi K. 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 95:351–358.
- Rai G, Yadav AK, Jain NK, Agrawal GP. 2016. Eudragit-coated dextran microspheres of 5-fluorouracil for site-specific delivery to colon. Drug Deliv. 23:328–337.
- Shahi A, Pandey B, Gopal R. 2014. PEG mediated solvothermal synthesis of fine ZnS sub-micro and microspheres and their optical properties. Mater Lett. 116:112–115.
- Swamy HK, Krishna V, Shankarmurthy K, Rahiman BA, Mankani K, Mahadevan K, Harish B, Naika HR. 2007. Wound healing activity of embelin isolated from the ethanol extract of leaves of Embelia ribes Burm. J Ethnopharmacol. 109:529–534.
- Thippeswamy BS, Mahendran S, Biradar MI, Raj P, Srivastava K, Badami S, Veerapur VP. 2011. Protective effect of embelin against acetic acid induced ulcerative colitis in rats. Eur J Pharmacol. 654:100–105.
- Tozaki H, Odoriba T, Okada N, Fujita T, Terabe A, Suzuki T, et al. 2002. Chitosan capsules for colon-specific drug delivery: enhanced localization of 5-aminosalicylic acid in the large intestine accelerates healing of TNBS-induced colitis in rats. J Control Release. 82:51–61.
- Xu CT, Meng SY, Pan BR. 2004. Drug therapy for ulcerative colitis. World J Gastroenterol. 10:2311–2317.